Abstract
During the last 20 years, the management of heart failure has significantly improved by means of new pharmacotherapies, more timely invasive treatments and device assisted therapies. Indeed, advances in mechanical support, namely with the development of more efficient left ventricular assist devices (LVADs), and the total artificial heart have reduced mortality and morbidity in patients awaiting transplantation, so much so, that LVADs are now approved of as a strategy for destination therapy. In this review, the authors describe in detail the current basic indications, functioning modalities, main limitations of surgical LAVDs, total artificial heart development, and percutaneous assist devices, trying to clarify this complex, but fascinating topic.
Keywords: Heart failure, Devices, Cardiocirculatory support, Heart transplant, Cardiac surgery
1. Introduction
The prevalence of heart failure is increasing and the prognosis of advanced heart failure remains dismal.[1] The current gold-standard therapy in advanced heart failure remains cardiac transplantation, but the eligible candidates far outnumber the available donor organs. During the last 20 years, significant progress has been made in the treatment of heart failure, related not only to new pharmacotherapies [Angiotensin converting enzyme (ACE) inhibitors and beta-blockers], but also to device therapy and invasive treatment.[2]–[6] These advances have resulted in an improved prognosis and also quality of life in patients with severe advanced heart failure. Advances in mechanical support, the development of the left ventricular assist devices (LVADs), and the total artificial heart have reduced mortality and morbidity in patients awaiting transplantation. Furthermore, LVADs are now approved for use as a strategy for destination therapy.
In this comprehensive review, we present the current knowledge on cardiocirculatory assist devices: basic knowledge of their functioning principles and results which should be the armamentarium of cardiovascular professionals and/ or general physicians who may be occasionally involved in the management of patients bearing such technology.
2. Considerations about advanced heart failure
In United States, the one year mortality rate among patients with advanced heart failure still remains high: in 1994–1997, it was about 20%, with a 3-year mortality of 67%.[7] In 1999–2001, corresponding mortality rates were 12% and 79%, respectively.[7] The effects of treatment depend on the age of patients. Prognosis in patients aged over 75 years is significantly worse with one-year mortality among patients more than 75 years of age about 37%, and three-year mortality about 60%.[7]–[10] These outcomes are not dissimilar from those in Europe. Heart transplantation remains the preferred strategy in eligible patients. Outcomes after heart transplantation improved substantially with the introduction of modern treatment, mainly with calcineurin inhibitors, such as cyclosporine. Data from the International Society for Heart and Lung Transplantation (ISHLT) registry show between 1993–2000, survival at 1, 3, 5, and 10 years was about 85%, 75%, 70%, and 50%, respectively,[11],[12] with some further improvement occurring in the last 10 years.
Unfortunately, in Europe, only less that one half of the eligible patients undergo timely transplantation, and therefore chronic LVADs is becoming an option. Nevertheless, data from Europe show that, among all patients receiving ventricular assist devices (VADs) as a temporary procedure, only 25% eventually receive a heart within one year.[13] A pool of patients in a chronic circulatory support state for an indefinite period of time is thus created representing an emerging health care issue.[14] A strategy of heart transplantation versus LVADs should be balanced against advantages and disadvantages (Table1).
Table 1. Advantages and disadvantages of heart transplantation versus cardiac assistance systems.
| Heart transplantation | Cardiac assistance systems | |
| Advantages | Definitive treatment | Immediately available |
| Normal physical activity | Planned intervention | |
| Good long-term prognosis | Good level of physical activity achievable | |
| Recovery of native heart possible | ||
| Disadvantages | Lack of donor organs | Dependency on device |
| Disease can arise in the donor organ | Device must be continually supplied with power | |
| Risk of transplant vasculopathy | Anticoa Cardiac arrhythmia | |
| Immune suppression | gulation (hemorrhage, stroke) | |
| Renal failure | Risk of infection | |
| Neoplasia | ||
| Susceptibility to infection | ||
| Diabetes mellitus | ||
| Hypertension |
3. Past and present of the mechanical circulatory support
Forty years ago, the functional replacement of the human heart was already a topic of intense public interest, not just because the first heart transplantation had been performed in December 1967, but also because the development and clinical applications of artificial hearts were already underway. A pneumatically driven, two-chambered apparatus was then being developed for total heart replacement.[15] Unlike heart transplantation, however, the artificial heart failed to become established in clinical practice in that early period,[16] and the type of artificial heart that was then under development is in clinical use today in only a few special situations.
The original type of artificial heart exhibited major technical flaws, and the need to power it with a very large, extracorporeal console made it utterly impractical for permanent therapy as we know it today.[17]
In order to address these technical problems, the artificial ventricles were moved outside the body: this step meant that what had originally been intended as an artificial heart was transformed into a ventricular assist device.[18] This technology was successfully applied and is still in use today. Experience has shown that the functional replacement of both cardiac ventricles is not necessary in every case; rather, left ventricular assistance alone usually suffices.[19]
In the 1990s, implantable, electrically powered left ventricular assist devices came onto the market. These large and heavy displacement pumps were implanted in a pocket below the diaphragm[20] and connected to the apex of the left ventricle and to the ascending aorta. They were connected to an external electric power supply through a percutaneous cable: this was the origin of the configuration that is still the most common one in use today,[21] i.e., an implanted pump with a transcutaneous connection to a power supply. The unsatisfactory aspects of this solution were the size of the apparatus, the noise it made, the risk of infection, embolization, and the limited mechanical durability. Using such a device as the sole method of treatment (destination therapy) was found to give patients a significantly higher chance of survival at one year when compared to conservative treatment [Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart failure (REMATCH) trial.[22] Again, the limited mechanical durability of the apparatus at two years became a critical issue when the survival advantage appeared to be no more than marginal.
The second generation of LVADs consisted of axial pumps. The new technology opened up new possibilities: these LVADs provide continuous blood flow without valves, are relatively small and light, and remain mechanically stable for years. When they were first used, there were major problems related to coagulation,[23] but technical modifications and improved anticoagulation schemes have largely circumvented these difficulties. The incidence of infections and neurological complications is also much lower than before: first-generation LVADs were found in the REMATCH trial to be associated with 0.39 severe neurological disturbances per patient-year, while the comparable figure in a study of axial pumps (second-generation LVADs) was only 0.18 strokes per patient-year.[24] The latter results of an incidence of 0.18 strokes per patient-year were duplicated in a trial of a third-generation device (Ventracor).[25]
Moreover, it has now become clear that continuous blood flow, with loss of the pulse, is physiologically[26] entirely unproblematic.[27] The neurocognitive disturbances associated with advanced heart failure can be improved with pulseless LVADs, just as they can with pulsatile devices.[28] The patient also remains able to compensate for the increasing physical stress of exercise after the native heart has been relieved of its functional burden.[29] Because of potential problems in the non-invasive measurement of blood pressure, as well as the inability to record a capillary pulse, the usual treatment modalities need to be altered for patients bearing pulseless LVADs. In particular, when medical or surgical treatments are needed independently of the cardiac situation, consultation with an implanting institution is recommended. These patients also have an elevated tendency to bleed, e.g., during dental or surgical procedures, partly due to necessary anticoagulation, partly because of acquired platelet dysfunction, and perhaps also as a consequence of acquired von Willebrand syndrome.[30] It follows that such procedures should only be performed in specialized centres.
4. Assist device systems
Many devices have been developed as replacement therapy, either as a pump or as a stable electrical system, with the aim of supporting the failing heart in critically ill patients with advanced heart failure. Depending on the particular device used, both the right and left ventricle can be assisted with the same concept, i.e., blood is removed from the failing ventricle into a pump and delivered to either aorta or pulmonary artery. Assist cardiac devices can be classified as total artificial heart (TAH) and VADs depending on their application (Table 2). Pulsatile devices were the first generation of pumps introduced clinically, but have a large size, multiple moving parts and can be implanted intra-corporeally or para-corporeally. The second generation of continuous flow pumps are miniaturized and have a single moving part: they permit intra-corporeal placement due to their small size. A third generation of blood pumps with mechanical non-contact magnetic bearings have been more recently evaluated in clinical studies. In addition, blood pumps can be grouped into either pulsatile or non-pulsatile based on their operational mechanism. Pulsatile pumps utilize inflow and outflow valves to pump the blood in one direction, whereas non-pulsatile pumps create an unidirectional flow without using valves. The features of the two different philosophies are summarized in Table 3.
Table 2. Classification of cardiocirculatory devices.
| Generation pump | TAH | VAD |
| First | Jarvick 7 | HeartMate I |
| Abiocor | Novacor | |
| Lion Heart | ||
| Second | CardioWest | MicroMed DeBakey |
| Jarvick 2000 | ||
| HeartMate II | ||
| Third | HeartMate III |
TAH: total artificial heart; VAD: ventricular assist devices.
Table3. Differences between pulsatile and non-pulsatile devices.
| Pulsatile | Non-pulsatile | |
| Size | Large | Smaller |
| Mechanism | Complex | Simpler |
| Control | Complex | Simpler |
| Valves | Two | None |
| Compliance | One | None |
| Implantability | Complex | Simpler |
| Overall system | Same | Same |
| Cost | Higher | Lower |
4.1. LAVD devices and their indications
VADs have been used in three clinical situations: as a bridge to transplantation, when clinical status of patients who are listed for transplantation is deteriorating rapidly before a suitable donor heart becomes available; as a bridge to recovery, in patients who are expected to recover left ventricular function, e.g., post-cardiotomy shock and fulminant myocarditis; or as alternative to heart transplantation, in patients not considered candidates for this procedure (‘destination’ therapy) (Table 4,5).[31]
Table 4. Indications for mechanical circulatory support.
| Bridge to transplantation: patients with such severe reductions in cardiac output or non–cardiac co-morbidities that survival and successful cardiac transplantation are unlikely without mechanical circulatory support |
| Impending cardiogenic shock despite inotropic support + IABP acute renal dysfunction (creatinine > 2.0) that is deemed secondary to insufficient renal blood flow and is poorly responsive to inotropic support. |
| Pulmonary hypertension (PA systolic pressure > 60 mmHg) that persist despite optimal medical and inotropic therapy and is presumed to have a reactive component that would respond to prolonged normalization of left atrial pressure (with mechanical circulatory support). |
| Bridge to recovery (for patients with otherwise fatal low cardiac output in situations where recovery is possible or probable) |
| Acute myocardial infarction complicated by cardiogenic shock. |
| Acute myocarditis with shock. |
| Acute cardiac failure following cardiac surgery. |
| Long-term device therapy (for patients with one or more major contraindications to cardiac transplant) |
| Class IV heart failure with chronic refractory and disabling heart failure symptoms despite optimal therapy: |
| 1. Peak oxygen consumption < 12–14 mL (kg/min) with cardiac limitation |
| 2. Dependence on intravenous inotropic support |
| Class IV heart failure with expected mortality exceeding 50% in one year |
IABP: intra-aortic balloon pump.
Table 5. Summary of the typical uses of the more investigated devices in the current era.
| Bridge to transplantation | Bridge to recovery | Destination therapy |
| Novacor LVAS (World Heart Corporation) | Abiomed BVS | HeartMate XVE1 |
| HeartMate VE (Thoratec Corporation) | Levitronix CentriMag | HeartMate II2 |
| HeartMate II | Jarvik 20002 | |
| Jarvik 2000 | DeBakey VAD2 | |
| Micro-Med DeBakey VAD | VentrAssist left ventricular assist system (Ventracor)2 | |
| INCOR (Berlin Heart AG, Berlin, Germany) LVAD | ||
| Levitronix CentriMag maglev pump |
1 the only one approved as destination therapy; 2 subjects of ongoing clinical evaluation to test the use as heart failure destination therapy. BVS: biventricular system; LVAD: left ventricular assist devices; LVAS: left ventricular assist; VAD: ventricular assist device.
Several devices have been tested and validated for use as a bridge to transplantation including the first generation devices, Novacor left ventricular assist (LVAS) (World Heart Corporation),[32],[33] the HeartMate XVE (Thoratec Corporation),[34] and the Thoratec para-corporeal and intra-corporeal devices.[35]
Newer second generation devices used as a bridge to transplantation include intra-corporeal devices, as the axial flow pumps HeartMate II,[36] Jarvik 2000,[37] and the Micro-Med DeBakey VAD.[38] These pumps are small and generate flows of more than 5 L/min. These devices produce a non-pulsatile flow and have potential advantages of improved durability, simple management and silent functioning. Such VADs have been successfully used in patients who are expected to recover sufficient myocardial function. The first condition is related to patients with compromised left ventricular function who have undergone long operations. Because of the severity of the post-operative circulatory shock, short term mechanical support as a bridge to recovery is often needed. An additional indication for VADs implantation as a bridge to recovery is post acute myocardial infarction shock, when traditional inotropic support and intra-aortic balloon pump counter-pulsation appear insufficient for good hemodynamic status and too weak to reverse the stunning of damaged myocardium. Patients affected by fulminant myocarditis with severe hemodynamic compromise recover fully in the majority of cases after VAD implantation. Finally, the last ‘bridge to recovery’ indication is the transient support of the right ventricle failure following LVAD therapy or heart transplantation. In these cases, right ventricular assist device (RVAD) support for a few days is sufficient to improve right ventricular function. The Abiomed biventricular system (BVS) is one of the devices employed as a short term bridge to recovery. This device, not widely used in Europe, is simple to use, able to support both ventricles and useful for inter-hospital transfer.[39]
Finally, VADs are selectively deployed in ‘destination’ therapy as alternatives to heart transplantation in patients not considered candidates for this procedure. To date, the HeartMate XVE has been approved for this purpose, but several newer devices are being evaluated for destination therapy.
Third generation devices utilizing magnetic and/or hydrodynamic levitation of the impeller without any contact bearings with the pump include the extra-corporeal systems such as Levitronix CentriMag maglev pump,[40] the magnetically suspended axial flow INCOR (Berlin Heart AG, Berlin, Germany) LVAD,[41] the HeartWare which uses hydrodynamic and magnetic levitation for suspension,[42] the HeartMate III which uses full magnetic suspension[43] and the DuraHeart (Terumo Somerset, USA).[44]
Recently, the Synergy Pocket Micro-pump device (CircuLite, Inc., Saddle Brook, New Jersey) has been investigated as partial cardiac support. It pumps approximately 3.0 L/min, is implanted (off pump) via a mini-thoracotomy, and is positioned in a right subclavicular subcutaneous pocket (like a pacemaker). The inflow cannula inserts into the left atrium while the outflow graft connects to the right subclavian artery.[45]
Simulations demonstrated that partial support (2 to 3 L/min) with this device increased total cardiac output (left ventricular assist device output plus native heart output) by more than 1 L/min and decreased left ventricular end-diastolic pressure by 7 to 10 mmHg in the presence of moderate-to-advanced heart failure. Analyses showed that the hemodynamic benefits of increased cardiac output and decreased left ventricular end-diastolic pressure are greater in lessdilated and less-dysfunctional hearts. Both the relationships between ventricular assist device flow and cardiac output and ventricular assist device flow and left atrial pressure predicted by the model closely approximated the same relationships obtained during the hemodynamic study in a bovine heart failure model.
4.2. Special case: post-cardiotomy failure
Post-cardiotomy failure occurs in 2%–6% of all cardiac surgical procedures, most commonly as a result of myocardial stunning or hibernation during cardiopulmonary bypass.[46] Post-cardiotomy failure is associated with > 75% mortality. Most of cases respond to conventional pharmacological and mechanical support, i.e., intra-aortic balloon pump, but sometimes the myocardium may be refractory to these therapies and would require an additional mechanical support. In the past, the use of LAVDs in post-cardiotomy failure was associated with very poor outcomes, but recently its use has been gaining some renewed acceptance due to > 60% survival with the newest devices. LVAD, RVAD or BiVAD may be implanted depending on operative assessment and hemodynamic impairment.
4.3. LVAD Outcomes
First generation HeartMate had an average implant duration of 80 to 100 days with a probability of device failure of 35% at two years.[47] The only prospective, randomized study comparing maximum medical therapy to implantation of an early generation of HeartMate VAD in a group of extremely ill patients with end-stage heart disease and not eligible for transplantation is the REMATCH study.[48] Survival of VAD and medical patients at one year was 52% versus 25%, and at two years 23% versus 8%. In addition, VAD patients had improvement in quality of life compared to the medically treated cohort. Recent modifications of the technique and in peri-operative care have decreased the high VAD related morbiddity and mortality reported in the REMATCH trial, including the high incidence of septic complications and their limited long-term reliability.[48]
To date, the HeartMate II is the most successful second-generation LVAD with over 6000 implants worldwide and improved survival rates, as experience was gained from the initial clinical trial results,[49] to the post-approval study[50] from 89% to 96% at 30 days; from 89% to 90% at six months; and from 68% to 85% at one year. The incidence of thromboembolic events ranged from three to six events per 100 patient-years,[51] somewhat lower than other second-generation devices, such as DuraHeart. Fatal outcomes associated with device related infections were 13%–27% (driveline or pump), when the second generation device infection was compared to first-generation device infection.[52]
The European BTT trial with the HeartWare third generation LAVDs appears to be similar to the HeartMate trial results.[53] Survival to transplant or ongoing support is 92% after 180 days in the United States bridge to transplant clinical trial (ADVANCE) presented at the 2010 AHA meeting.[54] Currently, reported third generation device-related infections range from 10.7%–6.4%. Additionally, ischemic stroke occurs at a rate of 0.11 events per patient-year and 0.05 for hemorrhagic stroke.
Finally, the first trial in humans of the Synergy Pocket Micro-pump device suggested that continuous aortic flow augmentation improved cardiac performance by improving cardiac index and pulmonary capillary wedge pressure, but the statistical significance for the primary efficacy end point was not attained.[45]
4.4. TAH: indications and outcomes
The most radical therapy for the treatment of end-stage heart disease also includes the complete orthotopic replacement of the heart with a total artificial heart (Table 6).
Table 6. Summary of the main features of all current assist devices.
| Thoratec | Indications: right, left, or biventricular support |
| Advantages: fits in a wide range of patient sizes (body surface area from 0.73 to 2.5 m2) | |
| Pump can be changed without invasive surgery | |
| Can replace the entire function of the supported ventricle | |
| Disadvantages: need for strict anticoagulation with risk of bleeding or thromboembolism | |
| Large lines crossing the skin with a high risk of infection | |
| Limited patient mobility | |
| Not approved for home use | |
| HeartMate | Indications: left ventricular support |
| Advantages: No need for anticoagulation | |
| Portability of controller and batteries permits good patient mobility and hospital discharge | |
| Can replace the entire function of the supported ventricle | |
| Disadvantages: Drive line crossing the skin poses a risk of infection | |
| Left ventricle support only | |
| Does not fit in patients with body surface area > 1.5 m2 | |
| Novacor | Indications: Left ventricular support |
| Advantages: Portability of controller and batteries permits good patient mobility and hospital discharge | |
| Can replace the entire function of the supported ventricle | |
| Disadvantages: Need for strict anticoagulation with higher risk of bleeding or thromboembolism | |
| Drive line crossing the skin poses a high risk of infection | |
| Left ventricle support only | |
| Does not fit in patients with body surface area (m2) > 1.5 m2 | |
| Continuous-flow pumps | Indications: left ventricular support |
| Advantages: small size permits greater patient mobility | |
| Quiet | |
| Fit in a wide range of patient body habitus | |
| Disadvantages: Need anticoagulation; risk of bleeding and thromboembolism unknown | |
| May not replace the entire function of the supported ventricle | |
| Non-pulsatile flow | |
| Total artificial hearts | Indications: advanced biventricular dysfunction |
| Pulmonary hypertension? | |
| Cardiac tumors? | |
| Advantages: complete replacement of the heart function | |
| Disadvantages: need anticoagulation; risk of bleeding or thromboembolism | |
| Due to the size of equipment, they only fit in patients with larger body habitus |
The CardioWest pneumatic TAH (TAH-t; SynCardia, Inc., Tucson, AZ, USA) has been approved as a temporary device for a bridge to cardiac transplantation. It is an orthotopic pneumatic biventricular device that replaces the ventricles, all four valves and the proximal portion of each great vessel. Its main limitations remain the external power source and a large control console that is not portable, thereby restricting patients to a hospital setting.
More than 700 CardioWest devices have been implanted worldwide. Several reports have suggested that the safety of the CardioWest TAH-t is comparable to currently used biventricular assist devices and LVADs.[55]
Extra-corporeal BiVADs have been associated with high risk with increased age, previous mediastinal operation, elevated blood urea nitrogen, elevated bilirubin levels, and mechanical ventilation before implantation. Recently, Copeland et al.[56] have reported their study on risk factor analysis for bridge to transplantation with the CardioWest TAH-t. By multivariate analysis, none of the previously reported risk factors related to VAD implantation influenced survival of patients bridged with the CardioWest. The authors concluded that this device can fill ‘a therapeutic gap that is not covered by LVADs or extra-corporeal BiVADs’.
The AbioCor TAH was designed as a completely implantable device intended exclusively as an alternative to transplantation. The design permits its complete implantation in the body and thus, patients are not tethered to a large air-pumping console, nor do they have transcutaneous wires or tubes. The system is powered by a small external battery that transmits power across the skin wirelessly. The initial clinical experience suggests that the AbioCor TAH might be effective as destination therapy in patients with biventricular end-stage congestive heart failure,[57],[58] even if further investigation is warranted. The company is proceeding with the development of a second generation of AbioCor that will be smaller and designed to perform well over five years.
5. Limitations of assist devices
Implantation of a mechanical support with a VAD is associated with significant mortality and morbidity. The most frequent early complications remain perioperative bleeding and right ventricular failure. The former is often the result of a prolonged heart operation and is related to perioperative coagulopathy and platelet dysfunction induced by the extracorporeal circulation. Moreover, bleeding is increased by routine use of anticoagulant and antiplatelet drugs in these patients affected by heart failure associated with frequent hepatic dysfunction secondary to right heart failure. This latter complication, present in patients who underwent LVAD implantation, occurs in 30% of patients and its etiology is still a matter of debate. Elevated pulmonary vascular resistance induced by cardiopulmonary bypass, changes in interventricular dependence, right coronary ischemia, and other causes have been put forward to explain this catastrophic complication associated with a very high mortality.[59] Late complications of VAD implantation are device related infection and limited reliability. Driveline infections can be successfully eradicated with wound treatment and antibiotic administration, while device-related infection often requires device exchange or heart transplantation. Device reliability is variable depending on the specific characteristics of the mechanical support device: the first generation of large pulsatile pumps performed well for only one to three years, due to the presence of several moving parts and the strain of mechanical bearings over time. The recent miniaturized axial flow pumps continue to work in humans for a duration of 5.5 years.[59] The third generation of maglev pumps allow suspension of a moving element without any mechanical contact, thus eliminating heat generation and wear that will take place at the contact surface. Nevertheless, durability and stability of the sensors and control system could be a problem in the sophisticated and expensive maglev system. All the equipment operates in a biological environment at a body temperature of 37°C and high humidity; these conditions can affect electronic performance and sealing of body fluid entering the package and can lead to an increase in the probability of failure. A simple maglev system is seen as required for making a long-life device.
6. Medical management of patients with assist devices
The proper clinical management of patients with assist devices is becoming increasingly important for optimizing outcomes.[60]
Pre-implant optimization of co-morbid conditions is very important in minimizing the incidence and severity of post-operative adverse events and enhancing survival. In particular, it is important: (1) to improve nutritional status (malnutrition is very common in candidates for an assist device, and if not improved, it enhances the risk of infection as well as the capability to recover after surgery. A body mass index (BMI) of < 20 kg/m2 is a marker of severe malnutrition (targets should be an albumin > 3 g/dL and a transferrin > 250 mg/dL); (2) to lower pulmonary vascular resistance to optimize right-heart function and to reduce right atrial pressure and secondary hepatic congestion (targets should be a right atrial pressure of < 15 mmHg and a pulmonary capillary wedge pressure of < 24 mmHg); (3) to aggressively manage volume to minimize right ventricular workload and hepatic congestion; (4) to optimize coagulation International normalized ratio (INR) < 1.2, hemoglobin > 10 g/dL, and platelets > 150,000/mm; (5) to optimize renal (target < 2.5 mg/dL), hepatic (total bilirubin > 2.5 mg/dl and AST/ ALT < 2 times normal), pulmonary, and neurologic functions; and (6) to treat any infection or provide prophylactic antibiotic therapy. Moreover, the patient's support system, psychosocial status, compliance with care, and ability to operate and care for external system components all warrant consideration in the patient selection process.
Post-operative care should include complete reversal of anticoagulation therapy after the surgical procedure, treatment of hypertension with blood pressure that cannot exceed 90 mmHg, and instauration of proper anticoagulation with an INR 1.5 to 2.5 in order to avoid bleeding which is very frequent and to protect from thrombotic events which are usually common. Aspirin 81 mg to 325 mg daily is used for antiplatelet therapy.[61]
Early pre-operative identification and post-operative surveillance for right heart dysfunction should be seriously undertaken because of the risk of increased mortality: echocardiography is a useful tool to monitor the right ventricle behaviour during LVAD support.[62] A change in right ventricle shape and dimension may contribute to right dysfunction, since this change in right ventricle geometry is a result of disturbed interaction between left and right ventricle during LVAD support. Pathophysiology includes a rapid off-loading of the left ventricle resulting in shifting of the septum from the right to the left which reduces the contribution of the septum to the right heart contractility. The severity of septal deviation and right ventricle shape together with the grade of tricuspid valve insufficiency may help to identify patients at high risk for right ventricle failure and also help to identify patients who would benefit from biventricular support.[63]
7. Financial implications of assist devices
A broader use of assist devices is limited by the financial impact of the global procedure. One of the most complete economic assessments, a systematic review conducted in the UK in 2005, demonstrated that LAVDs are clinically effecttive as a bridge-to-transplant, but they are not cost effective. Similarly, the only few studies on patients receiving LVADs as long-term cardiac support showed some indubitable clinical benefits for patients, but again their use did not appear cost effective.[64] Larger epidemiologic surveys should be undertaken in order to assess the real cost effectiveness of assist devices in the real world.
8. Percutaneous partial support devices
Recently, newer mini-invasive percutaneous devices have been used as LVAD for ‘rescue’ therapy.
8.1. Extra-corporeal membrane oxygenation (ECMO) as temporary circulatory support
This kind of support is becoming an important tool for treating acute heart failure in all age groups. In the setting of profound cardiogenic shock or cardiac arrest, ECMO is a viable option for resuscitation and initiating circulatory assist. Existing systems requiring only few minutes of priming time are commercially available for treating post-cardiotomy heart failure, high-risk percutaneous coronary interventions, or respiratory tract operations.[63] Overall survival is approximately 50%,[65] but their use is limited to short term useage because of the high rate of device-related complications, including stroke, bleeding, infection, thromboembolic events and vascular complications that lead to limb ischemia or bleeding from the access site or due to vascular injury.
8.2. The Tandem Heart Percutaneous VAD
The Tandem Heart Percutaneous VAD[66] is a continuous flow centrifugal assist device with an inflow cannula inserted percutaneously through the femoral vein and advanced across the intra-atrial septum into the left atrium. The extracorporeal pump receives blood from left atrium and returns it to femoral artery via outflow cannula. The Tandem-heart device incorporates 9-17 F arterial cannulae and a unique 21 F trans-septal cannula and centrifugal blood pump. Operating at 7500 r/min, the pump withdraws oxygenated blood from the left atrium and delivers at a rate of up to 4 L/min to the arterial circulation. It provides satisfactory short term hemodynamic support for patients affected by post-cardiotomy shock and for patients undergoing high-risk coronary percutaneous procedures. Unfortunately, this device has not demonstrated significant survival improvement in comparison with intra-aortic balloon pump.
8.3. The Impella
Another device used in the setting of severe ventricular dysfunction and cardiogenic shock following percutaneous procedures, or post-cardiac surgery, is the Impella (Impella Technologies of Abiomed Corporation).[67] This system uses a microaxial pump mounted on a 9 F catheter, inserted diretly in the heart chambers or percutaneously through the femoral artery and positioned under radioscopy across the aortic valve to unload the failing left ventricle. This device has the potential for being used also as a bridge to heart transplantation, or to another permanent destination support device.
9. Indications, results and limitations of percutaneous partial support systems
The Tandem Heart and the Impella have been used successfully in several different clinical settings ranging from a bridge to transplant, to high-risk percutaneous and surgical coronary revascularization,[68]–[71] severe allograft rejection[72] during high-risk abdominal surgery,[73] and recovery from fulminant miocarditis,[74] or as a rescue in critical aortic valve stenosis.[75] These two kinds of pump mainly suffered from technical and constructive drawbacks, including the interatrial septal course for the Tandem Heart and the low pump power with 2.5 L/min of flow for the Impella. This last device has been designed also for surgical implantation which increases flow to 5 L/min. Table 5 summarizes the main features and applications of the most frequently used TAHs.
From the brief overview of the current available literature discussed above, it appears to be clear that a completely percutaneous, long-term assist device suffers from many limitations due to current construction materials and miniaturization capabilities.
Surgical VADs are voluminous pulsatiles and, in most cases, need large French size cannula and surgical implantation that are very costly, although they have the advantage of ensuring a large flow (about 4.5 to 5 L/min), thus providing greater cardiac assistance compared to percutaneous VADs. Due to their capabilities, they are the best option for advanced heart failure patients both for bridge and destination therapy.
We must recognize that current percutaneous devices, such as the Impella and the Tandem Heart have substantial drawbacks, which are the arterial access in the former and the mixed percutaneous/surgical implantation technique in the latter. Both these limitations make long-term use unsuitable due to the risk of infection and problems associated to surgical access. Moreover, the power source is external and both devices require a console for proper functioning. These features limit the upright position compatible with real life, and discharge from the hospital. Because of the size of the cannula, ranging from 9 to 10 F, providing no more than 2.5 to 3 L/min, they cannot be an effective option for advanced heart failure patients, except as a bridge to recovery, or to transplantation, and they cannot be employed for long-term treatment.
The power source and its location is a key issue for percutaneous long-term assistance. External power sources already in use for surgical VADs, such as NOVACOR offer possible options but are unreliable for very long-term assistance and offer poor quality of life. Otherwise, power sources inside the body, as in the case of pacemakers, for example, may be preferable, but inevitably offer low power and a low degree of cardiac assistance.
Recently, the Synergy Micro-Pump (Circulite Inc., Hackensack, NJ, USA) has been implanted in humans[45],[46] and is approximately the size of an AA battery, weighting only 25 g, and can pump up to 2.5 to 3.0 L/min to provide partial left ventricular support. Because of its small size, it can be inserted via a right-sided mini-thoracotomy to withdraw the flow from the left atrium with blood returning to the subclavian artery. The pump is placed subcutaneously in the pectoral region similar to a pacemaker. Although this system is far from being percutaneous, it demonstrates the feasibility of the conceptual design of having a power source, in this case, the pump itself, implanted subcutaneously.
10. Future developments
Miniaturization of the pumps and surgical devices and improvement in overall efficacy are the next areas of continuing technological advancement. With current scientific technology, we are still far from a totally percutaneously, implantable supporting device, which would be a logical consequence of the research to provide less invasiveness, but probably in the next 20 years with the implementation of nano-technology, such efforts can offer simpler and definite support devices for advanced heart failure (Figure 1).
Figure 1. Futuristic totally percutaneous implantable permanent left ventricle assist device (virtual technology design).
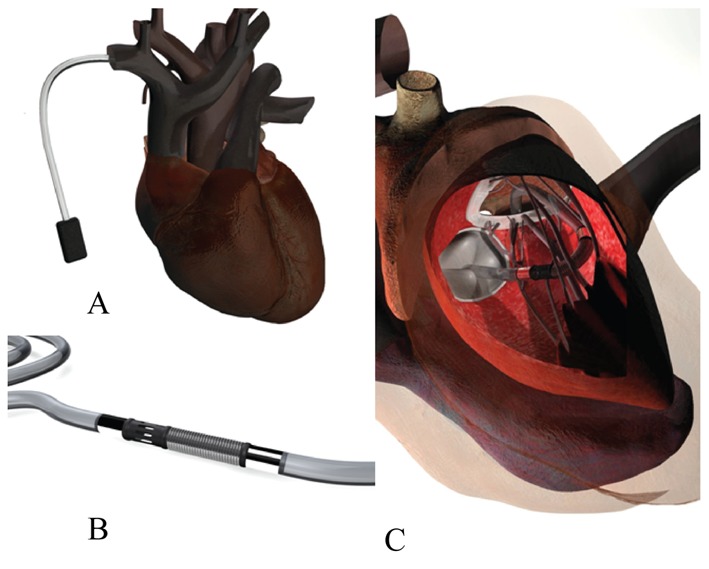
(A): Battery in subclavian pocket; (B): Pump; (C): Interatrial septal approach.
11. Conclusions
The history of cardiocirculatory assist devices is long with an abundance of big mistakes, but not without extraordinary successes. It is clear that these kinds of devices still require a very invasive surgical procedure of quite complicated percutaneous interventions, but offer a chance of survival to many patients regarded temporarily, or indefinitely, as not suitable for cardiac transplant. While awaiting future advances, the knowledge of current devices should be part of the armamentarium of cardiovascular professsionals who, although not specialist in the field, may be occasionally become involved with the management of patients with such devices.
References
- 1.Stewart S, MacIntyre K, Hole DJ, et al. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3:315–322. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 2.Klotz S, Loeher A, Drees G, et al. Surgical therapy of endstage heart failure. State of the art 2006. Herz. 2006;31:445–454. doi: 10.1007/s00059-006-2840-8. [DOI] [PubMed] [Google Scholar]
- 3.Allen LA, Felker GM. Advances in the surgical treatment of heart failure. Curr Opin Cardiol. 2008;23:249–253. doi: 10.1097/HCO.0b013e3282f54fea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) Am J Cardiol. 1988;62:60A–66A. doi: 10.1016/s0002-9149(88)80087-0. [DOI] [PubMed] [Google Scholar]
- 5.Domanski MJ, Krause-Steinrauf H, Massie BM, et al. A comparative analysis of the results from 4 trials of beta-blocker therapy for heart failure: BEST, CIBIS-II, MERIT-HF, and COPERNICUS. J Card Fail. 2003;9:354–363. doi: 10.1054/s1071-9164(03)00133-7. [DOI] [PubMed] [Google Scholar]
- 6.The SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;1:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 7.Cleland JGF, Khand A, Clark AC. The heart failure epidemiology: Exactly how big is it? Eur Heart J. 2001;22:623–665. doi: 10.1053/euhj.2000.2493. [DOI] [PubMed] [Google Scholar]
- 8.Gardner R, Mc Donagh T, Mac D, et al. Who needs a heart transplant? Eur Heart J. 2006;27:770–772. doi: 10.1093/eurheartj/ehi759. [DOI] [PubMed] [Google Scholar]
- 9.Butler J, Khadim G, Paul KM, et al. Selection of patients for heart transplantation in the current era of heart failure therapy. J Am Coll Cardiol. 2004;43:787–793. doi: 10.1016/j.jacc.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 10.Taylor DO, Edwards LB, Boucek MM, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-second official adult heart transplant report—2005. J Heart Lung Transplant. 2005;24:945–955. doi: 10.1016/j.healun.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Almenar-Bonet L Spanish Working Groups on Heart Transplantation. Spanish heart transplantation registry. 18th official report of the Spanish Society of Cardiology working group on heart failure, heart transplantation and associated therapies (1984–2006) Rev Esp Cardiol. 2007;60:1177–1187. [PubMed] [Google Scholar]
- 12.Metra M, Ponikowski P, Dickstein K, et al. Advanced chronic heart failure: a position statement from the Study Group on advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2007;9:689–694. doi: 10.1016/j.ejheart.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Komoda T, Hetzer R, Lehmkuhl HB. Destiny of candidates for heart transplantation in the Eurotransplant heart allocation system. Eur J Cardiothor Surg. 2008;34:301–306. doi: 10.1016/j.ejcts.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Strüber M, Sander K, Lahpor J, et al. Heart Mate II left ventricular assist device; early European experience. Eur J Cardiothoracic Surg. 2008;34:289–294. doi: 10.1016/j.ejcts.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Cooley DA. The total artificial heart. Nat Med. 2003;9:108–111. doi: 10.1038/nm0103-108. [DOI] [PubMed] [Google Scholar]
- 16.Bücherl ES, Henning E, Baer P, et al. Status of the artificial heart program in Berlin. World J Surg. 1985;9:103–115. doi: 10.1007/BF01656261. [DOI] [PubMed] [Google Scholar]
- 17.Cooley DA, Liotta D, Hallman GL, et al. Orthotopic cardiac prosthesis for two-staged cardiac replacement. Am J Cardiol. 1969;24:723–730. doi: 10.1016/0002-9149(69)90460-3. [DOI] [PubMed] [Google Scholar]
- 18.Pennington DG, Kanter KR, McBride LR, et al. Seven years experience with the Pierce-Donachy ventricular assist device. J Thorac Cardiovasc Surg. 1988;96:9014–9021. [PubMed] [Google Scholar]
- 19.Pennington DG, Reedy JE, Swartz MT, et al. Univentricular versus biventricular assist device support. J Heart Lung Transplant. 1991;10:258–263. [PubMed] [Google Scholar]
- 20.Frazier OH. First use of an untethered, vented electric left ventricular assist device for long term support. Circulation. 1994;89:2908–2914. doi: 10.1161/01.cir.89.6.2908. [DOI] [PubMed] [Google Scholar]
- 21.Vetter HO, Kaulbach HG, Schmitz C, et al. Experience with the Novacor left ventricular assist System as a bridge to cardiac transplantation, including a new wearable system. J Thorac Cardiovasc Surg. 1995;109:74–80. doi: 10.1016/S0022-5223(95)70422-1. [DOI] [PubMed] [Google Scholar]
- 22.Rose EA, Moskowitz AJ, Heitjan DF, et al. Long term mechanical left ventricular assistance for end stage heart failure. N Engl J Med. 2001;345:1434–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 23.Rothenburger M, Wilhelm MJ, Hammel D, et al. Treatment of thrombus formation associated with the MicroMed DeBakey VAD using recombinant tissue plasminogen activator. Circulation. 2002;106:I189–I192. [PubMed] [Google Scholar]
- 24.Miller LW, Pagani FD, Russell SD, et al. Use of continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 25.Esmore D, Spratt P, Larbalestier R, et al. VentrAssist left ventricular assist device: clinical trial results and clinical development plan update. Eur J Cardiothorac Surg. 2007;32:735–744. doi: 10.1016/j.ejcts.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 26.Wieselthaler GM, Riedl M, Schima H, et al. Endocrine function is not impaired in patients with a continuous MircoMed-DeBakey axial flow pump. J Thorac Cardiovasc Surg. 2007;133:2–6. doi: 10.1016/j.jtcvs.2003.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Radovancevic B, Vrtovec B, de Kort E, et al. End-organ function in patients on long term circulatory support with continuous—or pulsatile—flow assist devices. J Heart Lung Transplant. 2007;26:815–818. doi: 10.1016/j.healun.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Zimpfer D, Wieselthaler G, Czerny M, et al. Neurocognitive function in patients with ventricular assist devices: a comparison of pulsatile and continuous blood flow devices. Asaio J. 2006;52:24–27. doi: 10.1097/01.mat.0000191334.51375.7e. [DOI] [PubMed] [Google Scholar]
- 29.Akimoto T, Yamazaki K, Litwak P, et al. Rotary blood pump flow spontaneously increases during exercise under constant pump speed: results of a chronic study. Artif Organs. 1999;23:797–801. doi: 10.1046/j.1525-1594.1999.06426.x. [DOI] [PubMed] [Google Scholar]
- 30.Geisen U, Heilmann C, Beyersdorf F, et al. Non-surgical bleeding in patients with ventricular assist devices could be explained by acquired von Willebrand disease. Eur J Cardiothorac Surg. 2008;33:679–684. doi: 10.1016/j.ejcts.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 31.Kirklin JK, Naftel DC. Mechanical circulatory support: registering a therapy in evolution. Circ Heart Fail. 2008;1:200–205. doi: 10.1161/CIRCHEARTFAILURE.108.782599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holman WL, Davies JE, Rayburn BK, et al. Treatment of end-stage heart disease with outpatient ventricular assist devices. Ann Thorac Surg. 2002;73:1489–1493. doi: 10.1016/s0003-4975(02)03502-6. [DOI] [PubMed] [Google Scholar]
- 33.Frazier OH, Rose EA, Oz MC, et al. HeartMate LVAS Investigators Left ventricular assist system. Multicenter clinical evaluation of the HeartMate vented electric left ventricular assist system in patients awaiting heart transplantation. J Thorac Cardiovasc Surg. 2001;122:1186–1195. doi: 10.1067/mtc.2001.118274. [DOI] [PubMed] [Google Scholar]
- 34.Strauch JT, Spielvogel D, Haldenwang PL, et al. Recent improvements in outcome with the Novacor left ventricular assist device. J Heart Lung Transplant. 2003;22:674–680. doi: 10.1016/s1053-2498(02)01182-8. [DOI] [PubMed] [Google Scholar]
- 35.Slaughter MS, Tsui SS, El-Banayosy A, et al. IVAD Study Group Results of a multicenter clinical trial with the Thoratec implantable ventricular assist device. J Thorac Cardiovasc Surg. 2007;133:1573–1580. doi: 10.1016/j.jtcvs.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 36.Miller LW, Pagani FD, Russell SD, et al. HeartMate II Clinical Investigators Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 37.Frazier OH, Myers TJ, Westaby S, et al. Clinical experience with an implantable, intracardiac, continuous flow circulatory support device: physiologic implications and their relationship to patient selection. Ann Thorac Surg. 2004;77:133–142. doi: 10.1016/s0003-4975(03)01321-3. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein DJ. Worldwide experience with the MicroMed DeBakey ventricular assist device as a bridge to transplantation. Circulation. 2003;108(Suppl. I):272–277. doi: 10.1161/01.cir.0000087387.02218.7e. [DOI] [PubMed] [Google Scholar]
- 39.Samuels LE, Holmes EC, Garwood P, et al. Initial experience with the Abiomed AB 5000 ventricular assist device system. Ann Thorac Surg. 2005;80:309–312. doi: 10.1016/j.athoracsur.2004.07.086. [DOI] [PubMed] [Google Scholar]
- 40.Hoshi H, Shinshi T, Takatani S. Third-generation blood pumps with mechanical noncontact magnetic bearings. Artif Organs. 2006;30:324–338. doi: 10.1111/j.1525-1594.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 41.Kirsch M, Vermes E, Boval B, et al. Four years of circulatory support with the INCOR axial pump from Berlin Heart. Arch Mal Coeur Vaiss. 2007;100:967–970. [PubMed] [Google Scholar]
- 42.LaRose JA, Tamez D, Ashenuga M, et al. Design concepts and principle of operation of the heartware ventricular assist system. ASAIO J. 2010;56:285–289. doi: 10.1097/MAT.0b013e3181dfbab5. [DOI] [PubMed] [Google Scholar]
- 43.Farrar DJ, Bourque K, Dague CCP, et al. Design features, developmental status, and experimental results with the heart mate III centrifugal left ventricular assist system with a magnetically levitated rotor. ASAIO J. 2007;53:310–315. doi: 10.1097/MAT.0b013e3180536694. [DOI] [PubMed] [Google Scholar]
- 44.Morshuis M, el-Banayosy A, Arusoglu L, et al. European experience of DuraHeart magnetically levitated centrifugal left assist system. Eur J Cardiothorac Surg. 2009;35:1020–1028. doi: 10.1016/j.ejcts.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 45.Meyns B, Ector J, Rega F, et al. First human use of partial left ventricular heart support with the Circulite synergy micro-pump as a bridge to cardiac transplantation. Eur Heart J. 2008;29:2582. doi: 10.1093/eurheartj/ehn202. [DOI] [PubMed] [Google Scholar]
- 46.Nasir A, Bonde P, Graham ANJ. Ventricular assist device therapy in post-cardiotomy cardiogenic shock: historical outcomes and current trends. Interact Cardiovasc Thorac Surg. 2012;14:585–587. doi: 10.1093/icvts/ivr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frazier OH, Rose EA, McCarthy P, et al. Improved mortality and rehabilitation of transplant candidates treated with a long-term implantable left ventricular assist system. Ann Surg. 1995;222:327–338. doi: 10.1097/00000658-199509000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richenbacher WE, Naka J, Raines EP, et al. REMATCH Investigators Surgical management of patients in the REMATCH Trial. Ann Thorac Surg. 2003;75(6 Suppl.):S86–S92. doi: 10.1016/s0003-4975(03)00485-5. [DOI] [PubMed] [Google Scholar]
- 49.Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients waiting heart transplantation. N Engl J Med. 1007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 50.Starling RC, Naka Y, Boyle AJ. Results of the post-FDA-approval study with continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) J Am Coll Cardiol. 2011;10(57):1890–1898. doi: 10.1016/j.jacc.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 51.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 52.Holman WL, Kormos RL, Naftel DC, et al. Predictors of death and transplant in patients with a mechanical circulatory support device: a multi-institutional study. J Heart Lung Transplant. 2009;28:44–50. doi: 10.1016/j.healun.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 53.Wieselthaler GGM, O'Driscoll G, Jansz P, et al. Initial clinical experience with a novel left ventricular assist device with a magnetically levitated rotor in a multi-institutional trial. J Heart Lung Transplant. 2010;29:822–824. doi: 10.1016/j.healun.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 54.Aronson K. Evaluation of Heartware HVAD left ventricular assist device system for the treatment of advanced heart failure: results of the ADVANCE bridge-to-transplant Trial. 2010. Presentation at the AHA meeting, Chicago, Illinois, USA, 13-17 November.
- 55.Copeland JG, Smith RG, Arabia FA, et al. Comparison of the CardioWest total artificial heart, the Novacor left ventricular assist system, and the Thoratec ventricular assist system in bridge to transplantation. Ann Thorac Surg. 2001;71:S92–S97. doi: 10.1016/s0003-4975(00)02625-4. [DOI] [PubMed] [Google Scholar]
- 56.Copeland JG, Smith RG, Bose RK, et al. Risk factors analysis for bridge to transplantation with the CardioWest total artificial heart. Ann Thorac Surg. 2008;85:1639–1645. doi: 10.1016/j.athoracsur.2008.01.052. [DOI] [PubMed] [Google Scholar]
- 57.Samuels L. The AbioCor totally implantable replacement heart. Am Heart Hosp J. 2003;1:91–96. doi: 10.1111/j.1541-9215.2003.02090.x. [DOI] [PubMed] [Google Scholar]
- 58.Dowling RD, Gray LA, Jr, Etoch SW, et al. Initial experience with the AbioCor implantable replacement heart system. J Thorac Cardiovasc Surg. 2004;127:131–141. doi: 10.1016/j.jtcvs.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 59.Frazier OH, Dowling RD, Gray LA, Jr, et al. The total artificial heart: where do we stand. Cardiology. 2004;101:117–121. doi: 10.1159/000075992. [DOI] [PubMed] [Google Scholar]
- 60.Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist device in advanced heart failure. J Heart Lung Transplant. 2010;29:S1–S39. doi: 10.1016/j.healun.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 61.Rossi M, Serraino GF, Jiritano F, et al. What is the optimal anticoagulation in patients with a left ventricular assist device? Inter Cardiovasc Thor Surg. 2012:1–8. doi: 10.1093/icvts/ivs297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neragi-Miandoab S, Goldstein D, Bello R, et al. Right ventricular dysfunction following continuous flow left ventricular assist device placement in 51 patients: predicators and outcomes. J Cardiothorac Surg. 2012;7:60. doi: 10.1186/1749-8090-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Potapov EV, Stpanenko A, Dandel M, et al. Tricuspid incompetence and geometry of the right ventricle as predictors of right ventricular dysfunction after implantation of a left ventricular assist device. J Heart Lung Transplant. 2008;27:1275–1281. doi: 10.1016/j.healun.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 64.Clegg AJ, Scott DA, Loveman E, et al. The clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failure: a systematic review and economic evaluation. Heath Technol Assess. 2005;9:1–132. doi: 10.3310/hta9450. [DOI] [PubMed] [Google Scholar]
- 65.Matsumiya G, Saitoh S, Sawa Y. Extracorporeal assist circulation for heart failure. Cir J. 2009;73(Suppl A):42–47. doi: 10.1253/circj.cj-09-0071. [DOI] [PubMed] [Google Scholar]
- 66.Smedira NG, Moazami N, Golding CM, et al. Clinical experience with 202 adults receiving extracorporeal membrane oxygenation for cardiac failure: survival at 5 years. J Thorac Cardiovasc Surg. 2001;122:92–102. doi: 10.1067/mtc.2001.114351. [DOI] [PubMed] [Google Scholar]
- 67.Thiele H, Lauer B, Hambrecht R, et al. Reversal of cardiogenic shock by percutaneous left atrial-to-femoral arterial bypass assistance. Circulation. 2001;104:2917–2922. doi: 10.1161/hc4901.100361. [DOI] [PubMed] [Google Scholar]
- 68.LaRocca GM, Shimbo D, Rodriguez CJ, et al. The Impella recover LP 5.0 left ventricular assist device: a bridge to coronary artery bypass grafting and cardiac transplantation. J Am Soc Echocardiogr. 2006;19:468.e5–e7. doi: 10.1016/j.echo.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 69.Sjauw KD, Konorza T, Erbel R, et al. Supported high-risk percutaneous coronary intervention with the Impella 2.5 device the Europella registry. J Am Coll Cardiol. 2009;54:2430–2434. doi: 10.1016/j.jacc.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 70.Vranckx P, Schultz CJ, Eindhoven JA, et al. Assisted circulation using the TandemHeart during very high-risk PCI of the unprotected left main coronary artery in patients declined for CABG. Catheter Cardiovasc Interv. 2009;74:302–310. doi: 10.1002/ccd.22011. [DOI] [PubMed] [Google Scholar]
- 71.Kucukaksu S, Ergenoglu MU, Yildiz CE, et al. High-risk left main coronary artery bypass surgery supported by the Impella Recover LP 2.5 assist device: an alternative insertion technique. Heart Surg Forum. 2009;12:E324–E326. doi: 10.1532/HSF98.20091076. [DOI] [PubMed] [Google Scholar]
- 72.Rajagopal V, Steahr G, Wilmer CI, et al. A novel percutaneous mechanical biventricular bridge to recovery in severe cardiac allograft rejection. J Heart Lung Transplant. 2010;29:93–95. doi: 10.1016/j.healun.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 73.Atoui R, Samoukovic G, Al-Tuwaijri F, et al. The use of the Impella LP 2.5 percutaneous microaxial ventricular assist device as hemodynamic support during high-risk abdominal surgery. J Card Surg. 2009;25:238–240. doi: 10.1111/j.1540-8191.2009.00951.x. [DOI] [PubMed] [Google Scholar]
- 74.Andrade JG, Al-Saloos H, Jeewa A, et al. Facilitated cardiac recovery in fulminant myocarditis: pediatric use of the Impella LP 5.0 pump. J Heart Lung Transplant. 2010;29:96–97. doi: 10.1016/j.healun.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 75.Gregoric ID, Loyalka P, Radovancevic R, et al. TandemHeart as a rescue therapy for patients with critical aortic valve stenosis. Ann Thorac Surg. 2009;88:1822–1826. doi: 10.1016/j.athoracsur.2009.08.002. [DOI] [PubMed] [Google Scholar]


