Abstract
Herpesviruses are capable of several types of infection of a host cell. To investigate the early events which ultimately determine the nature of the virus-host cell interaction, a system was established utilizing temperature-sensitive mutants of herpes simplex virus type 2. Four mutants have been isolated which fail to induce cytopathic effects and do not replicate at 39 C in hamster embryo fibroblast cells. At least one mutant is virus DNA negative. Since intracellular complementation is detectable between pairs of mutants, a virus function is known to be temperature sensitive. However, all four mutants induce cytopathic effects and replicate to parental virus levels in rabbit kidney cells at 39 C. This suggests that a host cell function, lacking or nonfunctional in HEF cells but present in rabbit kidney cells at 39 C, is required for the replication of these mutants in hamster embryo fibroblasts cells at 39 C. Therefore, we conclude that these mutants are both temperature sensitive and exhibit host range properties.
Full text
PDF

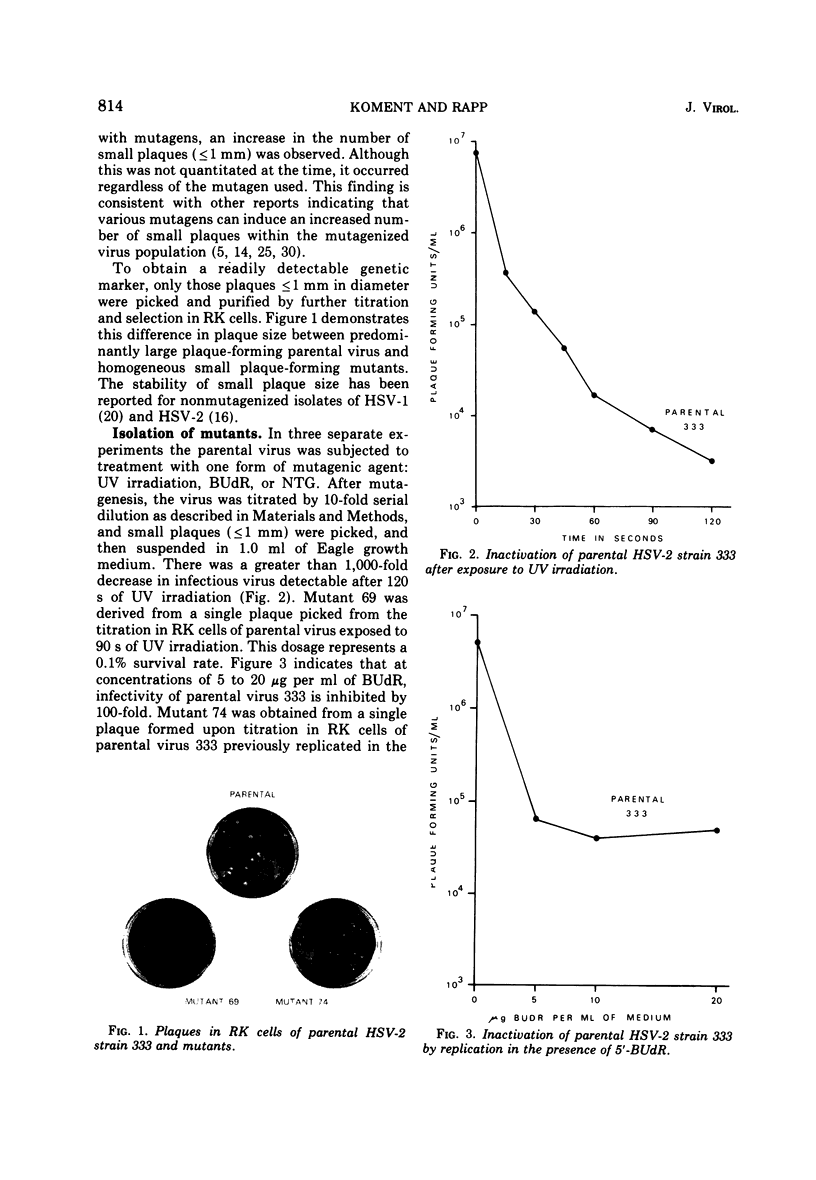
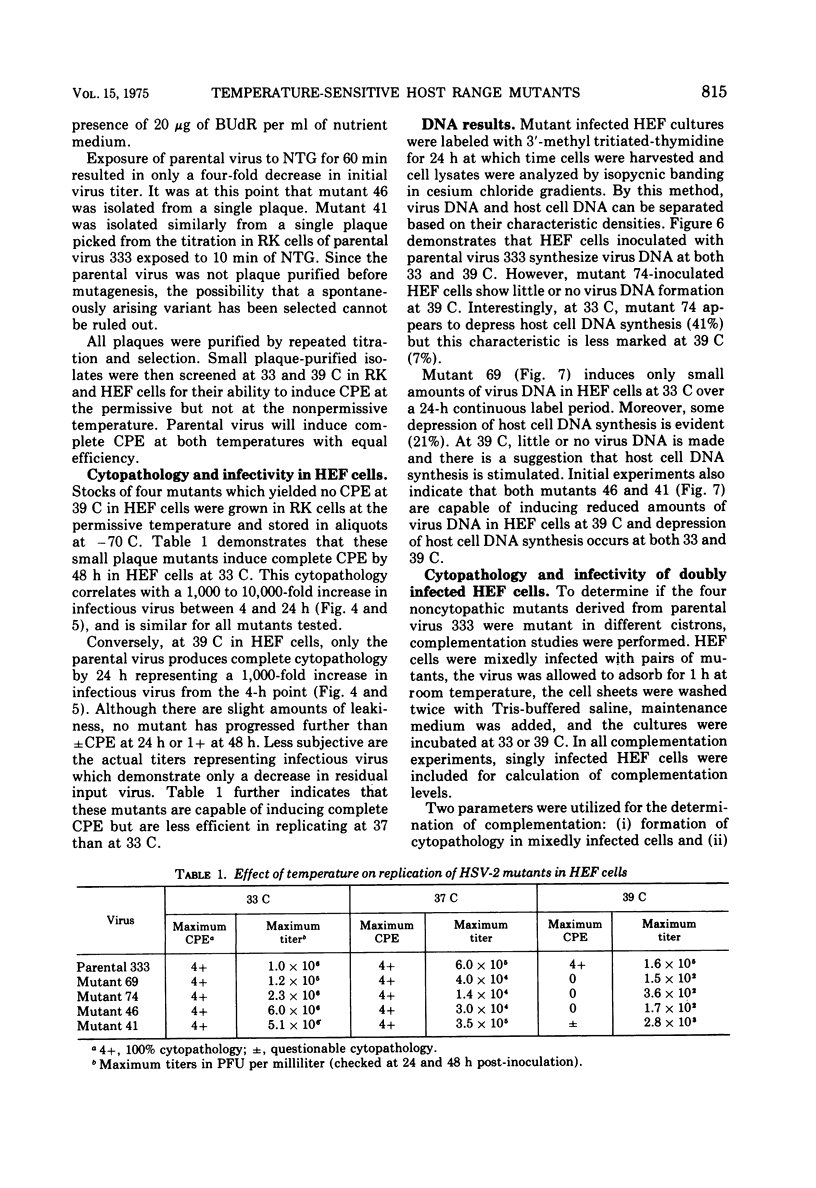
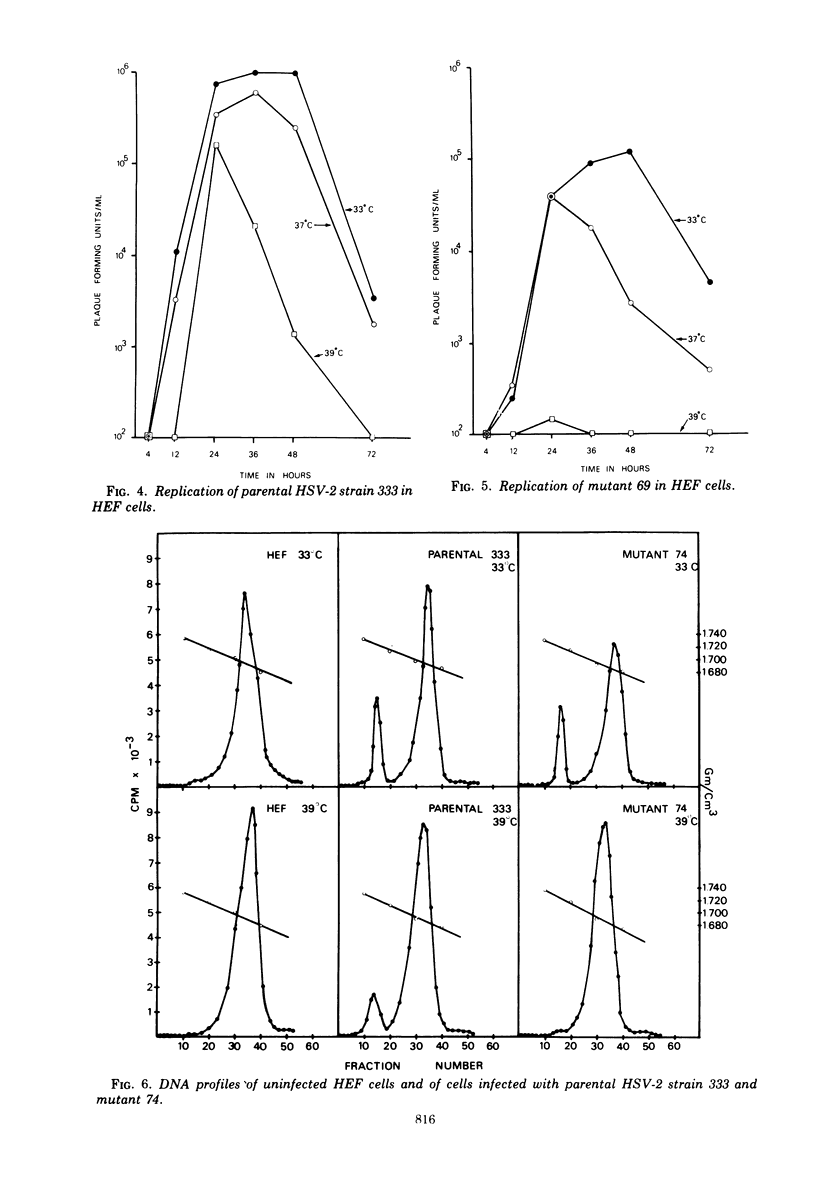
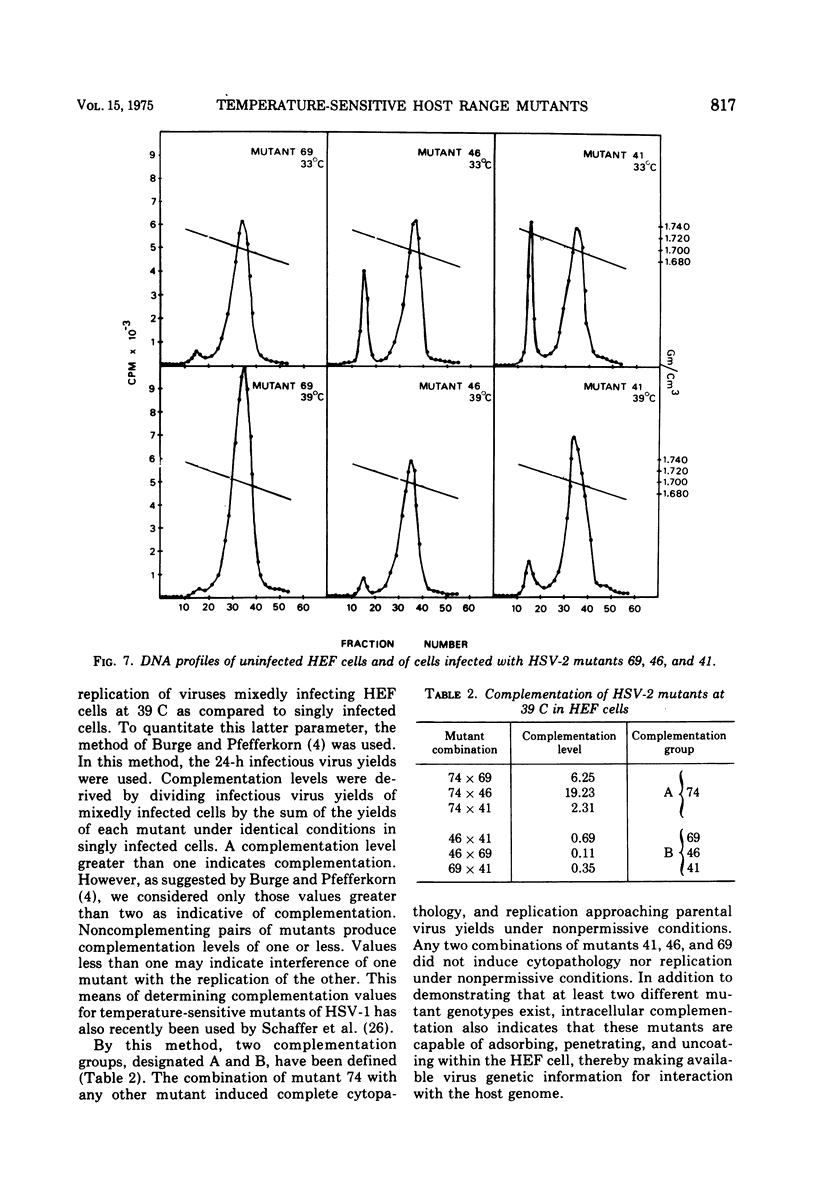


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baringer J. R., Swoveland P. Recovery of herpes-simplex virus from human trigeminal ganglions. N Engl J Med. 1973 Mar 29;288(13):648–650. doi: 10.1056/NEJM197303292881303. [DOI] [PubMed] [Google Scholar]
- Bastian F. O., Rabson A. S., Yee C. L., Tralka T. S. Herpesvirus hominis: isolation from human trigeminal ganglion. Science. 1972 Oct 20;178(4058):306–307. doi: 10.1126/science.178.4058.306. [DOI] [PubMed] [Google Scholar]
- Benda R., Cinátl J., Petrovic S., Roubal J., Plaisner V. Cultivation of ganglia on monofil fabric, a suitable method for demonstration of latent herpesvirus infection. Acta Virol. 1973 Jul;17(4):305–309. [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Complementation between temperature-sensitive mutants of Sindbis virus. Virology. 1966 Oct;30(2):214–223. doi: 10.1016/0042-6822(66)90097-3. [DOI] [PubMed] [Google Scholar]
- CARP R. I., KOPROWSKI H. Mutation of type 3 poliovirus with nitrous acid. Virology. 1962 May;17:99–109. doi: 10.1016/0042-6822(62)90086-7. [DOI] [PubMed] [Google Scholar]
- Darai G., Munk K. Human embryonic lung cells abortively infected with Herpes virus hominis type 2 show some properties of cell transformation. Nat New Biol. 1973 Feb 28;241(113):268–269. doi: 10.1038/newbio241268a0. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Oncogenic transformation of hamster cells after exposure to herpes simplex virus type 2. Nat New Biol. 1971 Sep 8;233(36):48–50. doi: 10.1038/newbio233048a0. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Properties of hamster embryo fibroblasts transformed in vitro after exposure to ultraviolet-irradiated herpes simplex virus type 2. J Virol. 1971 Oct;8(4):469–477. doi: 10.1128/jvi.8.4.469-477.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANOFF A. Induction of Newcastle disease virus mutants with nitrous acid. Virology. 1961 Apr;13:402–408. doi: 10.1016/0042-6822(61)90270-7. [DOI] [PubMed] [Google Scholar]
- Garfinkle B., McAuslan B. R. Non-cytopathic, non-productive infection by herpes simplex viruses types 1 and 2. Intervirology. 1973;1(5-6):362–375. doi: 10.1159/000148865. [DOI] [PubMed] [Google Scholar]
- Garfinkle B., McAuslan B. R. Transformation of cultured mammalian cells by viable herpes simplex virus subtypes 1 and 2. Proc Natl Acad Sci U S A. 1974 Jan;71(1):220–224. doi: 10.1073/pnas.71.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gönczöl E., Váczi L. Cytomegalovirus latency in cultured human cells. J Gen Virol. 1973 Feb;18(2):143–151. doi: 10.1099/0022-1317-18-2-143. [DOI] [PubMed] [Google Scholar]
- Kutinová L., Vonka V., Broucek J. Increased oncogenicity and synthesis of herpesvirus antigens in hamster cells exposed to herpes simplex type-2 virus. J Natl Cancer Inst. 1973 Mar;50(3):759–766. doi: 10.1093/jnci/50.3.759. [DOI] [PubMed] [Google Scholar]
- Munk K., Ludwig G. Properties of plaque variants of herpes virus hominis strains of genital origin. Arch Gesamte Virusforsch. 1972;37(4):308–315. doi: 10.1007/BF01241453. [DOI] [PubMed] [Google Scholar]
- Munyon W., Kraiselburd E., Davis D., Mann J. Transfer of thymidine kinase to thymidine kinaseless L cells by infection with ultraviolet-irradiated herpes simplex virus. J Virol. 1971 Jun;7(6):813–820. doi: 10.1128/jvi.7.6.813-820.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naib Z. M., Nahmias A. J., Josey W. E., Kramer J. H. Genital herpetic infection. Association with cervical dysplasia and carcinoma. Cancer. 1969 Apr;23(4):940–945. doi: 10.1002/1097-0142(196904)23:4<940::aid-cncr2820230432>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- O'Neill F. J., Goldberg R. J., Rapp F. Herpes simplex virus latency in cultured human cells following treatment with cytosine arabinoside. J Gen Virol. 1972 Feb;14(2):189–197. doi: 10.1099/0022-1317-14-2-189. [DOI] [PubMed] [Google Scholar]
- RAPP F. VARIANTS OF HERPES SIMPLEX VIRUS: ISOLATION, CHARACTERIZATION, AND FACTORS INFLUENCING PLAQUE FORMATION. J Bacteriol. 1963 Nov;86:985–991. doi: 10.1128/jb.86.5.985-991.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F., Duff R. Transformation of hamster embryo fibroblasts by herpes simplex viruses type 1 and type 2. Cancer Res. 1973 Jun;33(6):1527–1534. [PubMed] [Google Scholar]
- Rapp F., Li J. L., Jerkofsky M. Transformation of mammalian cells by DNA-containing viruses following photodynamic inactivation. Virology. 1973 Oct;55(2):339–346. doi: 10.1016/0042-6822(73)90173-6. [DOI] [PubMed] [Google Scholar]
- Ross L. J., Wildy P., Cameron K. R. Formation of small plaques by herpes viruses irradiated with ultraviolet light. Virology. 1971 Sep;45(3):808–812. doi: 10.1016/0042-6822(71)90201-7. [DOI] [PubMed] [Google Scholar]
- Schaffer P. A., Aron G. M., Biswal N., Benyesh-Melnick M. Temperature-sensitive mutants of herpes simplex virus type 1: isolation, complementation and partial characterization. Virology. 1973 Mar;52(1):57–71. doi: 10.1016/0042-6822(73)90398-x. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971 Aug 27;173(3999):843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Latent infections induced by herpes simplex viruses. Cancer Res. 1973 Jun;33(6):1399–1401. [PubMed] [Google Scholar]
- Stevens J. G., Nesburn A. B., Cook M. L. Latent herpes simplex virus from trigeminal ganglia of rabbits with recurrent eye infection. Nat New Biol. 1972 Feb 16;235(59):216–217. doi: 10.1038/newbio235216a0. [DOI] [PubMed] [Google Scholar]
- THIRY L. Chemical mutagenesis of Newcastle disease virus. Virology. 1963 Feb;19:225–236. doi: 10.1016/0042-6822(63)90012-6. [DOI] [PubMed] [Google Scholar]
- Walz M. A., Price R. W., Notkins A. L. Latent ganglionic infection with herpes simplex virus types 1 and 2: viral reactivation in vivo after neurectomy. Science. 1974 Jun 14;184(4142):1185–1187. doi: 10.1126/science.184.4142.1185. [DOI] [PubMed] [Google Scholar]




