Summary
Wnt/β-catenin signaling plays multiple roles in liver development including hepatoblast proliferation and differentiation, hepatocyte differentiation, and liver zonation. A positive role for Wnt/β-catenin signaling in liver specification was recently identified in zebrafish; however, its underlying cellular mechanisms are unknown. Here, we present two cellular mechanisms by which Wnt/β-catenin signaling regulates liver specification. First, using lineage tracing we show that ectopic hepatoblasts, which form in the endoderm posterior to the liver upon activation of Wnt/β-catenin signaling, are derived from the direct conversion of non-hepatic endodermal cells, but not from the posterior migration of hepatoblasts. We found that endodermal cells at the 4–6th somite levels, which normally give rise to the intestinal bulb or intestine, gave rise to hepatoblasts in Wnt8a-overexpressing embryos, and that the distribution of traced endodermal cells in Wnt8a-overexpressing embryos was similar to that in controls. Second, by using an endoderm-restricted cell-transplantation technique and mosaic analysis with transgenic lines that cell-autonomously suppress or activate Wnt/β-catenin signaling upon heat-shock, we show that Wnt/β-catenin signaling acts cell-autonomously in endodermal cells to induce hepatic conversion. Altogether, these data demonstrate that Wnt/β-catenin signaling can induce the fate-change of non-hepatic endodermal cells into a liver fate in a cell-autonomous manner. These findings have potential application to hepatocyte differentiation protocols for the generation of mature hepatocytes from induced pluripotent stem cells, supplying a sufficient amount of hepatocytes for cell-based therapies to treat patients with severe liver diseases.
Key words: Hepatoblast, Liver specification, Hepatic conversion, Endoderm, Zebrafish, Fate-change
Introduction
The liver develops from the foregut endoderm in response to hepatic inducing signals secreted from adjacent mesodermal tissues. Fgf from the cardiac mesoderm (Jung et al., 1999) and Bmp from the septum transversum mesenchyme (Rossi et al., 2001) were first identified in mice as hepatic inducing signals. Fgf ligands, FGF1 and FGF2, could replace the cardiac mesoderm to induce the hepatic marker albumin from the foregut endoderm in explant cultures, whereas the soluble extracellular domain of Fgf receptors blocked its induction (Jung et al., 1999). A Bmp inhibitor, noggin, blocked albumin induction from the foregut endoderm co-cultured with the cardiac mesoderm (Rossi et al., 2001). Furthermore, the role of Fgf and Bmp as hepatic inducing signals has been confirmed in chick (Zhang et al., 2004) and zebrafish (Shin et al., 2007). Chick explant culture studies showed that several Fgfs, including FGF1 and FGF2, could replace the cardiac mesoderm to induce hepatic markers HHEX and albumin in the anterior lateral endoderm, whereas noggin blocked the expression of these markers (Zhang et al., 2004). Moreover, implantation of noggin-expressing cells repressed HHEX expression in anterior lateral endodermal cells close to the implantation site, whereas that of BMP2-containing beads induced ectopic HHEX expression (Zhang et al., 2004). In zebrafish, the inhibition of Fgf and Bmp signaling after gastrulation via the overexpression of the dominant-negative forms of their receptors blocks the expression of the hepatoblast markers hhex and prox1 in the liver-forming region (Shin et al., 2007).
In addition to Bmp and Fgf signaling, Wnt signaling has relatively recently been implicated in liver specification. wnt2 (Poulain and Ober, 2011) and wnt2bb (Ober et al., 2006) are expressed in the anterior lateral plate mesoderm adjacent to the liver-forming region. The expression of the hepatoblast markers hhex and Prox1 is greatly reduced in wnt2bb mutant embryos, which exhibit very small liver buds (Ober et al., 2006). Importantly, wnt2 knockdown in wnt2bb mutants appears to completely block hhex and Prox1 expression in the liver-forming region and results in embryos without liver buds (Poulain and Ober, 2011), indicating that Wnt signaling is required for liver specification. The positive role of Wnt signaling in liver specification has been further supported from Xenopus data suggesting hepatic conversion. When β-catenin signaling was activated from Stage 30, a time after normal liver specification, the expression of a hepatic marker for1 greatly expanded and was ectopically detected in endodermal regions posterior to the original liver (McLin et al., 2007).
Recent zebrafish studies (Poulain and Ober, 2011; Shin et al., 2011) suggest Wnt as a hepatic inducing signal. Global overexpression of Wnt2bb (Poulain and Ober, 2011) or Wnt8a (Shin et al., 2011) results in ectopic hepatoblasts and subsequently hepatocytes in the posterior endodermal region, which normally gives rise to the intestinal bulb or intestine, whereas global activation of Bmp or Fgf signaling does not result in ectopic liver formation (Shin et al., 2011). However, it is not clear whether the ectopic hepatoblasts are derived from the direct conversion of non-hepatic endodermal cells into hepatoblasts or from the posterior migration of hepatoblasts in the liver-forming region or both. Determining this issue will clearly reveal whether Wnt is a hepatic inducing signal or not. To address the issue, we traced the lineage of endodermal cells, and found that ectopic hepatoblasts are derived from the direct conversion of non-hepatic endodermal cells, demonstrating that Wnt is a bona fide hepatic inducing signal. In addition, we addressed whether Wnt/β-catenin signaling regulates hepatic induction directly, cell-autonomously, or by controlling signaling from surrounding cells, non-cell-autonomously. To determine the cell-autonomy, we used mosaic analysis with the transgenic lines that cell-autonomously activate or suppress Wnt/β-catenin signaling upon heat-shock, and found that Wnt/β-catenin signaling acts cell-autonomously in endodermal cells. Altogether, our data demonstrate that Wnt/β-catenin signaling cell-autonomously induces non-hepatic endodermal cells to a liver fate.
Materials and Methods
Zebrafish strains
Embryos and adult fish were raised and maintained under standard laboratory conditions (Westerfield, 2000). We used the following transgenic lines: Tg(hsp70l:wnt8a-GFP)w34 (Weidinger et al., 2005) [referred to here as Tg(hs:wnt8a)], Tg(hsp70l:Mmu.Axin1-YFP)w35 (Kagermeier-Schenk et al., 2011) [referred to here as Tg(hs:axin1)], Tg(hse:ca-β-catenin-MYC,EGFP)w75 (Martin and Kimelman, 2012) [referred to here as Tg(hse:ca-β-catenin-MYC)], Tg(fabp10:RFP,ela3l:EGFP)gz12 (Korzh et al., 2008) [referred to here as Tg(fabp10:RFP)], and Tg(ptf1a:EGFP)jh1 (Godinho et al., 2005).
Heat-shock conditions
Embryos were heat-shocked at various stages by transferring them into egg water pre-warmed to 38°C [Tg(hs:wnt8a) and Tg(hs:axin1)] or 39.5°C [Tg(hse:ca-β-catenin-MYC)] and kept at this temperature for 25 or 15 minutes, respectively.
Whole-mount immunostaining
Whole-mount immunostaining was performed as previously described (Dong et al., 2007), using the following antibodies: rabbit polyclonal anti-Prox1 (1:1000; Chemicon), guinea pig polyclonal anti-Pdx1 (1:200; generous gift from C. Wright, Vanderbilt University), mouse monoclonal anti-MYC (1:200; Sigma), Alexa Fluor 488-, 568-, and 647-conjugated secondary antibodies (1:500; Invitrogen), and DyLight 405-conjugated secondary antibody (1:200; Jackson laboratories).
Transplantation
Wild-type or Tg(hs:axin1) embryos were used as donors. Donor embryos were co-injected with sox32 mRNA and rhodamine dextran at the one- to four-cell stage, and donor cells were transplanted along the blastoderm margin of wild-type embryos at 4 hpf as previously described (Chung and Stainier, 2008). Transplants were heat-shocked at 17 or 26 hpf, and harvested at 36 hpf.
Lineage tracing
50 pg of kikume and 25 pg of sox32 mRNA were co-injected into a single cell at the 32-cell stage, resulting in the mosaic expression of Kikume in the endoderm. The injected embryos were anesthetized at 25 hpf with 0.01% tricaine in egg water, and subsequently mounted in 1% low-melting agarose. Endodermal regions at each somite level from the 1st to 6th somite were excited by the 405-nm laser using the Zeiss LSM700 confocal microscope. After the photoconversion, embryos were released from the agarose, subsequently heat-shocked, and harvested at 40 hpf.
Results and Discussion
Enhancing Wnt/β-catenin signaling directly converts non-hepatic endodermal cells into hepatoblasts
To globally activate Wnt/β-catenin signaling, we used the Tg(hs:wnt8a) line that expresses Wnt8a under the heat-shock promoter (Weidinger et al., 2005). As previously reported (Shin et al., 2011), Wnt8a overexpression via heat-shock at 26 hours post-fertilization (hpf) resulted in ectopic hepatoblasts in the endoderm posterior to the liver-forming region at 40 hpf (Fig. 1B, bracket). The hepatoblast marker Prox1+ domain greatly expanded posteriorly, whereas the non-hepatic endodermal marker Pdx1+ domain was concomitantly restricted (Fig. 1B). These expression patterns suggest that the ectopic hepatoblasts may be derived from Pdx1+ non-hepatic endodermal cells. An alternative explanation is that hepatoblasts in the liver-forming region migrate posteriorly upon Wnt8a overexpression since Wnt/β-catenin signaling is implicated in migration in other tissues (Yook et al., 2006; Aman and Piotrowski, 2008). To distinguish these possibilities, we sought to determine the origin of ectopic hepatoblasts upon Wnt8a overexpression. We traced the lineage of endodermal cells using the photoconversion of Kikume protein, which can be irreversibly converted from green (KikGreen) to red (KikRed) fluorescence upon UV irradiation (Tsutsui et al., 2005). To restrict the Kikume to endodermal cells, kikume mRNA was co-injected with sox32 mRNA into a single cell at the 32-cell stage (diIorio et al., 2007) and the Kikume was photoconverted in a defined region just prior to heat-shock. Because Sox32 is a transcription factor sufficient for endoderm induction in zebrafish (Dickmeis et al., 2001; Kikuchi et al., 2001; Sakaguchi et al., 2001), sox32 mRNA injection converts cells to an endodermal fate (Kikuchi et al., 2001). To carefully define the endodermal region that does not express Prox1, we examined Prox1 and Pdx1 expression at 26 hpf in detail. Prox1 is expressed in endodermal cells at the 1st to 3rd somite levels, whereas Pdx1 is expressed at the 2nd to 5th somite levels (Fig. 1C,D). Pdx1+ cells at the 2nd to 3rd somite levels are located dorsally to Prox1+ cells at these levels. Since endodermal cells at the 5th somite level are Pdx1+ and Prox1−, this region was selected for photoconversion. KikRed+ cells became Prox1+ in Wnt8a-overexpressing embryos (Fig. 1G–H′, open arrowheads), whereas they were Prox1− in controls (Fig. 1F, arrowheads). Moreover, all KikRed+ endodermal cells were Prox1+ and Pdx1− in Wnt8a-overexpressing embryos (Fig. 1I). These data suggest that all endodermal cells at the 5th somite level possess hepatic competence, the ability to respond to hepatic inducing signals, and demonstrate that Pdx1+ non-hepatic endodermal cells were directly converted into Prox1+ hepatoblasts upon Wnt8a overexpression.
Fig. 1. Non-hepatic endodermal cells are directly converted into hepatoblasts upon Wnt8a overexpression.
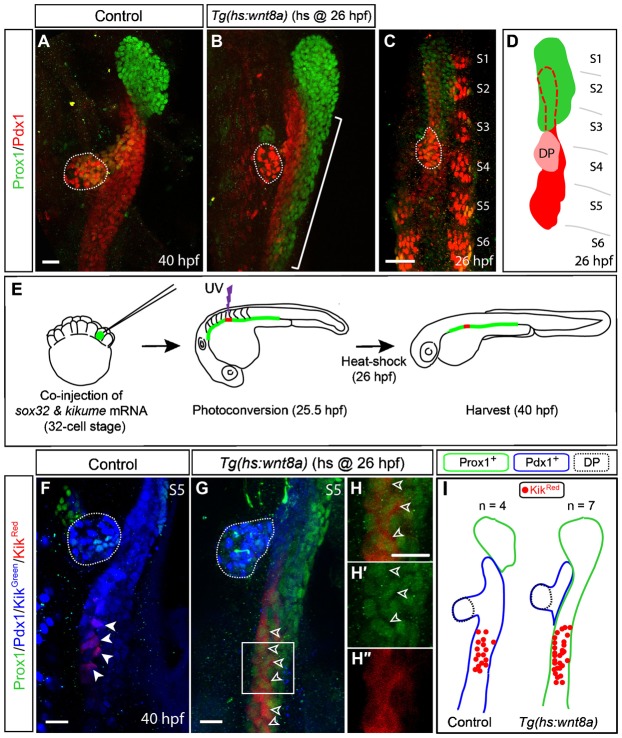
(A,B) Wild-type or Tg(hs:wnt8a) embryos were heat-shocked at 26 hpf, harvested at 40 hpf, and processed for immunostaining with anti-Prox1 (green) and anti-Pdx1 (red) antibodies. Ectopic hepatoblasts were present in the endoderm posterior to the liver-forming region in Wnt8a-overexpressing embryos (B, bracket). (C,D) anti-Prox1 and anti-Pdx1 staining of wild-type embryos at 26 hpf and a schematic representation. Prox1 is expressed in endodermal cells at the 1st to 3rd somite levels, but not at the 4th to 6th somite levels. S1–S6 denotes the 1st–6th somite; the pink region indicates the dorsal pancreas (DP). Dashed lines in D outline the Pdx1+ region beneath the liver. (E) Cartoons illustrating cell-lineage tracing experiments. (F,G) Wild-type or Tg(hs:wnt8a) embryos were co-injected with sox32 and kikume mRNA into a single cell at the 32-cell stage, resulting in the mosaic expression of Kikume in the endoderm. Endodermal cells at the 5th somite level were photoconverted at 25.5 hpf and then heat-shocked. Excited cells emit red fluorescence (KikRed) instead of green (KikGreen). The embryos were harvested at 40 hpf and processed for immunostaining. To reveal the entire endoderm morphology including the dorsal pancreas (dotted lines), both Pdx1 and Kikgreen expression, which was detected using the same laser, are shown. KikRed+ cells expressed Prox1 (green) in Wnt8a-overexpressing embryos (G, open arrowheads), but not in controls (F, arrowheads). (H–H″) Higher magnification images of the square region in G. (I) Cartoon incorporating all lineage-tracing data. Green and blue lines outline Prox1+ and Pdx1+ domains, respectively, and n indicates the number of embryos. Dotted lines outline the dorsal pancreas (DP). All confocal images are ventral views with anterior up. Scale bars, 20 µm.
Enhancing Wnt/β-catenin signaling does not induce the posterior migration of hepatoblasts
Although we showed that non-hepatic endodermal cells were converted into hepatoblasts upon Wnt8a overexpression, it is still possible that both the direct conversion and the posterior migration of hepatoblasts could contribute to ectopic hepatoblast formation upon Wnt8a overexpression. To test this possibility, endodermal regions at the 1st, 2nd, and 3rd somite levels, which contain hepatoblasts (Fig. 1D), were excited for photoconversion and the distribution of KikRed+ endodermal cells were compared between Wnt8a-overexpressing and control embryos. Their distribution in Wnt8a-overexpressing embryos was similar to that in controls although the Prox1+ domain greatly expanded posteriorly in Wnt8a-overexpressing embryos (supplementary material Fig. S1A–C; Fig. 2A–H). We also examined the distribution of endodermal cells from the 4th and 6th somite levels, and found that their distribution in Wnt8a-overexpressing embryos was similar to that in controls (Fig. 2I–M; supplementary material Fig. S1D–G). These lineage-tracing data indicate that the posterior migration of hepatoblasts did not contribute to ectopic hepatoblast formation upon Wnt8a overexpression.
Fig. 2. Wnt8a overexpression does not induce the posterior migration of hepatoblasts.
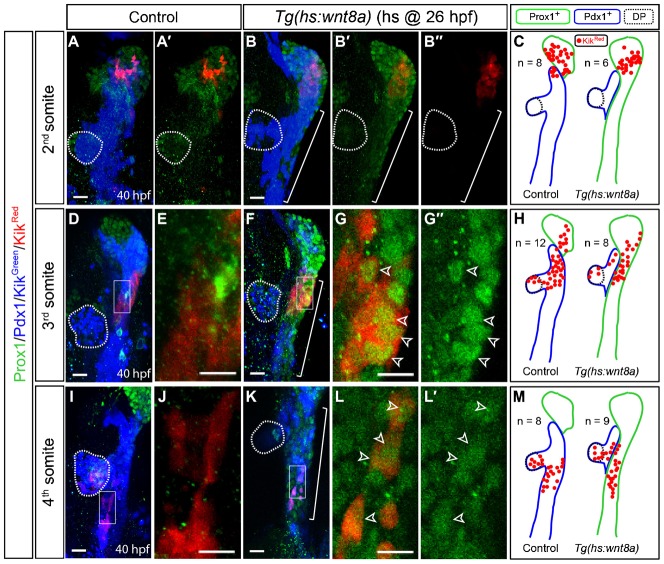
The lineage of endodermal cells at the 2nd, 3rd, or 4th somite level was traced. (A–C) Lineage tracing of endodermal cells at the 2nd somite level. The distribution of KikRed+ cells in control is similar to that in Wnt8a-overexpressing embryos. Cartoons incorporating all lineage-tracing data (C). (D–H) Lineage tracing of endodermal cells at the 3rd somite level. Most KikRed+ cells expressed Prox1 (green) in Wnt8a-overexpressing embryos (G, open arrowheads), whereas a small number of KikRed+ cells did in controls (E). Higher magnification images of the square regions in D and F are shown in E and G, respectively, without Pdx1 and Kikgreen expression. (I–M) Lineage tracing of endodermal cells at the 4th somite. Many KikRed+ cells expressed Prox1 (green) in Wnt8a-overexpressing embryos (L, open arrowheads), whereas none of the cells did in controls (J). Higher magnification images of the square regions in I and K are shown in J and L, respectively, without Pdx1 and Kikgreen expression. Green and blue lines outline Prox1+ and Pdx1+ domains, respectively, and n indicates the number of embryos (C,H,M). Brackets delineate the endodermal region in which ectopic hepatoblasts are present (B–B″,F,K). Dotted lines outline the dorsal pancreas (DP). Ventral views, anterior up. Scale bars, 20 µm.
Upon comparing the distribution of endodermal cells, we found that all KikRed+ endodermal cells from the 2nd and 6th somite levels became Prox1+ hepatoblasts in Wnt8a-overexpressing embryos, whereas 82% (n = 34) and 0% (n = 33) from the 2nd and 6th somite levels, respectively, were Prox1+ hepatoblasts in controls (Fig. 2C; supplementary material Fig. S1G). However, some KikRed+ endodermal cells from the 3rd and 4th somite levels still expressed Pdx1, but not Prox1, in Wnt8a-overexpressing embryos (Fig. 2H,M). These data suggest that all endodermal cells at the 2nd, 5th and 6th somite levels possess hepatic competence, at least, by 26 hpf, and that some endodermal cells at the 3rd and 4th somite levels have already been committed to non-hepatic endodermal cells at 26 hpf. In particular, the dorsal pancreas is located at the 3rd and 4th somite levels at 26 hpf (Fig. 1D); these dorsal pancreatic cells were not converted into hepatoblasts upon Wnt8a overexpression (Fig. 2H,M, dotted circles). Altogether, these lineage-tracing data demonstrate that ectopic hepatoblasts are derived from the direct conversion of non-hepatic endodermal cells, not from the migration of hepatoblasts, upon Wnt8a overexpression (supplementary material Fig. S2).
Wnt/β-catenin signaling cell-autonomously induces hepatoblasts
Since Wnt8a was globally overexpressed in our studies and Wnt8a is a secreted protein that can activate Wnt/β-catenin signaling in neighboring cells, the lineage-tracing data cannot help one determine whether Wnt/β-catenin signaling acts cell-autonomously or non-cell-autonomously in liver specification. To address this question, we first performed loss-of-function experiments using an endoderm-restricted cell-transplantation technique. To cell-autonomously suppress Wnt/β-catenin signaling, we used the Tg(hs:axin1) line that expresses Axin1, a central component of the β-catenin destruction complex, under the heat-shock promoter (Kagermeier-Schenk et al., 2011). When Axin1 was globally overexpressed via heat-shock at 17 hpf, liver size was greatly reduced in Axin1-overexpressing embryos compared to controls (supplementary material Fig. S3), as also seen in embryos that overexpressed Dkk1, a secreted inhibitor of the Wnt/β-catenin signaling pathway (Shin et al., 2011). This indicates that Axin1 overexpression blocks Wnt/β-catenin signaling as efficiently as Dkk1 overexpression. Donor embryos were co-injected with a lineage tracer, rhodamine dextran, and sox32 mRNA to convert donor cells into endoderm. Since wnt2bb starts to be expressed in the anterior lateral plate mesoderm from 18 hpf (Ober et al., 2006) and liver specification appears to occur at 22 hpf (Field et al., 2003; Wallace and Pack, 2003; Ober et al., 2006; Noël et al., 2008), the transplants were heat-shocked at 17 hpf, prior to wnt2bb expression and liver specification. Axin1-overexpressing donor cells failed to contribute to the liver (Fig. 3C, arrows), whereas wild-type donor cells significantly contributed to the liver (40%, n = 173) (Fig. 3B, arrowheads). Although a few Axin1-overexpressing donor cells (4%, n = 104) were Prox1+, they were located exclusively at the margin of the liver (Fig. 3D, open arrowheads). When Wnt/β-catenin signaling was blocked via heat-shock at 26 hpf, the stage after liver specification, Axin1-overexpressing donor cells significantly contributed to the liver (43%, n = 77) (Fig. 3E, arrowheads), suggesting that Wnt/β-catenin signaling is not required for the maintenance of Prox1 expression in hepatoblasts and their location in the liver. These transplantation data indicate that Wnt/β-catenin signaling is cell-autonomously required for liver specification.
Fig. 3. Endodermal cells with Wnt/β-catenin signaling repressed fail to contribute to the liver.
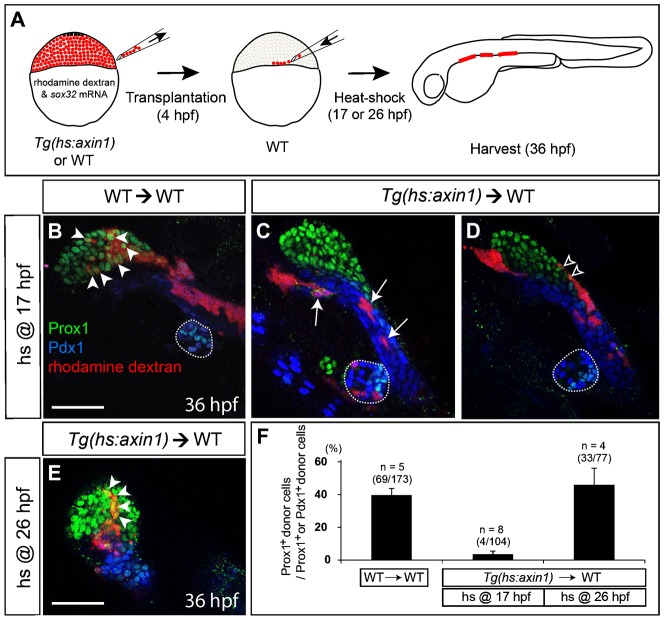
(A) Cartoons illustrating transplantation experiments. Wild-type or Tg(hs:axin1) embryos were co-injected with sox32 mRNA and rhodamine dextran at the 1- to 4-cell stage. Donor cells from the injected embryos were transplanted into a wild-type embryo at 4 hpf. The transplants were heat-shocked at 17 (B–D) or 26 (E) hpf, harvested at 36 hpf, and processed for immunostaining. (B,C) Wild-type donor cells expressed Prox1 (green) in the liver-forming region (B, arrowheads), whereas Axin1-overexpressing donor cells heat-shocked at 17 hpf expressed Pdx1 (blue) but not Prox1 (C, arrows). (D) A few Axin1-overexpressing donor cells were located at the margin of the liver-forming region (open arrowheads). (E) Axin1-overexpressing donor cells heat-shocked at 26 hpf expressed Prox1 and were located in the liver-forming region (arrowheads). (F) Quantification of the transplantation experiments. Percentages of Prox1+ donor cells relative to Prox1+ or Pdx1+ donor cells are shown. Error bars represent the standard deviation; n indicates the number of embryos. Dotted lines outline the dorsal pancreas. Ventral views, anterior up (B–E). Scale bars, 50 µm.
We next performed gain-of-function experiments using mosaic analysis. To cell-autonomously activate Wnt/β-catenin signaling, we used the Tg(hse:ca-β-catenin-MYC) line that expresses both MYC-tagged constitutive-active β-catenin and EGFP under a bidirectional promoter with the multimerized heat-shock elements (Martin and Kimelman, 2012). The MYC epitope tag contains six copies of 13 amino acids from the human MYC gene that is commonly used to reveal the location of tagged proteins. The highly mosaic expression of ca-β-catenin and EGFP in this transgenic line allowed us to perform mosaic analysis without transplantation. ca-β-catenin-expressing cells were detected using antibodies that recognize the MYC epitope tag fused to ca-β-catenin. Because endodermal cells around and in the dorsal pancreas are not affected by Wnt8a overexpression, we examined the endodermal region posterior to the dorsal pancreas since those cells were converted from a Pdx1+ to a Prox1+ hepatoblast fate in Wnt8a-overexpressing embryos (Fig. 1G). In this region, most ca-β-catenin-expressing endodermal cells expressed Prox1 (83%, n = 59) (Fig. 4), whereas none of the ca-β-catenin-negative cells expressed Prox1, demonstrating that Wnt/β-catenin signaling cell-autonomously acts in hepatoblast induction. ca-β-catenin-expressing cells in the region (n = 59) can be divided into three groups in terms of Prox1 and Pdx1 expression (Fig. 4E): Prox1+/Pdx1− (76%) (Fig. 4B,D, arrows), Prox1+/Pdx1+ (7%) (Fig. 4C, arrowheads), and Prox1−/Pdx1+ (17%) (Fig. 4D, open arrowheads). By comparing MYC expression levels among these groups, we found that strong MYC+ cells were Prox1+/Pdx1−, whereas weak MYC+ cells were Prox1−/Pdx1+ (Fig. 4D, arrow versus open arrowheads). Since the MYC expression level represents the expression level of ca-β-catenin, this correlation and the presence of the double-positive cells suggest that the threshold of Wnt/β-catenin signaling for Prox1 induction is lower than that for Pdx1 repression in non-hepatic endodermal cells. Altogether, both the loss- and gain-of-function data demonstrate that Wnt/β-catenin signaling cell-autonomously induces hepatoblasts. A recent report that the Wnt receptor fzd5 is expressed in the endoderm, including the liver-forming region and the non-hepatic endodermal region, and interacts genetically with wnt2 and wnt2bb (Poulain and Ober, 2011) also supports the cell-autonomous role of Wnt/β-catenin signaling in liver specification.
Fig. 4. Wnt/β-catenin signaling cell-autonomously induces hepatoblasts.
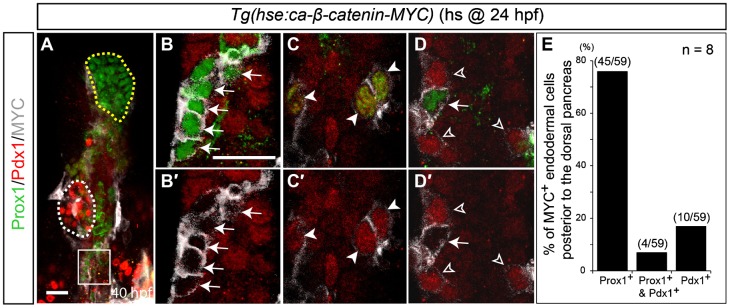
Tg(hse:ca-β-catenin-MYC) embryos were heat-shocked at 24 hpf, harvested at 40 hpf, and processed for anti-Prox1 (green), anti-Pdx1 (red) and anti-MYC (gray) staining. ca-β-catenin-expressing cells were detected by anti-MYC antibodies. (A,B) Most MYC+ cells in the endoderm posterior to the dorsal pancreas expressed Prox1 (B, arrows) but not Pdx1. Since the Prox1 antibody has a background staining problem, cytoplasmic or membrane-like staining is background staining, whereas nuclear Prox1 staining is real staining. White and yellow dotted lines in A outline the dorsal pancreas and liver, respectively. Higher magnification images of the square region in A are shown in B,B′. (C,C′) Some MYC+ cells expressed both Prox1 and Pdx1 (arrowheads). (D,D′) Weak MYC+ cells were Prox1−/Pdx1+, whereas strong MYC+ cells were Prox1+/Pdx1− (open arrowheads versus arrow). Ventral views, anterior up. Scale bars, 20 µm. (E) Quantification of MYC+ cells in the endoderm posterior to the dorsal pancreas based on Prox1 and Pdx1 expression. n indicates the number of embryos.
In our studies, Wnt8a was overexpressed at 26 hpf, a time after liver specification, thereby hepatic conversion of non-hepatic endodermal cells, but not liver specification of non-committed endodermal cells, was investigated. However, the findings here can be applied to earlier stages because of previous reports showing that Wnt8a overexpression at 14 hpf (Goessling et al., 2008) and Wnt2bb or Wnt2 overexpression at 18 hpf (Poulain and Ober, 2011), a time prior to liver specification, increased the expression of liver marker genes and induced ectopic liver formation. In order to determine whether Wnt/β-catenin signaling induces the fate-change of endodermal cells into hepatoblasts, we chose 26 hpf, a time when the non-hepatic endodermal region can be clearly defined, thereby allowing for the unambiguous labeling of non-hepatic endodermal cells for lineage tracing. Data from this lineage tracing (Figs 1, 2) together with the previous reports (Goessling et al., 2008; Poulain and Ober, 2011) indicate that Wnt/β-catenin signaling can induce hepatic conversion of both non-committed and committed endodermal cells, revealing its role in liver specification.
Although the positive role of Wnt/β-catenin signaling in liver specification has been reported in zebrafish (Ober et al., 2006; Goessling et al., 2008; Poulain and Ober, 2011; Shin et al., 2011) and our data demonstrate the fate-change of non-hepatic endodermal cells into hepatoblasts upon Wnt8a overexpression, such findings have not been reported in mouse yet. Mouse genetic studies showed that the conditional knockout of β-catenin in hepatoblasts, at least, from embryonic day (E) 9.5 results in underdeveloped livers after E12, indicating the role of Wnt/β-catenin signaling in hepatoblast expansion (Tan et al., 2008). In this study, it was not examined whether β-catenin deletion occurred in foregut endodermal cells before E8.25, the stage at which liver specification occurs in mouse (Gualdi et al., 1996; Jung et al., 1999). To determine the role of Wnt/β-catenin signaling in liver specification, it is critical to delete β-catenin before liver specification since in our studies Axin1 overexpression before, but not after, liver specification prevented Axin1-overexpressing endodermal cells from giving rise to hepatoblasts. Another mouse genetic study showed that the activation of Wnt/β-catenin signaling in Shh+ endodermal cells could convert the esophagus and stomach endoderm to lung progenitor cells (Goss et al., 2009; Harris-Johnson et al., 2009). In this study, the activation achieved by the Cre-loxP system occurred in the endoderm anterior to the liver, but not in the posterior endoderm, because the Shh:Cre line expresses Cre in the anterior endoderm. In our study, Wnt8a overexpression after liver specification induced ectopic hepatoblasts in the posterior, but not anterior, endoderm. In addition, a Xenopus study showed that activation of β-catenin signaling after liver specification resulted in enhanced liver buds and ectopic liver cells in the endoderm posterior to the original liver (McLin et al., 2007). Therefore, future experiments activating Wnt/β-catenin signaling in the posterior endoderm after liver specification using other Cre lines, such as the Foxa3:Cre or Pdx1:Cre line, will determine whether Wnt/β-catenin signaling can induce hepatic conversion in mice as well.
In contrast to its role in liver specification, an inhibitory role for Wnt/β-catenin signaling in anterior endoderm pattering, whose proper pattering is prerequisite for later liver specification, has been reported in other systems. Wnt/β-catenin signaling has to be suppressed during early somitogenesis to maintain the identity of the foregut. In Xenopus, the suppression of Wnt/β-catenin signaling during early somitogenesis caused posterior endodermal cells to adopt a foregut fate, resulting in the ectopic expression of the foregut marker hhex and later liver markers in the intestine (McLin et al., 2007). In contrast, activation of Wnt/β-catenin signaling caused anterior endodermal cells to adopt a hindgut fate, resulting in a failure of liver formation (McLin et al., 2007). Based on this role of Wnt/β-catenin signaling in the anterior–posterior (A–P) patterning of the endoderm, an in vitro differentiation protocol for the generation of intestinal tissues from human embryonic and induced pluripotent stem cells was established. In this protocol, WNT3a together with FGF4 was used to induce posterior endoderm patterning after activin-induced definitive endoderm formation, which allows for subsequent hindgut specification (Spence et al., 2011).
After the A–P pattering of the endoderm, Wnt/β-catenin signaling plays a positive role in hepatoblast proliferation. Repression of β-catenin signaling via β-catenin antisense oligonucleotides reduced hepatoblast proliferation in mouse (Monga et al., 2003). Similarly in chicken, overexpression of constitutively active β-catenin significantly increased liver size, whereas blocking β-catenin activity reduced liver size (Suksaweang et al., 2004). Although Wnt8a overexpression at 26 hpf did not significantly increase hepatoblast proliferation at 36 hpf in zebrafish (Shin et al., 2011), its overexpression at 14 hpf greatly increased liver size (Goessling et al., 2008) and Wnt2 or Wnt2bb overexpression at 18 hpf resulted in a two-fold increase in hepatoblast proliferation at 26 hpf (Poulain and Ober, 2011), indicating a role for Wnt/β-catenin signaling in hepatoblast proliferation in zebrafish as well.
In zebrafish, the timing of the A–P patterning is well separated from that of liver specification. The A–P patterning appears to occur in early somitogenesis (10–11 hpf) (Goessling et al., 2008), and liver specification occurs after the 18-somite stage (18 hpf) (Ober et al., 2006). Wnt8a overexpression at the one-somite stage (10 hpf) blocked liver development, whereas its overexpression at the 10-somite stage (14 hpf) increased liver size and greatly induced the expression of liver marker genes (Goessling et al., 2008). This temporal separation of these two events allows one to manipulate Wnt/β-catenin signaling in a specific developmental time-window, such as after the A–P patterning but before liver specification, thereby leading to the identification of the role of Wnt/β-catenin signaling in liver specification. However, the two events do not appear to be well separated from each other in mouse since liver specification occurs at the 7-somite stage (Gualdi et al., 1996), which may make it hard to manipulate Wnt/β-catenin signaling in the specific developmental time-window. As discussed above, it is not clear yet whether the role of Wnt/β-catenin signaling in liver specification is unique to zebrafish or common in mouse.
Wnt/β-catenin signaling is unique among the three hepatic inducing signaling pathways because its activation induces the conversion of non-hepatic endodermal cells into hepatoblasts even after normal liver specification occurs. In zebrafish, Bmp2b overexpression prior to liver specification induces ectopic hhex expression at the expense of pdx1 expression, but Bmp2b overexpression after liver specification does not induce hhex expression (Chung et al., 2008). Chick implantation studies showed that Bmp2-containing beads induced ectopic HHEX expression in endodermal cells if they were implanted at stage 5, before somitogenesis (Zhang et al., 2004), but it was not addressed whether later implantations could induce ectopic HHEX expression. This unique feature of Wnt/β-catenin signaling, the ability to directly convert endodermal cells outside the normal liver region to a liver fate, may allow for the improvement of current protocols to differentiate hepatocytes from human embryonic or induced pluripotent stem cells in vitro. Understanding of embryonic liver development has significantly contributed to these types of protocols (Behbahan et al., 2011). The stem cells are first differentiated into endodermal cells (D'Amour et al., 2005), subsequently into hepatoblasts, and finally into hepatocytes, mimicking liver developmental processes. BMP2/4 and FGF2/4 are currently used to induce hepatoblasts from endodermal cells in this process (Gouon-Evans et al., 2006; Cai et al., 2007). The Duncan group has recently established a hepatocyte differentiation protocol that can elicit the efficient and reproducible generation of hepatocytes from human embryonic or induced pluripotent stem cells (Si-Tayeb et al., 2010). This protocol allowed them to obtain more than 80% definitive endodermal cells, subsequently more than 80% hepatoblasts, and finally about 80% albumin-expressing hepatocytes. However, further improvement of the protocol is needed for cell-based therapies such as transplantation, because undifferentiated cells could cause tumor formation. In addition, the differentiated hepatocytes obtained are not fully mature, requiring further improvement. Our results suggest that appropriate applications of Wnt ligands and/or agonists into culture conditions after the A–P patterning of the definitive endoderm may induce better and/or more hepatoblasts, which could be further differentiated into mature hepatocytes suitable for transplantation to treat patients with severe liver diseases.
Supplementary Material
Acknowledgments
We thank Gilbert Weidinger for the Tg(hs:axin1) line, Lori Simmons-Stalter for fish care, Jian Zou for assistance with cell-transplantation, Tae-Young Choi and Michael Tsang for discussions, and Mehwish Khaliq for critical reading of the manuscript. This work was supported in part by grants from the American Liver Foundation and the UPMC Competitive Medical Research Fund to D.S.
Footnotes
Competing interests: The authors have no competing interests to declare.
References
- Aman A., Piotrowski T. (2008). Wnt/β-catenin and Fgf signaling control collective cell migration by restricting chemokine receptor expression. Dev. Cell 15, 749–761 10.1016/j.devcel.2008.10.002 [DOI] [PubMed] [Google Scholar]
- Behbahan I. S., Duan Y. Y., Lam A., Khoobyari S., Ma X. C., Ahuja T. P., Zern M. A. (2011). New approaches in the differentiation of human embryonic stem cells and induced pluripotent stem cells toward hepatocytes. Stem Cell Reviews and Reports 7, 748–759 10.1007/s12015-010-9216-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Zhao Y., Liu Y. X., Ye F., Song Z. H., Qin H., Meng S., Chen Y. Z., Zhou R. D., Song X. J.et al. (2007). Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology 45, 1229–1239 10.1002/hep.21582 [DOI] [PubMed] [Google Scholar]
- Chung W. S., Stainier D. Y. R. (2008). Intra-endodermal interactions are required for pancreatic β cell induction. Dev. Cell 14, 582–593 10.1016/j.devcel.2008.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W. S., Shin C. H., Stainier D. Y. (2008). Bmp2 signaling regulates the hepatic versus pancreatic fate decision. Dev. Cell 15, 738–748 10.1016/j.devcel.2008.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amour K. A., Agulnick A. D., Eliazer S., Kelly O. G., Kroon E., Baetge E. E. (2005). Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 23, 1534–1541 10.1038/nbt1163 [DOI] [PubMed] [Google Scholar]
- Dickmeis T., Mourrain P., Saint–Etienne L., Fischer N., Aanstad P., Clark M., Strähle U., Rosa F. (2001). A crucial component of the endoderm formation pathway, CASANOVA, is encoded by a novel sox-related gene. Genes Dev. 15, 1487–1492 10.1101/gad.196901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- diIorio P., Alexa K., Choe S. K., Etheridge L., Sagerström C. G. (2007). TALE-family homeodomain proteins regulate endodermal sonic hedgehog expression and pattern the anterior endoderm. Dev. Biol. 304, 221–231 10.1016/j.ydbio.2006.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong P. D., Munson C. A., Norton W., Crosnier C., Pan X., Gong Z., Neumann C. J., Stainier D. Y. (2007). Fgf10 regulates hepatopancreatic ductal system patterning and differentiation. Nat. Genet. 39, 397–402 10.1038/ng1961 [DOI] [PubMed] [Google Scholar]
- Field H. A., Ober E. A., Roeser T., Stainier D. Y. (2003). Formation of the digestive system in zebrafish. I. Liver morphogenesis. Dev. Biol. 253, 279–290 10.1016/S0012-1606(02)00017-9 [DOI] [PubMed] [Google Scholar]
- Godinho L., Mumm J. S., Williams P. R., Schroeter E. H., Koerber A., Park S. W., Leach S. D., Wong R. O. (2005). Targeting of amacrine cell neurites to appropriate synaptic laminae in the developing zebrafish retina. Development 132, 5069–5079 10.1242/dev.02075 [DOI] [PubMed] [Google Scholar]
- Goessling W., North T. E., Lord A. M., Ceol C., Lee S., Weidinger G., Bourque C., Strijbosch R., Haramis A. P., Puder M.et al. (2008). APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Dev. Biol. 320, 161–174 10.1016/j.ydbio.2008.05.526 [DOI] [PubMed] [Google Scholar]
- Goss A. M., Tian Y., Tsukiyama T., Cohen E. D., Zhou D., Lu M. M., Yamaguchi T. P., Morrisey E. E. (2009). Wnt2/2b and β-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev. Cell 17, 290–298 10.1016/j.devcel.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouon–Evans V., Boussemart L., Gadue P., Nierhoff D., Koehler C. I., Kubo A., Shafritz D. A., Keller G. (2006). BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat. Biotechnol. 24, 1402–1411 10.1038/nbt1258 [DOI] [PubMed] [Google Scholar]
- Gualdi R., Bossard P., Zheng M. H., Hamada Y., Coleman J. R., Zaret K. S. (1996). Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev. 10, 1670–1682 10.1101/gad.10.13.1670 [DOI] [PubMed] [Google Scholar]
- Harris–Johnson K. S., Domyan E. T., Vezina C. M., Sun X. (2009). β-Catenin promotes respiratory progenitor identity in mouse foregut. Proc. Natl. Acad. Sci. USA 106, 16287–16292 10.1073/pnas.0902274106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J., Zheng M., Goldfarb M., Zaret K. S. (1999). Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science 284, 1998–2003 10.1126/science.284.5422.1998 [DOI] [PubMed] [Google Scholar]
- Kagermeier–Schenk B., Wehner D., Ozhan–Kizil G., Yamamoto H., Li J., Kirchner K., Hoffmann C., Stern P., Kikuchi A., Schambony A.et al. (2011). Waif1/5T4 inhibits Wnt/β-catenin signaling and activates noncanonical Wnt pathways by modifying LRP6 subcellular localization. Dev. Cell 21, 1129–1143 10.1016/j.devcel.2011.10.015 [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Agathon A., Alexander J., Thisse C., Waldron S., Yelon D., Thisse B., Stainier D. Y. (2001). casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev. 15, 1493–1505 10.1101/gad.892301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzh S., Pan X., Garcia–Lecea M., Winata C. L., Pan X., Wohland T., Korzh V., Gong Z. (2008). Requirement of vasculogenesis and blood circulation in late stages of liver growth in zebrafish. BMC Dev. Biol. 8, 84 10.1186/1471-213X-8-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. L., Kimelman D. (2012). Canonical Wnt signaling dynamically controls multiple stem cell fate decisions during vertebrate body formation. Dev. Cell 22, 223–232 10.1016/j.devcel.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin V. A., Rankin S. A., Zorn A. M. (2007). Repression of Wnt/β-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development 134, 2207–2217 10.1242/dev.001230 [DOI] [PubMed] [Google Scholar]
- Monga S. P., Monga H. K., Tan X., Mulé K., Pediaditakis P., Michalopoulos G. K. (2003). β-catenin antisense studies in embryonic liver cultures: role in proliferation, apoptosis, and lineage specification. Gastroenterology 124, 202–216 10.1053/gast.2003.50000 [DOI] [PubMed] [Google Scholar]
- Noël E. S., Casal–Sueiro A., Busch–Nentwich E., Verkade H., Dong P. D. S., Stemple D. L., Ober E. A. (2008). Organ-specific requirements for Hdac1 in liver and pancreas formation. Dev. Biol. 322, 237–250 10.1016/j.ydbio.2008.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober E. A., Verkade H., Field H. A., Stainier D. Y. (2006). Mesodermal Wnt2b signalling positively regulates liver specification. Nature 442, 688–691 10.1038/nature04888 [DOI] [PubMed] [Google Scholar]
- Poulain M., Ober E. A. (2011). Interplay between Wnt2 and Wnt2bb controls multiple steps of early foregut-derived organ development. Development 138, 3557–3568 10.1242/dev.055921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J. M., Dunn N. R., Hogan B. L., Zaret K. S. (2001). Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 15, 1998–2009 10.1101/gad.904601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T., Kuroiwa A., Takeda H. (2001). A novel sox gene, 226D7, acts downstream of Nodal signaling to specify endoderm precursors in zebrafish. Mech. Dev. 107, 25–38 10.1016/S0925-4773(01)00453-1 [DOI] [PubMed] [Google Scholar]
- Shin D., Shin C. H., Tucker J., Ober E. A., Rentzsch F., Poss K. D., Hammerschmidt M., Mullins M. C., Stainier D. Y. (2007). Bmp and Fgf signaling are essential for liver specification in zebrafish. Development 134, 2041–2050 10.1242/dev.000281 [DOI] [PubMed] [Google Scholar]
- Shin D., Lee Y., Poss K. D., Stainier D. Y. R. (2011). Restriction of hepatic competence by Fgf signaling. Development 138, 1339–1348 10.1242/dev.054395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si–Tayeb K., Noto F. K., Nagaoka M., Li J. X., Battle M. A., Duris C., North P. E., Dalton S., Duncan S. A. (2010). Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 51, 297–305 10.1002/hep.23354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence J. R., Mayhew C. N., Rankin S. A., Kuhar M. F., Vallance J. E., Tolle K., Hoskins E. E., Kalinichenko V. V., Wells S. I., Zorn A. M.et al. (2011). Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109 10.1038/nature09691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suksaweang S., Lin C. M., Jiang T. X., Hughes M. W., Widelitz R. B., Chuong C. M. (2004). Morphogenesis of chicken liver: identification of localized growth zones and the role of β-catenin/Wnt in size regulation. Dev. Biol. 266, 109–122 10.1016/j.ydbio.2003.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X., Yuan Y., Zeng G., Apte U., Thompson M. D., Cieply B., Stolz D. B., Michalopoulos G. K., Kaestner K. H., Monga S. P. (2008). β-catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development. Hepatology 47, 1667–1679 10.1002/hep.22225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H., Karasawa S., Shimizu H., Nukina N., Miyawaki A. (2005). Semi-rational engineering of a coral fluorescent protein into an efficient highlighter. EMBO Rep. 6, 233–238 10.1038/sj.embor.7400361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace K. N., Pack M. (2003). Unique and conserved aspects of gut development in zebrafish. Dev. Biol. 255, 12–29 10.1016/S0012-1606(02)00034-9 [DOI] [PubMed] [Google Scholar]
- Weidinger G., Thorpe C. J., Wuennenberg–Stapleton K., Ngai J., Moon R. T. (2005). The Sp1-related transcription factors sp5 and sp5-like act downstream of Wnt/β-catenin signaling in mesoderm and neuroectoderm patterning. Curr. Biol. 15, 489–500 10.1016/j.cub.2005.01.041 [DOI] [PubMed] [Google Scholar]
- Westerfield M. (2000). The Zebrafish Book: A Guide For The Laboratory Use Of Zebrafish (Danio rerio). Eugene, OR: University of Oregon Press. [Google Scholar]
- Yook J. I., Li X. Y., Ota I., Hu C., Kim H. S., Kim N. H., Cha S. Y., Ryu J. K., Choi Y. J., Kim J.et al. (2006). A Wnt-Axin2-GSK3β cascade regulates Snail1 activity in breast cancer cells. Nat. Cell Biol. 8, 1398–1406 10.1038/ncb1508 [DOI] [PubMed] [Google Scholar]
- Zhang W., Yatskievych T. A., Baker R. K., Antin P. B. (2004). Regulation of Hex gene expression and initial stages of avian hepatogenesis by Bmp and Fgf signaling. Dev. Biol. 268, 312–326 10.1016/j.ydbio.2004.01.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


