Abstract
Background
Pulmonary arterial systolic pressure (PASP) can be estimated with transthoracic echocardiography. However, the significance of raised PASP on routine echocardiography is uncertain. In this study, we evaluated the mortality and hospitalization rates of subjects with raised PASP in a cohort of patients referred directly by their general practitioners for routine outpatient (open access) echocardiography for further analysis of suspected heart failure.
Results
A total of 485 subjects were referred for open access echocardiography at our hospital in 2002. A cohort of 209/485 (43%) consecutive subjects with measurable tricuspid regurgitation were followed for a minimum of five years investigating hospitalization rates and survival. Some 62 of 209 (30%) subjects had pulmonary hypertension (PH). Subjects with PH were significantly more likely to have four or more hospital admissions (22% vs. 8%; P < 0.01) and > 30 days of cumulative hospital stay over five years (29% vs. 13%; P < 0.01). PH was significantly associated with mortality (P = 0.003), while moderate to severe PH was an independent predictor of mortality (hazard ratio: 4.31; 95% confidence interval (95% CI): 1.51–12.30). Records from the Office of National Statistics revealed that subjects with PH were more likely to have chronic lung diseases recorded as immediate or contributory causes of death (50% vs. 14%; P < 0.05).
Conclusions
PASP ≥ 36 mmHg on routine echocardiography is associated with recurrent hospital admissions, prolonged hospitalizations and increased cause of mortality. Therefore, the diagnosis of PH on echocardiography deserves further clinical evaluation, with future studies designed at defining a suitable diagnostic strategy.
Keywords: Dyspnoea, Pulmonary hypertension, Transthoracic echocardiography, Chronic lung disease, Heart failure
1. Introduction
Pulmonary hypertension (PH) is not a disease, but a clinical condition for which there could be a multitude of attributable causes. The World Health Organization (WHO) classification divides PH into five categories: pulmonary arterial hypertension; PH with left heart disease; PH secondary to lung disease and chronic hypoxemia; chronic thromboembolic PH (CTEPH); and miscellaneous.[1] Pulmonary arterial hypertension, which includes both idiopathic PH and PH associated with connective tissue diseases, is rare with a prevalence of 15–50/million person years.[2],[3] The prevalence of CTEPH is 4%.[4] The prevalence of PH secondary to chronic lung disease, or left-sided heart conditions, is unknown, since patients with these conditions are not routinely screened for PH. Pulmonary hypertension and therefore is under detected.[5]
Pulmonary arterial systolic pressure (PASP) can be estimated with transthoracic echocardiography (TTE) by measuring the tricuspid regurgitant (TR) jet velocity. Echocardiographic measurements of PASP correlates well with values obtained with right heart catheterisation.[6] Right heart catheterisation may, however, still be required to confirm the diagnosis due to the low positive predictive value of TTE.[7] Nevertheless, TTE is now considered an essential screening tool for PH.[8] TTE is also widely used as an indispensable diagnostic test for left ventricular systolic dysfunction (LVSD). The presence of marginally raised PASP appears to be a common finding in individuals investigated with TTE for suspected heart failure by clinical observation. The significance of PH in this context has not been well characterized.[9] The finding of otherwise unexplained PH on TTE could, therefore, potentially lead to a series of additional investigations leading to mounting healthcare costs, increased patient burden, or exposure to invasive and potentially harmful tests. To ignore such a finding, on the other hand, feels counterintuitive to a clinician, as idiopathic PH is a deadly disease,[10] while secondary PH is often considered a negative prognostic marker for the underlying disorder.[11],[12]
We conducted a prospective study to quantify the prevalence of PH in patients undergoing TTE for suspected heart failure and to determine the morbidity and mortality associated with PH in these subjects over a five year period.
2. Methods
2.1. Subjects
From 1 January 2002 to 31 December 2002, consecutive patients referred by their general practitioners for open access echocardiography at Sunderland Royal Hospital, United Kingdom, a large general hospital, as an investigation for suspected heart failure were considered for the study. Subjects who did not have detectable TR jets were excluded. The echocardiography reports of all consecutive patients were reviewed for clinical and echocardiographic features.
2.2. Clinical features
Referring clinicians were required to indicate whether their patients had medical histories of myocardial infarction (MI), ischemic heart disease (IHD), chronic obstructive pulmonary disease (COPD), hypertension, cerebrovascular disease (CVD), peripheral vascular disease (PVD), diabetes and other relevant disorders. They were also asked to supply a complete list of their patients' current medication.
2.3. Transthoracic echocardiography
Transthoracic echocardiography was performed by three experienced sonographers using a HP echocardiography system (HPSONOS 55, Hewlett Packard®, USA). The peak TR velocity was measured using continuous wave Doppler echocardiography, and the transtricuspid pressure gradient (TTPG) was determined by applying the modified Bernoulli's equation (4V2). The PASP was then calculated by adding the estimated right atrial pressure (RAP) to TTPG (PASP = TTPG + RAP). Right atrial pressure was estimated as 5 mm Hg if the right atrial (RA) size is normal, 10 mmHg if RA appears dilated, or 15 mm Hg in the presence of massive RA dilatation. If present, LVSD was classified as mild [ejection fraction (EF) = 45%–54%], moderate (EF = 30%–44%) or severe impairment (EF ≤ 30%). Heart valve integrity and other structural heart abnormalities were assessed systematically and recorded. The presence of RA dilatation and right ventricular (RV) dilatation were determined by qualitative assessment. PH was defined as PASP ≥ 36 mmHg; with PASP ≥ 36 mmHg but < 50 mmHg considered mild PH; and PASP ≥ 50 mmHg considered moderate to severe PH.[7]
2.4. Hospitalization
All patients referred for echocardiography resided within the local geographical area with secondary care almost exclusively provided by one large hospital trust. The number of hospital episodes and total lengths of hospital stay could, therefore, be reliably determined using the hospital patient administration system.
2.5. Mortality
The hospital patient administration system and computerized hospital records were first examined to determine the survival of each subject. The survival of the remaining subjects were verified by contacting their referring primary care physicians. Copies of death certificates were obtained for deceased subjects. The hospital case notes of deceased subjects were examined whenever possible to verify causes of death.
2.6. Data analysis
All continuous variables were non-normally distributed and therefore expressed as medians with inter-quartile range (IQR) in parenthesis. Categorical variables were expressed as numbers with percentages. Comparisons were made using the Student's t-test for normally distributed, and the Mann-Whitney U-test for non-normally distributed continuous variables. The Chi-squared test was employed for categorical variables. Unadjusted odds ratios (OR) with 95% confidence intervals (CI) were determined for hospitalization and causes of death. A Kaplan-Meier survival curve was first obtained for mortality for individuals with, and without PH. A Cox proportional hazard multivariate analysis was then performed using a backward stepwise method to generate a predictor model for mortality. All statistical analyses were performed using the SPSS® 15.0 statistical software package.
Ethical approval had been obtained from the Sunderland Local Research Ethics Committee prior to commencement of the study.
3. Results
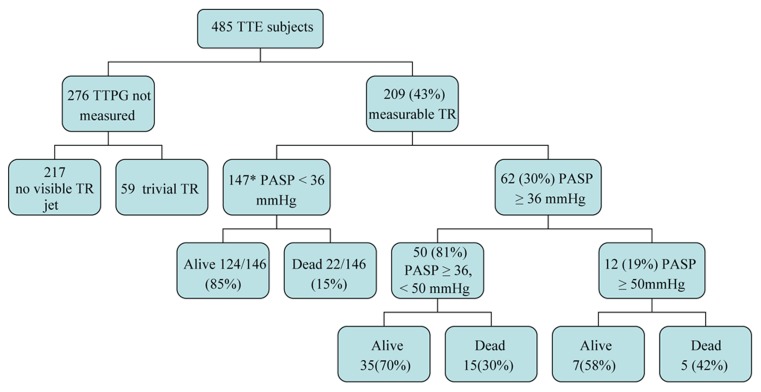
Four hundred and eighty-five patients were referred by their general practitioners for investigation with transthoracic echocardiography for suspected heart failure at Sunderland Royal Hospital, United Kingdom in the year 2002. Estimation of PASP (measurable TR) was possible in 209 of 485 (43%) patients. Subjects with measurable TR jets were older (P < 0.001), more likely to have dilated right heart chambers (P < 0.001), pulmonary regurgitation (P < 0.001), mitral regurgitation (P < 0.001) and aortic regurgitation (P < 0.001), (Table 1).
Table 1. Characteristics of subjects with and without measurable TR.
| Measureable TR | P value | ||
| Absent (n = 276) | Present (n = 209) | ||
| Age (Median (IQR)), yr(s) | 67 (58,74) | 71 (65,77) | < 0.001 |
| Male sex, n (%) | 117 (42) | 79 (38) | 0.361 |
| COPD, n (%) | 64 (23) | 35 (17) | 0.081 |
| Myocardial infarction, n (%) | 62 (22) | 38 (18) | 0.248 |
| Ischaemic heart disease, n (%) | 105 (38) | 77 (37) | 0.787 |
| Hypertension, n (%) | 132 (48) | 114 (54) | 0.143 |
| Right atrial dilatation, n (%) | 16 (6) | 54 (25) | < 0.001 |
| Right ventricular dilatation, n (%) | 13 (5) | 29 (14) | < 0.001 |
| Pulmonary regurgitation, n (%) | 18 (6) | 39 (19) | < 0.001 |
| Mitral regurgitation, n (%) | 88 (32) | 120 (57) | < 0.001 |
| Mitral stenosis, n (%) | 1 (0) | 6 (3) | 0.022 |
| Left ventricular systolic dysfunction, n (%) | 51 (18) | 49 (24) | 0.181 |
| Left ventricular diastolic dysfunction, n (%) | 23 (8) | 12 (6) | 0.275 |
| Aortic stenosis, n (%) | 41 (15) | 38 (18) | 0.326 |
| Aortic regurgitation, n (%) | 13 (5) | 28 (13) | 0.001 |
COPD: chronic obstructive pulmonary disease; IQR: inter-quartile range; TR: tricuspid regurgitation.
Sixty-two of the 209 subjects (30%) had PH defined as PASP ≥ 36 mmHg. Of these, fifty subjects (81%) had mild PH, and 12 (19%) had moderate or severe PH (Figure 1). Unadjusted OR suggest that PH was associated with increasing age (75 years vs. 71 years, P = 0.016); LVSD [OR: 2.16, 95% CI: 1.11–4.21]; mitral regurgitation (OR: 2.06, 95% CI: 1.10–3.86); right atrial dilatation (OR: 7.59, 95% CI: 3.82–15.10); right ventricular dilatation (OR: 4.25, 95% CI: 1.89–9.59); or the use of angiotensin converting enzyme (ACE) inhibitors (OR: 2.53, 95% CI: 1.33–4.81) (Table 2).
Figure 1. Outcomes for patients with measureable tricuspid regurgitation.
Flowchart demonstrating the echocardiographic outcomes of subjects investigated with routine outpatient echocardiography. *one subject untraceable. PASP: pulmonary arterial systolic pressure; TR: tricuspid regurgitation; TTE: transthoracic echocardiography; TTPG: transtricuspid pressure gradient.
Table 2. Clinical and echocardiographic features associated with PH.
| PH (n = 62) | No PH (n = 147) | OR (95% CI) | P value | |
| Age (Median (IQR)), yr(s) | 75 (69,78) | 71 (63,77) | 0.016 | |
| Male sex, n (%) | 23 (37) | 56 (38) | 0.96 (0.52–1.77) | 0.892 |
| Myocardial infarction, n (%) | 15 (24) | 23 (16) | 1.71 (0.82–3.55) | 0.150 |
| Ischemic Heart Disease, n (%) | 26 (42) | 51 (35) | 1.35 (0.73–2.47) | 0.339 |
| COPD, n (%) | 11 (18) | 24 (16) | 1.10 (0.50–2.42) | 0.802 |
| Hypertension, n (%) | 37 (60) | 77 (53) | 1.33 (0.73–2.42) | 0.358 |
| ACE-inhibitors, n (%) | 25 (40) | 31 (21) | 2.53 (1.33–4.81) | 0.004 |
| Diuretics, n (%) | 26 (42) | 62 (42) | 0.99 (0.54–1.81) | 0.974 |
| Other antihypertensives, n (%) | 29 (47) | 65 (44) | 1.11 (0.61–2.01) | 0.734 |
| Inhalers, n (%) | 9 (14) | 21 (14) | 1.02 (0.44–2.37) | 0.965 |
| LVSD, n (%) | 21 (34) | 28 (19) | 2.16 (1.11–4.21) | 0.022 |
| LVDD, n (%) | 2 (3) | 10 (7) | 0.45 (0.10–2.13) | 0.305 |
| Aortic stenosis, n (%) | 12 (19) | 26 (18) | 1.12 (0.52–2.39) | 0.775 |
| Aortic regurgitation, n (%) | 9 (14) | 19 (13) | 1.14 (0.49–2.69) | 0.758 |
| Mitral regurgitation, n (%) | 43 (69) | 77 (52) | 2.06 (1.10–3.86) | 0.023 |
| Mitral stenosis, n (%) | 3 (5) | 3 (2) | 2.44 (0.48–12.44) | 0.269 |
| Right atrial dilatation, n (%) | 34 (55) | 20 (14) | 7.59 (3.82–15.10) | < 0.001 |
| Right ventricular dilatation, n (%) | 17 (28) | 12 (8) | 4.25 (1.89–9.59) | < 0.001 |
| Pulmonary regurgitation, n (%) | 11 (18) | 28 (19) | 0.95 (0.44–2.05) | 0.888 |
ACE: angiotensin converting enzyme; IQR: inter-quartile range; LVDD: left ventricular diastolic dysfunction; LVSD: left ventricular systolic dysfunction; PH: pulmonary hypertension.
Hospitalization and mortality data were available for 208 subjects. The location of one subject could not be traced. Median hospitalization rate for individuals with no PH was one (IQR: 0–2), while individuals with PH had a median hospitalization rate of one (IQR: 0–3). The median total length of hospital stays for the two groups was 1.5 (IQR: 0–15) for the no PH group, compared to 8 (IQR: 0–44.25) for the PH group. When comparisons were made for severity of PASP, the median hospitalization rate and total hospital days for the mild PH group were 1 (IQR: 0–3) and 6.5 (IQR: 0–44.25) respectively, and the moderate to severe PH group were 2 (0.25–3) and 15 (IQR: 0.5–54.25) respectively. Of the 62 subjects with PH, 37 (60%) had experienced at least one hospital admission during the follow-up period, while 80 (55%) of the remainning 146 subjects without PH had been admitted to hospital. Fourteen of the sixty-two (22%) individuals with PH had four or more admissions compared to only 11/146 (8%) of individuals without PH (OR: 3.58, 95% CI: 1.52–8.42; P = 0.002). Eighteen (29%) subjects with PH and 19 (13%) of subjects with no PH had a cumulative length of hospital stay of over 30 days (OR: 2.73, 95% CI: 1.32–5.68; P = 0.006).
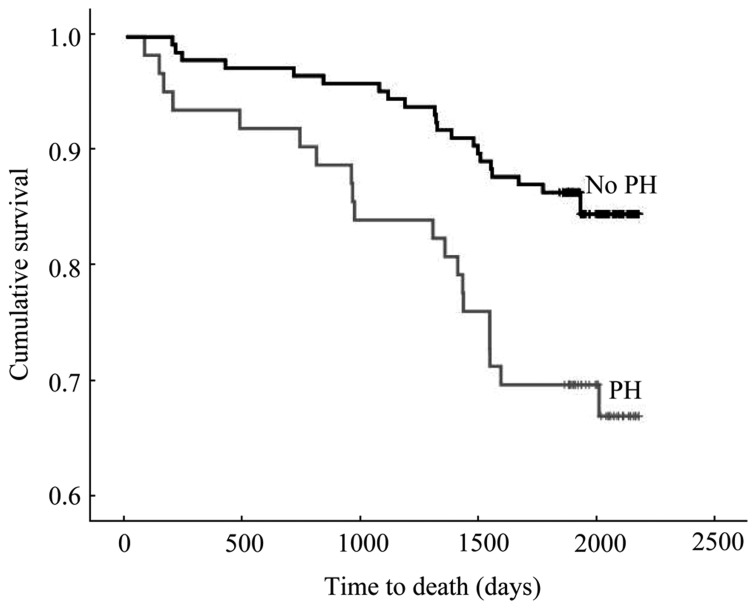
The Kaplan Meier survival plot in Figure 2 illustrates the mortality rate of subjects with PH, 20 (32%), compared to subjects without PH, 22 (15%), by the end of the median follow-up period of 5.5 years (inter-quartile range: 5.2–5.8, log rank: 8.70, P = 0.003). The survival plot in Figure 3 further illustrates the mortality rate of individuals with moderate to severe PH, mild PH and no PH (Figure 3). A stepwise Cox proportional hazards survival analysis suggested that the best predictor model for mortality consisted of PH (P = 0.032), age (P < 0.001), male sex (P = 0.012) and a reported history of hypertension (P = 0.098). If the presence of PH was subdivided into two categories of mild PH, or moderate to severe PH (P = 0.006), age (P < 0.001), COPD (P = 0.032) and previous myocardial infarction (P = 0.011) were independent predictors of mortality (Table 3). The above two models were generated using backward stepwise methods. When the presence of LVSD was then added by the method of forced entry, LVSD was not significantly associated with mortality [hazard ratio (HR): 1.035, 95% CI: 0.51–2.08; P = 0.924], while PH (HR: 2.36, 95% CI: 1.24–4.50; P = 0.009), male gender (HR: 0.034, 95% CI: 0.17–0.65; P = 0.001), mitral regurgitation (HR: 0.32, 95% CI: 0.11–0.97; P = 0.04), mitral stenosis (HR: 4.05, 95% CI: 0.92–7.64; P = 0.063) and age (HR: 1.13, 95% CI: 1.08–1.19; P < 0.001) remained relevant predictors for mortality.
Figure 2. Survival in individuals with and without PH.
Kaplan-Meier survival plot of individuals with PH compared to individuals without PH. Overall survival in individuals with PH was significantly lower (P = 0.003).
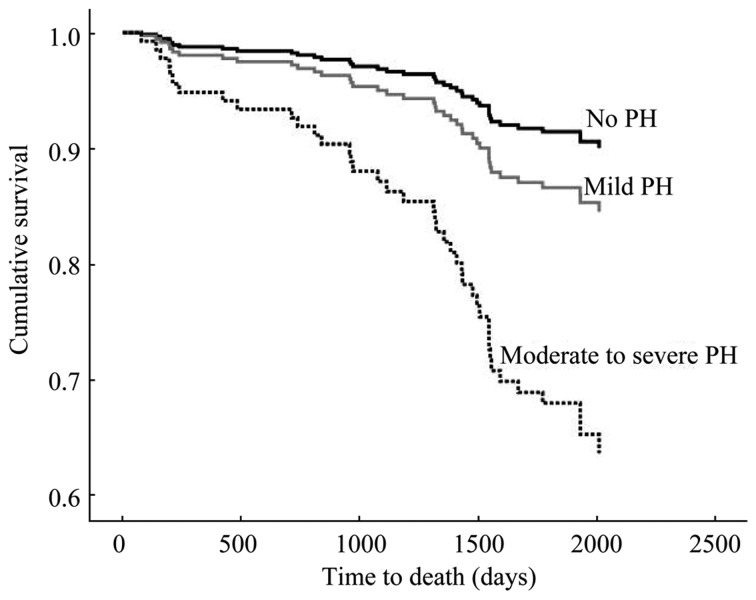
Figure 3. Survival plot for raised pulmonary arterial pressure by severity.
Cox regression survival plot for individuals with normal pulmonary arterial systolic pressure, mild pulmonary hypertension (PH), and moderate to severe PH, following adjustment for age, gender and presence of systemic hypertension. Moderate to severe PH was significantly associated with increased mortality (P = 0.006), while there was no significant difference in mortality between mild PH and no PH (P = 0.172).
Table 3. Cox regression models for mortality of all causes.
| Hazard ratio (95% CI) | P value | |
| Model 1 | ||
| Age* | 1.13 (1.08–1.18) | < 0.001 |
| Male sex | 2.23 (1.20–4.17) | 0.012 |
| Hypertension | 0.59 (0. 31–1.10) | 0.098 |
| PH | 1.98 (1.06–3.68) | 0.032 |
| Model 2# | ||
| Age* | 1.13 (1.08–1.19) | < 0.001 |
| COPD | 2.15 (1.07–4.33) | 0.032 |
| Myocardial infarction | 2.55 (1.07–5.25) | 0.011 |
| Mitral regurgitation | 0.48 (0.23–1.01) | 0.053 |
| Mild PH | 1.61 (0.82–3.17) | 0.172 |
| Moderate to severe PH | 4.31 (1.51–12.30) | 0.006 |
COPD: chronic obstructive pulmonary disease; PH: pulmonary hypertension. *per year increase; #PH divided into two subcategories of mild or moderate to severe.
Information on the causes of death derived from the Office of National Statistics Registry are listed in Table 4. Sixteen of the twenty deceased subjects (80%) who had PH on routine TTE experienced a cardiorespiratory death, compared to 12 (55%) of 22 deceased subjects without PH (OR: 3.33, 95% CI: 0.84–13.2; P = 0.081). Subjects with PH were more likely to have chronic lung diseases (OR: 6.33, 95% CI: 1.31–28.39) and COPD (OR: 5.38, 95% CI: 0.97–30.1) as immediate causes of death or conditions contributing towards death (Table 4). The other chronic lung diseases reported in death certificates included pulmonary fibrosis due to connective tissue disease in two cases, and one case each of cryptogenic fibrosing alveolitis, asbestosis and pneumoconiosis, respectively.
Table 4. Causes of death as recorded in death certificates.
| PH (n = 20) | No PH (n = 22) | OR (95% CI) | P value | ||
| Primary cause of death, n (%) | |||||
| Cardiac | 8 (40) | 4 (18) | 3.00 (0.74–12.23) | 0.118 | |
| Respiratory | 8 (40) | 8 (36) | 1.17 (0.33–4.06) | 0.808 | |
| Cerebrovascular accident | 2 (10) | 2 (9) | 1.11 (0.14–8.73) | 0.920 | |
| Malignancy | 2 (10) | 4 (18) | 0.50 (0.08–3.08) | 0.449 | |
| Others | 0 (0) | 4 (18) | - | 0.045 | |
| Conditions contributing to death, % | |||||
| Ischemic heart disease or myocardial infarction | 7 (35) | 6 (27) | 1.44 (0.39–5.34) | 0.588 | |
| Heart failure | 2 (10) | 4 (18) | 0.50 (0.08–3.08) | 0.449 | |
| Chronic lung disease | 10 (50) | 3 (14) | 6.33 (1.31–28.39) | 0.011 | |
| COPD | 7 (35) | 2 (9) | 5.38 (0.97–30.1) | 0.041 | |
| Other respiratory disorders | 4 (20) | 1 (5) | 5.25 (0.54–51.6) | 0.122 | |
COPD: chronic obstructive pulmonary disease; PH: pulmonary hypertension.
4. Discussion
PASP of 36 mmHg, or greater, was associated with recurrent hospitalization, prolonged hospital stay and increased mortality. Our subjects comprised predominantly of symptomatic elderly patients, mean age 71, for whom a rational approach to diagnosis is of vital importance, as they are less likely to tolerate multiple tests. There is also a potential for escalating costs arising from pursuing a number of abnormal initial tests. Our study, however, suggests that additional clinical assessments should be considered for individuals with a coincidental finding of increased PASP on routine TTE. In addition, moderate to severe PH was an independent predictor of mortality together with age, COPD and previous MI. While the latter finding should be interpreted with caution, as only a small number of subjects fell within the category of moderate to severe PH, the presence of a PASP of 50 mmHg, or greater, should add to the urgency of further investigations.
Our study evaluated long term survival in subjects with the incidental finding of PH on TTE. The subjects in our study consisted of ambulatory patients referred for outpatient echocardiography by their primary care physician for the symptoms of dyspnea and peripheral edema with, and without, other clinical features suggestive of heart failure. The non-specific clinical presentation of heart failure is also similar to that of PH and respiratory disorder, and can be clinically indistinguishable. Only sixteen percent of 254 patients referred to a similar open access echocardiography service reported by Francis, et al.[13] had LVSD confirmed by TTE. However, echocardiography led to important changes in management in 70% of their subjects.[13] Therefore, while at first glance, the diagnostic rate of LVSD of 20% in our subjects seems low, it actually emphasizes the importance of confirming the diagnosis of heart failure due to the non-specific nature of its presentation. A negative test is of equal significance as a positive test, to allow withdrawal of unnecessary treatment and to draw physicians towards alternative diagnoses, such as valvular heart disease, COPD or pulmonary fibrosis.
The estimation of PASP by TTE is dependent on the detection of a measurable TR jet. Transtricuspid pressure was estimated in 43% of our subjects. The rate of PASP estimation varies greatly between studies and is dependent on the conditions studied. Tricuspid regurgitation detection rates as low as 26% are reported in subjects with advanced COPD,[14] and as high as 87% are reported in cardiac patients.[15] The largest published study, involving 374 lung transplant candidates, reported an estimation rate of 44%, which was comparable to our study.[16] PASP is also more likely to be measurable and estimated in subjects with PH, whereas 64% of subjects with PH confirmed by right heart catheter had measurable TR compared to only 38% of subjects with no PH on right heart catheterisation.[16]
Thirty percent of our subjects with measureable TR jets had PH, defined according to the European Society of Cardiology Guidelines, as PASP ≥ 36 mmHg.[7] Twelve (19%) of the sixty two subjects had a PASP of greater than 50 mmHg, with the majority, 50 of 62 (81%), having only mild PH. PH in our cohort of subjects was associated with age, mitral regurgitation, LVSD, and (or) right heart chamber dilatation. Angiotensin converting enzyme inhibitors were also associated with PH in our study, but this may be confounded by the use of ACE-inhibitors as a treatment for heart failure, or hypertension. Finkelhor, et al.[17] reported that subjects with otherwise unexplained PH on TTE had significantly higher systolic blood pressure and were more likely to be taking antihypertensive medication than normal control subjects. Other studies have found a significant association between raised PASP with age, male sex, obesity, LVSD, diastolic dysfunction, mitral regurgitation and severity of airway obstruction.[18]–[20]
Chronic lung disease contributed significantly towards the higher mortality associated with PH in our study. Fifty percent of deaths in our cohort with PH were associated with chronic lung disease and 40% were associated with COPD, compared to 14% and 9%, respectively, in patients without PH. However, our subjects are likely to represent a more heterogenous group of subjects with multiple, potential causes of PH. Therefore, while the order of examinations to be undertaken in subjects with incidental PH on echocardiography remains unclear, screening for respiratory disorders should be featured prominently in any diagnostic strategy for raised PASP on TTE. In a study involving patients with established heart disease, increased TR gradient was associated with a significant increase in heart failure hospitalizations and cardiovascular mortality.[21] Our study differs in the absence of pre-existing heart disease, and the majority of subjects were not known to have heart disease, or LVSD.
The wider applicability of this study is limited by the exclusion of subjects without measureable TR. We were unable to make any comparisons based on PASP measurements for individuals without measureable TR who were younger, and less likely to have right heart chamber dilaation and valvular heart diseases with no differences in clinical features compared to subjects with measureable TR. PH was significantly associated with right heart chamber dilatation in our study and in a previous study which also showed that PH is less likely to occur in subjects in whom PASP could not be estimated.[16] Therefore, we anticipate that the mortality rate of subjects without measurable TR is likely to be no higher, if not lower, than subjects with measurable TR. This relationship would be a topic for future studies. The findings of our study is also only applicable to the individuals referred for further investigations having presented with the non-specific symptoms of dyspnea to their general practitioners, which is a common occurrence in clinical practice. The pulmonary arterial pressure obtained by TTE in our study was not verified with right heart catheterisation. While we accept that PASP measured on TTE can be inaccurate for individuals, it was difficult to justify the exposure of subjects with normal PASP or mild PH of uncertain significance to an invasive and potentially harmful test.
In conclusion, PH was associated with increasing age, mitral regurgitation, LVSD, recurrent hospital admissions, prolonged hospitalisation and increased mortality in patients investigated for suspected heart failure. Moderate to severe PH was an independent predictor of mortality in a Cox proportional hazard model. The excess mortality due to PH is associated with chronic respiratory disorders among other causes. The finding of raised PASP on TTE should therefore not be trivialized, and further clinical evaluations are justified.
Acknowledgments
The Significance of Pulmonary Hypertension in Echocardiography (SOPHIE) study was funded by a Specialist Registrar Start-Up Grant from the British Geriatrics Society. We are grateful to all the staff of the Medical Ambulatory Care Unit and Echocardiography Department at the Sunderland Royal Hospital for providing administrative and technical support. Special thanks to Mrs Sue Berry for data support.
References
- 1.Simonneau G, Galie N, Rubin LJ, et al. Clinical Classifical of Pulmonary Hypertension. J Am Coll Cardiol. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 2.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 3.Peacock AJ, Murphy NF, McMurray JJ, et al. An epidemiological study of pulmonary arterial hypertension. Eur Respir J. 2007;30:104–109. doi: 10.1183/09031936.00092306. [DOI] [PubMed] [Google Scholar]
- 4.Tapson VF, Humbert M. Incidence and prevalence of chronic thromboembolic pulmonary hypertension: from acute to chronic pulmonary embolism. Proc Am Thorac Soc. 2006;3:564–567. doi: 10.1513/pats.200605-112LR. [DOI] [PubMed] [Google Scholar]
- 5.Humbert M. The burden of pulmonary hypertension. Eur Respir J. 2007;30:1–2. doi: 10.1183/09031936.00055407. [DOI] [PubMed] [Google Scholar]
- 6.Skjaerpe T, Hatle L. Noninvasive estimation of systolic pressure in the right ventricle in patients with tricuspid regurgitation. Eur Heart J. 1986;7:704–710. doi: 10.1093/oxfordjournals.eurheartj.a062126. [DOI] [PubMed] [Google Scholar]
- 7.Galie N, Torbicki A, Barst R, et al. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The task force on diagnosis and treatment of pulmonary arterial hypertension of the European Society of Cardiology. Eur Heart J. 2004;25:2243–2278. doi: 10.1016/j.ehj.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Bossone E, Citro R, Blasi F, et al. Echocardiography in pulmonary arterial hypertension: An essential tool. Chest. 2007;131:339–341. doi: 10.1378/chest.06-2475. [DOI] [PubMed] [Google Scholar]
- 9.Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:40S–47S. doi: 10.1016/j.jacc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 10.Carrington M, Murphy NF, Strange G, et al. Prognostic impact of pulmonary arterial hypertension: a population-based analysis. Int J Cardiol. 2008;124:183–187. doi: 10.1016/j.ijcard.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 11.Trad S, Amoura Z, Beigelman C, et al. Pulmonary arterial hypertension is a major mortality factor in diffuse systemic sclerosis, independent of interstitial lung disease. Arthritis Rheum. 2006;54:184–191. doi: 10.1002/art.21538. [DOI] [PubMed] [Google Scholar]
- 12.Ghio S, Gavazzi A, Campana C, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 13.Francis CM, Caruana L, Kearney P, et al. Open access echocardiography in management of heart failure in the community. BMJ. 1995;310:634–636. doi: 10.1136/bmj.310.6980.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bach DS, Curtis JL, Christensen PJ, et al. Preoperative echocardiographic evaluation of patients referred for lung volume reduction surgery. Chest. 1998;114:972–980. doi: 10.1378/chest.114.4.972. [DOI] [PubMed] [Google Scholar]
- 15.Currie PJ, Seward JB, Chan KL, et al. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985;6:750–756. doi: 10.1016/s0735-1097(85)80477-0. [DOI] [PubMed] [Google Scholar]
- 16.Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167:735–740. doi: 10.1164/rccm.200210-1130OC. [DOI] [PubMed] [Google Scholar]
- 17.Finkelhor RS, Yang SX, Bosich G, et al. Unexplained pulmonary hypertension is associated with systolic arterial hypertension in patients undergoing routine Doppler echocardiography. Chest. 2003;123:711–715. doi: 10.1378/chest.123.3.711. [DOI] [PubMed] [Google Scholar]
- 18.Enriquez-Sarano M, Rossi A, Seward JB, et al. Determinants of pulmonary hypertension in left ventricular dysfunction. J Am Coll Cardiol. 1997;29:153–159. doi: 10.1016/s0735-1097(96)00436-6. [DOI] [PubMed] [Google Scholar]
- 19.Higham MA, Dawson D, Joshi J, et al. Utility of echocardiography in assessment of pulmonary hypertension secondary to COPD. Eur Respir J. 2001;17:350–355. doi: 10.1183/09031936.01.17303500. [DOI] [PubMed] [Google Scholar]
- 20.McQuillan BM, Picard MH, Leavitt M, et al. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects.[see comment] Circulation. 2001;104:2797–2802. doi: 10.1161/hc4801.100076. [DOI] [PubMed] [Google Scholar]
- 21.Ristow B, Ali S, Ren X, et al. Elevated pulmonary artery pressure by Doppler echocardiography predicts hospitalization for heart failure and mortality in ambulatory stable coronary artery disease: the Heart and Soul Study. J Am Coll Cardiol. 2007;49:43–49. doi: 10.1016/j.jacc.2006.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]