Abstract
Sphingomyelin and cholesterol can assemble into domains and segregate from other lipids in the membranes. These domains are reported to function as platforms for protein transport and signalling. Do similar domains exist in the Golgi membranes and are they required for protein secretion? We tested this hypothesis by using D-ceramide-C6 to manipulate lipid homeostasis of the Golgi membranes. Lipidomics of the Golgi membranes isolated from D-ceramide-C6-treated HeLa cells revealed an increase in the levels of C6-sphingomyelin, C6-glucosylceramide, and diacylglycerol. D-ceramide-C6 treatment in HeLa cells inhibited transport carrier formation at the Golgi membranes without affecting the fusion of incoming carriers. The defect in protein secretion as a result of D-ceramide-C6 treatment was alleviated by knockdown of the sphingomyelin synthases 1 and 2. C6-sphingomyelin prevented liquid-ordered domain formation in giant unilamellar vesicles and reduced the lipid order in the Golgi membranes of HeLa cells. These findings highlight the importance of a regulated production and organization of sphingomyelin in the biogenesis of transport carriers at the Golgi membranes.
Keywords: ceramide, fission, Golgi apparatus, lipid raft, transport
Introduction
The majority of ceramide synthesized at the endoplasmic reticulum (ER) is transported to the trans-Golgi network (TGN) by the ceramide transport protein, CERT (Hanada et al, 2003). At the cytosolic leaflet of the TGN, ceramide is converted into glucosylceramide (Coste et al, 1986). In addition, at the luminal leaflet of the TGN, sphingomyelin synthases 1 and 2 (SMS1 and SMS2) consume ceramide and phosphatidylcholine (PC) to produce sphingomyelin (SM) and diacylglycerol (DAG), respectively (Huitema et al, 2004).
SM and cholesterol are enriched in lipid rafts or the liquid-ordered membrane domains that are important for the organization of the plasma membrane into distinct regions (Simons and Ikonen, 1997; Simons and Gerl, 2010; Simons and Sampaio, 2011). These lipid rafts reportedly serve as platforms for a variety of functions that include T cell receptor signalling (Zech et al, 2009) or the budding of enveloped viruses (Scheiffele et al, 1999). A role for the lipid rafts in intracellular protein transport stems from the findings that, in yeast, both sterols and sphingolipids are enriched in the membranes of immuno-isolated Golgi-derived transport carriers containing apical cargo (Klemm et al, 2009). Addition of short-chain ceramide inhibits trafficking of newly synthesized vesicular stomatitis virus glycoprotein (VSV-G) across the stack of Golgi membranes (Rosenwald and Pagano, 1993), and Brefeldin A (BFA)-mediated fusion of Golgi membranes with the ER (Fukunaga et al, 2000). However, it is not known whether short-chain ceramide treatment affects the generation of transport carriers or their fusion with the target membrane. It is also not known whether ceramide homeostasis is important for the function of the Golgi membranes.
We have addressed these issues by testing the effects of exogenously added short-chain ceramide-C6 on the structure and the function of Golgi membranes in mammalian cells. We have found that exogenously added short-chain ceramide-C6 was incorporated into the Golgi membranes and locally increased the levels of short-chain SM, short-chain glucosylceramide, and DAG. This paralleled inhibition in the ability of the Golgi membranes to produce transport carriers. Surprisingly, the fusion of incoming carriers to the Golgi apparatus was not affected. We have also found that short-chain SM prevented the formation of phase-separated liquid-ordered domains in vitro. Based on our findings, we suggest that the formation of liquid-ordered SM-rich domains at the Golgi membranes is essential for transport carrier formation but not for the fusion of incoming transport carriers at the Golgi apparatus. The description of our findings follows.
Results
Treatment with D-ceramide-C6 increases the DAG content of the Golgi membranes
To determine whether ceramide-C6 was metabolized by sphingomyelin synthases localized in the Golgi membranes, HeLa cells were incubated with D-ceramide-C6 (N-hexanoyl-D-erythro-sphingosine) or its non-metabolizable enantiomer L-ceramide-C6 (N-hexanoyl-L-erythro-sphingosine) (Figure 1A). We monitored the DAG levels by fluorescence microscopy in HeLa cells expressing the DAG-binding GST-tagged C1a domain of protein kinase D1 (GST-C1a-PKD1) (Maeda et al, 2001; Baron and Malhotra, 2002). This sensor is mostly cytosolic and only a minor fraction is localized to the TGN under steady-state conditions (Maeda et al, 2001; Baron and Malhotra, 2002). Treatment with 20 μM D-ceramide-C6 for 30 min increased the levels of GST-C1a-PKD1 at the TGN in HeLa cells (Figure 1B). L-ceramide-C6 was ineffective in the recruitment of GST-C1a-PKD1 to the TGN (Figure 1B). We then examined the effect of ceramide-C6 treatment on the localization of full-length GST-tagged PKD2 to the TGN (Baron and Malhotra, 2002; Pusapati et al, 2010). In D-ceramide-C6-treated HeLa cells, substantially more GST-PKD2 was recruited to the TGN than in L-ceramide-C6-treated cells (Figure 1C). To quantify the increase in PKD recruitment to the TGN, GST-PKD2 expressing HeLa cells were treated with ethanol, L-ceramide-C6, or D-ceramide-C6, homogenized and separated by centrifugation into cytosolic and membrane fractions that were analysed by western blotting (Figure 1D). Quantification of the blot revealed that, compared with cells treated with L-ceramide-C6, the membrane-associated pool of GST-PKD2 in D-ceramide-C6-treated cells was more than two-fold higher (Figure 1E). These findings suggest that exogenously added D-ceramide-C6 is converted, together with PC, into SM and DAG at the TGN. The newly generated DAG recruits PKD to the TGN. L-ceramide-C6, as expected, has no effect on the DAG levels at the TGN.
Figure 1.
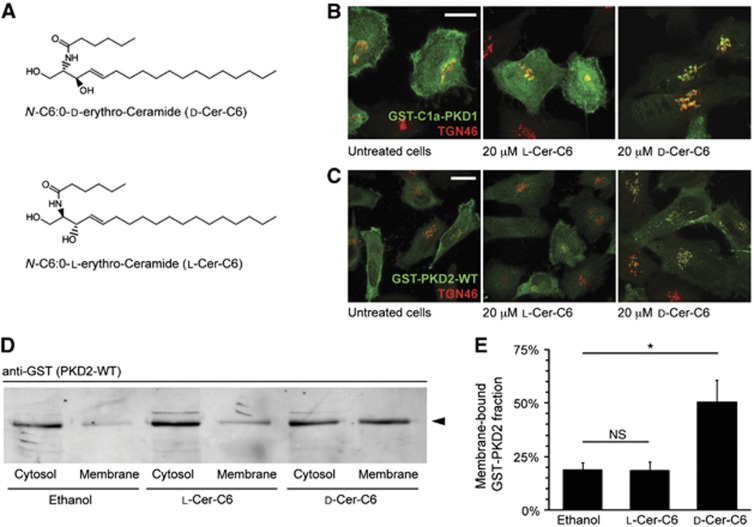
D-Ceramide-C6 causes recruitment of PKD to the Golgi apparatus. (A) Chemical structure of D-ceramide-C6 (top) and L-ceramide-C6 (bottom). (B) HeLa cells were transfected with GST-tagged C1a-PKD1 and treated with 20 μM L-ceramide-C6 or D-ceramide-C6 for 30 min. Localization of GST-C1a-PKD1 and the organization of TGN membranes, before and after ceramide-C6 incubation, were monitored by fluorescence microscopy with anti-GST and anti-TGN46 antibodies. (C) HeLa cells were transfected with full-length GST-PKD2-wild type (WT) and treated with 20 μM L-ceramide-C6 or D-ceramide-C6 for 30 min, and immunostained with anti-GST and anti-TGN46 antibodies. (D) HeLa cells were transfected with GST-PKD2-WT, treated with ethanol (vehicle), 20 μM L-ceramide-C6, or D-ceramide-C6 for 30 min, and homogenized. The homogenates were separated into a membrane and cytosolic fraction and both were western blotted with an anti-GST antibody. (E) Results in (D) were quantified and average values±s.e.m. are plotted as bar graphs (N=3). Compared data sets were considered as statistically significant when P<0.05 (*). Source data for this figure is available on the online supplementary information page.
Measurement of lipid levels by mass spectrometry in ceramide-C6-treated cells and Golgi membranes
HeLa cells were treated with ethanol (control), 20 μM L-ceramide-C6 or 20 μM D-ceramide-C6 for 30 min, after which lipids were extracted for mass-spectrometric analysis. The increase in ceramide levels after treatment with L-ceramide-C6 or D-ceramide-C6 indicates that both lipids were equally incorporated into cells (Figure 2A). As expected, L-ceramide-C6 was not metabolized, as none of its potential metabolites were found in the lipid profile of the treated cells (Figure 2B). However, a six-fold increase in the levels of glucosylceramide, the predominant form of hexosylceramide, was observed in D-ceramide-C6-treated cells (Figure 2A). Although there was no net increase in total SM levels in D-ceramide-C6-treated cells, C6-SM amounts to 10% of the cellular SM, but was undetected in ethanol or L-ceramide-C6-treated cells (Figure 2B; Supplementary Figure S1). This indicates that D-ceramide-C6 and PC are converted into C6-SM and DAG, respectively, by the enzyme SMS. Under these conditions, the levels of the physiological long-chain SM are reduced without affecting the net levels of SM (Supplementary Figure S1).
Figure 2.
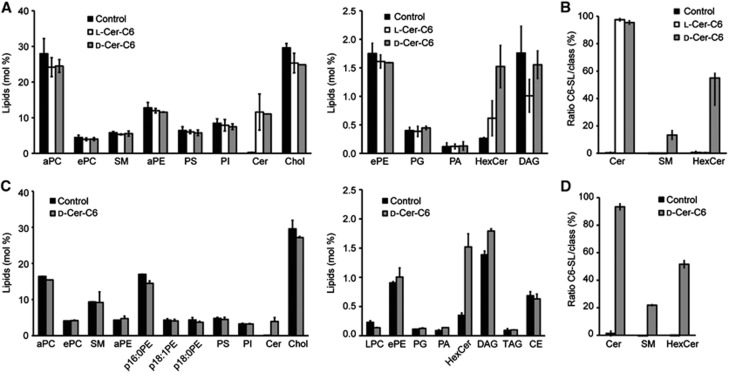
Lipidomics of ceramide-C6-treated HeLa cells and isolated Golgi membranes. (A) Relative amount of total lipids measured by mass spectrometry of whole HeLa cells treated with ethanol (control), 20 μM L-ceramide-C6, or 20 μM D-ceramide-C6 for 30 min. For clarity, high and low abundant lipids are separated in the two graphs, respectively. (B) Ratio of C6-containing sphingolipids (SL) to total SL population in control, L-ceramide-C6, or D-ceramide-C6-treated cells. (C) Relative amount of total lipids measured by mass spectrometry of Golgi membranes isolated from HeLa cells treated with ethanol (control) or 20 μM D-ceramide-C6 for 30 min. (D) Ratio of C6-containing SL to total SL population in Golgi membranes enriched from control or D-ceramide-C6-treated cells. Average values±s.d. (N=3) are represented in all panels. PC, phosphatidylcholine; SM, sphingomyelin; PE, phosphatidylethanolamine; PS, phosphatidylserine; PI, phosphatidylinositol; Cer, ceramide; Chol, cholesterol; PG, phosphatidylglycerol; PA, phosphatidic acid; HexCer, hexosylceramide; DAG, diacylglycerol; LPC, lyso-PC; TAG, triacylglycerol; CE, cholesteryl ester; a, acyl-linked; e, ether-linked; and p, plasmalogen.
To further quantitate the changes in lipids in the Golgi membranes, we isolated Golgi membranes from ethanol (control) or D-ceramide-C6-treated HeLa cells. The isolated membranes were western blotted with antibodies to the TGN-specific integral membrane proteins SPCA1 and TGN46. The results reveal a six-fold enrichment of these membrane proteins in the isolated membrane fraction compared with the total homogenate (Supplementary Figure S2). Lipids from these isolated Golgi membranes were then analysed by mass spectrometry and the data show a 30% increase in the levels of DAG and a four-fold increase in the levels of glucosylceramide (Figure 2C). Although the total levels of SM remained unchanged (Figure 2C), C6-SM makes up for >20% of total SM (Figure 2D). Treatment with D-ceramide-C6 thus causes a dramatic change in the levels of DAG, glucosylceramide and C6-SM in the Golgi membranes.
D-ceramide-C6 inhibits cargo export from the Golgi apparatus
We first tested the effect of ceramide-C6 on general protein secretion in HeLa cells. HeLa cells were treated with ethanol, L-ceramide-C6, D-ceramide-C6 or BFA, pulsed with 35S-methionine for 15 min and chased in non-labelled methionine-containing medium for 2 h. Proteins in the medium were then precipitated and analysed by SDS–PAGE and autoradiography, which revealed that D-ceramide-C6 treatment caused an overall reduction in the secretion of newly synthesized proteins compared with control or L-ceramide-C6 treatment (Figure 3A).
Figure 3.
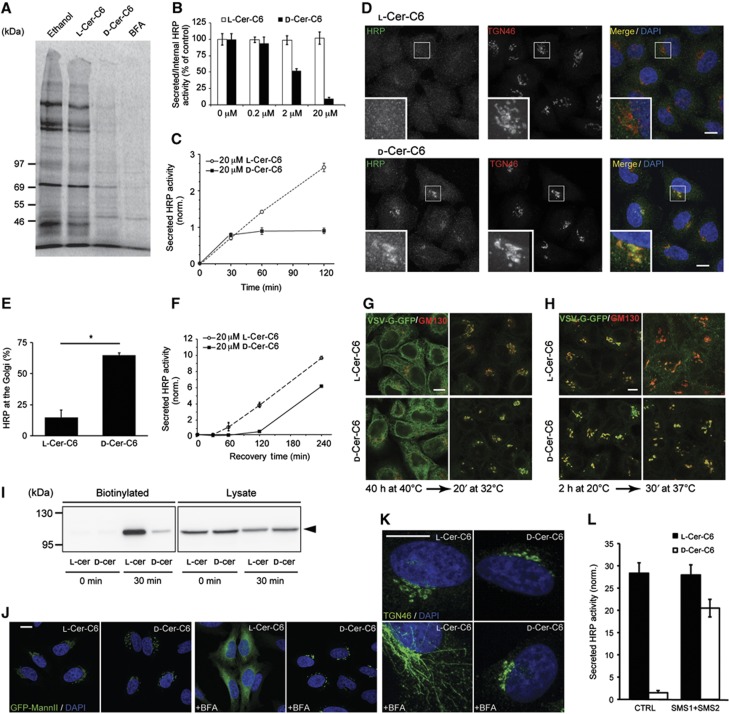
D-Ceramide-C6 inhibits cargo export from the Golgi apparatus. (A) HeLa cells were pulsed with 35S-methionine for 15 min and chased for 2 h in complete medium containing ethanol, 20 μM L-ceramide-C6, 20 μM D-ceramide-C6 or 5 μg/ml BFA. The medium from the cells was collected and analysed by SDS–PAGE and autoradiography. (B) HeLa-ssHRP cells were treated with indicated concentrations of L-ceramide-C6 or D-ceramide-C6 for 2 h, and the secretion of HRP was monitored by chemiluminescence and normalized to internal HRP activity. (C) HeLa-ssHRP cells were treated with 20 μM L-ceramide-C6 or D-ceramide-C6; the HRP activity in the medium was measured by chemiluminescence and normalized to the total cellular protein content, at the indicated time points. (D) HeLa-ssHRP cells were treated for 30 min with 20 μM L-ceramide-C6 or D-ceramide-C6, washed, incubated in complete medium with 100 μM cycloheximide for 60 min, and then fixed for immunofluorescence. HRP was detected with an anti-HRP antibody (green) and the TGN was visualized using an anti-TGN46 (red) antibody. Zooms of marked sections are shown in the bottom panels. (E) At least 100 randomly selected cells from each sample (L-ceramide-C6 or D-ceramide-C6-treated cells) in three different experiments as described in (D) were observed to quantify intracellular HRP location. Average values±s.e.m. (N=3) are represented. Compared data sets were considered as statistically significant when P<0.05 (*). (F) HeLa-ssHRP cells were treated with 20 μM L-ceramide-C6 or D-ceramide-C6 for 30 min, and then washed in complete medium. HRP secretion was measured by chemiluminiscence at indicated time points and normalized to the total cellular protein content. (G) The transport of VSV-G-GFP was monitored in L-ceramide-C6 and D-ceramide-C6-treated cells. HeLa-VSV-G-GFP cells stably transfected with a plasmid encoding for VSV-G (ts045)-GFP were incubated at 40°C for 40 h to arrest VSV-G-GFP at the ER. 20 μM D-ceramide-C6 or L-ceramide-C6 was added during the last 30 min of the incubation. The medium was then replaced by ceramide-C6-free medium containing 100 μM cycloheximide and 25 mM HEPES pH 7.4 buffer, and cells were shifted to the permissive temperature of 32°C for 20 min. (H) Cells were incubated at 40°C, and then shifted to 20°C for 2 h in the presence of 100 μM cycloheximide and 25 mM HEPES pH 7.4 buffer to arrest and accumulate VSV-G-GFP in the TGN. Cells were treated with L-ceramide-C6 or D-ceramide-C6 during the last 10 min of the 20°C block, and then shifted to 37°C to allow transport of VSV-G-GFP along the secretory pathway to the plasma membrane. Samples were fixed at the indicated time points, after which VSV-G-GFP and GM130 were visualized by immunofluorescence microscopy. (I) HeLa-VSV-G-GFP cells in (H) were biotinylated after the shift to 37°C at indicated time points. The biotinylated fraction and 6.6% of the total cell lysate were analysed by western blotting using an antibody to GFP. (J) D-ceramide-C6 inhibits BFA-induced Golgi apparatus to ER transport. HeLa-GFP-MannII cells were grown on coverslips in the presence of 20 μM L- or D-ceramide-C6 for 30 min at 37°C. The cells were then incubated with BFA at 5 μg/ml for 10 min, fixed and processed for immunofluorescence. (K) HeLa-GFP-MannII cells were incubated with 20 μM L- or D-ceramide-C6 for 30 min and then with BFA (5 μg/ml) for 5 min. The cells were fixed and stained with anti-TGN46 antibody. (L) HeLa-ssHRP cells were transfected with control or SMS1- and SMS2-specific siRNA for 4 days, and then assayed for HRP secretion for 2 h in the presence of 20 μM L-ceramide-C6 or D-ceramide-C6. Secreted HRP activity was measured by chemiluminescence and normalized to total protein content in the lysate. Scale bar is 10 μm in all panels. Source data for this figure is available on the online supplementary information page.
To reveal the site at which newly synthesized secretory proteins accumulate upon treatment with D-ceramide-C6, we tested its effects on specific secretory cargoes. HeLa cells stably expressing horseradish-peroxidase containing a signal sequence (HeLa-ssHRP) were treated with L-ceramide-C6, D-ceramide-C6, or ethanol, after which the secretion of HRP was measured as described previously (von Blume et al, 2011). As expected, treatment with D-ceramide-C6, but not L-ceramide-C6, inhibited HRP secretion in a concentration- (Figure 3B) and time-dependent manner (Figure 3C). HeLa-ssHRP cells treated with L- or D-ceramide-C6 were fixed and stained with antibodies to HRP and the TGN-specific protein TGN46, revealing that D-ceramide-C6 treatment arrested HRP in the TGN (Figure 3D and E). Is the effect of D-ceramide-C6 on protein secretion reversible? This is an important concern because long-term ceramide treatment promotes apoptosis (Pettus et al, 2002). HeLa-ssHRP cells were treated for 30 min with D-ceramide-C6, washed, incubated in ceramide-C6-free complete medium and then assayed for HRP secretion. The results show that after 2 h incubation in ceramide-C6-free medium, the rate of HRP secretion was restored (Figure 3F). This indicates that cells are viable under our experimental procedures and treatment with D-ceramide-C6 is reversible with respect to protein secretion. Based on these findings, we chose 20 μM D-ceramide-C6 treatment for 30 min as a suitable procedure for all subsequent experiments described here, unless otherwise stated.
A temperature-sensitive mutant G protein of the VSV (VSV-G-ts045) is retained in the ER at the non-permissive temperature of 40°C because of a folding defect. However, shifting cells to 32–37°C alleviates the defect in folding and VSV-G is exported from the ER to the Golgi membranes (Bergmann, 1989). HeLa cells stably expressing the mutant VSV-G-ts045 fused to GFP (HeLa-VSV-G-GFP) were incubated at 40°C for 40 h to arrest the newly synthesized VSV-G-GFP in the ER. The cells were treated with L- or D-ceramide-C6 at 40°C for 30 min, after which the cells were shifted to 32°C for 20 min and visualized by fluorescence microscopy. VSV-G-GFP was transported from the ER to the Golgi membranes in both L- and D-ceramide-C6-treated cells (Figure 3G). We then arrested VSV-G-GFP at the TGN by incubating the cells at 20°C for 2 h by the established procedures (Griffiths et al, 1985). The cells were then treated with L- or D-ceramide-C6 for 15 min, after which they were shifted to 37°C for 30 min. The trafficking of VSV-G-GFP to the cell surface was monitored by fluorescence microscopy and cell surface biotinylation. Under these experimental procedures, D-ceramide-C6 treatment arrested VSV-G-GFP at the TGN (Figure 3H). Cell surface biotinylation revealed a clear defect in the trafficking of VSV-G-GFP to the cell surface in D-ceramide-C6-treated cells compared to L-ceramide-C6-treated cells (Figure 3I).
Glycosylphosphatidylinositol (GPI)-anchored proteins traffic through the secretory pathway to the SM-rich apical cell surface, where they are thought to be enriched in lipid rafts (Lingwood and Simons, 2010). We tested the effect of D-ceramide-C6 treatment on the trafficking of GFP-GPI in HeLa cells. HeLa cells expressing GFP-GPI were incubated with cycloheximide for 10 min, treated with either ethanol or D-ceramide-C6 for 30 min and then fixed and stained for visualization by immunofluorescence microscopy. In control cells, GFP-GPI was found at the TGN and the cell surface, whereas D-ceramide-C6-treated cells revealed that GFP-GPI was only visible in the Golgi apparatus (Supplementary Figure S3A). This suggests that export of GFP-GPI from the TGN is blocked in cells treated with D-ceramide-C6. Taken together, these results indicate that D-ceramide-C6 treatment causes a general block in cargo export from the TGN to the cell surface.
Short-term treatment with BFA tubulates Golgi membranes: the tubules from the cis/medial/trans cisternae fuse with the ER, whereas the TGN-derived tubules fuse with the endosomes (Lippincott-Schwartz et al, 1991). What is the effect of D-ceramide-C6 on BFA-dependent Golgi membrane reorganization? HeLa cells stably expressing a GFP chimera of the cis/medial Golgi apparatus resident protein Mannosidase II (HeLa-GFP-MannII) were treated with L- or D-ceramide-C6 for 30 min followed by a 10-min incubation with BFA. Fluorescence microscopy revealed that in L-ceramide-C6-treated cells, GFP-MannII relocated to the ER upon BFA treatment. However, D-ceramide-C6 treatment inhibited the BFA-induced relocation of GFP-MannII to the ER (Figure 3J). BFA-induced tubulation of the TGN was also inhibited upon D-ceramide-C6 treatment (Figure 3K). Next, we tested the effect of ceramide-C6 treatment on the reassembly of the Golgi apparatus after BFA washout. HeLa-GFP-MannII cells were treated with BFA for 30 min and then with L- or D-ceramide-C6 for 30 min. Cells were then washed extensively and incubated in BFA-free medium for 60 min. Incubation with either L-ceramide-C6 or D-ceramide-C6 during the BFA washout did not affect the reassembly of the Golgi apparatus (Supplementary Figure S3B). Thus, D-ceramide-C6 blocks BFA-dependent tubulation and fusion of the Golgi membranes with the ER, it affects tubulation of the TGN, but not the fusion events leading to reassembly of the Golgi apparatus after BFA washout.
The conversion of D-ceramide-C6 to C6-SM is required for its inhibitory effect on HRP secretion
The SMS enzymes convert ceramide and PC into SM and DAG, respectively. SMS1 is localized at the Golgi membranes, whereas SMS2 is predominantly found at the cell surface (Huitema et al, 2004). HeLa-ssHRP cells were transfected with siRNA for both SMS1 and SMS2, and the knockdown efficiency by RT–PCR revealed a 50 and 60% reduction in the mRNA levels of SMS1 and SMS2, respectively (Supplementary Figure S4A). The cells were assayed for HRP secretion in the presence of either L- or D-ceramide-C6. The results show that depletion of SMS has no obvious effect in HRP secretion in the presence of L-ceramide-C6. However, it alleviates the secretion defect induced by D-ceramide-C6 treatment (Figure 3L; Supplementary Figure S4B). Thus, conversion of D-ceramide-C6 to C6-SM causes the defect in HRP export from the Golgi membranes.
The addition of D-ceramide-C6 also increased the levels of C6-glucosylceramide in both HeLa cells and the isolated Golgi membranes (Figure 2). To test whether excess C6-glucosylceramide affected HRP secretion, we performed the following experiment. HeLa-ssHRP cells were incubated for 2 h at 20°C to arrest HRP at the TGN and then shifted to 37°C in the presence of L- or D-ceramide-C6 and of DMSO or the glucosylceramide synthase inhibitor DL-threo-1-Phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP). This incubation was carried out in the presence of cycloheximide as PDMP is known to inhibit protein synthesis (Rosenwald et al, 1992). The results show that D-ceramide-C6-induced inhibition of HRP secretion was not rescued by PDMP treatment (Supplementary Figure S4C). We also measured SM, ceramide, and hexosylceramide levels in HeLa cells treated with D-ceramide-C6 and PDMP by mass spectrometry. The results show that PDMP treatment effectively reduced D-ceramide-C6-induced increase in the glucosylceramide levels (Supplementary Figure S4D). Taken together, these results indicate that production of C6-glucosylceramide upon D-ceramide-C6 treatment does not affect HRP secretion.
D-Ceramide-C6 treatment blocks early events in vesicle biogenesis
Overexpression of a dominant-negative kinase-dead mutant of PKD (PKD-KD) inhibits the fission of transport carriers at the TGN and, as a result, cargo-containing tubules are found attached to the TGN (Liljedahl et al, 2001). A HeLa cell line that expresses PKD1-KD-GST-Flag at low levels (GF17 cells) (Liljedahl et al, 2001) shows no defect in Golgi membrane organization or traffic at 32–37°C. Upon a shift to 20°C, PKD-KD concentrates at the TGN and long tubules containing TGN-to-cell surface specific cargo are found attached to the TGN (Maeda et al, 2001). GF17 cells were incubated with L-ceramide-C6 or D-ceramide-C6 for 30 min at 37°C, shifted to 20°C, and PKD1-KD-GST-Flag and TGN46 were visualized by immunofluorescence microscopy. Tubulation of the TGN was observed in cells treated with L-ceramide-C6; however, D-ceramide-C6 treatment inhibited the tubule formation (Figure 4A).
Figure 4.
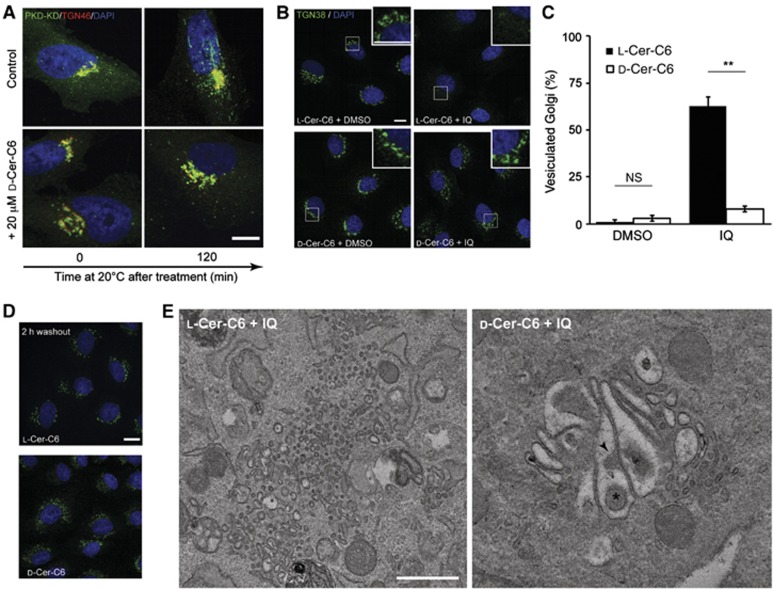
D-Ceramide-C6 inhibits membrane fission. (A) GF17 cells stably expressing PKD1-KD-GST-Flag were placed at 20°C to induce tubulation of the TGN. Cells were pretreated with ethanol (control) or D-ceramide-C6 for 30 min at 37°C, then shifted to 20°C and, at indicated time points, fixed and stained with anti-TGN46 and anti-GST antibodies. (B) NRK cells were pretreated for 30 min with 20 μM L- or D-ceramide-C6, then washed and incubated with 140 μM IQ or DMSO for 1 h at 37°C. The cells were fixed with PFA and visualized by fluorescence microscopy with anti-TGN38 antibody. (C) Quantitation of cells with a fragmented (vesiculated) Golgi apparatus in 100 randomly selected cells per experiment. Bars show the mean±s.e.m. of three independent experiments (N=3) P<0.01 (**). (D) Post IQ treatment, NRK cells were treated with 20 μM L- or D-ceramide-C6. After 30 min, IQ was removed and the cells incubated in medium containing L- or D-ceramide-C6, respectively, and observed by fluorescence microscopy. (E) NRK cells were treated as in (B), fixed, and thin sections visualized by electron microscopy. Size scale bars (A), (B), and (D)=10 μm and (E)=500 nm.
The sponge metabolite ilimaquinone (IQ) vesiculates the Golgi membranes by overactivating a PKD-dependent membrane fission reaction (Takizawa et al, 1993; Campelo and Malhotra, 2012). Does ceramide-C6 affect IQ-mediated Golgi membrane vesiculation? Normal Rat Kidney (NRK) cells were incubated for 30 min with L- or D-ceramide-C6 followed by incubation with IQ for 60 min. The organization of the TGN was monitored by immunofluorescence microscopy using an anti-TGN38-specific antibody. In L-ceramide-C6-treated cells, the Golgi apparatus was completely vesiculated after incubation with IQ. D-ceramide-C6 treatment, however, inhibited IQ-mediated vesiculation of the Golgi membranes (Figure 4B and C). Upon removal of IQ from the cells, the vesiculated Golgi membranes fuse and reassemble into an intact functional Golgi apparatus in the pericentriolar region (Takizawa et al, 1993). To test whether ceramide-C6 treatment had an effect on this membrane fusion and reassembly pathway, NRK cells were first treated with IQ to vesiculate Golgi membranes. The cells were then washed and incubated in the presence of L- or D-ceramide-C6 for 60 min. The results reveal that neither L-ceramide-C6 nor D-ceramide-C6 treatment affected the fusion and reassembly of the vesiculated Golgi membranes into an intact Golgi apparatus (Figure 4D).
To gain further insight into the effect of ceramide-C6 and IQ treatment on the organization of the Golgi membranes, NRK cells were pretreated with L- or D-ceramide-C6 for 30 min, followed by treatment with IQ for 60 min. The morphology of the Golgi membranes was analysed by electron microscopy. In L-ceramide-C6-treated cells, the Golgi membranes were extensively vesiculated. (Figure 4E, left panel). There was no obvious vesiculation of the Golgi cisternae in D-ceramide-C6-treated cells; however, the Golgi membranes were highly distended and swollen (Figure 4E, right panel). Interestingly, there were inward invaginations in Golgi membranes (arrowhead, Figure 4E) and vesicles inside the swollen cisternae (asterisk, Figure 4E).
In the absence of IQ, the Golgi membranes of HeLa cells that were treated with L- or D-ceramide-C6 for 30 min showed no obvious morphological changes (Supplementary Figure S5A). However, treatment with D-ceramide-C6 for 30 min followed by incubation in ceramide-C6-free medium for 90 min caused the curling of Golgi cisternae (Supplementary Figure S5B). We suggest that these changes are a consequence of the effect of D-ceramide-C6 on membrane fission versus membrane fusion at the Golgi membranes. In the presence of D-ceramide-C6, transport carriers fuse with the Golgi membranes, however, membrane export is blocked. This increases the surface area-to-volume ratio of the Golgi cisternae. This is similar to morphological changes in model membranes as a result of an increase in the surface area-to-volume ratio (Berndl et al, 1990; Seifert et al, 1991).
C6-SM prevents the formation of liquid-ordered domains in GUVs and reduces lipid order in Golgi membranes
Model membranes composed of SM, PC, and cholesterol are known to separate into liquid-disordered and liquid-ordered phases (Dietrich et al, 2001; Goñi et al, 2008). Although the inability of short-chain ceramide (Chiantia et al, 2007) or fluorescently labelled C6-SM (Wang and Silvius, 2000) to form liquid-ordered phases has been reported, it is not known whether non-labelled C6-SM can form or perturb liquid-ordered domains. To investigate this, we generated giant unilamellar vesicles (GUVs) composed of PC:C16-SM:Cholesterol at a mole ratio of 2:1:1, and also GUVs in which C16-SM was replaced with C6-SM, and probed for the presence of liquid-ordered and liquid-disordered domains using naphthopyrene and Lissamine-Rh-PE, respectively. The long-chain C16-SM induced the formation of liquid-ordered domains both at 37°C and at 22°C, as expected. However, no phase separation was observed in C6-SM-containing GUVs at both temperatures (Figure 5A; Supplementary Figure S6A). GUVs containing equal amounts of both SMs (PC:C6-SM:C16-SM:Cholesterol at 2:0.5:0.5:1) showed an intermediate phenotype, where phase separation was observed, but impaired, indicating that C6-SM is functionally distinct from C16-SM and does not contribute to phase separation (Supplementary Figure S6B).
Figure 5.
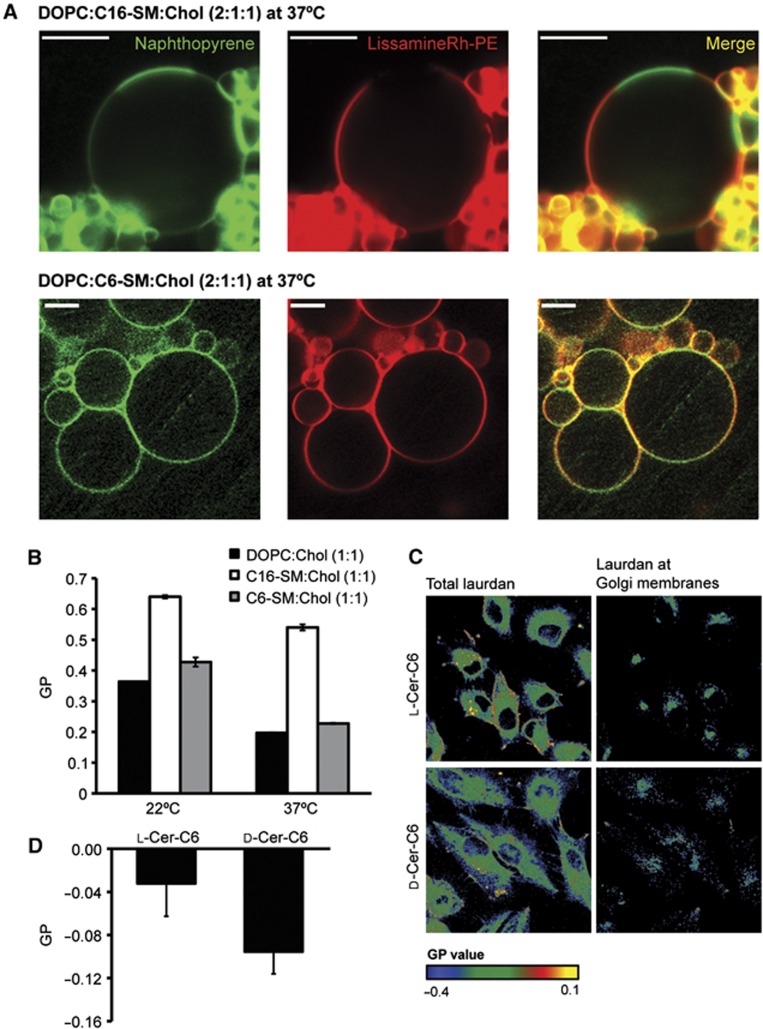
C6-SM prevents the formation of liquid-ordered domains in GUVs and reduces order in the Golgi membranes. (A) GUVs made of DOPC (dioleoylphosphatidylcholine):C16-SM:Chol (2:1:1) (top panels), and DOPC:C6-SM:Chol (2:1:1) (bottom panels) were heated to 37°C, and visualized by fluorescence microscopy using the dyes naphthopyrene (green) that preferentially stains liquid-ordered membrane regions, and Lissamine-Rh-PE (red) that preferentially stains liquid-disordered regions. Scale bar is 10 μm. (B) Generalized polarization (GP) measured in DOPC, C16-SM, or C6-SM containing LUVs in a 1:1 molar ratio to cholesterol at 20 or 37°C. Average values±s.d. (N=3) are represented. (C) HeLa-MannII-RFP cells were treated with 20 μM L- or D-ceramide-C6 for 30 min and then washed and incubated in laurdan-containing FCS-free medium for 90 min before fixation. Cells were observed by two-photon fluorescence microscopy and the laurdan GP was measured and plotted in the whole cell (left) and also in the MannII-RFP-positive area (right). Colour code represents the measured GP value. (D) Laurdan GP from different cells in (C) was measured. Average values±s.d. (N=17) are represented.
We then asked what ratio of C6-SM to total SM affected the formation liquid-ordered domains. Our results show that when 30% of C16-SM is substituted for C6-SM, phase separation in GUVs is inhibited. However, substitution of 10% C16-SM with C6-SM does not affect the formation of lipid-ordered domains (Supplementary Figure S6C). We then prepared GUVs with PC:C6-SM:C16-SM:Cholesterol at 1:1:1:1 molar ratio, which revealed a phase separation similar to GUVs of PC:C16-SM:Cholesterol at a mole ratio of 2:1:1, suggesting that C6-SM is functionally similar to PC (Supplementary Figure S6D). The emission wavelength of laurdan (6-lauryl-2-dimethylamino-napthalene) is dependent on the degree of order in the surrounding lipids, and can be used to quantify the lipid order in the membranes (Kaiser et al, 2009). Using laurdan, we measured the relative membrane order of the binary mixtures of C6-SM:Cholesterol (1:1), C16-SM:Cholesterol (1:1), and PC:Cholesterol (1:1) in large unilamellar vesicles (LUVs). Our results indicate that both C6-SM and PC-containing LUVs form a low-order lipid phase, in contrast to the high-order membrane that is generated by C16-SM (Figure 5B).
Next, we used laurdan to measure lipid order in cellular membranes (Gaus et al, 2003; Owen et al, 2012). HeLa cells stably expressing Mannosidase II-RFP (HeLa-MannII-RFP) were treated with L-ceramide-C6 or D-ceramide-C6 for 30 min, washed, and incubated with laurdan for 90 min, after which the generalized polarization (GP) of laurdan in the Mannosidase II-RFP-positive area was measured (Figure 5C). In the Mannosidase II-RFP-positive area of D-ceramide-C6-treated cells, less polarization of the probe was measured than in L-ceramide-C6-treated cells (Figure 5D). This indicates that D-ceramide-C6 treatment causes a reduced membrane order in the Golgi membranes. Taken together, these experiments show that, in contrast to endogenous long-chain SM, short-chain C6-SM is unable to form liquid-ordered phase-separated domains, and that treatment with short-chain D-ceramide-C6 reduces the overall lipid order in the Golgi membranes.
Discussion
The SM- and cholesterol-rich liquid-ordered domains are reported to compartmentalize the plasma membrane into functionally distinct domains (Simons and Ikonen, 1997; Kusumi et al, 2004; Goswami et al, 2008; Roduit et al, 2008; Brameshuber et al, 2010; Simons and Gerl, 2010; Sezgin and Schwille, 2011; Simons and Sampaio, 2011). As SM is generated in the TGN, it has long been suggested that similar raft-like domains exist in the Golgi membranes (Simons and van Meer, 1988; Gkantiragas et al, 2001). There is some evidence that supports this proposal; for example, SM-enriched domains at the TGN are required for the sorting of apical cargo, and apical-like vesicles that form at the late Golgi membranes in yeast are enriched in SM and ergosterol (Klemm et al, 2009; Surma et al, 2011). However, the size, composition, and existence of lipid rafts are contentious issues (Munro, 2003; Goswami et al, 2008). Among the numerous issues that challenge the existence of lipid rafts, the major reasons are the inability to visualize these domains and a non-toxic means to disrupt them to monitor the subsequent effects on membrane organization. The situation is exacerbated with respect to the Golgi membranes because of their relative small surface area and accessibility to visual and biochemical manipulations (Cao et al, 2012).
Our first goal was to test whether short-chain ceramide-C6 was metabolized into short-chain C6-SM by the Golgi membrane-specific SMS enzymes. The fact that D-ceramide-C6 caused a two-fold increase in the recruitment of DAG-binding PKD to the TGN suggested that D-ceramide-C6 was converted into C6-SM. To obtain quantitative data on the lipid changes induced by ceramide-C6 treatment, we analysed by mass spectrometry the lipidome of both HeLa cells and the isolated Golgi membranes. The results show that, after D-ceramide-C6 treatment, there was an increase in the levels of glucosylceramide and, although there was no obvious change in the total levels of SM, short-chain SM was generated by SMS enzymes at the TGN. Thus, short-chain ceramide was converted into short-chain SM and glucosylceramide at the TGN. We then asked whether short-chain SM affected the ability of endogenous long-chain SM to assemble into a liquid-ordered domain. Our findings revealed that short-chain SM does not assemble into a liquid-ordered domain. The polarization of the laurdan probe at the Golgi membranes, which is dependent on the lipid order and packing of the membrane where it is incorporated, was also decreased by D-ceramide-C6 treatment in HeLa cells. We suggest that replacing long-chain SM with short-chain SM, locally prevents the formation of a raft-like liquid-ordered domain in the Golgi membranes.
We also tested the effect of short-chain SM, generated by the treatment of HeLa cells with D-ceramide-C6, on the structure and the function of Golgi membranes. Our findings reveal that short-chain SM inhibits the biogenesis of transport carriers at the Golgi apparatus. IQ, which vesiculates Golgi membranes, or BFA, which tubulates the Golgi membranes to promote rapid fusion with the ER, was ineffective in D-ceramide-C6-treated cells. Thus, short-chain SM causes a general block in the ability of Golgi membranes to generate transport carriers. It is noteworthy that SMS generates SM in the luminal leaflet of the TGN membrane (Huitema et al, 2004). Unlike DAG, SM cannot spontaneously flip across the lipid bilayer; SM-rich liquid-ordered domains therefore form in the luminal leaflet of the TGN. We suggest that preventing the packing of endogenous long-chain SM by short-chain SM inhibits membrane compartmentation required for cargo sorting and the segregation of components necessary for events such as membrane bending. These events therefore inhibit the process of transport carrier formation without affecting the fusion of incoming carriers (Figure 6). An equally plausible explanation of our results is the possibility that change in lipid composition due to D-ceramide-C6 treatment modifies other biophysical properties of Golgi membranes, such as bending rigidity, membrane thickness, or spontaneous curvature. These, in turn, could affect membrane curvature or localization of proteins that are required for transport carrier biogenesis.
Figure 6.

A working hypothesis of selective lipid raft disruption by C6-SM. SMS localizes to the TGN membrane and catalyses the formation of DAG and SM from PC and ceramide at the luminal membrane leaflet. SM and cholesterol are enriched in liquid-ordered domains or lipid rafts. Upon addition of short-chain ceramide-C6, and its incorporation into the TGN membrane, it is metabolized to generate C6-SM. The excess of C6-SM disrupts the ability of SM to assemble into lipid rafts in the luminal leaflet of the bilayer. DAG, diacylglycerol; PC, phosphatidylcholine; SM, sphingomyelin; Cer, ceramide; Chol, cholesterol; SMS, sphingomyelin synthase.
How are the levels of SM and cholesterol controlled at the Golgi membranes? We suggest that PKD plays a major role in this process. PKD is recruited to the TGN by binding DAG and Arf1 (Baron and Malhotra, 2002; Pusapati et al, 2010). The known functions of activated PKD bound to the TGN include: to increase the production of phosphatidylinositol 4-phosphate (PI4P) by phosphorylation of PI4KIIIβ (Hausser et al, 2005) and to control the binding of CERT and OSBP (oxysterol-binding protein) (Fugmann et al, 2007; Nhek et al, 2010). In other words, PKD-dependent location of CERT controls the amount of ceramide available for the production of SM, DAG, and glucosylceramide at the TGN. This could explain the role of PKD in the events leading to the biogenesis of transport carriers at the TGN (Malhotra and Campelo, 2011; Campelo and Malhotra, 2012). These findings, however, also raise a new challenge: PKD is required, predominantly, for the biogenesis of carriers that contain the basolateral surface-specific cargoes, whereas short-chain SM inhibits the trafficking of many different exit routes from the Golgi membranes. Is PKD involved in the biogenesis of other transport carriers that form at the Golgi apparatus?
Altogether, these findings suggest the existence of liquid-ordered domains in the Golgi membranes and their specific role in events leading to transport carrier biogenesis.
Materials and methods
Reagents
N-Hexanoyl-D-erythro-sphingosine (D-ceramide-C6) and N-Hexanoyl-L-erythro-sphingosine (L-ceramide-C6) were from Matreya, and dissolved in pure ethanol (Merck) as stock solution. Ilimaquinone was from Calbiochem and stock solution was made in DMSO (Sigma). Cycloheximide was from AG Scientific and BFA was from Sigma. 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC), N-palmitoyl-D-erythro-sphingosylphosphorylcholine (C16-SM), Cholesterol (Chol), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(Lissamine rhodamine B sulfonyl) (Lissamine-Rh-PE) and N-hexanoyl-D-erythro-sphingosylphosphorylcholine (C6-SM) were from Avanti Polar Lipids. Naphtho[2,3a-]pyrene (NAP) was from Sigma. 2-dimethylamino-6-lauroylnaphthalene (laurdan) was from Molecular Probes. DL-threo-1-Phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP) was from Enzo Life Sciences.
Antibodies, plasmids, RNAi, and cell culture
Mouse monoclonal antibody against HRP (clone HP-03) was purchased from Abcam and sheep anti-human TGN46 from AbD Serotec. Goat anti-GST used for western blot was from GE Healthcare, and rabbit anti-GST for immunofluorescence from Sigma. Mouse anti-rat TGN38 and mouse anti-GM130 were from BD Biosciences. Rabbit anti-GFP was from Santa Cruz. Alexa-labelled secondary antibodies for immunofluorescence microscopy were from Invitrogen. GST-PKD1-C1a (Maeda et al, 2001) and GST-PKD2-WT (Yeaman et al, 2004) were described previously. The GFP-GPI plasmid was kindly provided by Dr K Simons (MPI-CBG, Dresden, Germany). HeLa cells, including those stably expressing ssHRP (von Blume et al, 2011), VSV-G (von Blume et al, 2009). Mannosidase II-RFP (Tafesse et al, 2007), and Mannosidase II-GFP (Sutterlin et al, 2005), GF17 cells (Liljedahl et al, 2001), and NRK cells were cultured in DMEM (PAA) containing 10% fetal calf serum (FCS). HeLa-ManII-RFP cells were kindly provided by Dr JCM Holthuis (Utrecht University, The Netherlands). Cells were transfected using Lipofectamine 2000 (Invitrogen), FuGENE 6 (Roche), or TransIT-HeLaMonster (Mirus) following manufacturer’s recommendations. siRNA transfection was performed using HiPerFect transfection reagent (QIAGEN) following manufacturer’s protocol. The non-targeting control siRNA oligonucleotide sequence was 5′-AAUUGCGUAGUCUAAGUUAAAGUGG-3′ (Invitrogen); siRNA against SMS1 and SMS2 was Silencer Select Predesigned siRNA from Ambion with IDs s48915 and s46644, respectively.
Membrane fractionation
Separation of homogenates into a membrane and a cytosolic fraction was carried out as described in Maeda et al (2001). Briefly, cells were scraped and resuspended in hypotonic buffer (20 mM Tris pH 7.4, 25 mM NaF, 5 mM Na4P2O7, and protease inhibitors) and were incubated on ice for 10 min. After homogenization by passage through a needle, NaCl was added to 150 mM final concentration. The homogenate was centrifuged at 10 000 g for 10 min at 4°C, after which the resulting supernatant was centrifuged at 100 000 g for 1 h at 4°C in a tabletop ultracentrifuge (Beckman-Coulter) with a TLA120.2 rotor. The cytosolic (supernatant) and membrane (pellet) fractions were boiled in Laemmli buffer and analysed by western blotting.
Isolation of Golgi membranes
Golgi membranes were isolated from HeLa cells as described before (Balch et al, 1984) with the following modifications. Briefly, after treatment with 20 μM D-ceramide-C6 or ethanol for 30 min, the cells were washed three times with PBS and once with cell breaking buffer (250 mM sucrose, 10 mM Tris pH 7.4). The cells were harvested by scraping and centrifugation for 10 min, 600 g. The cells were resuspended in cell breaking buffer and homogenized by 12 passages through an EMBL cell cracker bearing a ball of 8.002 mm diameter. After adjusting the homogenate to a final concentration of 37% (w/w) sucrose, 1 mM EDTA, and 10 mM Tris, pH 7.4, 4 ml of the homogenate was placed into an ultracentrifuge tube which was then overlayed with 4.5 ml 35% (w/w) and 4 ml 29% (w/w) sucrose. The samples were centrifuged in a SW40 Ti rotor (Beckman-Coulter) for 2.5 h at 30 000 r.p.m. The band between 35 and 29% sucrose was collected and characterized by western blotting with antibodies to the TGN-specific membrane proteins SPCA1 and TGN46.
Immunofluorescence microscopy
Samples were fixed with 4% formaldehyde in PBS, permeabilized with 0.2% TX-100 in PBS and blocked in 2% BSA in PBS prior to antibody staining. For localization of internal HRP, after fixation, cells were permeabilized with 0.5% SDS, 0.2% TX-100 in 4% BSA in PBS, and incubated with anti-HRP antibody overnight at 4°C. Fixed samples were analysed either with a confocal microscope (SPE; Leica) using the × 63 Plan Apo NA 1.3 objective, or with an inverted Nikon Eclipse ED-2000 equipped with a CoolSnap CCD Camera (Princeton Instruments). For detection with the confocal microscope, the following laser lines were applied: DAPI, 405 nm; Alexa Fluor 594, 532 nm; and Alexa Fluor 488 and GFP, 488 nm. Pictures were acquired using the Leica software and converted to TIFF files using ImageJ (version 1.43; National Institutes of Health).
HRP secretion assay
HRP secretion assay was performed using HeLa-ssHRP cells as described previously (von Blume et al, 2011). To measure secretion recovery after treatment, equal amounts of HeLa-ssHRP cells in triplicates were treated with L-ceramide-C6, D-ceramide-C6, or vehicle for 30 min at 37°C, 7% CO2 on a 12-well plate. After that, cells were washed in complete medium and 1 ml of prewarmed complete medium was added to each well. In all, 50 μl aliquots were taken at selected time points and assayed for HRP activity using ECL substrate (Thermo Scientific) and measured using Wallac 1420 counter (Perkin-Elmer).
Cell surface biotinylation
HeLa-VSV-G-GFP cells were cultured at 40°C for 40 h. The cells were then incubated at 20°C for 105 min in the presence of 100 μM cycloheximide and 25 mM HEPES pH 7.4, after which the incubation was continued for 15 min in presence of 20 μM L- or D-ceramide-C6. The cells were shifted to 32°C for 0 or 30 min, after which the cells were washed three times with ice-cold PBS+ (PBS with 0.1 mM CaCl2 and 0.1 mM MgCl2). The cells were biotinylated with 1 mg/ml sulfo-NHS-LC-Biotin (Pierce) in PBS+ for 30 min on ice. The cells were washed twice with PBS+ and biotin was quenched by incubation with 100 mM glycine in PBS+ for 30 min on ice. After washing twice with PBS+, the cells were lysed with PBS containing 2% NP-40, 0.2% SDS, and protease inhibitors for 10 min on ice. The lysates were centrifuged for 15 min at 16 000 g and the resulting supernatants were incubated with equilibrated Neutravidin agarose resin (Thermo Scientific) overnight at 4°C while rotating. The resins were washed twice with PBS containing 2% NP-40 and 0.2% SDS and twice with PBS. The resin was eluted with SDS sample buffer. VSV-G-GFP was detected by western blotting with an anti-GFP antibody.
Metabolic labelling
Pulse-chase experiments were performed as described previously (von Blume et al, 2009). Briefly, HeLa cells were cultured in DMEM without L-methionine and L-cysteine (Gibco) for 1 h. Starting from the last 30 min of starvation until the end of the experiment, the cells were incubated with ethanol, 20 μM L-ceramide-C6, 20 μM D-ceramide-C6, or 5 μg/ml BFA. The cells were pulsed for 15 min with 100 μCi [35S]-methionine (Perkin-Elmer), and then chased for 2 h with medium supplemented with 10 mM methionine. Proteins in the medium were collected by precipitation by trichloroacetic acid and analysed by SDS–PAGE and autoradiography. Cells were lysed in 0.5% TX-100 in PBS for 10 min on ice, and label incorporation was determined for normalization.
Electron microscopy
Cells were fixed with 1% glutaraldehyde in 0.2 M HEPES buffer, washed, incubated subsequently with 1% uranyl acetate and 1% OsO4. Cells were then dehydrated, embedded in Epon and sectioned using Leica EM UC7 ultramicrotome (Leica Microsystems). EM images were acquired from thin sections using a JEM-1011 electron microscope (JEOL) equipped with an MORADA CCD digital camera (Soft Imaging Systems GmbH).
GUV preparation
GUVs were prepared using the electroformation method developed by Angelova and Tsoneva (1999). For GUV formation, a homemade chamber was used, which allows direct GUV visualization under the microscope (Fidorra et al, 2006). Stock solutions of lipids (0.2 mg/ml total lipid containing either 0.2 mol % Lissamine-Rh-PE or 0.5 mol % NAP) were prepared in a chloroform:methanol (9:1, v/v) solution. In all, 3 μl of the appropriate lipid stocks was added on the surface of Pt electrodes and the solvent traces were removed by evacuating the chamber under high vacuum for at least 2 h.
The Pt electrodes were covered with 400 μl of 20 mM PIPES, 150 mM NaCl, 1 mM EDTA, pH 7.4 previously heated at 60°C. The Pt wires were connected to an electric wave generator (TG330 function generator, Thurlby Thandar instruments, Huntington, UK) under AC field conditions: (1) frequency 500 Hz, amplitude 30 mV for 5 min; (2) frequency 500 Hz, amplitude 300 mV for 20 min; and (3) frequency 500 Hz, amplitude 900 mV for 90 min at 60°C.
Microscopy of GUVs
After GUV formation, the chamber was assembled in a heating system (Ibiti HT200, Ibidi Integrated BioDiagnostics) that allows temperature control and the system was placed in an inverted confocal fluorescence microscope (Nikon D-ECLIPSE C1, Nikon Inc.). To visualize GUVs at 37°C, the objective was heated with an objective heater (Bioptech, Chromaphor Anaysen-Technik GmbH). The excitation wavelengths were 457 nm for NAP and 561 nm for Lissamine-Rh-PE, respectively. The images were collected through two different channels using band-pass filters of 485±20 nm for the NAP and 593±20 nm for the Lissamine-Rh-PE. Image treatment and quantification was performed using the software EZ-C1 3.20 (Nikon Inc.). No difference in domain size, formation, or distribution was observed in the GUVs along the observation period or after laser exposure.
LUV preparation
The appropriate lipids were mixed in chloroform:methanol (2:1, v/v) and evaporated thoroughly. Lipids were hydrated in 20 mM PIPES, 150 mM NaCl, 1 mM EDTA, pH 7.4, and the LUVs were prepared by the extrusion method (Mayer et al, 1986), using polycarbonate filters with a pore size of 0.1 μm (Nuclepore). Quantitative analysis of the lipid composition of our LUV preparations, as described by Ruiz-Arguello et al (1996), showed that it did not differ significantly from the initial lipid mixture. Lipid phosphorus was measured to determine the final phospholipid concentration.
Fluorescence spectroscopy
The GP of laurdan was measured in an SLM-AMINCO 8100 spectrofluorometer, equipped with thermoregulated cell holders. The excitation GPEX parameter was calculated according to:
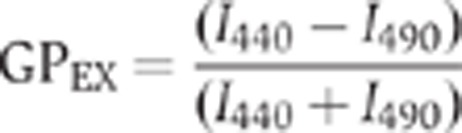 |
where I440 and I490 are the emission intensities obtained at 440 and 490 nm, respectively, exciting at 360 nm. The final probe/lipid molar ratio was 1/1000.
Laurdan cell microscopy
HeLa-MannII-RFP cells grown on glass coverslips were stained for 90 min at 37°C with 5 μM laurdan (Invitrogen) in serum-free medium, fixed and mounted after two rinses in PBS as described previously (Gaus et al, 2003; Rentero et al, 2008). Laurdan imaging was performed with a TCS SP5 inverted confocal microscope (Leica, Germany) equipped with a near infrared laser (Mai Tai Broad Band 710–990 nm) and an APO × 63 glycerol immersion objective (1.3 NA). Confocal RFP signal (excitation 561 nm; emission 570–620 nm) was recorded, followed by two-photon laurdan images, which were acquired at 800 nm excitation, with emission ranges collected simultaneously at 400–460 nm and 470–530 nm. Laurdan intensity images were converted into GP images, with each pixel calculated in ImageJ from the two laurdan intensity images according to the equation:
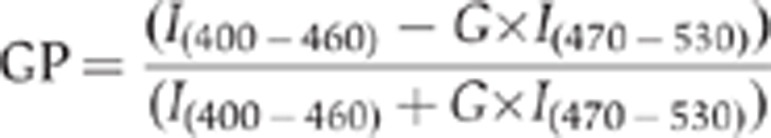 |
GP distributions were obtained from the histogram values of RFP-masked GP images and non-linear fitted to one or two Gauss distributions using a custom built macro in ImageJ (Owen et al, 2012).
Lipidomics
Lipid extractions and mass spectrometric analysis of lipids by nano-electrospray ionization tandem mass spectrometry were performed as described (Brügger et al, 2006; Haag et al, 2012). Cholesterol was quantified by the published procedure (Liebisch et al, 2006).
Statistics
Statistical significance was tested using an unpaired Student’s t-test. Different data sets were considered to be statistically significant when P-value was <0.05 (*) or <0.01 (**).
Supplementary Material
Acknowledgments
We thank all members of the Malhotra Laboratory for valuable discussions. We would like to acknowledge Simona Iacobacci for EM specimen preparation, Telethon Electron Microscopy Core Facility (TeEMCoF, IBP, CNR, Naples; Telethon project #GTF08001) and Integrated Microscopy Facility (IGB, CNR, Naples) for EM support to ME and RP. FW and BB are supported by the German Research Foundation (DFG, SFB/TRR 83) and by CellNetworks Heidelberg. We acknowledge grants from the Spanish Ministerio de Ciencia e Innovación (BFU 2007/62062) and from the University of the Basque Country (IT 461-07) to FMG. Laurdan-two photon images were obtained at Centres Científics i Tecnològics, Universitat de Barcelona/IDIBAPS with the technical assistance of Maria Calvo. FC is funded by a Juan de la Cierva postdoctoral fellowship. CR and CE acknowledge funding from Consolider (CSD2009-00016). VM is an Institució Catalana de Recerca i Estudis Avançats (ICREA) professor at the Center for Genomic Regulation, and the work in his laboratory is funded by grants from Plan Nacional (BFU2008-00414), Consolider (CSD2009-00016), Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR) Grups de Recerca Emergents (SGR2009-1488; AGAUR-Catalan Government), and European Research Council (268692). The project has received research funding from the European Union. This paper reflects only the author’s views. The Union is not liable for any use that may be made of the information contained therein.
Author contributions: JMD, FC, JVG, and VM designed the experiments and wrote the paper. JMD, FC, and JVG performed experiments and analysed the data. RSP and MVE performed and analysed electron microscopy samples. TS, BB, and FW performed and analysed lipidomic analyses. JS and FMG performed and analysed liposome experiments. CR and CE performed and analysed the measurement of laurdan polarization in cells.
Footnotes
The authors declare that they have no conflict of interest.
References
- Angelova MI, Tsoneva I (1999) Interactions of DNA with giant liposomes. Chem Phys Lipids 101: 123–137 [DOI] [PubMed] [Google Scholar]
- Balch WE, Dunphy WG, Braell WA, Rothman JE (1984) Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell 39: 405–416 [DOI] [PubMed] [Google Scholar]
- Baron CL, Malhotra V (2002) Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science 295: 325–328 [DOI] [PubMed] [Google Scholar]
- Bergmann JE (1989) Using temperature-sensitive mutants of VSV to study membrane protein biogenesis. Methods Cell Biol 32: 85–110 [DOI] [PubMed] [Google Scholar]
- Berndl K, Käs J, Lipowsky R, Sackmann E, Seifert U (1990) Shape transformations of giant vesicles - Extreme sensitivity to bilayer asymmetry. Europhys Lett 13: 659–664 [Google Scholar]
- Brameshuber M, Weghuber J, Ruprecht V, Gombos I, Horvath I, Vigh L, Eckerstorfer P, Kiss E, Stockinger H, Schutz GJ (2010) Imaging of mobile long-lived nanoplatforms in the live cell plasma membrane. J Biol Chem 285: 41765–41771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brügger B, Glass B, Haberkant P, Leibrecht I, Wieland FT, Krausslich HG (2006) The HIV lipidome: a raft with an unusual composition. Proc Natl Acad Sci USA 103: 2641–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campelo F, Malhotra V (2012) Membrane fission: the biogenesis of transport carriers. Annu Rev Biochem 81: 407–27 [DOI] [PubMed] [Google Scholar]
- Cao X, Surma MA, Simons K (2012) Polarized sorting and trafficking in epithelial cells. Cell Res 22: 793–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiantia S, Kahya N, Schwille P (2007) Raft domain reorganization driven by short- and long-chain ceramide: a combined AFM and FCS study. Langmuir 23: 7659–7665 [DOI] [PubMed] [Google Scholar]
- Coste H, Martel MB, Got R (1986) Topology of glucosylceramide synthesis in Golgi membranes from porcine submaxillary glands. Biochim Biophys Acta 858: 6–12 [DOI] [PubMed] [Google Scholar]
- Dietrich C, Bagatolli LA, Volovyk ZN, Thompson NL, Levi M, Jacobson K, Gratton E (2001) Lipid rafts reconstituted in model membranes. Biophys J 80: 1417–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidorra M, Duelund L, Leidy C, Simonsen AC, Bagatolli LA (2006) Absence of fluid-ordered/fluid-disordered phase coexistence in ceramide/POPC mixtures containing cholesterol. Biophys J 90: 4437–4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugmann T, Hausser A, Schoffler P, Schmid S, Pfizenmaier K, Olayioye MA (2007) Regulation of secretory transport by protein kinase D-mediated phosphorylation of the ceramide transfer protein. J Cell Biol 178: 15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga T, Nagahama M, Hatsuzawa K, Tani K, Yamamoto A, Tagaya M (2000) Implication of sphingolipid metabolism in the stability of the Golgi apparatus. J Cell Sci 113: 3299–3307 [DOI] [PubMed] [Google Scholar]
- Gaus K, Gratton E, Kable EP, Jones AS, Gelissen I, Kritharides L, Jessup W (2003) Visualizing lipid structure and raft domains in living cells with two-photon microscopy. Proc Natl Acad Sci USA 100: 15554–15559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkantiragas I, Brügger B, Stuven E, Kaloyanova D, Li XY, Lohr K, Lottspeich F, Wieland FT, Helms JB (2001) Sphingomyelin-enriched microdomains at the Golgi complex. Mol Biol Cell 12: 1819–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goñi FM, Alonso A, Bagatolli LA, Brown RE, Marsh D, Prieto M, Thewalt JL (2008) Phase diagrams of lipid mixtures relevant to the study of membrane rafts. Biochim Biophys Acta 1781: 665–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami D, Gowrishankar K, Bilgrami S, Ghosh S, Raghupathy R, Chadda R, Vishwakarma R, Rao M, Mayor S (2008) Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell 135: 1085–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Pfeiffer S, Simons K, Matlin K (1985) Exit of newly synthesized membrane proteins from the trans cisterna of the Golgi complex to the plasma membrane. J Cell Biol 101: 949–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag M, Schmidt A, Sachsenheimer T, Brügger B (2012) Quantification of signaling lipids by nano-electrospray ionization tandem mass spectrometry (Nano-ESI MS/MS). Metabolites 2: 57–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M (2003) Molecular machinery for non-vesicular trafficking of ceramide. Nature 426: 803–809 [DOI] [PubMed] [Google Scholar]
- Hausser A, Storz P, Martens S, Link G, Toker A, Pfizenmaier K (2005) Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase IIIbeta at the Golgi complex. Nat Cell Biol 7: 880–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitema K, van den Dikkenberg J, Brouwers JF, Holthuis JC (2004) Identification of a family of animal sphingomyelin synthases. EMBO J 23: 33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser HJ, Lingwood D, Levental I, Sampaio JL, Kalvodova L, Rajendran L, Simons K (2009) Order of lipid phases in model and plasma membranes. Proc Natl Acad Sci U S A 106: 16645–16650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm RW, Ejsing CS, Surma MA, Kaiser HJ, Gerl MJ, Sampaio JL, de Robillard Q, Ferguson C, Proszynski TJ, Shevchenko A, Simons K (2009) Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J Cell Biol 185: 601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi A, Koyama-Honda I, Suzuki K (2004) Molecular dynamics and interactions for creation of stimulation-induced stabilized rafts from small unstable steady-state rafts. Traffic 5: 213–230 [DOI] [PubMed] [Google Scholar]
- Liebisch G, Binder M, Schifferer R, Langmann T, Schulz B, Schmitz G (2006) High throughput quantification of cholesterol and cholesteryl ester by electrospray ionization tandem mass spectrometry (ESI-MS/MS). Biochim Biophys Acta 1761: 121–128 [DOI] [PubMed] [Google Scholar]
- Liljedahl M, Maeda Y, Colanzi A, Ayala I, Van Lint J, Malhotra V (2001) Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell 104: 409–420 [DOI] [PubMed] [Google Scholar]
- Lingwood D, Simons K (2010) Lipid rafts as a membrane-organizing principle. Science 327: 46–50 [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan L, Tipper C, Amherdt M, Orci L, Klausner RD (1991) Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell 67: 601–616 [DOI] [PubMed] [Google Scholar]
- Maeda Y, Beznoussenko GV, Van Lint J, Mironov AA, Malhotra V (2001) Recruitment of protein kinase D to the trans-Golgi network via the first cysteine-rich domain. EMBO J 20: 5982–5990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra V, Campelo F (2011) PKD regulates membrane fission to generate TGN to cell surface transport carriers. Cold Spring Harb Perspect Biol 3: pii: a005280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer LD, Hope MJ, Cullis PR (1986) Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim Biophys Acta 858: 161–168 [DOI] [PubMed] [Google Scholar]
- Munro S (2003) Lipid rafts: elusive or illusive? Cell 115: 377–388 [DOI] [PubMed] [Google Scholar]
- Nhek S, Ngo M, Yang X, Ng MM, Field SJ, Asara JM, Ridgway ND, Toker A (2010) Regulation of oxysterol-binding protein golgi localization through protein kinase D-mediated phosphorylation. Mol Biol Cell 21: 2327–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DM, Rentero C, Magenau A, Abu-Siniyeh A, Gaus K (2012) Quantitative imaging of membrane lipid order in cells and organisms. Nat Protoc 7: 24–35 [DOI] [PubMed] [Google Scholar]
- Pettus BJ, Chalfant CE, Hannun YA (2002) Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta 1585: 114–125 [DOI] [PubMed] [Google Scholar]
- Pusapati GV, Krndija D, Armacki M, von Wichert G, von Blume J, Malhotra V, Adler G, Seufferlein T (2010) Role of the second cysteine-rich domain and Pro275 in protein kinase D2 interaction with ADP-ribosylation factor 1, trans-Golgi network recruitment, and protein transport. Mol Biol Cell 21: 1011–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentero C, Zech T, Quinn CM, Engelhardt K, Williamson D, Grewal T, Jessup W, Harder T, Gaus K (2008) Functional implications of plasma membrane condensation for T cell activation. PLoS ONE 3: e2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roduit C, van der Goot FG, De Los Rios P, Yersin A, Steiner P, Dietler G, Catsicas S, Lafont F, Kasas S (2008) Elastic membrane heterogeneity of living cells revealed by stiff nanoscale membrane domains. Biophys J 94: 1521–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald AG, Machamer CE, Pagano RE (1992) Effects of a sphingolipid synthesis inhibitor on membrane transport through the secretory pathway. Biochemistry 31: 3581–3590 [DOI] [PubMed] [Google Scholar]
- Rosenwald AG, Pagano RE (1993) Inhibition of glycoprotein traffic through the secretory pathway by ceramide. J Biol Chem 268: 4577–4579 [PubMed] [Google Scholar]
- Ruiz-Arguello MB, Basanez G, Goni FM, Alonso A (1996) Different effects of enzyme-generated ceramides and diacylglycerols in phospholipid membrane fusion and leakage. J Biol Chem 271: 26616–26621 [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Rietveld A, Wilk T, Simons K (1999) Influenza viruses select ordered lipid domains during budding from the plasma membrane. J Biol Chem 274: 2038–2044 [DOI] [PubMed] [Google Scholar]
- Seifert U, Berndl K, Lipowsky R (1991) Shape transformations of vesicles: Phase diagram for spontaneous- curvature and bilayer-coupling models. Phys Rev A 44: 1182–1202 [DOI] [PubMed] [Google Scholar]
- Sezgin E, Schwille P (2011) Fluorescence techniques to study lipid dynamics. Cold Spring Harb Perspect Biol 3: a009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Gerl MJ (2010) Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol 11(10): 688–699 [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387: 569–572 [DOI] [PubMed] [Google Scholar]
- Simons K, Sampaio JL (2011) Membrane organization and lipid rafts. Cold Spring Harb Perspect Biol 3: a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, van Meer G (1988) Lipid sorting in epithelial cells. Biochemistry 27: 6197–6202 [DOI] [PubMed] [Google Scholar]
- Surma MA, Klose C, Klemm RW, Ejsing CS, Simons K (2011) Generic sorting of raft lipids into secretory vesicles in yeast. Traffic 12: 1139–1147 [DOI] [PubMed] [Google Scholar]
- Sutterlin C, Polishchuk R, Pecot M, Malhotra V (2005) The Golgi-associated protein GRASP65 regulates spindle dynamics and is essential for cell division. Mol Biol Cell 16: 3211–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafesse FG, Huitema K, Hermansson M, van der Poel S, van den Dikkenberg J, Uphoff A, Somerharju P, Holthuis JC (2007) Both sphingomyelin synthases SMS1 and SMS2 are required for sphingomyelin homeostasis and growth in human HeLa cells. J Biol Chem 282: 17537–17547 [DOI] [PubMed] [Google Scholar]
- Takizawa PA, Yucel JK, Veit B, Faulkner DJ, Deerinck T, Soto G, Ellisman M, Malhotra V (1993) Complete vesiculation of Golgi membranes and inhibition of protein transport by a novel sea sponge metabolite, ilimaquinone. Cell 73: 1079–1090 [DOI] [PubMed] [Google Scholar]
- von Blume J, Alleaume AM, Cantero-Recasens G, Curwin A, Carreras-Sureda A, Zimmermann T, van Galen J, Wakana Y, Valverde MA, Malhotra V (2011) ADF/cofilin regulates secretory cargo sorting at the TGN via the Ca2+ ATPase SPCA1. Dev Cell 20: 652–662 [DOI] [PubMed] [Google Scholar]
- von Blume J, Duran JM, Forlanelli E, Alleaume AM, Egorov M, Polishchuk R, Molina H, Malhotra V (2009) Actin remodeling by ADF/cofilin is required for cargo sorting at the trans-Golgi network. J Cell Biol 187: 1055–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TY, Silvius JR (2000) Different sphingolipids show differential partitioning into sphingolipid/cholesterol-rich domains in lipid bilayers. Biophys J 79: 1478–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C, Ayala MI, Wright JR, Bard F, Bossard C, Ang A, Maeda Y, Seufferlein T, Mellman I, Nelson WJ, Malhotra V (2004) Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network. Nat Cell Biol 6: 106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zech T, Ejsing CS, Gaus K, de Wet B, Shevchenko A, Simons K, Harder T (2009) Accumulation of raft lipids in T-cell plasma membrane domains engaged in TCR signalling. EMBO J 28: 466–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


