Abstract
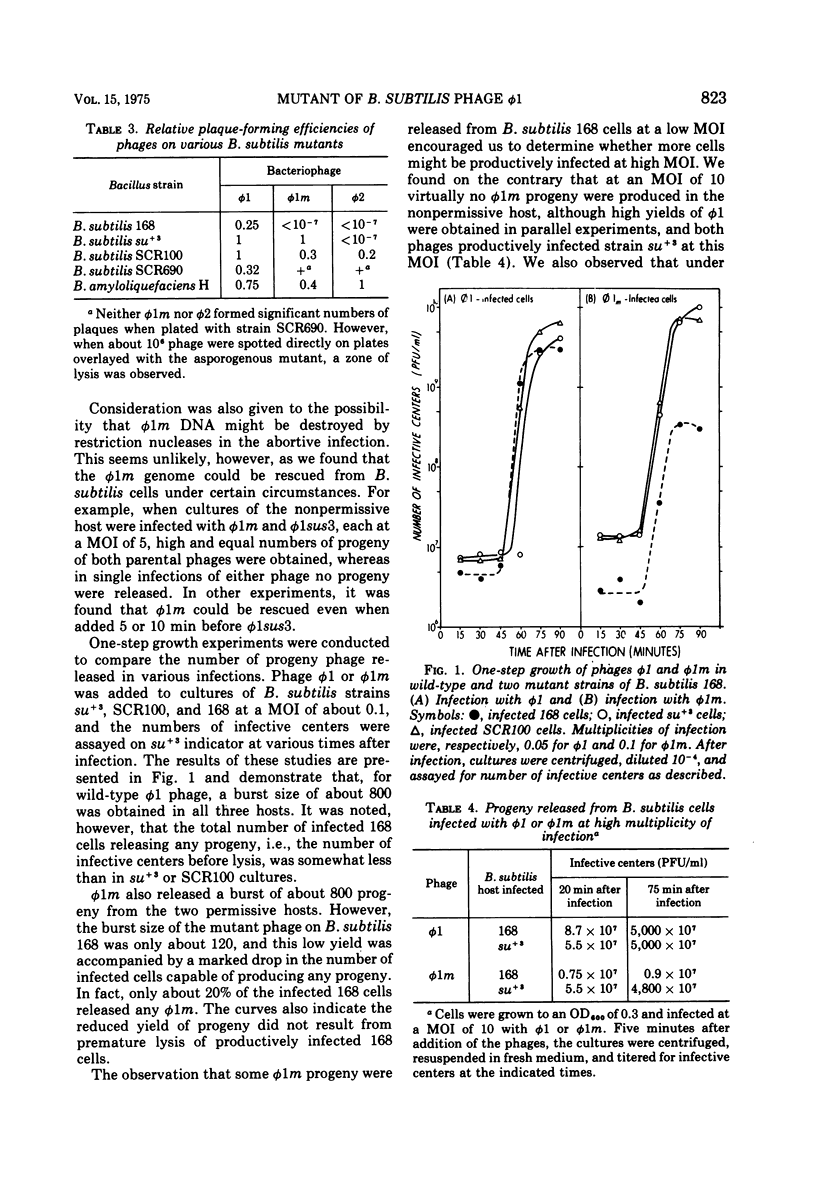
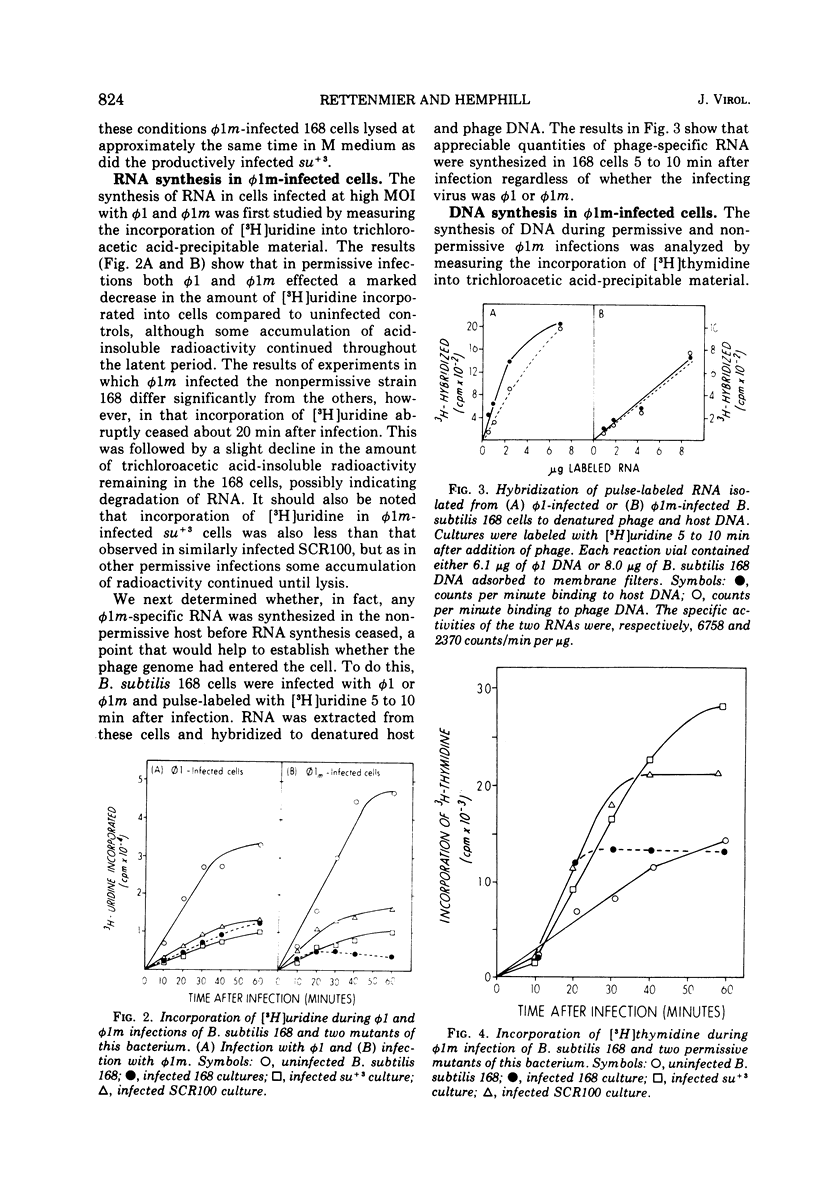
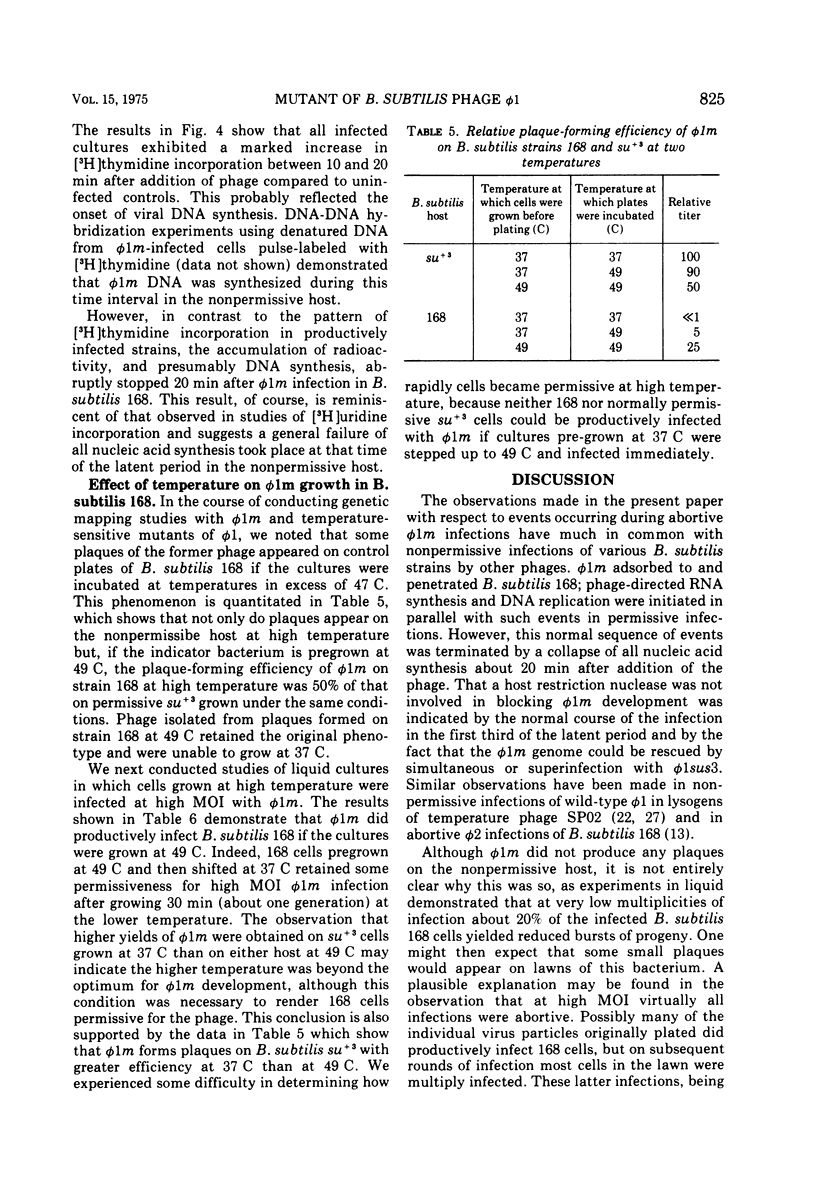
Bacillus subtilis bacteriophage phi 1m, a host-range variant, was isolated after mutagenesis of virulent bacteriophage phi 1. Unlike its wild-type antecedent, phi 1m could not form plaques on lawns of B subtilis 168 at 37 C, although it adsorbed to, penetrated, and killed this bacterium. Experiments conducted in liquid medium at 37 C showed that B. subtilis 168 cells allowed reduced levels of phi 1m development at low multiplicities of infection, whereas high multiplicity infections of this strain by the phage were abortive. Certain mutants, derived originally from B. subtilis 168, were observed to be permissive for phi 1m at 37 C; moreover, their permissive phenotype could be duplicated by growing wild-type B. subtilis 168 cells at temperatures above 47 C. Studies on phi 1m and host nucleic acid synthesis under nonpermissive conditions demonstrated that transciption and DNA synthesis proceeded up to 20 min after infection, after which time there was a cessation of all nucleic acid production. These observations are discussed with respect to other abortive bacteriophage infections in B. subtilis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baptist J. N., Tevethia M. J., Mandel M., Shaw C. R. Altered proteins with triosephosphate isomerase activity in suppressor-containing strains of Bacillus subtilis. J Bacteriol. 1974 Sep;119(3):976–985. doi: 10.1128/jb.119.3.976-985.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm S. P., Staal S. P., Hoch J. A. Phenotypes of pleiotropic-negative sporulation mutants of Bacillus subtilis. J Bacteriol. 1973 Sep;115(3):1063–1070. doi: 10.1128/jb.115.3.1063-1070.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho A., Moreno F., Carrascosa J. L., Viñuela E., Salas M. A suppressor of nonsense mutations in Bacillus subtilis. Eur J Biochem. 1974 Aug 15;47(1):199–205. doi: 10.1111/j.1432-1033.1974.tb03683.x. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P. Suppressor system in Bacillus subtilis 168. J Bacteriol. 1969 Mar;97(3):1397–1402. doi: 10.1128/jb.97.3.1397-1402.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Goldberg I. D., Bryan T. Productive infection of Bacillus subtilis 168, with bacteriophage SP-10, dependent upon inducing treatments. J Virol. 1968 Aug;2(8):805–812. doi: 10.21236/ad0686354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn D. D., Lawton W. D. Alteration of host specificity in Bacillus subtilis. Bacteriol Rev. 1968 Dec;32(4 Pt 1):297–301. [PMC free article] [PubMed] [Google Scholar]
- Havender W. R., Trautner T. A. Genetic and transfection studies with B. subtilis phage SP50. II. Temperature sensitive mutants and the establishment of a linkage map. Mol Gen Genet. 1970;108(1):61–69. doi: 10.1007/BF00343185. [DOI] [PubMed] [Google Scholar]
- Hemphill H. E., Whiteley H. R. Nucleic acid synthesis in Bacillus subtilis infected with bacteriophage beta-22. J Virol. 1970 Oct;6(4):381–392. doi: 10.1128/jvi.6.4.381-392.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Meinke W., Hathaway G., Spizizen J. Studies on Bacillus subtilis bacteriophage phi 15. Virology. 1973 Nov;56(1):110–122. doi: 10.1016/0042-6822(73)90291-2. [DOI] [PubMed] [Google Scholar]
- Ito J. Pleiotropic nature of bacteriophage tolerant mutants obtained in early-blocked asporogenous mutants of Bacillus subtilis 168. Mol Gen Genet. 1973 Aug 10;124(2):97–106. doi: 10.1007/BF00265143. [DOI] [PubMed] [Google Scholar]
- Ito J., Spizizen J. Abortive infection of sporulating Bacillus subtilis 168 by phi 2 bacteriophage. J Virol. 1971 Apr;7(4):515–523. doi: 10.1128/jvi.7.4.515-523.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMontagne J. R., McDonald W. C. A bacteriophage of Bacillus subtilis which forms plaques only at temperatures above 50 C. I. Physical and chemical characteristics of TSP-1. J Virol. 1972 Apr;9(4):646–651. doi: 10.1128/jvi.9.4.646-651.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMontagne J. R., McDonald W. C. A bacteriophage of Bacillus subtilis which forms plaques only at temperatures above 50 C. II. Reduction of TSP-1-specific receptor sites on cells grown at 37 C or 45 C, and the temperature-dependent inactivation of replicating phage. J Virol. 1972 Apr;9(4):652–658. doi: 10.1128/jvi.9.4.652-658.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMontagne J. R., McDonald W. C. A bacteriophage of bacillus subtilis which forms plaques only at temperatures above 50 C. 3. Inhibition of TSP-1-specific deoxyribonucleic acid synthesis at 37 C and 45 C. J Virol. 1972 Apr;9(4):659–663. doi: 10.1128/jvi.9.4.659-663.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire J. C., Pène J. J., Barrow-Carraway J. Gene expression during the development of bacteriophage phi 29. 3. Analysis of viral-specific protein synthesis with suppressible mutants. J Virol. 1974 Mar;13(3):690–698. doi: 10.1128/jvi.13.3.690-698.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palefski S., Hemphill H. E., Kolenbrander P. E., Whiteley H. R. Dominance relationships in mixedly infected Bacillus subtilis. J Virol. 1972 Apr;9(4):594–601. doi: 10.1128/jvi.9.4.594-601.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly B. E., Zeece V. M., Anderson D. L. Genetic study of suppressor-sensitive mutants of the Bacillus subtilis bacteriophage phi 29. J Virol. 1973 May;11(5):756–760. doi: 10.1128/jvi.11.5.756-760.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Hemphill H. E. Abortive infection of lysogenic Bacillus subtilis 168(SPO2) by bacteriophage phi 1. J Virol. 1974 Apr;13(4):870–880. doi: 10.1128/jvi.13.4.870-880.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Hemphill H. E. Prophage-mediated interference affecting the development of Bacillus subtilis bacteriophage phi e. J Virol. 1973 Mar;11(3):372–377. doi: 10.1128/jvi.11.3.372-377.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottländer E., Trautner T. A. Genetic and transfection studies with B, subtilis phage SP 50. I. Phage mutants with restricted growth on B. subtilis strain 168. Mol Gen Genet. 1970;108(1):47–60. doi: 10.1007/BF00343184. [DOI] [PubMed] [Google Scholar]
- Shibata T., Ando T. Host controlled modification and restriction in Bacillus subtilis. Mol Gen Genet. 1974;131(4):275–280. doi: 10.1007/BF00264858. [DOI] [PubMed] [Google Scholar]
- Tevethia M. J., Baptist J. N., Mandel M. Pleiotropic effects of suppressor mutations in Bacillus subtilis. J Bacteriol. 1974 Sep;119(3):961–975. doi: 10.1128/jb.119.3.961-975.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautner T. A., Pawlek B., Bron S., Anagnostopoulos C. Restriction and modification in B. subtilis. Biological aspects. Mol Gen Genet. 1974;131(3):181–191. doi: 10.1007/BF00267958. [DOI] [PubMed] [Google Scholar]
- Yasbin R. E., Ganesan A. T., Young F. E. Bacteriophage interference in Bacillus subtilis 168. J Virol. 1974 Apr;13(4):916–921. doi: 10.1128/jvi.13.4.916-921.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Requirement of glucosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2377–2384. doi: 10.1073/pnas.58.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]


