Abstract
EMBO J (2012) 31 24, 4488–4501 doi:; DOI: 10.1038/emboj.2012.294; published online November 09 2012
The endoreplication programme consists of multiple rounds of full genome replication in the absence of any intervening mitosis. This leads to an increase in nuclear ploidy that has been linked to the initiation of cell differentiation and growth. Recent work (Breuer et al, 2012) has identified the GTL1 transcription factor as a master regulator of cell growth through two different mechanisms: one, endoreplication dependent where GTL1 acts as a repressor of the anaphase-promoting complex (APC), and another as a regulator of endoreplication-independent cell growth.
The series of unidirectional events that constitute the cell division cycle renders two cells with an identical genomic content, for example, a 2C diploid nucleus. One of the crucial events is genome replication that occurs faithfully during each S phase of the cell cycle. Later, the two identical genomic copies are transferred to each daughter cell during mitosis. This paradigmatic process is at the base of the necessary increase in cell number required for organogenesis, cell and tissue repair and development. There are, however, alternative cycles where genome replication is not followed by cell division. Instead, mitosis is actively prevented in such a way that nuclear ploidy increases, yielding nuclei (or cells) with a DNA content of 4C, 8C, 16C, 32C, 64C, and so on. The major mechanism whereby ploidy is increased in this manner is endoreplication (also termed as endoreduplication), although other pathways leading to polyploidy also exist in nature. It is worth noting that endoreplication consists of multiple rounds of full genome replication. Consequently, it is a process different from selective amplification of certain genomic regions or from re-replication, triggered in response to various DNA replication stress conditions and by altering the mechanisms controlling genome replication.
Endoreplication occurs across the evolutionary scale in many multicellular organisms, but it is far more frequent in plants. In fact, it is normally associated to the plant response to hormonal signals, developmental cues or environmental challenges. The increase in nuclear ploidy by endoreplication requires a very precise arrest of the cell cycle, the entry into the new endoreplication cycle (initiation) and also active mechanisms sustaining several endoreplication cycles (endocycles). A plethora of processes involved in controlling initiation of the endocycle programme have been identified, including cell patterning and differentiation, DNA replication, DNA damage, chromatin status, the retinoblastoma/E2F pathway, and hormonal signals, among others (Gutierrez, 2009; De Veylder et al, 2011). Progression through various endocycles has been linked to cell differentiation and growth. Nuclear ploidy level and size of a cell seem to be correlated in Arabidopsis epidermal cells, although multiple experiments have revealed that cell growth is controlled by different elements, being endoreplication only one of the numerous components that determine the final size of a cell.
This key issue has been addressed in a recent work published in The EMBO Journal by Breuer et al (2012). Breuer et al focussed their study on development of Arabidopsis trichomes, unicellular epidermal hairs that increase their cell volume >100-fold. After cell fate specification, trichomes enter a highly complex differentiation programme that proceeds through two major growth phases (Figure 1): first, protodermal cells that have taken the trichome fate undergo several rounds of endocycles and substantial increase in size and branching events, and second, occurrence of a rapid cell expansion driven mainly by vacuolization (Ishida et al, 2008). The first stage involves initiation of the endoreplication programme but crucial questions that still remain elusive include whether there are mechanisms that control termination of the endocycle programme and, as a consequence, arrest of cell differentiation-associated growth, and what are the mechanisms that couple endoreplication and cell growth. A previous study from the same laboratory identified a mutation in the GTL1 gene, encoding a member of the GT-2 family of trihelix transcription factors, that results in extended number of endocycles and increased trichome cell growth (Breuer et al, 2009). Here the authors combined cellular, molecular, genetic and genomic analysis that, together, establish GTL1 as a master regulator of a cohort of genes that control termination of the endoreplication programme and its coupling to cell growth.
Figure 1.
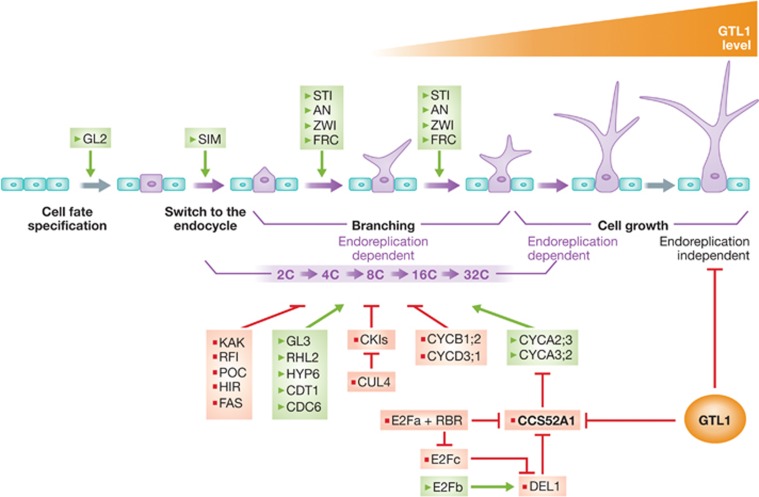
Coordination between the endoreplication programme and cell growth during trichome development. Trichome development starts with cell fate specification of some protodermal cells in the epidermis by expression of the homeobox gene GL2. These cells arrest cell division and switch to the endoreplication programme, which is modulated by various regulatory pathways and associated to the branching stage, genetically controlled by different genes. Late during trichome development, GTL1 accumulates leading to termination of the endocycle programme and to arrest of the endoreplication-independent trichome growth. Note that some of the endocycle control pathways have not been specifically demonstrated to operate in trichomes. Red and green fonts indicate activators and repressors of the pathway, respectively.
GTL1 is normally expressed in the late phases of trichome development when they have terminated their branching process (Breuer et al, 2009). To test its possible contribution to the temporal control of the endocycle programme, GTL1 was expressed at early stages of trichome development using cell type-specific promoters that are active either early in establishing the trichome fate of protodermal cells (GL2) or active in all leaf epidermal cells (ATML1). These experiments demonstrated that GTL1 is sufficient to inhibit endoreplication and ploidy-dependent cell growth and that its correct spatio-temporal expression is responsible for the termination of the endoreplication programme in trichomes (Figure 1). The fact that ectopic expression of GTL1 in pavement epidermal also inhibits growth suggests that other members of the trihelix transcription factor family may be involved in controlling cell growth arrest. Interestingly, expression of GTL1 in trichome cell precursors also inhibits the expression of the homeobox gene GL2, indicating that it may have an impact on cell fate establishment or maintenance.
To identify GTL1 target(s), the authors used a combination of ChIP/chip analysis and trichome-specific microarrays of the gtl1 mutant. Within the set of potential direct GTL1 targets identified, it was striking to find the CCS52A1 gene (Figure 1), encoding a Cdh1/Fzr-type of activators of the APC. This gene possesses a GTL1 binding GT3 box in its promoter and is upregulated in the gtl1 mutant. CCS52A genes were previously implicated in the onset of endoreplication during trichome development (Larson-Rabin et al, 2009). In fact, CCS52A1 cooperates with SIAMESE (SIM), a plant-specific CDK inhibitor, in preventing the accumulation of mitotic cyclins and triggering the endocycle in trichomes (Kasili et al, 2010) and is modulated by the retinoblastoma (RBR) pathway (Magyar et al, 2012). Using a reporter construct driving the expression of H2B-GFP under the control of the CCS52A1 promoter, the authors demonstrate that expression of GTL1 at late stages of trichome development represses CCS52A1, leading to endoreplication arrest. Furthermore, CCS52A1 and GTL1 interact genetically since the ccs52a1-2 mutation completely suppresses the typical increased ploidy phenotype of gtl1-1 trichomes, which in the double mutant are under-branched and under-endoreplicated. This is strong genetic evidence that CCS52A1 is acting directly downstream of GTL1 regulating endocycle and branching, the first phase of trichome growth.
It has been postulated that endoreplication could play a predominant role in cell size control setting up the approximate area of cell growth (that might vary between different cell types) and within this range, a DNA-independent growth mechanism is responsible for the final cell size (Schnittger et al, 2003; Harashima and Schnittger, 2010). In this context, Breuer et al (2012) found that trichome cells in the double gtl1-1 ccs52a1-2 mutant are significantly larger than those in the single ccs52a1-2 mutant, suggesting that the ccs52a1-2 mutation does not completely suppress the cell growth phenotype of gtl1-1. This indicates that ploidy and cell growth can be readily uncoupled and points to GTL1 as a transcription factor that controls endocycle-dependent cell growth by repressing CCS52A1 expression and endocycle-independent cell growth through additional targets (Figure 1). Thus, a challenging endeavour would be the identification of GTL1-regulated genes controlling cell growth in an endoreplication-independent manner.
Finally, these observations suggest that the extent of the endoreplication programme is established in a way that the cell is able to “count” the number of endocycles. The mechanism is not known but it is very remarkable that gtl1 mutants, as well as other endoreplication mutants (Gutierrez, 2009), do not normally loose completely the control of how many endoreplication cycles occur during trichome development since they extend their programme with one extra endocycle, but only one in most of the cases. This limitation in the number of endocycles is suggestive of one or more factors being synthesized de novo and necessary to initiate new endocycles (or made available in each endocycle), even after altering the counting mechanism, whatever it is in molecular terms. Once certain factors, for example, GTL1, come to play they may inhibit their synthesis, accumulation and/or accessibility thus limiting the number of extra endocycles. It is obvious that a number of questions are now open, for example, what are the windows where the various pathways activating or terminating the endocycle operate or how the various pathways impinging on the endocycle control redundantly or coordinately affect endoreplication progress (Gutierrez, 2009; Roodbarkelari et al, 2010; De Veylder et al, 2011). Also, what is the role of other GTL1-related members and their targets in controlling termination of cell growth and whether there are cell type-specific mechanisms involved. These and other unanswered questions remain to be addressed in the future before we can understand fully the mechanisms controlling the increase in ploidy through the endoreplication programme, the pathways impinging on the activation and termination and its role in cell growth and expansion during cell differentiation.
Acknowledgments
The CG laboratory is supported by BFU2009-9783 and CSD2007-0057 (MICCIN), and the EU (PIOF-GA-2009-234849). The CBMSO receives an institutional grant from Fundacion Ramon Areces. We apologize to colleagues whose work was not cited or cited indirectly due to space limitations.
Footnotes
The authors declare that they have no conflict of interest.
References
- Breuer C, Kawamura A, Ichikawa T, Tominaga-Wada R, Wada T, Kondou Y, Muto S, Matsui M, Sugimoto K (2009) The trihelix transcription factor GTL1 regulates ploidy-dependent cell growth in the Arabidopsis trichome. Plant Cell 21: 2307–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer C, Morohashi K, Kawamura A, Takahashi N, Ishida T, Umeda M, Grotewold E, Sugimoto K (2012) Transcriptional repression of the APC/C activator CCS52A1 contributes to the active termination of cell growth. EMBO J 31: 4488–4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L, Larkin JC, Schnittger A (2011) Molecular control and function of endoreplication in development and physiology. Trends Plant Sci 16: 624–634 [DOI] [PubMed] [Google Scholar]
- Gutierrez C (2009) The Arabidopsis cell cycle. In The Arabidopsis Book. American Society of Plant Biologists. (http://www.bioone.org/doi/full/10.1199/tab.0120) Vol. 7, pp e0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harashima H, Schnittger A (2010) The integration of cell division, growth and differentiation. Curr Opin Plant Biol 13: 66–74 [DOI] [PubMed] [Google Scholar]
- Ishida T, Kurata T, Okada K, Wada T (2008) A genetic regulatory network in the development of trichomes and root hairs. Annu Rev Plant Biol 59: 365–386 [DOI] [PubMed] [Google Scholar]
- Kasili R, Walker JD, Simmons LA, Zhou J, De Veylder L, Larkin JC (2010) SIAMESE cooperates with the CDH1-like protein CCS52A1 to establish endoreplication in Arabidopsis thaliana trichomes. Genetics 185: 257–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Rabin Z, Li Z, Masson PH, Day CD (2009) FZR2/CCS52A1 expression is a determinant of endoreduplication and cell expansion in Arabidopsis. Plant Physiol 149: 874–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar Z, Horvath B, Khan S, Mohammed B, Henriques R, De Veylder L, Bako L, Scheres B, Bogre L (2012) Arabidopsis E2FA stimulates proliferation and endocycle separately through RBR-bound and RBR-free complexes. EMBO J 31: 1480–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodbarkelari F, Bramsiepe J, Weinl C, Marquardt S, Novak B, Jakoby MJ, Lechner E, Genschik P, Schnittger A (2010) Cullin 4-ring finger-ligase plays a key role in the control of endoreplication cycles in Arabidopsis trichomes. Proc Natl Acad Sci USA 107: 15275–15280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittger A, Weinl C, Bouyer D, Schobinger U, Hulskamp M (2003) Misexpression of the cyclin-dependent kinase inhibitor ICK1/KRP1 in single-celled Arabidopsis trichomes reduces endoreduplication and cell size and induces cell death. Plant Cell 15: 303–315 [DOI] [PMC free article] [PubMed] [Google Scholar]


