Abstract
Lateral root (LR) formation is initiated when pericycle cells accumulate auxin, thereby acquiring founder cell (FC) status and triggering asymmetric cell divisions, giving rise to a new primordium. How this auxin maximum in pericycle cells builds up and remains focused is not understood. We report that the endodermis plays an active role in the regulation of auxin accumulation and is instructive for FCs to progress during the LR initiation (LRI) phase. We describe the functional importance of a PIN3 (PIN-formed) auxin efflux carrier-dependent hormone reflux pathway between overlaying endodermal and pericycle FCs. Disrupting this reflux pathway causes dramatic defects in the progress of FCs towards the next initiation phase. Our data identify an unexpected regulatory function for the endodermis in LRI as part of the fine-tuning mechanism that appears to act as a check point in LR organogenesis after FCs are specified.
Keywords: auxin, endodermis, founder cell, lateral root initiation, pericycle
Introduction
Unlike the majority of animals, plants have the potential to develop new organs post-embryonically. For instance, lateral roots (LRs) generate an entirely new organ from a small number of apparently differentiated root cells. LRs originate from a single concentric layer of pericycle cells within the root of the model plant Arabidopsis thaliana (Dubrovsky et al, 2000; Beeckman et al, 2001). This new root developmental program initiates when a restricted number of pericycle cells overlaying the xylem pole acquire founder cell (FC) identity and divide asymmetrically. Next, these pericycle derived initials undergo a sequence of cell divisions and differentiation events that ultimately results in the formation of an LR primordium. The new organ will eventually emerge through the overlaying cell layers of the primary root and form a new apical meristem that will control further growth of the mature LR (Malamy and Benfey, 1997; Swarup et al, 2008).
Accumulation of the plant hormone auxin in a restricted number of pericycle cells is one of the earliest events during FC specification that precedes LR initiation (LRI) (Dubrovsky et al, 2008). Interference with the auxin accumulation by chemical or genetic inhibition of auxin transport or downstream auxin responses leads to severe defects in FC establishment and LRI (Casimiro et al, 2001; Fukaki et al, 2002; Benková et al, 2003; Dharmasiri et al, 2005; Vanneste et al, 2005).
Several studies have addressed the auxin-based mechanism that regulates recurrent LRI. The observation that a periodic auxin response in the protoxylem of the basal meristem later coincides with the site of LRI led to the hypothesis that FCs are primed before auxin accumulation occurs in the defined pericycle cells (De Smet et al, 2007). Similar studies based on the oscillating behaviour of the auxin response marker DR5:LUC further suggested that regular fluctuations in auxin response along with complex oscillating gene expression patterns which determine competence for LRI, are required for specification of root branching sites (Moreno-Risueno et al, 2010). Other work proposed that auxin accumulation in the FC pericycle cells might result from gravitropic root bending and/or mechanical changes acting on expression of auxin carriers such as AUX1 (Laskowski et al, 2008). Indeed, the correlation between primary root gravity responses and LRI suggested that they are co-regulated and that root waving promotes an alternating left-right LRI positioning on the convex site of the root bent (De Smet et al, 2007; Lucas et al, 2008). In addition, experimental and computational modelling indicated that mechanical deformation of tissues during root bending increases the auxin levels on the convex side of the bent root, thereby driving LRI (Laskowski et al, 2008). Both gravistimulation and cell shape changes during root bending were shown to modulate local auxin transport dynamics by affecting the expression and polarity of auxin influx and efflux carriers AUX1 and PIN1 at the plasma membrane (Ditengou et al, 2008; Laskowski et al, 2008).
Despite these new insights, it remains unclear how an auxin threshold is reached in a subset of pericycle cells in order for LRs to initiate. In Arabidopsis, the PIN family of auxin efflux carrier proteins is part of the polar auxin transport machinery determining the direction of the auxin flow and thus, auxin levels within plant tissues (Wiśniewska et al, 2006). Among them, PIN3 was shown to play an important role during LRI (Benková et al, 2003; Laskowski et al, 2008). Here, we reveal that PIN3 is part of an auxin reflux pathway that is transiently established during early phases of LRI. This developmentally specific reflux pathway reinforces auxin flow from the endodermal cells to the FCs, enabling them to reach auxin threshold required to transit from founder to LRI phases. We show that the loss of PIN3 causes a dramatic delay in the onset of LRI after FCs are specified, which can be fully recovered by the expression of PIN3 exclusively in the endodermis. Our data demonstrate that the endodermis plays an active role in the regulation of LRI and acts as one of the check-point mechanisms acting during the transition between FC and LRI.
Results
PIN3 is transiently induced in the endodermis during LRI
To determine how PIN3 contributes to LRI, we initially examined its expression in the root. As previously reported, PIN3::PIN3-GFP (Žádníková et al, 2010) was expressed in stele tissues including pericycle cells (Benková et al, 2003) (Figure 1A; Supplementary Figure S1). However, we also observed that the PIN3-GFP signal was detected in endodermal cell overlaying FCs and initiating LR primordia (LRP) at stage I (Malamy and Benfey, 1997), while neighbouring endodermal cells lacked the signal (Figure 1A; Supplementary Figure S1C). Detailed monitoring of PIN3-GFP in the root endodermis revealed that PIN3 expression varied along the longitudinal axis (Dolan et al, 1993). Starting from the most distal end, through the root meristem (RM) and the elongation zone (EZ), a continuous PIN3 signal was detected (Supplementary Figure S1A). Unlike in the differentiation zone (DZ), where cells exhibit signs of terminal maturation encompassing xylem strand differentiation (Dolan et al, 1993), this signal disappeared and PIN3 was exclusively detectable in endodermal cells overlaying LRI sites (Supplementary Figure S1A and C).
Figure 1.
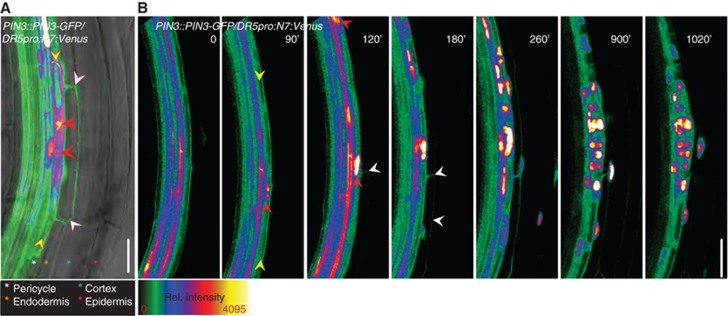
PIN3 is transiently induced in the endodermis during LRI. (A) PIN3::PIN3-GFP expression was detected in pericycle FCs (yellow arrowheads), and the overlaying endodermis cell (white arrowheads). Note that neighbouring endodermal cells are not labelled with the PIN3-GFP signal. Red arrowheads indicate the migrated nuclei expressing an enhanced DR5pro::N7:Venus auxin response reporter. A semiquantitative colour-coded heat-map of the GFP fluorescence intensity is provided. Colour-coded asterisks indicate the different root cell files. Scale bar: 25 μm. (B) Real-time analysis of PIN3::PIN3-GFP expression in the endodermis (white arrowheads) relative to FC establishment and LRI. FC establishment and LRI were followed by the accumulation of the nuclear DR5pro::N7:Venus signal (red arrowheads) in the pericycle FCs (yellow arrowheads) and the division of these nuclei. Image series depicted is a representative example from at least 10 observations, and time stated is relative to root bending. Scale bar: 20 μm.
To obtain a spatio-temporal view of the dynamics of PIN3 expression during pre-initiation and LRI phases, real-time analysis of LRI was performed using the previously established assay for induction of LRI through mechanical root bending (Laskowski et al, 2008; Marhavý et al, 2011) (for details, see Materials and methods). Local enhancement of auxin activity in the pericycle cells, monitored by the nuclear localized auxin response reporter DR5pro::N7:Venus (Heisler et al, 2005), was considered as the earliest sign of FC specification (Dubrovsky et al, 2008). Directly after bending, PIN3-GFP expression was exclusively observed in the vascular tissues (Figure 1B). At 90 min, an enhanced nuclear signal (Figure 1B; red arrowhead) in a restricted pericycle cell indicated FC establishment (Figure 1B; yellow arrowheads). Shortly thereafter (at 120 min), the PIN3-GFP signal was induced in the adjacent endodermal cells (Figure 1B; white arrowheads) and remained there during the entire LRI phase. No PIN3-GFP expression in the endodermis could be detected prior to this DR5 activation in the pericycle cells. As LRP progressed to the next developmental phase around 900 min, PIN3-GFP in the endodermis gradually disappeared and was absent from the endodermis once the developing primordium obtained three cell layers around 1020, min (Figure 1B). Thus, the transient PIN3 expression in the endodermis takes place in a narrow window encompassing FC establishment and LRI.
PIN3 promotes the transition from FCs to the LRI phase
To dissect the developmental role of this spatially and temporally confined pattern of PIN3 expression in the endodermis, we re-examined the LRI defect in the pin3 mutant, focusing specifically on the earliest stages of LR formation. The DR5rev::GFP auxin reporter (Friml et al, 2003) expressed from the FC stage onwards (Dubrovsky et al, 2008), was used to analyse densities of FCs (pericycle cells exhibiting DR5 expression, but no division scored) and LRI events in 5-day-old pin3 mutant and wild-type (WT) roots. As expected, pin3 mutants displayed a significant decrease in LRI density (Benková et al, 2003; Laskowski et al, 2008) (4.5±0.5 in WT; 2.3±0.6 in pin3; n=10) (Figure 2B). Surprisingly, FC density was dramatically increased in the pin3 background (2.1±0.3 in WT; 5.4±0.6 in pin3; n=10) (Figure 2A), whereas the total FC plus LRI density remained equal in both WT and the pin3 mutant (Figure 2C). To further corroborate these observations we used the CycB1;1::GUS reporter that is active from the G2 phase of the cell cycle until early M phase and therefore marks the FCs at the advent of LRI and the subsequent cell divisions during LR formation (Beeckman et al, 2001). Analysis of FC and LRI densities with the CycB1;1::GUS marker fully confirmed the results obtained with the DR5::GFP reporter (Supplementary Figure S2H–K). Scoring of FC and LRI in 7-day-old WT and pin3 seedlings using the DR5 auxin marker revealed similarly to 5-day-old roots significantly increased FC density (Supplementary Figure S2C–F), thus indicating the age-independent character of this phenotype. Finally, monitoring of FC and LRP along the acropetal root growth axis revealed that the accumulation of FCs mainly occurred at the distal root end (Supplementary Figure S2A and B), in accordance with the overall acropetal development of LR formation described in Arabidopsis (Dubrovsky et al, 2006).
Figure 2.
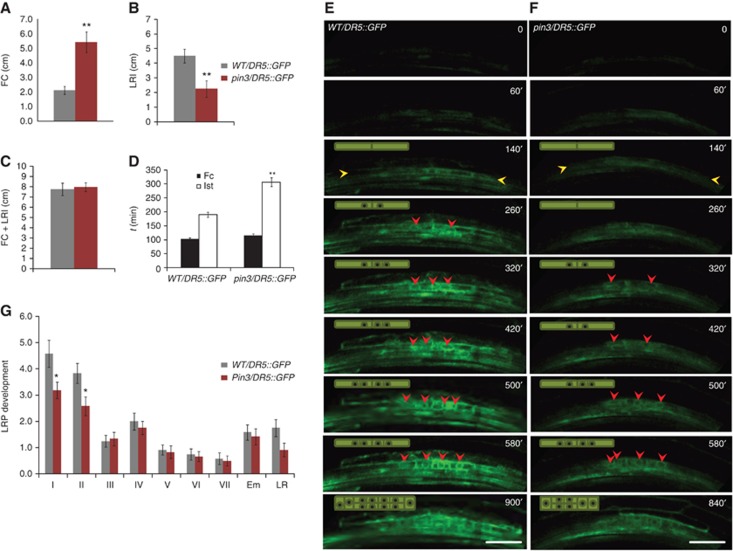
The pin3 mutation causes a delay in the transition from the FC stage to LRI. (A) FC density is increased in pin3 mutants compared to WT. (B, C) LRI density is decreased in pin3 mutants (B), whereas the sum of FC+LRI densities is similar to WT (C). (D) Timing of FC establishment and LRI after root bending indicates a delay between FC establishment and LRI in pin3 mutants compared to WT. (E, F) Real-time analysis of FC establishment and LRI in WT/DR5::GFP (E) and pin3/DR5::GFP (F) after bending. FC establishment was followed by the appearance of the DR5rev::GFP signal in the pericycle (orange arrowhead) and LRI was scored when nuclear (red arrowhead) division could be observed. Schematic representation of the observed cell divisions is shown in the left upper corner. Image series depicted is a representative example from at least 10 observations and time stated is relative to root bending (D–F). Scale bar: 30 μm. (G) Stage distribution of LRP shows a decrease in stage I and stage II LRP in pin3 mutants. Error bars represent standard error (s.e.m.) (n=10–20). P-values are *<0.05, **P<0.01; Student’s t-test.
The increased accumulation of FCs, together with the decrease in LRI events in pin3, suggests that this mutant is defective in the transition between the FC and LRI phase. To corroborate this hypothesis, LRI was followed in real time. Typically, in control roots, DR5 expression in individual pericycle cells, indicative of FC establishment, was induced around the same time after bending in both WT and pin3 backgrounds (Figure 2D–F). However, while the transition from FC to LRI required on average 100 min (103±10, n=10) in WT roots, the time taken in pin3 was double (206’±16’, n=10) (Figure 2D–F). Hence, pin3 mutants are delayed in the progression from FC establishment to LRI. To assess the impact of pin3 loss of function on the subsequent developmental phases of the LR organogenesis, we examined the number of the individual stages of LR development (Malamy and Benfey, 1997) in 7-day-old roots. Compared to WT, differences were mostly pronounced in the earliest developmental stages (Figure 2G; Supplementary Figure S2G). Notably, we observed a decrease in the number of stage I (4.6±0.5 in WT; 3.2±0.3 in pin3; n=20), whereas the following developmental stages were not significantly affected. We conclude from these data sets that PIN3 activity promotes the transition from the FC stage to LRI.
PIN7 controls LRI by a mechanism distinct to PIN3
PIN3 is most closely related to PIN7 in the Arabidopsis PIN family of auxin efflux carriers (Paponov et al, 2005). Both genes share common expression domains and have been shown to act redundantly during numerous plant developmental processes (Benková et al, 2003; Devlin et al, 2003; Friml et al, 2003; Blilou et al, 2005; Paponov et al, 2005). To explore the functional redundancy of PIN7 and PIN3 during the early phases of LRI, we examined the expression of pPIN7::PIN7-GFP (Blilou et al, 2005) in the root (Supplementary Figure S3A). As expected, PIN7-GFP was detected in the central root cap and the stele, similar to PIN3-GFP (Blilou et al, 2005) (Supplementary Figure S1A, compared to S3A). However, PIN7 expression was not detected in the neighbouring endodermal cells in the RM and EZ. The root was subsequently scanned for LRI events. No PIN7-GFP signal was observed in the overlaying endodermal cells (Supplementary Figure S3A). Hence, expression in the endodermis during LRI is specific to PIN3. It has been reported previously that the pin7 mutation affects the total number of initiated LRP (Benková et al, 2003). Although the density of FCs in the pin7 mutant was significantly increased (2.9±0.2 in WT; 4.6±0.4 in pin7; n=10) (Supplementary Figure S3C), real-time monitoring of the LRI showed that timing of FC specification (140′±11′ in WT; 174′±30′ in pin7; n=10) and the transition from FC to LRI (118.7′±15.4′ in WT; 138.3′±17.7′ in pin7; n=10) were not affected (Supplementary Figure S3B and E). This indicates that lack of PIN7 activity interferes with mechanisms underlying the frequency of FC specification, but not with the FC-LRI transition. The developmental consequences of the pin7 mutation were manifested by an accumulation of primordia at stage I (3.9±0.3 in WT; 4.8±0.5 in pin7; n=20), raising the density of LRI events (Supplementary Figure S3F). Hence, both the endodermal expression accompanying LRI and the control over FC-LRI transition are specific to PIN3.
To further verify whether other members of the PIN family might act along with PIN3 in the endodermis, we examined the expression of PIN1, PIN2 and PIN4 in the root. Expression of none of these PIN genes was detected in endodermal cells overlaying LRP in early developmental phases, neither under control condition, after treatment with auxin, which is shown to promote LRI, nor when expressed in pin3 mutant background (Supplementary Figure S3G). Thus, PIN3 seems to be the major auxin efflux carrier acting in the LRP adjacent endodermal cells.
PIN3 activity in the endodermis stimulates FC progression to LRI
The dual PIN3 expression pattern encompassing both the pericycle and endodermis during LRI raises the question about the functional contribution of PIN3 in the endodermal (versus pericycle) cells on the regulation of LRI that cannot be determined by studying the pin3 mutant phenotype. Instead, to discern whether PIN3 activity in endodermis cells is required for the proper progression of this developmental event, we employed a tissue-specific complementation assay. The endodermis-specific SCR promoter was selected to drive a PIN3-YFP fusion (Žádníková et al, 2010) in the pin3/DR5::GFP background. As expected, the SCR promoter restricted PIN3-YFP to the endodermal cells of the RM, EZ, and the DZ (Figure 3A). Importantly, the SCR promoter was active in those endodermal cells overlaying LRI, but no PIN3-YFP expression was detected in dividing pericycle cells in stage I LRP (Figure 3A). Only later, from developmental stage II on, could SCR::PIN3-YFP expression be detected in the outer layers of the LRP (Figure 3F). Expression of PIN3-YFP exclusively in the endodermis was sufficient to fully rescue FC (2.9±0.2 in WT/DR5::GFP; 5.8±0.3 in pin3/DR5::GFP; 6.8±0.5 and 3.2±0.3 in pin3/DR5::GFP/SCR::PIN3-YFP fluorescence – and +, respectively; n=10) and LRI densities (5.2±0.3 in WT/DR5::GFP; 3.6±0.3 in pin3/DR5::GFP; 3.8±0.3 and 7.1±0.5 in pin3/DR5::GFP/SCR::PIN3-YFP fluorescence – and +, respectively; n=10) (Figure 3B and C). Accordingly, the decrease in density of stage I LRP was eliminated (6.7±0.5 in WT/DR5::GFP; 4.5±0.5 in pin3/DR5::GFP; 3.9±0.4 and 5.7±0.5 in pin3/DR5::GFP/SCR::PIN3-YFP fluorescence – and +, respectively; n=20) (Figure 3E). To further corroborate our findings, the promoter of CASP1, a recently identified endodermis-specific gene (Roppolo et al, 2011), was also used. Unlike the SCR promoter, endodermis-specific expression of PIN3-YFP driven by the CASP1 promoter was restricted to the differentiating part of the root (Roppolo et al, 2011; Supplementary Figure S4A). Introduction of CASP1::PIN3-YFP in the pin3/DR5::GFP background completely restored the FC (3.7±0.3 in WT/DR5::GFP; 7.7±0.2 in pin3/DR5::GFP; 8.3±0.4 and 5.5±0.5 in pin3/DR5::GFP/CASP1::PIN3-YFP fluorescence – and +, respectively; n=10) and LRI densities (8.2±0.6 in WT/DR5::GFP; 4.5±0.3 in pin3/DR5::GFP; 5.3±0.7 and 9.4±0.7 in pin3/DR5::GFP/CASP1::PIN3-YFP fluorescence – and +, respectively; n=10) (Supplementary Figure S4B and C). Also the decrease in stage I LRP was rescued (5.0±0.4 in WT/DR5::GFP; 3.7±0.6 in pin3/DR5::GFP; 4.1±0.7 and 6.9±0.4 in pin3/DR5::GFP/CASP1::PIN3-YFP fluorescence – and +, respectively; n=20) (Supplementary Figure S4E). Altogether these data show that PIN3 activity in the endodermis adjacent to the FCs and LRI events stimulates FC progression to LRI. Furthermore, the data exclude a role of endodermal PIN3 in the distal root zones, encompassing the basal meristem, from contribution to this FC-LRI regulatory module.
Figure 3.
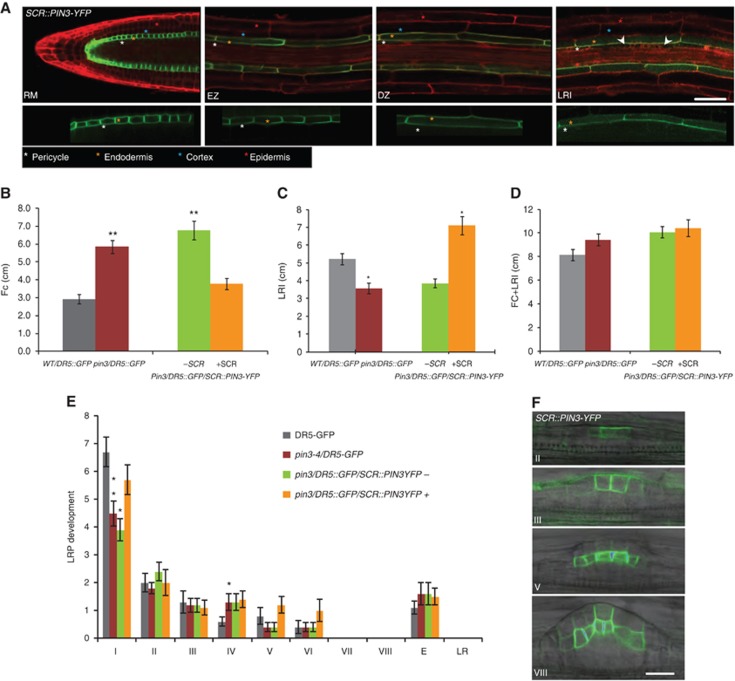
PIN3 activity in the endodermis promotes FC progression. (A) Monitoring of SCR::PIN3-YFP expression (green) in the RM, EZ, DZ and during LRI (white arrowhead). Propidium iodide (PI) counterstain (red) is shown in the upper panel. The lower panel reveals PIN3 polarity in the endodermal cells of the depicted root zones. Colour-coded asterisks indicate the different root cell files. Scale bar: 50 μm. (B, C) FC (B) and LRI (C) densities are rescued in the pin3/DR5::GFP roots expressing SCR::PIN3-YFP in the endodermis. (D) The total number of FC+LRI densities is similar to WT in all genetic backgrounds. (E) Stage distribution of LRP indicates that the decrease in stage I in pin3/DR5::GFP mutants is restored in the fluorescent-positive pin3/DR5::GFP/SCR::PIN3-YFP seedlings compared to WT/DR5::GFP. +SCR and –SCR refer to the pooled fluorescent-positive and -negative seedlings, respectively, from a segregating population in the stable pin3/DR5::GFP background (B–E). (F) The SCR promoter drives PIN3-YFP expression in the LR only from stage II onwards. Corresponding stages of LR development are indicated. Scale bar: 20 μm. Error bars represent s.e.m. (n=10–20). P-values are *<0.05, **P<0.01; Student’s t-test.
To determine whether PIN3 activity in the root stele participates in the regulation of this developmental event, the SHR promoter that is active in the root stele (Helariutta et al, 2000) was used to drive PIN3-YFP expression in the pin3/DR5GFP background. The SHR promoter activated PIN3-YFP in the stele of the RM, EZ and DZ, comprising the dividing pericycle cells (Lucas et al, 2011; Figure 4A and C). The PIN3-YFP signal in the stele masked any DR5::GFP signal in the pericycle, thus hindering the scoring of FCs and LRI events. A distribution of the different stages of LR development however clearly showed that expression of PIN3-YFP in the stele did not increase the number of stage I primordia in the pin3 background (5.7±0.4 in WT; 4.8±0.5 in pin3; 4.5±0.4 and 4.7±0.4 in pin3/DR5::GFP/SHR::PIN3-YFP fluorescence – and +, respectively) (Figure 4B). Hence, PIN3 action in the stele, including pericycle cells, does not significantly contribute to the transition from FC establishment to LRI.
Figure 4.
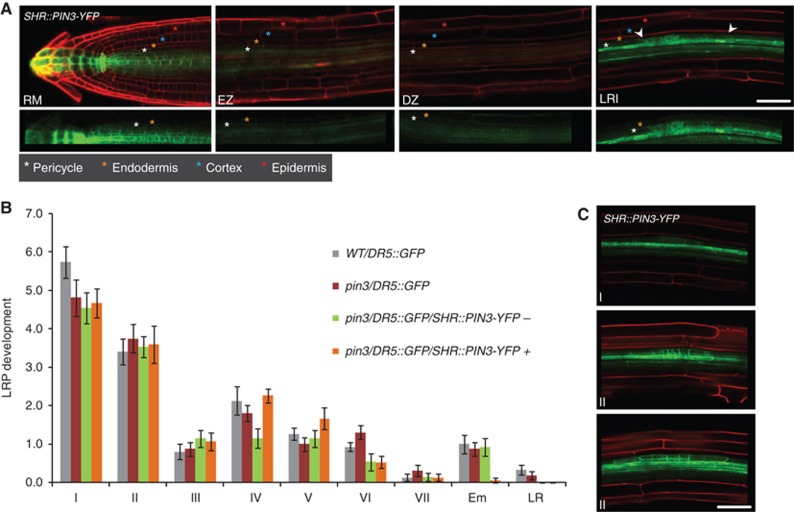
SHR-driven PIN3 stele expression is not sufficient to rescue pin3 LRI phenotype. (A) The SHR promoter drives PIN3-YFP expression (green) in the stele of the RM, EZ and DZ and during LRI (arrowheads) in the pin3/DR5::GFP background, with PI counterstain (red) in the upper panel. Colour-coded asterisks indicate the different root cell files. Note that PIN3-YFP is also present in the dividing pericycle cells during LRI. The fluorescent signal in the root cap is derived from the DR5::GFP marker. Scale bar: 50 μm. (B) Stage distribution of LR primordia indicates that the decrease in stage I is not restored in pin3 mutants when PIN3-YFP is expressed in the stele. +SHR and –SHR refer to the pooled fluorescent-positive and -negative seedlings, respectively, from a segregating population in the stable pin3/DR5::GFP background. Error bars represent s.e.m. (n=10–20). (C) The SHR promoter drives PIN3-YFP expression in the dividing pericycle cells from the first stage of LR development onwards. Corresponding stages of LR development are indicated. The PI counterstain is shown in red. Scale bar: 50 μm.
PIN3 defines an auxin reflux pathway between overlaying endodermal and FCs
The direction of auxin flow is largely determined by the polarity of PIN efflux carriers within cells (Wiśniewska et al, 2006). Thus, PIN3 polarity at the plasma membrane is indicative of the direction of PIN3 mediated auxin transport. Optical cross-sections of PIN3-GFP roots at the site of LRI indicated that PIN3 is localized to the inner side of the overlaying endodermal cells whereas no PIN3 signal was observed at the membranes of the remaining endodermal cells (Figure 5A and B). PIN3 promoter activity in the pericycle alongside the endodermal cells limits detailed examination of the PIN3 polarity in either cell type (Supplementary Figure S1C). Therefore, to study the membrane localization of PIN3 specifically in the endodermal cells, the SCR::PIN3-YFP line was analysed. Differently from the PIN3 promoter, whose expression is induced only transiently in the endodermis in the DZ during LRI, the SCR promoter is active continuously throughout the root zones encompassing RM, EZ and DZ. Along the root growth axis, PIN3 displayed a variable polarity in the endodermal cell file. While PIN3 exhibited basal polarity in RM cells (Figure 3A), this became apical in the EZ. In cells that had entered the DZ, PIN3 was first non-polarly distributed (Figures 3A and 5C). However, more proximal in the root, a dramatic change in PIN3 polarity was again observed where PIN3 was polarized to the inner lateral membrane facing the pericycle cells (Figure 5D). When the root was subsequently searched for LRIs, we found that these always occurred within the region of the DZ where PIN3 was polarized to the inner lateral wall (Figures 3A and 5D). This indicates that when PIN3 expression is induced in the endodermis during LRI, the PIN3 protein might polarize by default to the inner lateral membrane.
Figure 5.
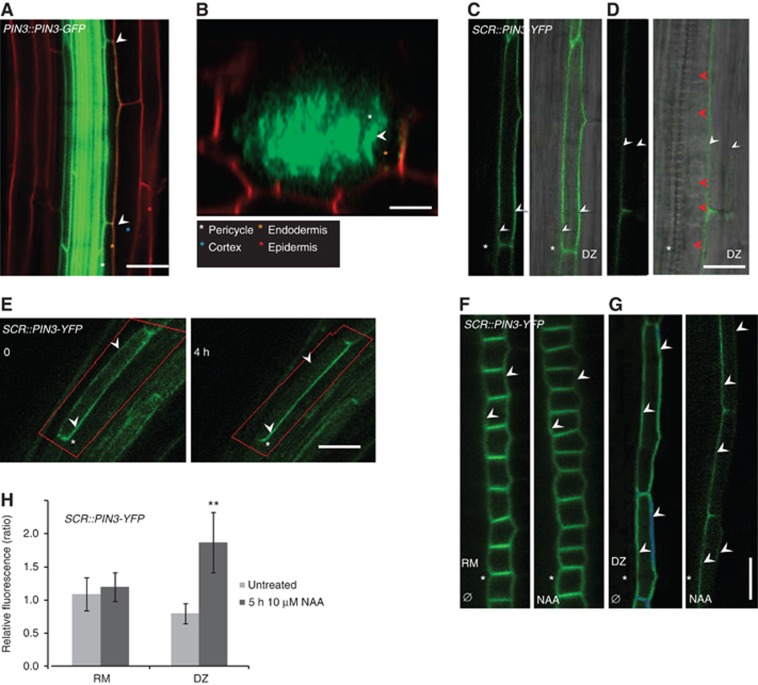
PIN3 polarizes to the inner membranes of endodermal cells overlaying LRI sites. (A, B) PIN3::PIN3-GFP (green) expressing root with PI counterstain in red (A) and the corresponding optical cross-section at the site of LRI (B) shows PIN3-GFP at the inner lateral membrane of the two overlaying endodermal cells (arrowheads), whereas PIN3-GFP is absent from the remaining endodermal cells in the cross-section. Colour-coded asterisks indicate the different root cell files. Scale bars: 40 μm. (C, D) SCR-driven PIN3-YFP in endodermal cells of DZ in close proximity of EZ (C) and in a more proximal region of the DZ where the LRIs take place (D). Note non-polar (C) and lateral (D) membrane localization of PIN3-YFP. Fluorescence image (left panel) and overlay with DIC (right panel). White arrowheads indicate the lateral membranes in the endodermis and red arrowheads indicate divided nuclei in the stage I LRP. Scale bars: 20 μm. (E–H) Manipulations that induce LRI and provoke PIN3 lateralization. Observations were done in the SCR::PIN3-YFP line, directly behind the RM, where PIN3 is apolarly distributed on all membranes. (E) After root bending, SCR-driven PIN3-YFP in the endodermal cell laterlizes in the course of 4 h. Time points are relative to root bending. (F–H) NAA (10 μM) treatment induces PIN3-YFP lateralization in the DZ but not in the RM. Fluorescent images (F, G) and graph (H) displaying the ratio between relative fluorescence intensity at the inner and outer cell membranes in the RM (F) and DZ (G) in control and 5-hour NAA-treated seedlings. White arrowheads indicate the lateral membranes in the endodermis and white asterisks indicate the neighbouring pericycle cell file. Error bars represent s.e.m. (n=30), P-values are **P<0.001; Student’s t-test. Scale bars: 50 μm.
Next, using the SCR::PIN3-YFP line, we checked whether inducing LRI provokes PIN3 lateralization in the region of the DZ where it is non-polarly distributed along all membranes. Strikingly, bending the root in this region triggered PIN3 lateralization (Figure 5E). In the course of 4 h (240 min), the originally non-polar PIN3 membrane distribution had changed and the prevailing part of PIN3 was detected on the inner membrane of the endodermal cells. Consistently, treatment of roots with auxin, a well-established enhancer of LRI, also induced PIN3 lateralization (Figure 5F–H). After 2 h, PIN3 lateralization was observed, with the strongest difference seen after 5 h (Figure 5G and H). This auxin-based lateralization of PIN3 was specific to the DZ and was not observed in the RM (Figure 5F and H). We conclude that PIN3, induced in the endodermal cell after FC specification, locates towards the inner membrane, presumably to direct auxin towards the underlying FC.
Discussion
New LRPs in Arabidopsis are derived exclusively from a subset of cells within the pericycle that is overlain by several additional root tissues encompassing the endodermis, cortex, and epidermis. This puts special demands on neighbouring tissues to adapt to the expansion of the newly developing organ. The interaction between LRP and overlaying cells is particularly critical for ensuring that the emergence of primordia through neighbouring tissues causes minimal damage. An important regulatory component of this tissue interaction is auxin, shown to promote cell separation in advance of the developing LRP through upregulation of cell wall remodelling genes (Swarup et al, 2008) and downregulation of water channels termed aquaporins (Péret et al, 2012).
Here, we reveal that controlled auxin transport between the site of LR formation and the overlaying tissues is instructive for LR formation at a much earlier stage. Expression of PIN3 in endodermal cells before LRI indicates a function during the developmental phase that precedes LRP dome formation.
During pre-initiation phases, PIN3 was transiently induced in endodermal cells overlaying FCs until LRP were initiated. At the same time, the auxin response marker was active in the same endodermal cells (Figure 1B; 180’). PIN3 is auxin inducible (Vieten et al, 2005) and auxin treatment induces PIN3-GFP in the endodermal and cortical cell files of the root (Supplementary Figure S5). Altogether these data suggest that after FC establishment, auxin induces PIN3 expression specifically in the overlaying endodermal cell (Figure 6A–C). Laterally localized PIN3 in these endodermal cells subsequently reinforces auxin movement towards the FCs and thus provides a local auxin reflux pathway important for further progress to LRI (Figure 6C and D).
Figure 6.
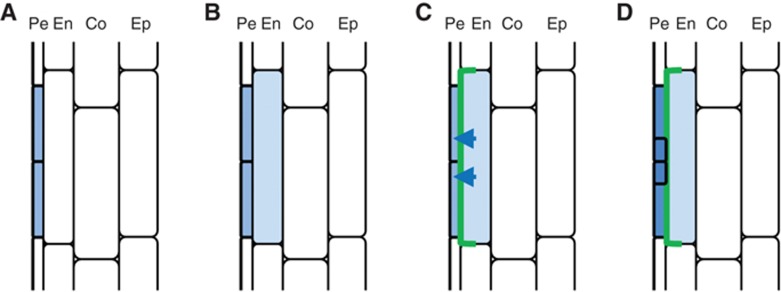
Model for endodermal PIN3 regulated transition from FC to LRI. (A) Auxin response activity (blue) in a restricted number of pericycle cells indicates FC establishment. (B, C) Soon after, auxin signalling is activated in the overlaying endodermal cell (B), inducing local and transient expression of PIN3 (green) (C). In the endodermis cell, the PIN3 protein is laterally localized to the inner membrane, thereby transporting auxin towards the FCs, providing a local auxin reflux pathway. (D) This PIN3-driven auxin reflux contributes to auxin accumulation in FCs important for further progress to LRI.
The identification of mutants in which, despite defects in LRI (Dubrovsky et al, 2008; De Rybel et al, 2010), FC establishment still occurs, indicates that specification of FCs does not lead by default to LRI and that these two events are not directly coupled. In addition, real-time imaging of LRI has shown that auxin continues to accumulate in the FCs, until it reaches a maximum just prior to the actual initiation event (De Rybel et al, 2010). These observations indicate that LRI might require additional input upon FC establishment and that there are extra checkpoints, requirements to be met before pericycle cells actually divide. Our data support a regulatory function for the endodermis during LRI and indicate that the interaction between FC and the adjacent endodermal cells defines a developmentally specific auxin reflux pathway, which supports FCs to reach auxin threshold levels necessary to initiate LR formation.
Materials and methods
Plant material
The transgenic Arabidopsis thaliana (L.) Heynh. lines have been described elsewhere: PIN7::PIN7-GFP (Blilou et al, 2005), PIN3::PIN3-GFP (Žádníková et al, 2010), pin3-4/DR5::GFP (Ding et al, 2011), DR5rev::GFP (Friml et al, 2003), DR5pro::N7:Venus (Heisler et al, 2005), pin7-1 (Benková et al, 2003). The PIN1::PIN1-GFP (Benková et al, 2003), PIN2::PIN2-GFP (Xu and Scheres, 2005), PIN4::PIN4-GFP (Blilou et al, 2005) lines were crossed into the pin3-4 mutant background.
Growth conditions
Seeds of Arabidopsis (ecotype Columbia-0) were plated on 0.5 MS medium (Duchefa) with 1% sucrose and 0.8% agar (pH 5.7) and stratified for 2 days at 4°C. Seedlings were grown on vertically oriented plates in growth chambers under a 16-h-light/8-h-dark photoperiod at 18°C.
Plasmid construction
Construction of the PIN3-YFP (Rakusová et al, 2011) and CASP1 (Roppolo et al, 2011) promoter entry vectors and the SCR::PIN3-YFP (Rakusová et al, 2011) expression vector has been described elsewhere. Entry clones containing the 2500-bp SHR promoter were cloned into the donor vector pDONRP4P1R. Expression clones were generated by recombining the promoter and PIN3-YFP fragments into the expression vector pB7m24GW, which were then directly transformed to pin3-4/DR5rev::GFP plants.
Pharmacological and hormonal treatments
In all, 5- or 6-day-old seedlings were transferred onto solid MS media with or without 1-naphthalene acetic acid (NAA) (10 μM) and were further incubated for the time indicated.
Confocal imaging and image analysis
For confocal microscopy images, the Zeiss LSM 510 or Olympus FV10 ASW confocal scanning microscopes were used. Fluorescence signals for GFP (excitation 488 nm, emission 507 nm) and PI (excitation 536 nm, emission 617 nm) were detected. YFP signals were observed using GFP settings. Sequential scanning was used to avoid any interference between fluorescence channels.
Timing of the transition from FC to LRI was done after root bending. In all, 5- or 6-day-old seedlings were placed on chambered cover glass (Nunc Lab-Tek) and manually curved by bending the root tip just above the RM using forceps. The seedlings were then covered with 0.2 mm thin square blocks of solid MS media. All the samples were analysed in the same area of the bended root with a focus on the beginning of xylem strains formation. LRPs were scanned in 3, 5, 10, or 20 min time intervals for 8–17 h by the FV10 ASW confocal microscope (Olympus) with a × 20 or × 60 (water immersion) objective.
For relative fluorescence intensity measurements on lateral plasma membranes, between 30 and 40 cells corresponding to the RM and DZ, where PIN3 is normally non-polarly distributed, were imaged with both inner and outer lateral membranes in focus. Later, images were analysed using the ImageJ software (NIH; http://rsb.info.nih.gov/ij), where the absolute fluorescence intensities of both lateral membranes were measured to calculate the relative fluorescence intensity of the inner over the outer lateral membrane. The statistical significance was evaluated with Student’s t-test.
Phenotypic analysis of LRI
For phenotypic analyses of FC and LRI, 10–20 roots of 5-day-old seedlings were analysed. The counting process was conducted in the direction from the root tip towards the root base. GFP settings were used to identify and count DR5rev::GFP-positive signals in the pericycle. GFP signals accompanied with nuclear divisions were scored as LRI, whereas FCs were counted when no cell divisions were observed.
For stage distribution of LR development, 15–20 roots from 7-day-old seedlings were processed. The LRP density was analysed as described (Malamy and Benfey, 1997). Root growth parameters (root length) were analysed with the ImageJ software (NIH; http://rsb.info.nih.gov/ij).
For all experiments, independent experiments were performed in triplicate with the same results, and representative images are shown. The statistical significance was evaluated with Student’s t-test.
Supplementary Material
Acknowledgments
We thank Agnieszka Bielach, Gieljan De Rop and Jerome Duclerq for technical assistance; Joseph Dubrovsky and Niko Geldner for discussions; and Martine De Cock and Annick Bleys for help in preparing the manuscript. This work was supported by the ERC starting independent research grant ERC-2007-Stg-207362-HCPO (PM and EB). MV is a postdoctoral fellow of the Research Foundation-Flanders. BDR was supported by a pre-doctoral grant provided by the Special Research Fund of Ghent University, MB was funded by a BBSRC IPA grant and both MB and TB were supported by the Interuniversity Attraction Poles Programme (IUAP VI/33), initiated by the Belgian State, Science Policy Office.
Author contributions: PM contributed to the acquisition of the data and analysis/interpretation of data. MV contributed to the study design, acquisition of the data, analysis/interpretation of data, and writing the manuscript. BDR contributed to the construction of the plasmids and assisted in critical revising of the manuscript. ZD contributed to the generation of plant material. MB and TB assisted in critical revising of the manuscript. EB contributed to the study design, data analysis/interpretation, and writing the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Beeckman T, Burssens S, Inzé D (2001) The peri-cell-cycle in Arabidopsis. J Exp Bot 52: 403–411 [DOI] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44 [DOI] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, Bennett M (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13: 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rybel B, Vassileva V, Parizot B, Demeulenaere M, Grunewald W, Audenaert D, Van Campenhout J, Overvoorde P, Jansen L, Vanneste S, Möller B, Wilson M, Holman T, Van Isterdael G, Brunoud G, Vuylsteke M, Vernoux T, De Veylder L, Inzé D, Weijers D et al. (2010) A novel aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr Biol 20: 1697–1706 [DOI] [PubMed] [Google Scholar]
- De Smet I, Tetsumura T, De Rybel B, Frey NFD, Laplaze L, Casimiro I, Swarup R, Naudts M, Vanneste S, Audenaert D, Inzé D, Bennett MJ, Beeckman T (2007) Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134: 681–690 [DOI] [PubMed] [Google Scholar]
- Devlin PF, Yanovsky MJ, Kay SA (2003) A genomic analysis of the shade avoidance response in Arabidopsis. Plant Physiol 133: 1617–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jürgens G, Estelle M (2005) Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell 9: 109–119 [DOI] [PubMed] [Google Scholar]
- Ding Z, Galván-Ampudia CS, Demarsy E, Łangowski Ł, Kleine-Vehn J, Fan Y, Morita MT, Tasaka M, Fankhauser C, Offringa R, Friml J (2011) Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat Cell Biol 13: 447–452 [DOI] [PubMed] [Google Scholar]
- Ditengou FA, Teale WD, Kochersperger P, Flittner KA, Kneuper I, van der Graaff E, Nziengui H, Pinosa F, Li X, Nitschke R, Laux T, Palme K (2008) Mechanical induction of lateral root initiation in Arabidopsis thaliana. Proc Natl Acad Sci USA 105: 18818–18823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B (1993) Cellular organisation of the Arabidopsis thaliana root. Development 119: 71–84 [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Doerner PW, Colón-Carmona A, Rost TL (2000) Pericycle cell proliferation and lateral root initiation in Arabidopsis. Plant Physiol 124: 1648–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky JG, Gambetta GA, Hernández-Barrera A, Shishkova S, González I (2006) Lateral root initiation in Arabidopsis: developmental window, spatial patterning, density and predictability. Ann Bot 97: 903–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, Celenza J, Benková E (2008) Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci USA 105: 8790–8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426: 147–153 [DOI] [PubMed] [Google Scholar]
- Fukaki H, Tameda S, Masuda H, Tasaka M (2002) Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J 29: 153–168 [DOI] [PubMed] [Google Scholar]
- Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM (2005) Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol 15: 1899–1911 [DOI] [PubMed] [Google Scholar]
- Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, Sena G, Hauser M-T, Benfey PN (2000) The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 101: 555–567 [DOI] [PubMed] [Google Scholar]
- Laskowski M, Grieneisen VA, Hofhuis H, ten Hove CA, Hogeweg P, Marée AFM, Scheres B (2008) Root system architecture from coupling cell shape to auxin transport. PLoS Biol 6: e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Guédon Y, Jay-Allemand C, Godin C, Laplaze L (2008) An auxin transport-based model of root branching in Arabidopsis thaliana. PLoS One 3: e3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Swarup R, Paponov IA, Swarup K, Casimiro I, Lake D, Peret B, Zappala S, Mairhofer S, Whitworth M, Wang J, Ljung K, Marchant A, Sandberg G, Holdsworth MJ, Palme K, Pridmore T, Mooney S, Bennett MJ (2011) SHORT-ROOT regulates primary, lateral, and adventitious root development in Arabidopsis. Plant Physiol 155: 384–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44 [DOI] [PubMed] [Google Scholar]
- Marhavý P, Bielach A, Abas L, Abuzeineh A, Duclercq J, Tanaka H, Pařezová M, Petrášek J, Friml J, Kleine-Vehn J, Benková E (2011) Cytokinin modulates endocytic trafficking of PIN1 auxin efflux carrier to control plant organogenesis. Dev Cell 21: 796–804 [DOI] [PubMed] [Google Scholar]
- Moreno-Risueno MA, Van Norman JM, Moreno A, Zhang J, Ahnert SE, Benfey PN (2010) Oscillating. gene expression determines competence for periodic Arabidopsis root branching. Science 329: 1306–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paponov IA, Teale WD, Trebar M, Blilou I, Palme K (2005) The PIN auxin efflux facilitators: evolutionary and functional perspectives. Trends Plant Sci 10: 170–177 [DOI] [PubMed] [Google Scholar]
- Péret B, Swarup K, Ferguson A, Seth M, Yang Y, Dhondt S, James N, Casimiro I, Perry P, Syed A, Yang H, Reemmer J, Venison E, Howells C, Perez-Amador MA, Yun J, Alonso J, GTS Beemster, Laplaze L, Murphy A et al. (2012) AUX/LAX genes encode a family of auxin influx transporters that perform distinct functions during Arabidopsis development. Plant Cell 24: 2874–2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakusová H, Gallego-Bartolomé J, Vanstraelen M, Robert HS, Alabadí D, Blázquez MA, Benková E, Friml J (2011) Polarization of PIN3-dependent auxin transport for hypocotyl gravitropic response in Arabidopsis thaliana. Plant J 67: 817–826 [DOI] [PubMed] [Google Scholar]
- Roppolo D, De Rybel B, Tendon VD, Pfister A, Alassimone J, Vermeer JEM, Yamazaki M, Stierhof Y-D, Beeckman T, Geldner N (2011) A novel protein family mediates Casparian strip formation in the endodermis. Nature 473: 380–383 [DOI] [PubMed] [Google Scholar]
- Swarup K, Benková E, Swarup R, Casimiro I, Péret B, Yang Y, Parry G, Nielsen E, De Smet I, Vanneste S, Levesque MP, Carrier D, James N, Calvo V, Ljung K, Kramer E, Roberts R, Graham N, Marillonnet S, Patel K et al. (2008) The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol 10: 946–954 [DOI] [PubMed] [Google Scholar]
- Vanneste S, De Rybel B, Beemster GTS, Ljung K, De Smet I, Van Isterdael G, Naudts M, Iida R, Gruissem W, Tasaka M, Inzé D, Fukaki H, Beeckman T (2005) Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. Plant Cell 17: 3035–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieten A, Vanneste S, Wiśniewska J, Benková E, Benjamins R, Beeckman T, Luschnig C, Friml J (2005) Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 132: 4521–4531 [DOI] [PubMed] [Google Scholar]
- Wiśniewska J, Xu J, Seifertová D, Brewer PB, Růžička K, Blilou I, Rouquié D, Benková E, Scheres B, Friml J (2006) Polar PIN localization directs auxin flow in plants. Science 312: 883. [DOI] [PubMed] [Google Scholar]
- Xu J, Scheres B (2005) Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epidermal cell polarity. Plant Cell 17: 525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žádníková P, Petrášek J, Marhavý P, Raz V, Vandenbussche F, Ding Z, Schwarzerová K, Morita MT, Tasaka M, Hejátko J, Van Der Straeten D, Friml J, Benková E (2010) Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana. Development 137: 607–617 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


