Abstract
Small heat shock proteins (sHSPs) play a central role in protein homeostasis under conditions of stress by binding partly unfolded, aggregate-prone proteins and keeping them soluble. Like many sHSPs, the widely expressed human sHSP, αB-crystallin (‘αB’), forms large polydisperse multimeric assemblies. Molecular interactions involved in both sHSP function and oligomer formation remain to be delineated. A growing database of structural information reveals that a central conserved α-crystallin domain (ACD) forms dimeric building blocks, while flanking N- and C-termini direct the formation of larger sHSP oligomers. The most commonly observed inter-subunit interaction involves a highly conserved C-terminal ‘IxI/V’ motif and a groove in the ACD that is also implicated in client binding. To investigate the inherent properties of this interaction, peptides mimicking the IxI/V motif of αB and other human sHSPs were tested for binding to dimeric αB-ACD. IxI-mimicking peptides bind the isolated ACD at 22°C in a manner similar to interactions observed in the oligomer at low temperature, confirming these interactions are likely to exist in functional αB oligomers.
Keywords: αB-crystallin, alpha-crystallin domain, HSPB5, IxI motif, small heat shock protein
Introduction
Small heat shock proteins (sHSPs) help to maintain protein homeostasis by interacting with partly unfolded, aggregate-prone proteins to prevent cell damage (Haslbeck et al, 2005; Bukau et al, 2006). In humans, there are 10 members of the ATP-independent sHSP family (HSPB1, HSPB2, etc.) of which αB-crystallin (‘αB’ or HSPB5) is a paradigm example (Horwitz, 1992). Though originally discovered in eye lens as a protein essential for maintaining lens transparency, αB is additionally known to be expressed in muscle and brain. Specific biological roles for αB continue to emerge. In mice, knockout of αB reduces life span by half whereas its overexpression protects cardiac tissue from necrosis and myocardial infarction following ischaemic stress (Brady et al, 2001; Ray et al, 2001). Dysfunctions of αB in humans are associated with the occurrence of neurodegenerative diseases such as Alzheimer’s disease and Alexander’s disease (Kato et al, 2001; Goldstein et al, 2003). Inherited missense mutations in αB are associated with desmin-related cardiomyopathy, congenital cataracts, and myopathy (Vicart et al, 1998; Selcen and Engel, 2003; Liu et al, 2006; Rajasekaran et al, 2007). Furthermore, high expression of αB in cancer cells confers resistance to chemotherapy and is correlated with poor clinical outcomes (Vargas-Roig et al, 1998; Moyano et al, 2006). Yet despite its critical roles in maintaining healthy cells, understanding of αB structure and function remains rudimentary.
As is common for mammalian sHSPs, αB exists as large polydisperse assemblies containing variable numbers of subunits (Aquilina et al, 2003). The inherent dynamics of the assemblies is a defining feature and is likely linked to αB function. For decades, high-resolution structural studies on functional, full-length αB and mammalian sHSPs in general were confounded by both polydispersity and dynamics. Developments in solid-state NMR (ssNMR), solution-state NMR, and electron microscopy are finally providing experimental means to obtain atomic-level information on αB. Each approach has its strengths and weaknesses and each requires quite different experimental conditions under which a measurement can be made. Thus, a unifying model should be congruent with all observations to the extent possible.
As a class, sHSPs are defined by the presence of a conserved α-crystallin domain (ACD) that mediates formation of dimeric building blocks. The central ACD is flanked by highly variable N- and C-termini that mediate formation of the larger oligomeric assemblies (Bagnéris et al, 2009; Jehle et al, 2009; Langanowsky et al, 2010; Baldwin et al, 2011). sHSP oligomers may be composed of a single type of subunit or of a combination of different subunits (Zantema et al, 1992; Kato et al, 1994; Sun and Liang, 1998; Sugiyama et al, 2000; Bukach et al, 2009; Engelsman et al, 2009). Atomic-level structures of ACDs from a number of mammalian sHSPs including αB have been determined by X-ray crystallography and ssNMR (Bagnéris et al, 2009; Jehle et al, 2010; Langanowsky et al, 2010; Baranova et al, 2011; Clark et al, 2011). Atomic-level structures are available for non-mammalian sHSP oligomers (Kim et al, 1998; van Montfort et al, 2001; Stamler et al, 2005; Hilario et al, 2011). Pseudo-atomic models for αB have been generated using data from a combination of methods including ssNMR, SAXS, negative-stain EM, and cryo-EM (Braun et al, 2011; Jehle et al, 2011). The growing database of structural information provides a rich resource for studies aimed at understanding how sHSP structure is related to function.
Structures of sHSP oligomers and of isolated ACDs reveal some common features. The ACD is a 6–8 stranded β-sandwich structure and two ACDs usually associate in an anti-parallel fashion to form homodimeric units that serve as the building block for higher order structures (see Supplementary Figure S1) (Bagnéris et al, 2009; Jehle et al, 2010; Langanowsky et al, 2010; Baranova et al, 2011; Clark et al, 2011; Jehle et al, 2011). In addition, sequences from outside the ACD have been observed to be in contact with the ACD both in crystalline samples and by ssNMR. The interactions involve three ACD regions: (1) the β3 strand, (2) a groove on the edge of the ACD structure known as the β4/β8 groove, and (3) the surface formed by the dimer interface (Kim et al, 1998; van Montfort et al, 2001; Bagnéris et al, 2009; Langanowsky et al, 2010; Jehle et al, 2011) (see Supplementary Figure S1 for strand nomenclature).
In what is by far the most frequently observed interaction, a small conserved sequence motif called the IxI/V motif found in the C-terminal region of many sHSPs is bound in the β4/β8 groove of an ACD. The motif is defined as two Ile (or Val) residues separated by one residue. The interaction has been observed both in the context of homo-oligomers such as αB-crystallin, MjHSP16.5, and wheat HSP16.9, and in the context of ACD crystals, where the interactions take place among neighbouring dimers in the crystal lattice (Kim et al, 1998; van Montfort et al, 2001; Langanowsky et al, 2010; Jehle et al, 2011). Heteromeric IxI/V-β4/β8 interactions have been detected in solution between human sHSPs, αB and αA-crystallin (αA) by FRET experiments (Pasta et al, 2004). That the ACD-IxI/V interaction is observed in so many contexts implies some shared structural and/or functional role. To varying degrees, the interaction has been implicated in oligomer formation (Studer et al, 2002; Pasta et al, 2004). Deletions spanning the C-terminal motif result in a loss of higher order oligomeric structure in multiple sHSPs and mutation of the conserved motif residues Ile/Val (IxI/V) to Gly (GxG), Ala (AxA), or to Phe (FxF) also appears to abrogate the interaction (Studer et al, 2002; Pasta et al, 2004; Hayes et al, 2008; Takeda et al, 2011). Altogether, the observations suggest that the ACD-IxI/V interaction has a role in oligomer structure. Solution-state NMR studies at 25°C designed to specifically detect highly flexible regions detect the IxI/V motifs in some sHSP oligomers but the same experiments fail to observe this region in αB, implying the region is either bound within oligomer or is exchanging among multiple states (Ghahghaei et al, 2009; Benesch et al, 2010; Treweek et al, 2010). In contrast, a 13C-methyl-TROSY solution-state NMR study on αB oligomers at temperatures ranging from 24 to 50°C contained signals assigned to the IxI/V motif congruent with the residues being highly flexible and in an unbound state (Baldwin et al, 2011). This observation led the authors of that study to propose that the IxI/V interactions observed in structural studies of sHSPs in the solid-state or crystalline form may not accurately reflect the oligomer in solution (Baldwin et al, 2011). Here, we assess the inherent binding properties of an ACD and an IxI/V motif outside an oligomeric or crystalline context. Towards this end, we have tested the ability of peptides that mimic sequences from human sHSPs to bind to αB-ACD and identify determinants for the interaction using solution-state NMR.
Results
The isolated ACD of αB (residues 64–152; called αB-ACD) forms well-behaved dimers that are amenable to solution-state NMR (Jehle et al, 2009). NMR resonance assignments for the αB-ACD have been previously reported and provide the ability to obtain residue-specific information regarding potential peptide binding to an isolated ACD dimer (Jehle et al, 2009). Solution-state 1H–15N HSQC NMR spectra provide both a peak position (15N and 1H chemical shift) and peak intensity for 15N-labelled proteins. Comparison of spectra collected on samples that contain increasing amounts of a putative binding partner will reveal perturbations in the position, the intensity of a peak, or both. Perturbations are caused by a change in the local environment of the nuclei responsible for the peak. In this way, events such as peptide or protein binding can be detected, quantified, and mapped onto known structures of the protein under investigation.
NMR binding experiments were performed by titrating peptides with sequences containing the C-terminal IxI motif of αB or other human sHSPs into NMR samples of 15N-αB-ACD. The effect of peptide addition was monitored in the 1H–15N HSQC NMR spectra of αB-ACD collected at 22°C. As illustrated in Figure 1, significant changes in specific peak positions are observed as a consequence of addition of C-terminal αB-IxI peptide (Ac-PERTIPITREEK-NH2). Thus, the spectra confirm that isolated ACD dimers in solution can interact with a C-terminal sequence outside an oligomeric context. Notably, only a subset of resonances is affected by the presence of peptide, indicating that binding is not accompanied by a significant conformational change in the ACD dimer. Inspection of individual peaks in spectra collected throughout a titration revealed that some peaks exhibit intermediate exchange behaviour: a phenomenon in which peaks broaden under conditions where the bound state is not saturated and reappear as observable resonances at saturating peptide concentrations (Supplementary Figure S2). In addition, a subset of affected peaks titrate to multiple resonance positions (up to 3), indicating that these nuclei exist in multiple environments as a result of peptide binding (Figure 2). The observation of multiple resonances rather than one averaged resonance indicates that distinct ACD-peptide complexes exist and that they do not interconvert rapidly. The observed exchange behaviour allows us to estimate the exchange rate, kex, from the magnitude of the chemical shift differences among species. The chemical shift perturbations (CSPs) between the free and bound states are all quite similar for the amide group resonance for residue L94, which has well-resolved multiple peaks. This indicates a similar rate of exchange for each observed species. Under conditions where distinct resonances are observed for different forms present, the exchange rate (kex=kon+koff) between two forms is given by kex<Δω, where Δω is chemical shift difference between the two forms. In the case of αB-IxI peptide bound to αB-ACD, we have determined the upper limit for kex to be 60 s−1 for the multiple states observed.
Figure 1.
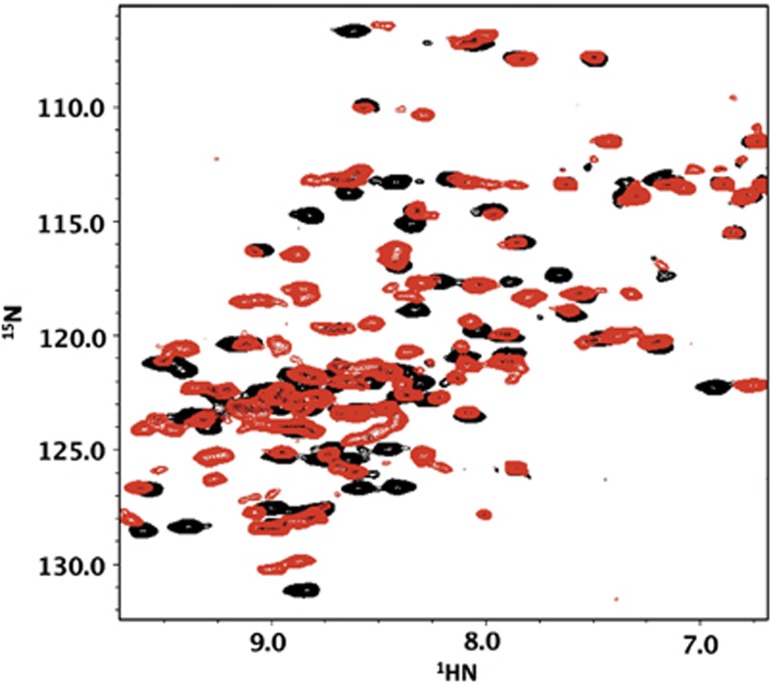
Overlay of 1H–15N HSQC spectra of 15N αB-ACD collected at 22°C, in the absence (black) and at saturating concentrations (five-fold) of αB-IxI peptide (red).
Figure 2.
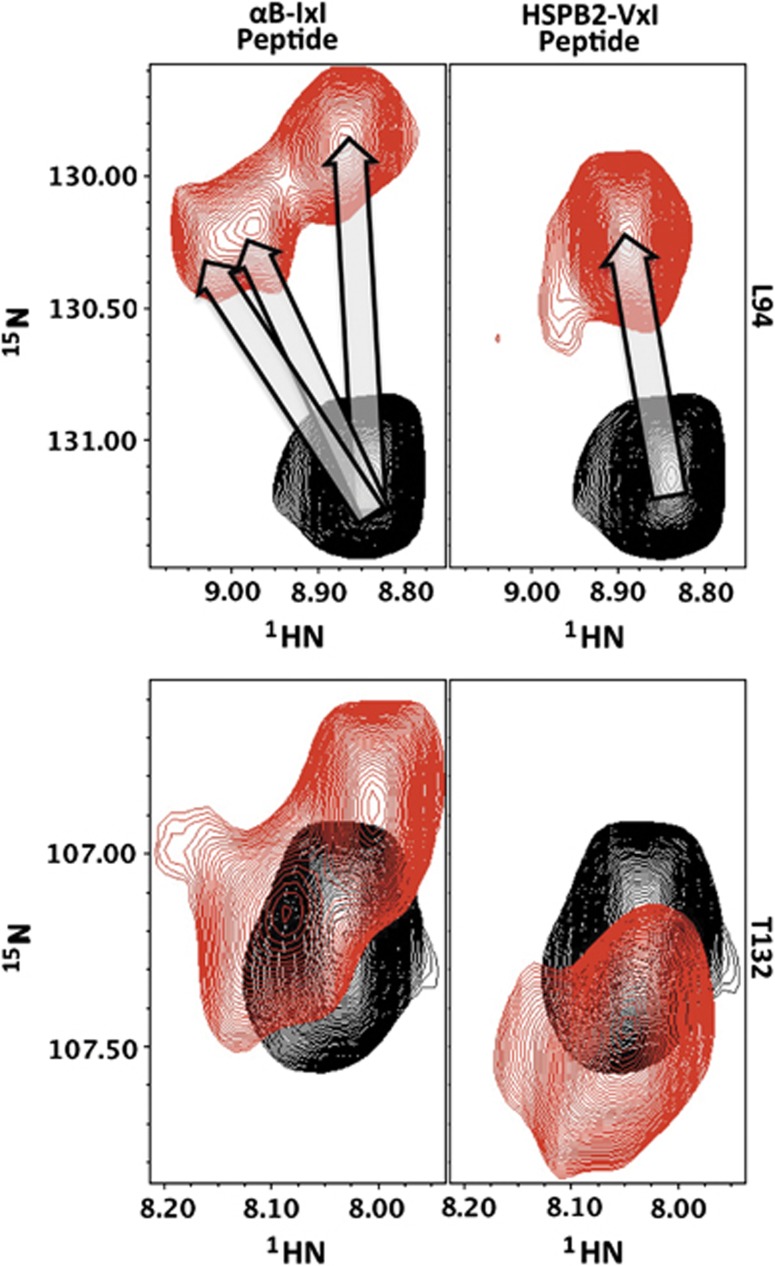
αB-ACD peaks titrate to multiple resonances at saturating concentrations of αB-IxI peptide. Three distinct resonances are observed for the amide of L94 in the presence of αB-IxI peptide (upper left arrows), while a single dominant peak is observed in the presence of the HSPB2-VxI peptide. The resonance for T132 also titrates to multiple positions in the presence of αB-IxI peptide (lower left), while a single dominant peak is observed in the presence of the HSPB2-VxI peptide. Spectra of the peptide-free and peptide-saturated ACD are shown in black and red, respectively.
As mentioned above, resonance assignments for peaks in the αB-ACD spectrum provide residue-specific information from the titration data. For each peak that could be followed through the titration series, the difference between its peptide-free chemical shift and its peptide-bound position was measured, as shown in the histogram in Figure 3. For peaks that titrate to multiple peptide-bound states, the position of the most intense peak in the peptide bound spectrum was used in this analysis. A histogram in which the CSPs are ranked according to their magnitude reveals a group of peaks with CSPs significantly larger than all others (>0.25 p.p.m.). For a subset of peaks, the final positions in the peptide-bound spectrum could not be readily assigned because their resonances could not be followed due to intermediate exchange behaviour or limited peak intensity. Nevertheless, the fact that they are in intermediate exchange indicates that their CSP values are even larger than those peaks whose positions shift gradually throughout the titration. The residues that experience the largest perturbations as a consequence of peptide binding reside in the β4/β8 groove and the loop between β4 and β5 (β4/5 loop) of the αB-ACD. Less affected peaks are clustered at the C-terminal end of the β3 strand (Figure 3). Peaks that are unaffected by the addition of peptide derive from residues outside these regions (Figure 3). The large CSPs observed within the β4/β8 groove are consistent with the peptide binding to a surface similar to that observed in ssNMR and X-ray crystallographic studies (Figure 4). Thus, there is an intrinsic affinity between the αB-ACD and the αB C-terminal sequence that promotes their binding even outside the context of solid-state or crystalline samples.
Figure 3.
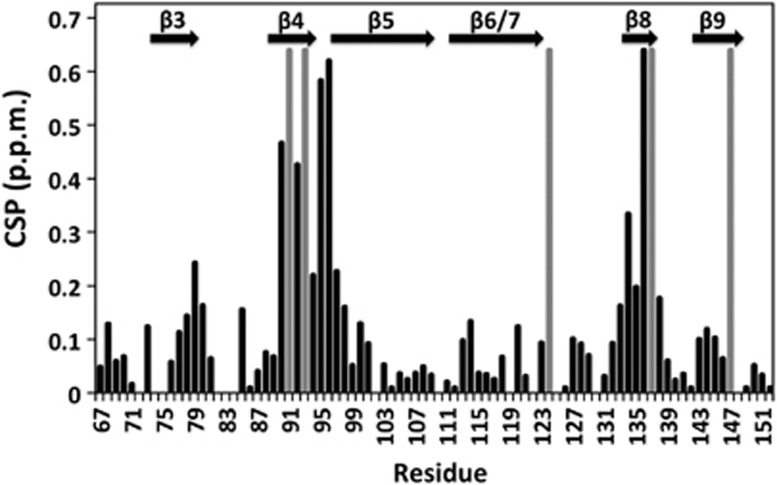
Histogram of calculated CSPs (black) for the αB-ACD in the presence of saturating concentrations of αB-IxI peptide. Positions where CSPs could not be determined due to exchange behaviour (and therefore have very large chemical shifts) are indicated in grey. Secondary structure elements are shown above.
Figure 4.
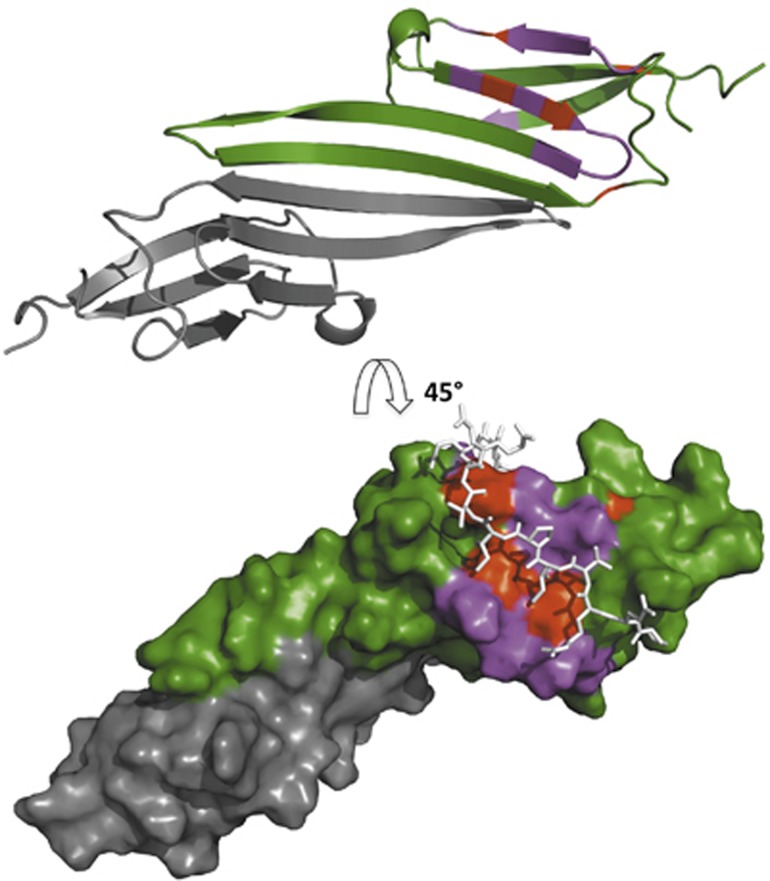
The chemical shift perturbations due to αB-IxI peptide binding map to the β4/β8 groove on the edge of the ACD. (Top) CSPs are mapped onto a secondary structure representation of the αB-ACD dimer (green and grey) (2klr). The most affected peaks (CSP>0.16) are shown in magenta on one monomer (green) and map to the β4/β8 groove and β4/5 loop. Residues where CSPs could not be quantitated due to exchange over large chemical shifts are shown in red. (Bottom) Surface representation of the αB-ACD dimer (2klr) rotated 45° with the same residue colouring as above. The C-terminal αB sequence PERTIPITREEK (white sticks) is shown as modelled previously with ssNMR restraints to illustrate the congruence between the chemical shifts observed for binding outside the oligomeric context and the structure observed in the context of αB oligomer.
The small CSPs (0.14–0.24 p.p.m.) observed for residues at the C-terminal end of the β3 strand may arise from two possible scenarios: (1) this region may comprise a second, lower affinity peptide binding site or (2) the observed CSPs may be due to small changes in the chemical environment of these residues caused by binding in the β4/β8 groove. To distinguish between the two possibilities, a point mutation in the β8 residue Ser135 was designed with the goal of disrupting the β4/β8-peptide interaction. In αB structures derived from ssNMR data and crystal structures, the side chain of Ser135 sits between two pockets into which the side chain of Ile159 and Ile161 of the IxI motif are bound (Langanowsky et al, 2010; Jehle et al, 2010). Substitution of Ser135 with a longer Gln side chain (S135Q) should occlude the binding pockets without significantly perturbing other elements of the structure. The 1H–15N HSQC spectrum of S135Q-αB-ACD is highly similar to the wt-ACD spectrum, indicating that the mutation has not significantly altered the overall structure (Supplementary Figure S3). Spectra from a peptide titration carried out for 15N-S135Q-αB-ACD reveal that S135Q-ACD binds peptide with significantly lower affinity: even a 10-fold molar excess does not achieve saturation with the mutant ACD whereas for the wt-ACD, saturation was attained at a four-fold excess. Furthermore, the observed CSPs are much smaller in the mutant ACD and all affected peaks exhibit fast exchange rather than intermediate/slow exchange (Figure 5). Altogether, the observations indicate that the C-terminal peptide binds with lower affinity to the S135Q-ACD mutant and has a shorter lifetime in the bound state. Notably, CSPs at the C-terminal end of the β3 strand also fail to reach saturation at 10-fold excess peptide in the S135Q mutant. If these perturbations were due to a second, independent binding event, then they would not be expected to be disrupted by the S135Q mutation. The apparent coupling of the CSPs observed within the β4/β8 groove and the β3 region is strong evidence that they arise from the same binding event. Together with our previously published ssNMR data, the data presented here lead us to conclude that the IxI motif within the C-terminal peptide has an intrinsic affinity for the β4/β8 groove of αB-ACD and that alterations to the groove can modulate the affinity of this interaction.
Figure 5.
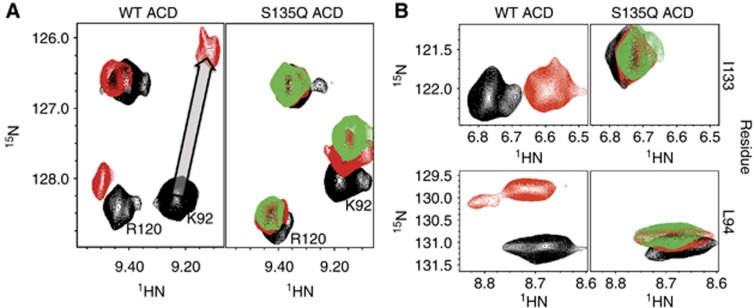
Mutation of residue S135 in the β4/β8 groove disrupts IxI peptide binding. (A) Selected resonances from 1H–15N HSQC of WT ACD (left) and the mutant S135Q ACD (right) in the absence and presence of αB-IxI peptide are compared. Spectra collected in the absence of peptide are shown in black. Spectra collected in the presence of 6-fold αB-IxI peptide (red) for the WT and S135Q ACD and 10-fold (green) peptide for S135Q are shown. In the S135Q mutant, residues K92 from the β4 strand and R120 (which is close to and behaves similarly to perturbed residues on the β3 strand) show a decrease in peptide-dependent chemical shift perturbation. The titration vector for the resonance of K92 in the WT spectra is shown as a grey arrow. (B) Peptide-induced CSPs observed for resonances of L94 and I133 are compared, with the same conditions and colouring as in (A).
Does the interaction between an isolated C-terminal peptide and an ACD dimer recapitulate that observed in the αB oligomer? To address this question, we compared the measured 15N chemical shifts for the isolated αB-ACD dimer in solution with those measured within the oligomer. The calculated difference is plotted for each residue in the histogram, shown as red bars in Figure 6. The largest differences occur at the termini of the excised ACD construct, the β3 strand, and at positions within the β4/β8 groove, suggesting that these regions differ in structure and/or environment between the ACD dimer alone in solution and in an oligomer. The calculation was repeated using 15N chemical shifts measured for the peptide-saturated state of the ACD in solution and the ssNMR oligomer, shown as black bars in Figure 6. Notably, in the presence of peptide all observable β4/β8 residues in the ACD in solution have shifted significantly towards their solid-state 15N chemical shifts (compare black and red bars). This remarkable concordance suggests the interaction between the αB-IxI peptide and the αB-ACD in the absence of oligomeric context recapitulates native intermolecular contacts within the αB oligomer. We note that a few peaks move away from their solid-state chemical shifts with the addition of peptide. The most significant of these deviations involves the perturbed positions at the C-terminal end of the β3 strand of the ACD. Thus, the secondary effects of peptide binding observed for this region in solution may not reflect a native effect and should be interpreted with caution.
Figure 6.
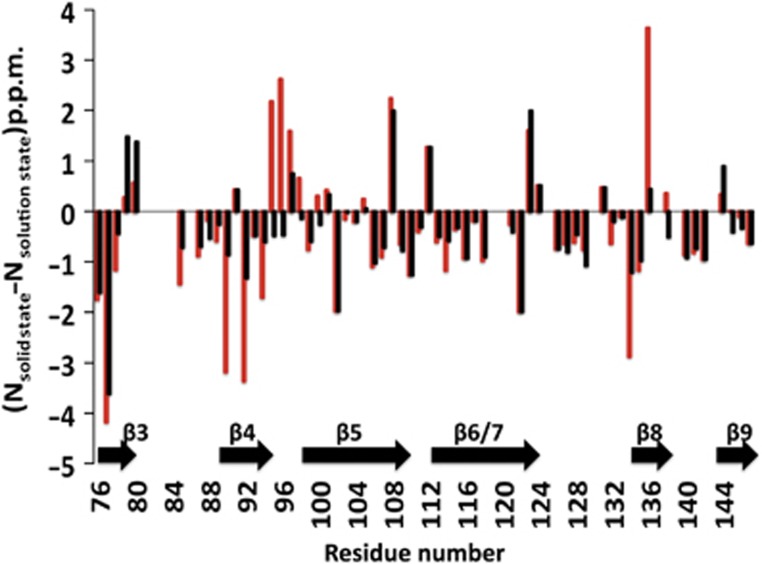
Comparison of 15N chemical shift between the αB-ACD in the context of the oligomer observed by ssNMR and isolated αB-ACD in solution. (15Nsolid state−15Nsolution state) differences were measured using the solution-state values observed both in the absence of peptide (red bars) and in the presence of saturating αB-IxI peptide (black bars). Note the greatly reduced values for (15Nsolid state-15Nsolution state) in the presence of peptide in the β4 strand and β4/5 loop (residues 89–98) and the β8 strand (residues 133–137). Positions where (15Nsolid state−15Nsolution state) could not be determined are assigned the value of 0.0 p.p.m. for clarity. For residues where multiple states were observed in the peptide saturated spectrum, the position of the most intense peak in the saturated spectrum was used.
To test the direct involvement of the IxI sequence in binding the β4/β8 groove, NMR experiments were performed using αB C-terminal peptides in which the IPI sequence was mutated either to GPG or APA. No detectable binding was observed for either mutant peptide even at high molar excess of peptide, consistent with the two Ile residues being the major determinants for the interaction (data not shown). This result raises the question as to whether other IxI/V sequences that exist (i.e., VxI or IxV) are capable of binding to the groove in αB. To address this question, NMR binding experiments were performed using peptides mimicking C-terminal sequences of HSPB1, HSPB2, and αA-crystallin (HSPB4), each of which contain an I/VxI or IxI/V motif (Table I). The NMR titrations confirm that each of these peptides binds to αB-ACD and produces CSPs that map to the β4/β8 groove. Differences in affinity and exchange behaviour are apparent in the heterologous titrations. For example, binding of the αA-IxV peptide and the HSPB2-VxI peptide exhibit fast exchange behaviour instead of the intermediate exchange observed with the αB-IxI peptide (Supplementary Figure S2). In a manner similar to the αB-IxI peptide titration, a subset of peaks in the αA-IxV peptide spectra titrates to multiple well-populated resonances throughout the titration. Throughout the HSPB2-VxI peptide titration, peaks titrate along a vector to a dominant saturated state. Additional multiple resonances are apparent at saturation, but are much more lowly populated than those observed in the αB-IxI or αA-IxV peptides (Figure 2). The observations permitted approximation of a Kd app value of ∼90 μM for HSPB2 peptide based on the dominant binding state CSPs (Supplementary Figure S4). This value should be viewed only as an upper limit estimate Kd app because alternative lowly populated bound states effectively serve as competitors to the dominant binding mode and therefore contribute to the observed binding isotherm. Further, our inability to detect additional binding modes for this peptide does not mean they do not exist, but rather that if they do exist they exchange rapidly. Altogether the data indicate that peptides that contain either an IxV or a VxI motif can bind the β4/β8 groove of the αB-ACD. In contrast, peptides that correspond to other regions of sHSPs that have been observed to make contact with an ACD in other structures display no detectable binding to αB-ACD under the conditions of our experiments (Table 1) (Bagnéris et al, 2009; Jehle et al, 2010). Thus, the binding observed in the solution-state NMR experiments is specific and cannot be attributed to a general promiscuity of αB-ACD for unstructured polypeptides.
Table 1. Summary of C-terminal peptide sequences from different sHSPs binding to αB-ACD.
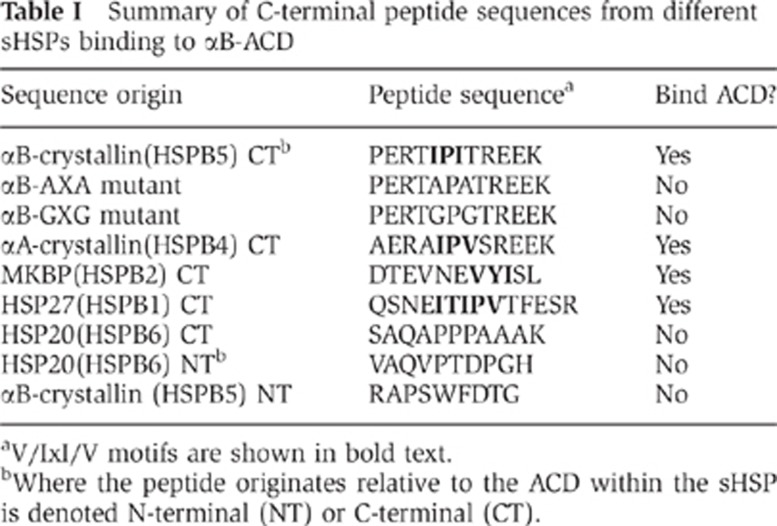
aV/IxI/V motifs are shown in bold text.
bWhere the peptide originates relative to the ACD within the sHSP is denoted N-terminal (NT) or C-terminal (CT).
Discussion
The αB-ACD binds αB C-terminal IxI motif outside the oligomeric context
Our results demonstrate that the αB-ACD has an inherent affinity for its C-terminal IxI motif and that the interaction can take place outside the context of either an oligomer or a crystal lattice. The major determinants for binding are the two Ile side chains, which interact directly with the β4/β8 groove of the ACD, yielding a bound state that is remarkably similar to that detected by ssNMR studies (Jehle et al, 2009). In addition to the β4/β8 groove, the β4/5 loop is strongly affected by peptide binding, while loops on the opposite end of the groove are not significantly perturbed. In the αB oligomer structure determined from ssNMR, the C-terminal region of a neighbouring dimer contacts the β4/5 loop but not the loops on the other end of the β4/β8 groove (Jehle et al, 2010). Similar binding modes are observed in crystal structures: the sequence N-terminal to the IxI motif is in close proximity to the β4/5 loop of a neighbouring subunit while sequences C-terminal to the motif exit the groove without making contact with β6+7/8 or β3/4 loops (Langanowsky et al, 2010; van Montfort et al, 2001; Kim et al, 1998; Jehle et al, 2010). Thus, the solution-state NMR data reported here are consistent with observations made for numerous sHSPs using multiple techniques. Solution-state NMR spectra that contain signals only from highly flexible and mobile residues failed to observe resonances from the IxI motif on αB-crystallin oligomers at 25°C. This is consistent with the sequence being less flexible than residues more C-terminal to it, whose signals were detected (Treweek et al, 2010). In contrast, the IxI motif was deemed highly flexible based on a solution-state NMR study on oligomeric αB in which Ile resonances were selectively detected by the methyl-TROSY approach (Baldwin et al, 2011). Altogether the available observations are most consistent with a model in which the IxI motif exists in an equilibrium between a free, flexible state, and a bound state. In the context of αB oligomers that contain between 24 and 32 subunits, free and bound IxI motifs, and empty and filled β4/β8 grooves, will exist simultaneously. Our results indicate a bound-state lifetime of 17 ms (kex≅60 s−1) and a dissociation constant below 90 μM for the binding of αB’s IxI C-terminal sequence. These numbers are for a bimolecular binding reaction. In the context of an oligomer, the effective local concentrations of both binding components will be significantly higher than the conditions of our bimolecular, peptide-based NMR experiments. So, it is reasonable to conclude that the population of bound C-termini at any given time will be significant. As we have previously proposed, the equilibrium-free and bound states may be shifted towards one state or the other by a change in conditions such as pH and temperature (Jehle et al, 2010).
Finally, we note the presence of multiple bounds states for αB IxI peptide at saturation in the NMR spectrum. Two IxI/V motif binding orientations in the β4/β8 groove have been observed in crystallographic studies (‘parallel’ and ‘antiparallel’ to the β8 strand; Langanowsky et al, 2010). Due to the presence of two β4/β8 grooves per ACD dimer, if the two peptide orientations are independent and of approximately similar affinity in solution, three bound species would be predicted: parallel/parallel, antiparallel/antiparallel, and parallel/antiparallel in a 1:1:2 ratio. It is notable that for the very limited number of examples where three resonances can be clearly and unambiguously observed, their ratios appear to be approximately as predicted. Thus, the simplest interpretation of the observed behaviour is that the αB-IxI peptide can bind in two orientations in solution. The slow exchange behaviour observed between binding modes indicates that a slow step, presumably peptide dissociation, governs the inter-conversion between binding modes. We cannot, however, rule out other possible explanations for the presence of multiple bound states. For example, if binding to the ACD is not restricted to a single peptide conformation, then the multiple bound states observed by NMR may originate from variability in the bound peptide conformation as opposed to its orientation. Whether the observed data result from one or a combination of these two possibilities cannot be unambiguously distinguished from the data at hand.
Binding to the β4/β8 groove requires an I/VxI/V motif
Of the peptides tested, only peptides with I/VxI/V motifs were observed to bind to the αB-ACD. Substitution of both Ile side chains with the smaller hydrophobic side chain Ala abolished all detectable binding. Whether all combinations of I/V in the two positions and whether Leu or other residues are also allowable in one or both positions will require a more thorough and systematic study. The structure of the β4/β8 groove is defined by the side chains that face into the groove from the two β strands. Substitution of the β8 residue, Ser135, with a larger side chain (Gln) significantly inhibited IxI binding. This result is consistent with the structure derived from ssNMR in which Ser135 resides between the two hydrophobic binding pockets and may provide a functional explanation to the strong conservation of small amino-acid residues observed at this position in sequence alignments (Kim et al, 1998; Taylor and Benjamin, 2005; Baldwin et al, 2011). Furthermore, the lowered affinity of the S135Q mutant for the IxI-peptide and the much shorter lifetime of the mutant bound state suggest that interpretation of studies in which this residue is mutated and/or modified should be approached with care (Baldwin et al, 2011).
Hetero-oligomers and C-terminal peptide binding
Hetero-oligomerization of αB with αA crystallin and HSPB1 has been reported and αB/HSP20 hetero-oligomers have been indirectly observed (Zantema et al, 1992; Kato et al, 1994; Sugiyama et al, 2000; Bukach et al, 2009; Engelsman et al, 2009). In contrast, attempts to observe αB/HSPB2 hetero-oligomers have failed to detect these species (Sugiyama et al, 2000; Engelsman et al, 2009). Such observations raise the question of what determines formation of sHSP hetero-oligomeric species. Given the high degree of conservation within the β4/β8 sequences of human sHSPs and the variability of sequences flanking the IxI motifs in the C-terminal regions, an appealing possibility is that β4/β8-to-IxI interactions help to define hetero-oligomers. If this were the case, then we would expect to detect interactions with the C-terminal peptides from αA and HSPB1, but not from HSPB2. As summarized in Table I, the prediction is not supported by our results. Nevertheless, interactions between the IxI motifs from αA-crystallin and HSPB1 and the αB-ACD suggest such an interaction is possible in hetero-oligomers. Whether and how the interaction contributes to hetero-oligomer formation or function must await further investigation.
Conclusion
It is possible to recapitulate interactions that normally occur within the context of an sHSP oligomer using a simplified system of isolated parts. The remarkable concordance of the NMR data acquired in solution on isolated ACD dimers and unstructured peptides with those data collected on oligomers in the solid-state indicate that important and relevant insights can be obtained using either approach. This provides a powerful strategy with which to study functionally relevant interactions involving sHSPs and their binding partners, be they other sHSPs or partly folded client proteins.
Materials and methods
Expression and purification of 15N labelled αB-ACD
Expression of a TEV cleavable N-terminally His-tagged αB-ACD (64–152) was carried out using methods and conditions previously described for the αB-ACD (Jehle et al, 2009). Cultures were pelleted then resuspended in a buffer solution containing 20 mM Tris (pH 8) 100 mM NaCl, 1 mM PMSF and EDTA-Free Protease Inhibitor Cocktail (Sigma-Aldrich) and were lysed by French Press. Lysate was diluted five-fold in 6 M Urea, 100 mM NaCl and centrifuged at 43 000 g. The supernatant was applied to a Ni-NTA Agarose column (Invitrogen). Protein bound to the column was refolded by three column volumes (CV) of 25 mM Tris, 150 mM NaCl (Buffer A), 10 mM Imidazole, pH 7.6 and His-tagged αB-ACD was eluted with the addition of Buffer A containing 250 mM Imidazole, pH 7.6. The TEV cleavable His tag was removed by dialysing overnight into Buffer A containing 5 mM DTT in the presence TEV protease. Following TEV digestion, the protein was reapplied to a Ni-NTA column and eluted with the addition of Buffer A containing 50 mM Imidazole pH 7.6. αB-ACD was further purified by anion exchange using a MonoQ column (GE) and SEC as previously described (Jehle et al, 2009). Purified αB-ACD was dialysed into water and lyophilized for storage.
Peptides
Peptides were generated by Life Tein Custom Peptide Synthesis Services with acetylated N-termini and amidated C-termini. Additional αB-IxI peptide was generously provided by the Gestwicki Laboratory (University of Michigan). Peptides were initially solubilized in water and the pH was adjusted to neutral, before the addition of phosphate and salt. Peptide concentrations were calculated based on the dry weight of lyophilized peptide and final peptide stock solutions ranged from 30 to 65 mM peptide, in 25 mM sodium phosphate and 150 mM NaCl pH 7.5.
NMR data collection and analysis
NMR experiments were performed on a Bruker 500 MHz Avance III spectrometer equipped with a 5-mm z axis gradient, triple resonance probe. 15N-labelled, lyophilized αB-ACD was resuspended in 25 mM sodium phosphate (pH 7.5), 150 mM NaCl. Peptide titration spectra were collected on 600 μM αB ACD (300 μM A) samples in 25 mM sodium phosphate, 150 mM NaCl, 10% D2O at 295K. Titration points at one-fold (600 μM), two-fold, and four-fold peptide were collected for all peptides that bound the ACD. Additional titration points were collected with the αB-IxI peptide and the αA-IxI peptide to confirm titration assignments and saturation points. Peptides that did not perturb the αB-ACD spectrum at four-fold excess were defined as peptides that do not bind the ACD and their presence in solution was confirmed by 1H–1D NMR. Because of the inherent acidity of peptide solutions used, the pH of all NMR samples was confirmed prior to data collection.
All spectra were processed using NMRPipe and data analysis was performed with NMRViewJ (Johnson and Blevins, 1994; Delaglio et al, 1995). Assignments for amide resonances in peptide-bound αB-ACD were obtained by following the resonances during the course of the titration. Some resonances in the spectra of αB-ACD bound to αB-IxI peptide could not be assigned unambiguously due to intermediate exchange behaviour where the resonances disappear during the intermediate titration points and reappear at the excess peptide concentration. To resolve ambiguities in αB-IxI peptide bound αB-ACD assignments, peak trajectories were compared to trajectories in αA-IxI and HSPB2 peptide titrations where the resonances could be followed unambiguously. CSPs were calculated based on the formula {(ΔδHN2+(ΔδN/5)2)}1/2 where ΔδHN and ΔδN are the chemical shift differences in amide proton and nitrogen resonances in p.p.m., respectively. ΔδN is divided by 5 since the spectral width in 15N-dimension is five-fold greater than the spectral width in the 1H dimension. CSPs were plotted onto a model of 2KLR using Pymol.
The Kd app for the HSPB2-VxI peptide binding to αB-ACD was determined using NMRViewJ (Johnson and Blevins, 1994). Six peaks from the HSPB2-VxI peptide titration were fit to a quadratic binding curve using a base 10 quadratic fit and 250 simulations and an average Kd app for all peaks fit was calculated.
Supplementary Material
Acknowledgments
We thank Jason Gestwicki and Leah Makley for their generous gift of peptide. We thank Ponni Rajagopal, Peter Brzovic, and Jonathan Pruneda for informative discussions and Ponni Rajagopal for critical evaluation of the manuscript. This work was funded by NIH grant 1R01 EY017370 (to REK). SD is supported in part by NIGMS 2T32 GM008268.
Author contributions: SPD, SJ, and REK conceived of and designed the experiments; SD performed the experiments; SD and REK analysed the results and wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aquilina JA, Benesch JL, Bateman OA, Slingsby C, Robinson CV (2003) Polydispersity of a mammalian chaperone: mass spectrometry reveals the population of oligomers in alphaB-crystallin. Proc Natl Acad Sci USA 19: 10611–10616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnéris C, Bateman OA, Naylor CE, Cronin N, Boelens WC, Keep NH, Slingsby C (2009) Crystal structures of alpha-crystallin domain dimers of alphaB-crystallin and Hsp20. J Mol Biol 392: 1242–1252 [DOI] [PubMed] [Google Scholar]
- Baldwin AJ, Hilton GR, Lioe H, Bagneris C, Benesch JLP, Kay LE (2011) Quaternary dynamics of αB-crystallin as a direct consequence of localised tertiary fluctuations in the C-terminus. J Mol Biol 413: 310–320 [DOI] [PubMed] [Google Scholar]
- Baldwin AJ, Lioe H, Hilton GR, Baker LA, Rubinstein JL, Kay LE, Benesch J (2011) The polydispersity of aB-crystallin is rationalized by an interconverting polyhedral architecture. Structure 19: 1855–1863 [DOI] [PubMed] [Google Scholar]
- Baranova EV, Weeks SD, Beelen S, Bukach OV, Gusev NB, Strelkov SV (2011) Three-dimensional structure of α-crystallin domain dimers of human small heat shock proteins HSPB1 and HSPB6. J Mol Biol 411: 110–122 [DOI] [PubMed] [Google Scholar]
- Benesch JLP, Aquilina JA, Baldwin AJ, Rekas A, Stengel F, Lindner RA, Basha E, Devlin GL, Horwitz J, Vierling E, Carver JA, Robinson CV (2010) The quaternary organization and dynamics of the molecular chaperone HSP26 are thermally regulated. Chem Biol 17: 1008–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JP, Garland DL, Green DE, Tamm ER, Giblin FJ, Wawrousek EF (2001) AlphaB-crystallin in lens development and muscle integrity: a gene knockout approach. Invest Ophthalmol Vis Sci 42: 2924–2934 [PubMed] [Google Scholar]
- Braun N, Zacharias M, Peschek J, Kastenmüller A, Zou J, Hanzlik M, Haslbeck M, Rappsilber J, Buchner J, Weinkauf S (2011) Multiple molecular architectures of the eye lens chaperone αB-crystallin elucidated by a triple hybrid approach. Proc Natl Acad Sci USA 108: 20491–20496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukach OV, Glukhova AE, Seit-Nebi AS, Gusev NB (2009) Heterooligomeric complexes formed by human small heat shock proteins HspB1 (Hsp27) and HspB6 (Hsp20). Biochim Biophys Acta 1794: 486–495 [DOI] [PubMed] [Google Scholar]
- Bukau B, Weissman J, Horwich A (2006) Molecular chaperones and protein quality control. Cell 125: 443–451 [DOI] [PubMed] [Google Scholar]
- Clark AR, Naylor CE, Bagnéris C, Keep NH, Slingsby C (2011) Crystal structure of R120G disease mutant of human αB-crystallin domain dimer shows closure of a groove. J Mol Biol 408: 118–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipeL a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6: 277–293 [DOI] [PubMed] [Google Scholar]
- Engelsman J, Boros S, Dankers PYW, Kamps B, Vree Egberts WT, Böde CS, Lane LA, Aquilina JA, Benesch J, Robinson CV, de Jong WW, Boelens WC (2009) The small heat-shock proteins HSPB2 and HSPB3 form well-defined heterooligomers in a unique 3 to 1 subunit ratio. J Mol Biol 393: 1022–1032 [DOI] [PubMed] [Google Scholar]
- Ghahghaei A, Rekas A, Carver JA, Augusteyn RC (2009) Structure/function studies of dogfish α-crystallin, comparison with bovine α-crystallin. Mol Vis 15: 2411–2420 [PMC free article] [PubMed] [Google Scholar]
- Goldstein LE, Muffat JA, Cherny RA, Moir RD, Ericsson MH, Huang X, Mavros C, Coccia JA, Faget KY, Fitch KA, Masters CL, Tanzi RE, Chylack LT Jr, Bush AI (2003) Cytosolic β-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer’s disease. Lancet 361: 1258–1265 [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Franzmann T, Weinfurtner D, Buchner J (2005) Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol 12: 842–846 [DOI] [PubMed] [Google Scholar]
- Hayes VH, Devlin G, Quinlan RA (2008) Truncation of alphaB-crystallin by the myopathy-causing Q151X mutation significantly destabilizes the protein leading to aggregate formation in transfected cells. J Biol Chem 283: 10500–10512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilario E, Javier F, Martin M, Bertolini MC, Fan L (2011) Crystal structures of xanthomonas small heat shock protein provide a structural basis for an active molecular chaperone oligomer. J Mol Biol 408: 74–86 [DOI] [PubMed] [Google Scholar]
- Horwitz J (1992) α-Crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA 89: 10449–10453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle S, Rajagopal P, Bardiaux B, Markovic S, Kühne R, Stout JR, Higman VA, Klevit RE, van Rossum BJ, Oschkinat H (2010) Solid-state NMR and SAXS studies provide a structural basis for the activation of alphaB-crystallin oligomers. Nat Strucl Mol Biol 17: 1037–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle S, van Rossum B, Stout JR, Noguchi SR, Falber K, Rehbein K, Oschkinat H, Klevit RE, Rajagopal P (2009) alphaB-crystallin: a hybrid solid-state/solution-state NMR investigation reveals structural aspects of the heterogeneous oligomer. J Mol Biol 385: 1481–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle S, Vollmar BS, Bardiaux B, Dove KK, Rajagopal P, Gonen T, Oschkinat H, Klevit RE (2011) N-terminal domain of alphaB-crystallin provides a conformational switch for multimerization and structural heterogeneity. Proc Natl Acad Sci USA 108: 6409–6414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Blevins RA (1994) NMR View: A computer program for the visualization and analysis of NMR data. J Biomol NMR 4: 603–614 [DOI] [PubMed] [Google Scholar]
- Kato K, Goto S, Inaguma Y, Hasegawa K, Morishita R, Asano T (1994) Purification and characterization of a 20-kDa protein that is highly homologous to alpha B crystallin. J Biol Chem 269: 15302–15309 [PubMed] [Google Scholar]
- Kato K, Inaguma Y, Ito H, Iida K, Iwamoto I, Kamei K, Ochi N, Ohta H, Kishikawa M (2001) Ser-59 is the major phosphorylation site in αB-crystallin accumulated in the brains of patients with Alexander’s disease. J Neurochem 76: 730–736 [DOI] [PubMed] [Google Scholar]
- Kim KK, Kim R, Kim SH (1998) Crystal structure of a small heat-shock protein. Nature 394: 595–599 [DOI] [PubMed] [Google Scholar]
- Langanowsky A, Benesch J, Landau M, Ding L, Sawaya M, Cascio D, Huang Q, Robinson C, Horwitz J, Eisenberg D (2010) Crystal structures of truncated alphaA and alphaB crystallins reveal structural mechanisms of polydispersity important for eye lens function. Protein Sci 19: 1031–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang X, Luo L, Wu M, Zeng R, Cheng G, Hu B, Liu B, Liang JJ, Shang F (2006) A novel αB-crystallin mutation associated with autosomal dominant congenital lamellar cataract. Invest Ophthalmol Vis Sci 47: 1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyano JV, Evans JR, Chen F, Lu M, Werner ME, Yehiely F, Diaz LK, Turbin D, Karaca G, Wiley E, Nielsen TO, Perou CM, Cryns VL (2006) AlphaB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J Clin Invest 116: 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasta SY, Raman B, Ramakrishna T, Rao CM (2004) The IXI/V motif in the C-terminal extension of alpha-crystallins: alternative interactions and oligomeric assemblies. Mol Vis 10: 655–662 [PubMed] [Google Scholar]
- Rajasekaran NS, Connell P, Christians ES, Yan LJ, Taylor RP, Orosz A, Zhang XQ, Stevenson TJ, Peshock RM, Leopold JA, Barry WH, Loscalzo J, Odelberg SJ, Benjamin IJ (2007) Human αB-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell 130: 427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PS, Martin JL, Swanson EA, Otani H, Dillmann WH, Das DK (2001) Transgene overexpression of alphaB crystallin confers simultaneous protection against cardiomyocyte apoptosis and necrosis during myocardial ischemia and reperfusion. FASEB J 15: 393–402 [DOI] [PubMed] [Google Scholar]
- Selcen D, Engel AG (2003) Myofibrillar myopathy caused by novel dominant negative α B-crystallin mutations. Ann Neurol 54: 804–810 [DOI] [PubMed] [Google Scholar]
- Stamler R, Kappe G, Boelens W, Slingsby C (2005) Wrapping the α-Crystallin Domain Fold in a Chaperone Assembly. J Mol Biol 353: 68–79 [DOI] [PubMed] [Google Scholar]
- Studer S, Obrist M, Lentze N, Narberhaus F (2002) A critical motif for oligomerization and chaperone activity of bacterial alpha-heat shock proteins. Eur J Biochem 269: 3578–3586 [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Suzuki A, Kishikawa M, Akutsu R, Hirose T, Waye MMY, Tsui SKW, Yoshida S, Ohno S (2000) Muscle develops a specific form of small heat shock protein complex composed of MKBP/ HSPB2 and HSPB3 during myogenic differentiation. Biochemistry 275: 1095–1104 [DOI] [PubMed] [Google Scholar]
- Sun TX, Liang JJ (1998) Intermolecular exchange and stabilization of recombinant human alphaA- and alphaB-crystallin. Biochemistry 273: 286–290 [DOI] [PubMed] [Google Scholar]
- Takeda K, Hayashi T, Abe T, Hirano Y, Hanazono Y, Yohda M, Miki K (2011) Dimer structure and conformational variability in the N-terminal region of an archaeal small heat shock protein, StHsp14.0. J Struct Biol 174: 92–99 [DOI] [PubMed] [Google Scholar]
- Taylor RP, Benjamin IKJ (2005) Small heat shock proteins: a new classification scheme in mammals. J Mol Cell Cardiol 38: 433–444 [DOI] [PubMed] [Google Scholar]
- Treweek TM, Rekas A, Walker MJ, Carver JA (2010) A quantitative NMR spectroscopic examination of the flexibility of the C-terminal extensions of the molecular chaperones, αA- and αB-crystallin. Exp Eye Res 91: 691–699 [DOI] [PubMed] [Google Scholar]
- van Montfort RL, Basha E, Friedrich KL, Slingsby C, Vierling E (2001) Crystal structure and assembly of a eukaryotic small heat shock protein. Nat Struct Biol 8: 1025–1030 [DOI] [PubMed] [Google Scholar]
- Vargas-Roig LM, Gago FE, Tello O, Aznar JC, Ciocca DR (1998) Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer 79: 468–475 [DOI] [PubMed] [Google Scholar]
- Vicart P, Caron A, Guicheney P, Li Z, Prévost MC, Faure A, Chateau D, Chapon F, Tomé F, Dupret JM, Paulin D, Fardeau M (1998) A missense mutation in the αB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet 20: 92–95 [DOI] [PubMed] [Google Scholar]
- Zantema A, Verlaan-De Vries M, Maasdam D, Bol S, van der Eb A (1992) Heat shock protein 27 and alpha B-crystallin can form a complex, which dissociates by heat shock. J Biol Chem 267: 12936–12941 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


