Summary
Goldfish have been used for cold acclimation studies, which have focused on changes in glycolytic and oxidative enzymes or alterations in lipid composition in skeletal muscle. Here we examine the effects of cold acclimation on the functional properties of isolated mitochondria and permeabilized fibers from goldfish white skeletal muscle, focusing on understanding the types of changes that occur in the mitochondrial respiratory states. We observed that cold acclimation promoted a significant increase in the mitochondrial oxygen consumption rates. Western blot analysis showed that UCP3 was raised by ∼1.5-fold in cold-acclimated muscle mitochondria. Similarly, we also evidenced a rise in the adenine nucleotide translocase content in cold-acclimated muscle mitochondria compared to warm-acclimated mitochondria (0.96±0.05 vs 0.68±0.02 nmol carboxyatractyloside mg−1 protein). This was followed by a 2-fold increment in the citrate synthase activity, which suggests a higher mitochondrial content in cold-acclimated goldfish. Even with higher levels of UCP3 and ANT, the effects of activator (palmitate) and inhibitors (carboxyatractyloside and GDP) on mitochondrial parameters were similar in both warm- and cold-acclimated goldfish. Thus, we propose that cold acclimation in goldfish promotes an increase in functional oxidative capacity, with higher mitochondrial content without changes in the mitochondrial uncoupling pathways.
Key words: Cold acclimation, Mitochondria, Skeletal muscle
Introduction
Ectothermic fish, which vary their body temperature according to the environmental temperature, are widespread in a variety of environments. Fish have evolved several strategies to deal with the hardships imposed by cold temperature. For example, Antarctic notothenioids express antifreeze glycoproteins in their body fluids at the same time that they also possess high densities of mitochondria (Harding et al., 2003; O'Brien and Mueller, 2010). Eurythermal freshwater fish are exposed to widely fluctuating water temperatures varying from 0–4°C during the winter to over 30°C in summer. Several studies have used these fish to understand how metabolic adaptation to low temperatures is achieved (for reviews, see Guderley, 2004; Somero, 2004; Seebacher et al., 2010; O'Brien, 2011). Cold acclimation can modify diverse metabolic parameters mainly related to the oxidative capacity of skeletal muscle. Changes in the expression and activities of mitochondrial enzymes have been well documented in rainbow trout (Oncorhynchus mykiss), carp (Cyprinus carpio), cod (Gadus morhua) and zebrafish (Danio rerio) (Battersby and Moyes, 1998; Gracey et al., 2004; Lucassen et al., 2006; McClelland et al., 2006). Furthermore, enhancement of mitochondrial volume density has been described in striped bass (Morone saxatilis), stickleback (Gasterosteus aculeatus) and European eel (Anguilla anguilla) (Egginton and Johnston, 1984; Egginton and Sidell, 1989; Orczewska et al., 2010). In some species, such as short-horned sculpin (Myoxocephalus scorpius) and rainbow trout (Oncorhynchus mykiss), adjustments in membrane phospholipids composition contribute for changes in mitochondrial oxidative capacity (Guderley and Johnston, 1996; Guderley et al., 1997). Altogether, these changes may enhance catalytic capacity and facilitate effusive processes (Egginton and Sidell, 1989).
Goldfish (Carassius auratus L.) have been used for studying metabolic responses to several environmental challenges. For instance, this eurythermal fish is able to be active even at low temperatures and to survive to hypoxia and anoxia. With regard to cold acclimation, the mechanisms underlying this thermal compensation of swimming performance at low temperatures are complex and involve changes in the central and peripheral nervous systems, muscles, and other tissues (Hazel and Prosser, 1974; Johnston and Dunn, 1987). Most of the changes related to skeletal muscle metabolism that have been reported in goldfish suggest a more aerobic phenotype, mainly through alterations in mitochondrial enzymes (Hazel, 1972b; Hazel, 1972a; Sidell, 1980; LeMoine et al., 2008), mitochondrial volume density (Tyler and Sidell, 1984) and the relative proportion of slow twitch fibers in the myotomes (Johnston and Lucking, 1978). Little is known about mitochondrial physiology, however, such as respiratory rates of oxygen consumption and substrate preferences. Van den Thillart and Modderkolk observed a higher phosphorylative state (state 3) in isolated mitochondria from cold-acclimated goldfish compared to their warm-acclimated counterparts (van den Thillart and Modderkolk, 1978). This difference was attributed to modifications in the apparent Arrhenius activation energies and in the phospholipid composition of the mitochondria isolated from white and red muscles.
The present study aims to examine the effects of cold acclimation on the functional properties of isolated mitochondria and permeabilized fibers from goldfish white skeletal muscle, focusing on coupled and uncoupled oxygen consumption. Because goldfish are particularly cold tolerant, we decided to use an acclimation protocol that has been recently described by our group (dos Santos et al., 2010) to understand what types of changes occur in the mitochondrial respiratory states and mechanisms that could be affecting ATP synthesis. We compared white muscle mitochondria isolated from goldfish acclimated to 25°C or 5°C for one month. After a cold acclimation period, measurements were made in mitochondria, and fibers were isolated from goldfish white skeletal muscle. We present evidence for an enhanced mitochondrial biogenesis after cold exposure in the goldfish white skeletal muscle. We are also the first to use isolated fibers to address this question. We observed that acclimation to 5°C promotes an increase in basically all respiratory states when using succinate (plus rotenone) as a substrate. In addition, oxygen consumption performed with permeabilized fibers showed an increase in all respiratory rates in cold-acclimated fish independent of the substrates used. We used different approaches to investigate if cold acclimation could promote mitochondrial uncoupling by adenine nucleotide translocase (ANT) and uncoupling proteins (UCPs). Palmitate (PA) was able to increase oxygen consumption in state 4o in mitochondria from warm-acclimated and cold-acclimated goldfish, and carboxyatractyloside (CAT), but not guanosine diphosphate (GDP), reduced palmitate-uncoupled respiration. The addition of bovine serum albumin free of fatty acid (BSAFFA), which chelates fatty acids, returned the oxygen consumption to the basal rate in both conditions. A similar effect was observed when the oxygen consumption rate was measured using permeabilized fibers. Both ANT content and uncoupling protein 3 (UCP3) expressions were higher in cold-acclimated goldfish, which may be associated with a greater mitochondrial content. Altogether, these results suggest that cold acclimation in goldfish promotes an increase in functional oxidative capacity, which may be attributed to higher rates of mitochondrial biogenesis. More importantly, we show that cold acclimation does not lead to mitochondrial uncoupling in goldfish.
Materials and Methods
Ethics statement
All experimental and holding procedures were approved by the Ethics Committee of the Carlos Chagas Filho Institute of Biophysics, Health Sciences Centre, Federal University of Rio de Janeiro (Protocol no. IBQM049).
Reagents and materials
Unless otherwise specified, all reagents were purchased from Sigma Chemical Co. (St. Louis, MO).
Animals and acclimation process
Goldfish were purchased from a local breeder (Duque de Caxias, RJ), maintained in a glass aquarium with aerated and dechlorinated water, and fed two to three times a day with commercial goldfish food pellets. Fish (20–30 g) were maintained at 25°C on a 12 hour:12 hour light/dark cycle and were generally used within 1–1.5 months of arrival. The acclimation was performed using a refrigeration unit (347 CDG-FANEM®, São Paulo, Brazil) as described previously (dos Santos et al., 2010). Fish were kept at least for three days in the lab before starting the experiment in order to minimize the potential stress caused by transportation. Subsequently, they were divided into two groups of five to six fish each and transferred to a new aquarium. One group (warm-acclimated) was kept at 25°C (±0.5°C), and the second group (cold-acclimated) was submitted to an acclimation process using the refrigeration unit above. For the cold-acclimated group, the temperature of the refrigeration unit was gradually reduced 3°C per day for one week until it reached 5°C (±0.5°C), and the animals were kept at this condition for four additional weeks.
Isolation of skeletal muscle mitochondria
After the acclimation period, animals were killed by decapitation and skeletal muscle mitochondria were isolated based on an adapted protocol from Chappell and Makinen (Chappell and Perry, 1954; Makinen and Lee, 1968). All manipulations were carried out at 4°C. White axial muscle (2–4 g) was rapidly removed and washed in Basic Medium (BM) containing 140 mmol l−1 KCl, 20 mmol l−1 HEPES, 5 mmol l−1 MgCl2 and 1 mmol l−1 EGTA, pH 7.0. After contaminants were removed, the tissue was sliced into small pieces in the same buffer (1:1 w/v), and Homogenization Medium (HM) containing 140 mmol l−1 KCl, 20 mmol l−1 HEPES, 5 mmol l−1 MgCl2, 2 mmol l−1 EGTA, 1 mmol l−1 ATP, 1% BSA and 2 mg g−1 muscle wet weight of trypsin, pH 7.0 (1:10 w/v) were added. The tissue was homogenized in a Teflon glass potter, and the homogenate was centrifuged at 500 g for 10 minutes. The supernatant was strained through cheesecloth, diluted twice with HM and re-centrifuged at 500 g for 10 minutes. The resultant supernatant was strained through cheesecloth and centrifuged at 10,000 g for 10 minutes. The pellet was resuspended in BM and incubated on ice for 3–5 minutes for myofibrillar re-polymerization. This fraction was centrifuged at 500 g for 10 minutes, and the retained supernatant was centrifuged at 10,000 g for 10 minutes. The final pellet obtained was resuspended in BM and kept at 4°C until assayed.
Preparation of skeletal muscle permeabilized fibers
The fibers from white axial muscle tissues obtained from both groups were prepared as previously described (Kuznetsov et al., 2004). Briefly, from a 100 mg tissue sample, fiber bundles were prepared and nearly all fibers were used for usually three replicate experiments. The relaxing solution contained 2.77 mmol l−1 CaK2EGTA, 7.23 mmol l−1 K2EGTA (free Ca2+ concentration 0.1 µmol l−1), 20 mmol l−1 imidazole, 20 mmol l−1 taurine, 6.56 mmol l−1 MgCl2, 5.77 mmol l−1 ATP, 15 mmol l−1 phosphocreatine, 0.5 mmol l−1 dithiothreitol, and 50 mmol l−1 K-MES, pH 7.1. Skeletal muscle fibers (∼10 mg) were permeabilized by gentle agitation for 30 minutes at 4°C in the relaxing solution supplemented with 50 µg ml−1 saponin. Fibers were washed in ice-cold respiration medium (see below) by agitation for 10–15 minutes and were kept in this medium (see below) until respirometric assay.
Protein determination
The protein concentration in the subcellular fractions and isolated mitochondria was determined by the Folin-Lowry method, using BSA as a standard (Lowry et al., 1951).
Oxygen consumption in isolated mitochondria
Two sets of experiments were performed using isolated mitochondria from warm-acclimated and cold-acclimated goldfish. In the first, oxygen consumption was measured using a Clark-type electrode (Hansatech Instruments, Norfolk, England) in a respiration medium (RM-A) containing 60 mmol l−1 Tris-HCl, pH 7.4, 110 mmol l−1 mannitol, 60 mmol l−1 KCl, 5 mmol l−1 KH2PO4, 5 mmol l−1 MgCl2, 0.5 mmol l−1 EDTA, 0.2 mg ml−1 BSA essentially fatty acid free (BSAFFA). Mitochondria (0.3 mg ml−1) were incubated with 0.5 ml of RM-A, and the cuvette was closed immediately before starting the experiments. Basal respiration (State 2) was measured after the addition of mitochondrial substrates (5 mmol l−1 pyruvate plus 5 mmol l−1 malate for complex I and 10 mmol l−1 succinate plus 1 µmol l−1 rotenone for complex II). The phosphorylating respiration rate (State 3) was measured with the addition of 0.15 mmol l−1 ADP; State 4o (proton leak) and State 3u (uncoupled state) were reached with the addition of 1 µg ml−1 oligomycin and 0.5 µmol l−1 FCCP, respectively.
In the second set of experiments, respiration was measured in titration-injection high-resolution respirometers (Oroboros, Oxygraph; Innsbruck, Austria) (Gnaiger, 2001). The respiration medium (RM-B) consisted of 110 mmol l−1 sucrose, 60 mmol l−1 K-MES, 0.5 mmol l−1 EGTA, 3 mmol l−1 MgCl2, 20 mmol l−1 taurine, 10 mmol l−1 KH2PO4, 20 mmol l−1 K-HEPES, pH 7.1 (Gnaiger et al., 2000). Mitochondria (0.3 mg ml−1) were incubated with 2.0 ml of RM-B, and the cuvette was closed immediately before starting the experiments. The same mitochondrial substrates were used as in the first set of experiments. Where indicated, 5 µmol l−1 CAT, 10 µmol l−1 palmitic acid (PA), 1 mmol l−1 GDP and 1 mg ml−1 BSAFFA were added to the respiration medium. All experiments with isolated mitochondria were carried out at 25°C with continuous stirring in respiration buffer.
Oxygen consumption in permeabilized fibers using high-resolution respirometry
Respiration was measured at 25°C in titration-injection respirometers (Oroboros, Oxygraph; Innsbruck, Austria) (Gnaiger, 2001). Permeabilized fibers (∼10 mg) were incubated with 2.0 ml of the RM-B plus 1 mg ml−1 BSAFFA, and the cuvette was closed immediately before starting the experiments. The types and concentrations of mitochondrial substrates used were the same as those in the experiments with isolated mitochondria, except for ADP, which was used at 2 mmol l−1. To evaluate proton leak, BSAFFA was left out of the respiration medium. Where indicated, 10 µmol l−1 PA, 1 mmol l−1 GDP, 5 µmol l−1 CAT and 1 mg ml−1 BSAFFA were added to the respiration medium.
The experiments using isolated mitochondria and muscle fibers were performed carefully. Drugs were injected sequentially and each injection was made based on the stabilization of oxygen consumption rate after every single injection. The oxygen concentration of the medium at the end of each experiment was never less than 100 nmol/ml in order to avoid a possible hypoxic condition.
Measurement of ANT content by CAT titration
Measurement and calculation of ANT content was performed as described previously (Brand et al., 2005) with slight modifications. Briefly, mitochondria (0.3 mg ml−1) were incubated with 2.0 ml of RM-B plus 1 mg ml−1 BSAFFA, and the cuvette was closed immediately before starting the experiments. After the addition of complex II substrates (10 mmol l−1 succinate plus 1 µmol l−1 rotenone), excess ADP (0.5 mmol l−1) was added, followed by CAT titration (0–0.5 µmol l−1), until state 4 was well established. After, more ADP (0.5 mmol l−1) was added to determine if the mitochondria were in state 4, followed by the addition of 0.5 µmol l−1 FCCP.
Immunoblotting of UCPs
Mitochondrial proteins (50 µg) from warm-acclimated and cold-acclimated goldfish were separated on 13% polyacrylamide gels and then transferred onto nitrocellulose membranes. Blots were blocked with 5% milk in Tris-buffered saline with 0.1% Tween 20 (TBS-T) for 2 hours and probed overnight at 4°C with antibodies raised against rat, mouse and human UCP3 (ab3477, Abcam, Cambridge, MA) at a dilution of 1:1000 in TBS-T. Blots were washed with TBS-T and incubated with affinity-purified goat anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody (GE Healthcare, Buckinghamshire, UK) for 1 hour at room temperature. The blots were washed again and developed using a standard ECL detection kit (Millipore Corporation, Billerica, MA, USA). Signal intensity was measured by densitometry using Un-Scan-it gel Version 6.1 software (Silk Scientific Corporation, Utah, USA). We tried unsuccessfully to measure the expression of COX4 as a loading control. We then normalized our signals to the amount of protein added.
Statistical analysis
All data were analyzed using PRISM software (GraphPad Software, Inc., San Diego, CA, USA) and are expressed as the mean ± s.e.m. Student's t-test was used for single comparisons between two groups. Symbols indicate a significant difference between warm- and cold-acclimated groups and the P values are presented in the figure legends. Statistical significance was defined as P<0.05.
Results
Oxygen consumption of mitochondria isolated from white muscle: effect of cold acclimation
Using a protocol for cold acclimation described previously by our group (dos Santos et al., 2010), we measured the different mitochondrial respiratory states in white muscle mitochondria isolated from warm-acclimated and cold-acclimated goldfish (Fig. 1).
Fig. 1. Changes in maximal mitochondrial oxidative capacity during cold acclimation: O2 consumption in isolated mitochondria from warm-acclimated and cold-acclimated goldfish.

Procedures for isolating the mitochondria and measuring the rates of oxygen consumption and respiratory states for the first set of experiments are described in Materials and Methods. (A) 5 mmol l−1 Pyruvate + 5 mmol l−1 Malate and (B) 10 mmol l−1 Succinate. White bars represent warm-acclimated, and gray bars represent cold-acclimated. Values are the mean ± s.e.m. of 5 fish per group. #P<0.05 and *P<0.01 compared to the warm-acclimated group.
As shown in Fig. 1A, the cold acclimation was found to promote a significant increase in the oxygen consumption rate during oligomycin-inhibited respiration (state 4o) when pyruvate and malate are used as respiratory substrates. The respiratory states 2 (basal), 3 (ADP-induced phosphorylating respiration rates) in mitochondria from cold-acclimated goldfish using these substrates showed a tendency to increase, but this result was not statistically significant.
During succinate oxidation (Fig. 1B), we found a significant increase in oxygen consumption in respiratory states 2, 3 and 4o in the mitochondria derived from cold-acclimated goldfish. Surprisingly, the rates of oxygen consumption in mitochondria from cold-acclimated animals in the presence of FCCP (state 3u) was almost twice as high, which might suggest a greater number of respiratory complexes.
Oxygen consumption of white muscle permeabilized fibers: effect of cold acclimation
To investigate mitochondrial function, one can either use isolated mitochondria or permeabilized fibers. As described previously (Sperl et al., 1997; Picard et al., 2011), there are some problems with using isolated mitochondria, including potentially poor yield, stability of function and morphological state. Thus, we decided to also investigate the effects of cold acclimation using saponin-permeabilized fibers from goldfish white skeletal muscle to more closely match the physiological state.
At first glance, the effects of cold acclimation were more pronounced in permeabilized fibers than in isolated mitochondria, except for state 4o (Fig. 2). As observed for isolated mitochondria (Fig. 1), cold acclimation promoted an increase in basal respiration rates when substrates for complexes I and II were added (state 2, Fig. 2). In addition, the rates of respiration in states 3 and 4o were ∼2-fold higher in cold-acclimated goldfish.
Fig. 2. Changes in maximal mitochondrial oxidative capacity during cold acclimation: O2 consumption in permeabilized fibers from warm-acclimated and cold-acclimated goldfish.
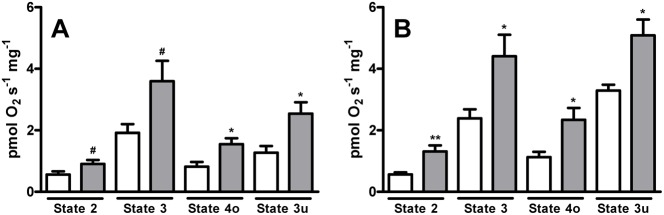
Procedures for the permeabilization of fibers and measurements of the rates of oxygen consumption and respiratory states are described in Materials and Methods. (A) 5 mmol l−1 Pyruvate + 5 mmol l−1 Malate and (B) 10 mmol l−1 Succinate. White bars represent warm-acclimated, and gray bars represent cold-acclimated. Values are the mean ± s.e.m. of 10 fish per group. #P<0.05, *P<0.01 and **P<0.001 compared to the warm-acclimated group.
Respiratory rates in permeabilized fibers are expressed by wet weight (in milligrams), which considers the total amount of mitochondria per weight of fiber added. Thus, the effect of cold acclimation that was observed in permeabilized fibers could be due to an increase in mitochondrial biogenesis. Citrate synthase activity, which is largely used as a mitochondrial content marker, was found to be ∼2-fold higher in cold-acclimated fish than warm-acclimated fish (0.44±0.02 vs 0.23±0.02 µmol citrate mg−1 minute−1, n = 5 fish per group; P<0.0001), suggesting that there is a higher mitochondrial content after cold acclimation.
Proton leak in warm-acclimated and cold-acclimated white-muscle-isolated mitochondria and permeabilized fibers
Cold acclimation was found to promote a strong increase in state 4o in goldfish white muscle; this effect was observed both in isolated mitochondria and permeabilized fibers (Figs 1, 2). Proton leakage, which partially uncouples the oxidative phosphorylation, may be associated with the presence of UCPs and ANT (Andreyev et al., 1989; Brand and Esteves, 2005; Krauss et al., 2005). However, the role of UCP3 as a source of proton leak is still under debate by several groups (for a review, see Nedergaard and Cannon, 2003). Thus, we asked whether the cold-induced proton leak could be due to the presence of such proteins. To answer this question, we measured the effect of an activator (PA) or inhibitors (GDP, CAT and BSA) of ANT and UCP upon oligomycin-inhibited respiration rate in isolated mitochondria and permeabilized fibers (Fig. 3). We found that all respiration conditions in mitochondria and fibers isolated from cold-acclimated fish were higher than those from warm-acclimated fish. Palmitate was able to increase oxygen consumption in both preparations (mitochondria and fibers). GDP did not have any effect on these rates, but CAT and BSA decreased the stimulation promoted by PA addition, with the most pronounced effect being observed with BSA.
Fig. 3. Effect of palmitate, GDP, carboxyatractyloside and BSAFFA on proton leak in isolated mitochondria and permeabilized fibers from warm-acclimated and cold-acclimated goldfish.
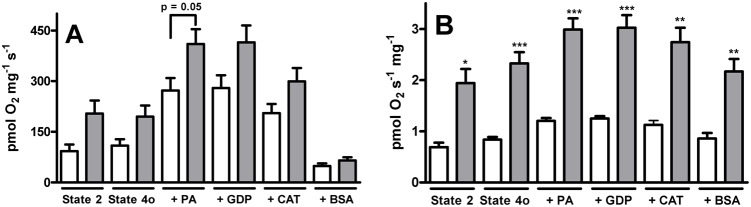
Procedures for the preparation of isolated mitochondria and permeabilized fibers and measurements of rates of oxygen consumption and respiratory states are described in Materials and Methods. After achieving state 4o using complex II substrates, 10 µmol l−1 palmitate (PA), 2 mmol l−1 GDP, 5 µmol l−1 CAT and 1 mg ml−1 BSAFFA were added into the respiratory chamber. (A) Isolated mitochondria (B) permeabilized fibers. White bars represent warm-acclimated, and gray bars represent cold-acclimated. Values are the mean ± s.e.m. of 3–5 fish per group. *P<0.01, **P<0.001 and ***P<0.0001 compared to the warm-acclimated group.
ANT and UCP3 content
White muscle mitochondria were isolated from warm-acclimated and cold-acclimated fish, and the amount of functional ANT was determined quantitatively by titration with CAT, an ANT high affinity inhibitor. The titration showed that the ANT content of cold-acclimated muscle mitochondria was 1.4-fold higher than that of warm-acclimated muscle mitochondria (0.96±0.05 vs 0.68±0.02 nmol CAT mg−1 protein, n = 4, P<0.01) (Fig. 4A). We were unable to perform western blots using a commercial rabbit polyclonal antibody against ANT. We did have success using a commercial rat polyclonal antibody against UCP3 in Western blots analysis. The antibody was found to effectively recognize a single band in a concentration curve of protein that was a few kilodaltons smaller than the positive control made with isolated mitochondria from mouse gastrocnemius muscle (data not shown). We determined that UCP3 is present in mitochondria from warm-acclimated and cold-acclimated fish. We found that cold acclimation promotes a 1.5-fold increase in the level of UCP3 in cold-acclimated fish.
Fig. 4. ANT and UCP3 content in warm-acclimated and cold-acclimated goldfish.

ANT content (A) and UCP3 expression (B) were measured as described in Materials and Methods. (A) ANT contents measured by CAT titration. Values are the mean ± s.e.m. of four fish per group; (B) representative gel and results from the densitometry expressed as arbitrary units. Values are the mean ± s.e.m. of three fish per group. *P<0.01 compared to the warm-acclimated group. White bars represent warm-acclimated (WA) and gray bars, cold-acclimated (CA).
Discussion
Cold acclimation promotes several changes in metabolic parameters not just in fish but also in mammals, birds, reptiles and amphibians (Bicudo et al., 2002; Trzcionka et al., 2008; Cannon and Nedergaard, 2011; Guderley and Seebacher, 2011). These metabolic responses play a crucial role in the thermal adaption to environmental change. In fish, multiple adaptive mechanisms can mediate survival at low temperatures, such as the synthesis of antifreeze proteins in Antarctic fish, endothermy in salmon sharks, the presence of a heater organ in swordfish and the enhancement of oxidative capacity in the striped bass and crucian carp (for a review, see Hochachka and Somero, 2002). The elevation of oxidative capacity is particularly important in the skeletal muscle, especially in cold-active species, which maintain their swimming activities even at low temperatures. Among these species are the cyprinids, which are eurythermal fish that have evolved compensatory responses to climatic changes that occur naturally in their habitat.
In fish, one of the most relevant survival strategies for cold acclimation is the concentration increase of aerobic metabolic enzymes, such as enzymes involved in the Krebs cycle and oxidative phosphorylation, which reside within the mitochondrion (for a review, see O'Brien, 2011). In certain cyprinid species such as goldfish, cold acclimation increases the oxidative capacity of skeletal muscle, mainly by increasing mitochondrial volume density, enzyme activity, and changes in the phospholipid composition (van den Thillart and Modderkolk, 1978; Tyler and Sidell, 1984; LeMoine et al., 2008). In addition, a shift in the relative distribution of different muscle fiber types has been observed (Johnston and Lucking, 1978). This increase in mitochondrial volume density upon cold acclimation is a result of mitochondrial biogenesis in cyprinids. This effect could be related to an increase in the expression of nuclear respiratory factor 1 (NRF-1) and peroxisome proliferator-activated receptor gamma coactivator (PGC)-1β (rather than PGC-1α), which are known to regulate nuclear and mitochondrial genes involved in mitochondrial assembly (LeMoine et al., 2008; Bremer et al., 2012). Because mitochondrial content, as well as the activity of enzymes involved in mitochondrial metabolism, increases after cold exposure we decided to investigate whether respiratory states are altered in the white muscle mitochondria from cold-acclimated goldfish.
We measured oxygen consumption in permeabilized fibers and found that cold acclimation promotes an increase in all respiratory states, independent of the substrate used (Fig. 2). It has been reported previously that cold-acclimated goldfish have a higher mitochondrial density, which can be observed in transmission electron microscopy and by measuring the activity of mitochondrial enzymes (Tyler and Sidell, 1984; LeMoine et al., 2008). Because our measurements using permeabilized fibers consider the whole mitochondrial content per fiber, the observed increase in respiratory states may be consequence of this higher mitochondrial volume density. Indeed, we also observed that citrate synthase activity, which is largely used as a marker for mitochondrial content, was 2-fold higher in cold-acclimated goldfish. Although this increase in the mitochondrial volume density after cold acclimation in goldfish has already been reported, we have now demonstrated that this increase in density reflects in the oxygen consumption.
In isolated mitochondria, we observed that cold acclimation promotes an increase in respiratory rates (Fig. 1). When pyruvate plus malate were used as complex I substrates, only oxygen consumption at state 4o was increased after cold acclimation (Fig. 1A). Conversely, the use of succinate (plus rotenone) as a substrate increased basically all respiratory states (states 3, 4o and 3u) after cold acclimation (Fig. 1B). In addition, a higher phosphorylative state (state 3) has been observed previously (van den Thillart and Modderkolk, 1978) and indicates an increase in the oxidative capacity of mitochondria after cold acclimation, which can be explained by higher ANT content (Fig. 4). These results show that cold acclimation increases mitochondrial content and respiratory rate.
In agreement with the results above, we observed that state 4o as well as ANT and UCP3 content are only proportionally increased with increased total mitochondrial yield, suggesting that there is no evidence for enhanced uncoupling. Calculation of RCR showed no difference between warm and cold-acclimated goldfish, confirming the absence of cold-induced mitochondrial uncoupling.
We propose that cold acclimation promotes changes in mitochondrial bioenergetics by affecting mitochondrial biogenesis, the expression/activity of mitochondrial enzymes and mitochondrial membrane composition. While an increase in the levels of enzymes related to energy synthesis (e.g. succinate dehydrogenase and cytochrome c oxidase) increases oxidative capacity, higher levels of ANT and UCP3 might constitute an antioxidant defense mechanism to attenuate the generation of reactive oxygen species and oxidative stress. However, there is no evidence for a physiological role for these proteins in goldfish and more studies should be performed to address this question.
In conclusion, we have shown here that cold acclimation process modulates a metabolic program in order to augment the oxidative capacity by increasing mitochondrial machinery.
Acknowledgments
We are grateful to Dr Leopoldo de Meis for the constant encouragement and to all of our lab colleagues for the discussions. R.S.S., W.S.S., and A.G. conceived and designed the experiments. R.S.S. performed the experiments. R.S.S., W.S.S., and A.G. analyzed the data. W.S.S. contributed reagents, materials, and analysis tools. R.S.S. and W.S.S. wrote the paper. Funding was provided by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro/Brazil (FAPERJ) Grants. R.S.S. and W.S.S. are fellows of CNPq.
Footnotes
Competing interests: The authors have no competing interests to declare.
References
- Andreyev A. Y., Bondareva T. O., Dedukhova V. I., Mokhova E. N., Skulachev V. P., Tsofina L. M., Volkov N. I., Vygodina T. V. (1989). The ATP/ADP-antiporter is involved in the uncoupling effect of fatty acids on mitochondria. Eur. J. Biochem. 182, 585–592 10.1111/j.1432-1033.1989.tb14867.x [DOI] [PubMed] [Google Scholar]
- Battersby B. J., Moyes C. D. (1998). Influence of acclimation temperature on mitochondrial DNA, RNA, and enzymes in skeletal muscle. Am. J. Physiol. 275, R905–R912. [DOI] [PubMed] [Google Scholar]
- Bicudo J. E., Bianco A. C., Vianna C. R. (2002). Adaptive thermogenesis in hummingbirds. J. Exp. Biol. 205, 2267–2273. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Esteves T. C. (2005). Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2, 85–93 10.1016/j.cmet.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Brand M. D., Pakay J. L., Ocloo A., Kokoszka J., Wallace D. C., Brookes P. S., Cornwall E. J. (2005). The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem. J. 392, 353–362 10.1042/BJ20050890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer K., Monk C. T., Gurd B. J., Moyes C. D. (2012). Transcriptional regulation of temperature-induced remodeling of muscle bioenergetics in goldfish. Am. J. Physiol. 303, R150–R158 10.1152/ajpregu.00603.2011 [DOI] [PubMed] [Google Scholar]
- Cannon B., Nedergaard J. (2011). Nonshivering thermogenesis and its adequate measurement in metabolic studies. J. Exp. Biol. 214, 242–253 10.1242/jeb.050989 [DOI] [PubMed] [Google Scholar]
- Chappell J. B., Perry S. V. (1954). Biochemical and osmotic properties of skeletal muscle mitochondria. Nature 173, 1094–1095 10.1038/1731094a0 [DOI] [PubMed] [Google Scholar]
- dos Santos R. S., Diniz L. P., Galina A., da–Silva W. S. (2010). Characterization of non-cytosolic hexokinase activity in white skeletal muscle from goldfish (Carassius auratus L.) and the effect of cold acclimation. Biosci. Rep. 30, 413–423 10.1042/BSR20090128 [DOI] [PubMed] [Google Scholar]
- Egginton S., Johnston I. A. (1984). Effects of acclimation temperature on routine metabolism muscle mitochondrial volume density and capillary supply in the elver (Anguilla anguilla L.). J. Therm. Biol. 9, 165–170 10.1016/0306-4565(84)90016-0 [DOI] [Google Scholar]
- Egginton S., Sidell B. D. (1989). Thermal acclimation induces adaptive changes in subcellular structure of fish skeletal muscle. Am. J. Physiol. 256, R1–R9. [DOI] [PubMed] [Google Scholar]
- Gnaiger E. (2001). Bioenergetics at low oxygen: dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. Respir. Physiol. 128, 277–297 10.1016/S0034-5687(01)00307-3 [DOI] [PubMed] [Google Scholar]
- Gnaiger E., Kuznetsov A. V., Schneeberger S., Seiler R., Brandacher G., Steurer W., Margreiter R. (2000). Mitochondria in the cold. Life In The Cold (ed. Heldmaier G, Klingenspor M.), pp. 431–442 Berlin: Springer. [Google Scholar]
- Gracey A. Y., Fraser E. J., Li W., Fang Y., Taylor R. R., Rogers J., Brass A., Cossins A. R. (2004). Coping with cold: An integrative, multitissue analysis of the transcriptome of a poikilothermic vertebrate. Proc. Natl. Acad. Sci. USA 101, 16970–16975 10.1073/pnas.0403627101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guderley H. (2004). Metabolic responses to low temperature in fish muscle. Biol. Rev. Camb. Philos. Soc. 79, 409–427 10.1017/S1464793103006328 [DOI] [PubMed] [Google Scholar]
- Guderley H., Johnston I. I. (1996). Plasticity of fish muscle mitochondria with thermal acclimation. J. Exp. Biol. 199, 1311–1317. [DOI] [PubMed] [Google Scholar]
- Guderley H., Seebacher F. (2011). Thermal acclimation, mitochondrial capacities and organ metabolic profiles in a reptile (Alligator mississippiensis). J. Comp. Physiol. B 181, 53–64 10.1007/s00360-010-0499-1 [DOI] [PubMed] [Google Scholar]
- Guderley H., St Pierre J., Couture P., Hulbert A. J. (1997). Plasticity of the properties of mitochondria from rainbow trout red muscle with seasonal acclimatization. Fish Physiol. Biochem. 16, 531–541 10.1023/A:1007708826437 [DOI] [Google Scholar]
- Harding M. M., Anderberg P. I., Haymet A. D. (2003). ‘Antifreeze’ glycoproteins from polar fish. Eur. J. Biochem. 270, 1381–1392 10.1046/j.1432-1033.2003.03488.x [DOI] [PubMed] [Google Scholar]
- Hazel J. R. (1972a). The effect of temperature acclimation upon succinic dehydrogenase activity from the epaxial muscle of the common goldfish (Carassius auratus L)—I. Properties of the enzyme and the effect of lipid extraction. Comp. Biochem. Physiol. 43B, 837–861 10.1016/0305-0491(72)90230-1 [DOI] [PubMed] [Google Scholar]
- Hazel J. R. (1972b). The effect of temperature acclimation upon succinic dehydrogenase activity from the epaxial muscle of the common goldfish (Carassius auratus L)—II. Lipid reactivation of the soluble enzyme. Comp. Biochem. Physiol. 43B, 863–882 10.1016/0305-0491(72)90231-3 [DOI] [PubMed] [Google Scholar]
- Hazel J. R., Prosser C. L. (1974). Molecular mechanisms of temperature compensation in poikilotherms. Physiol. Rev. 54, 620–677. [DOI] [PubMed] [Google Scholar]
- Hochachka P. W., Somero G. N. (2002). Cellular metabolism, regulation, and homeostasis. Biochemical Adaptation: Mechanism And Process In Physiological Evolution, pp. 290–450 New York: Oxford University Press. [Google Scholar]
- Johnston I. A., Dunn J. (1987). Temperature acclimation and metabolism in ectotherms with particular reference to teleost fish. Symp. Soc. Exp. Biol. 41, 67–93. [PubMed] [Google Scholar]
- Johnston I. A., Lucking M. (1978). Temperature induced variation in the distribution of different types of muscle fibre in the goldfish (Carassius auratus). J. Comp. Physiol. 124B, 111–116. [Google Scholar]
- Krauss S., Zhang C. Y., Lowell B. B. (2005). The mitochondrial uncoupling-protein homologues. Nat. Rev. Mol. Cell Biol. 6, 248–261 10.1038/nrm1592 [DOI] [PubMed] [Google Scholar]
- Kuznetsov A. V., Schneeberger S., Seiler R., Brandacher G., Mark W., Steurer W., Saks V., Usson Y., Margreiter R., Gnaiger E. (2004). Mitochondrial defects and heterogeneous cytochrome c release after cardiac cold ischemia and reperfusion. Am. J. Physiol. 286, H1633–H1641 10.1152/ajpheart.00701.2003 [DOI] [PubMed] [Google Scholar]
- LeMoine C. M., Genge C. E., Moyes C. D. (2008). Role of the PGC-1 family in the metabolic adaptation of goldfish to diet and temperature. J. Exp. Biol. 211, 1448–1455 10.1242/jeb.014951 [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275. [PubMed] [Google Scholar]
- Lucassen M., Koschnick N., Eckerle L. G., Pörtner H. O. (2006). Mitochondrial mechanisms of cold adaptation in cod (Gadus morhua L.) populations from different climatic zones. J. Exp. Biol. 209, 2462–2471 10.1242/jeb.02268 [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Lee C. P. (1968). Biochemical studies of skeletal muscle mitochondria. I. Microanalysis of cytochrome content, oxidative and phosphorylative activities of mammalian skeletal muscle mitochondria. Arch. Biochem. Biophys. 126, 75–82 10.1016/0003-9861(68)90561-4 [DOI] [PubMed] [Google Scholar]
- McClelland G. B., Craig P. M., Dhekney K., Dipardo S. (2006). Temperature- and exercise-induced gene expression and metabolic enzyme changes in skeletal muscle of adult zebrafish (Danio rerio). J. Physiol. 577, 739–751 10.1113/jphysiol.2006.119032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J., Cannon B. (2003). The ‘novel’ ‘uncoupling’ proteins UCP2 and UCP3: what do they really do? Pros and cons for suggested functions. Exp. Physiol. 88, 65–84 10.1113/eph8802502 [DOI] [PubMed] [Google Scholar]
- O'Brien K. M. (2011). Mitochondrial biogenesis in cold-bodied fishes. J. Exp. Biol. 214, 275–285 10.1242/jeb.046854 [DOI] [PubMed] [Google Scholar]
- O'Brien K. M., Mueller I. A. (2010). The unique mitochondrial form and function of Antarctic channichthyid icefishes. Integr. Comp. Biol. 50, 993–1008 10.1093/icb/icq038 [DOI] [PubMed] [Google Scholar]
- Orczewska J. I., Hartleben G., O'Brien K. M. (2010). The molecular basis of aerobic metabolic remodeling differs between oxidative muscle and liver of threespine sticklebacks in response to cold acclimation. Am. J. Physiol. 299, R352–R364 10.1152/ajpregu.00189.2010 [DOI] [PubMed] [Google Scholar]
- Picard M., Taivassalo T., Ritchie D., Wright K. J., Thomas M. M., Romestaing C., Hepple R. T. (2011). Mitochondrial structure and function are disrupted by standard isolation methods. PLoS ONE 6, e18317 10.1371/journal.pone.0018317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebacher F., Brand M. D., Else P. L., Guderley H., Hulbert A. J., Moyes C. D. (2010). Plasticity of oxidative metabolism in variable climates: molecular mechanisms. Physiol. Biochem. Zool. 83, 721–732 10.1086/649964 [DOI] [PubMed] [Google Scholar]
- Sidell B. D. (1980). Responses of goldfish (Carassius auratus, L.) muscle to acclimation temperature: alterations in biochemistry and proportions of different fiber types. Physiol. Zool. 53, 98–107. [Google Scholar]
- Somero G. N. (2004). Adaptation of enzymes to temperature: searching for basic “strategies”. Comp. Biochem. Physiol. 139B, 321–333 10.1016/j.cbpc.2004.05.003 [DOI] [PubMed] [Google Scholar]
- Sperl W., Skladal D., Gnaiger E., Wyss M., Mayr U., Hager J., Gellerich F. N. (1997). High resolution respirometry of permeabilized skeletal muscle fibers in the diagnosis of neuromuscular disorders. Mol. Cell. Biochem. 174, 71–78 10.1023/A:1006880529195 [DOI] [PubMed] [Google Scholar]
- Trzcionka M., Withers K. W., Klingenspor M., Jastroch M. (2008). The effects of fasting and cold exposure on metabolic rate and mitochondrial proton leak in liver and skeletal muscle of an amphibian, the cane toad Bufo marinus. J. Exp. Biol. 211, 1911–1918 10.1242/jeb.016519 [DOI] [PubMed] [Google Scholar]
- Tyler S., Sidell B. D. (1984). Changes in mitochondrial distribution and diffusion distances in muscle of goldfish upon acclimation to warm and cold temperatures. J. Exp. Zool. 232, 1–9 10.1002/jez.1402320102 [DOI] [Google Scholar]
- van den Thillart G., Modderkolk J. (1978). The effect of acclimation temperature on the activation energies of state III respiration and on the unsaturation of membrane lipids of goldfish mitochondria. Biochim. Biophys. Acta 510, 38–51 10.1016/0005-2736(78)90128-1 [DOI] [PubMed] [Google Scholar]


