Abstract
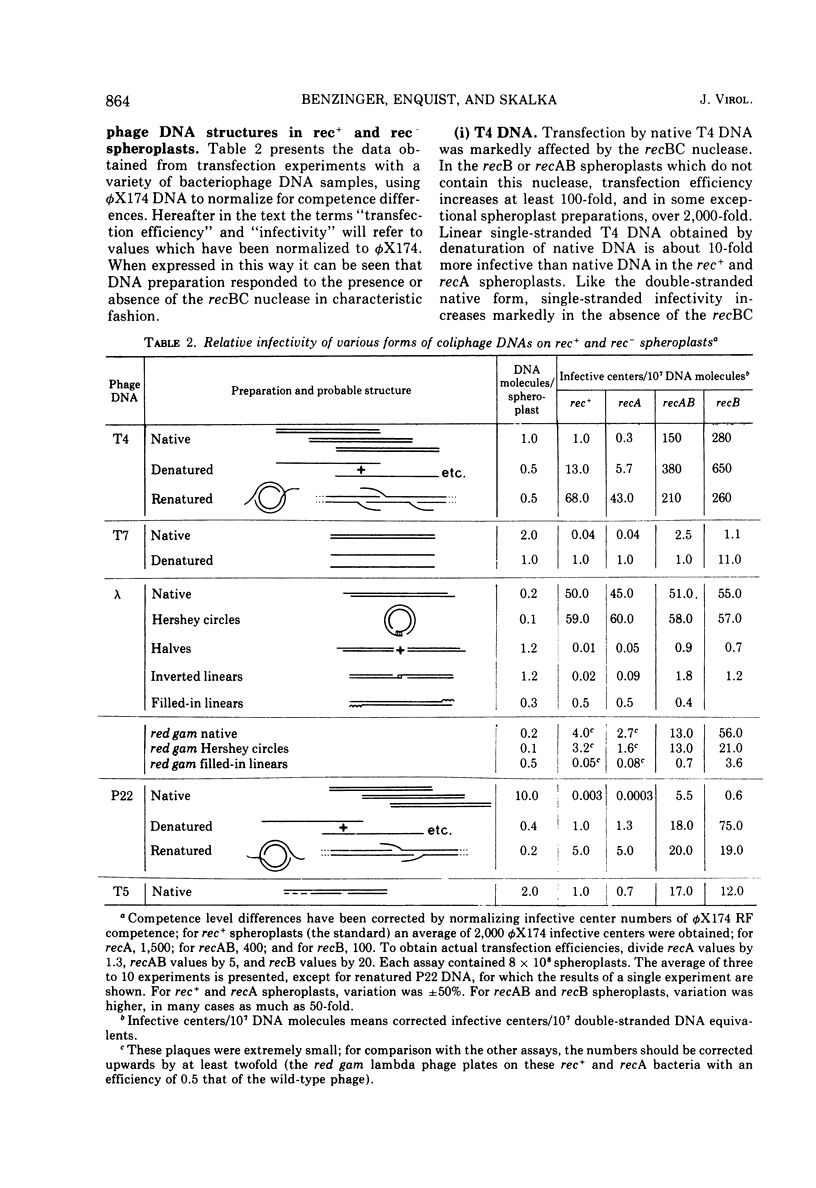
The in vivo activity of the recBC nuclease was assayed by transfection of isogenic rec+ and rec minus spheroplasts with bacteriophage DNA of various origin and structure. The results indicate that the recBC nuclease can limit transfection at several stages during the production of an infective center; such limitations depend primarily on whether the DNA is in, or assumes, a nuclease-sensitive structure. The first stage of limitation can occur when a nuclease-sensitive transfecting molecule enters the spheroplast. Other potential limitation points occur during replication and maturation of the bacteriophage DNA. The initial stage can be bypassed by using recBC nuclease-resistant molecules such as circular forms. Through analysis of results with other DNA structures, we found that in vivo the effects of the double-strand exonucleolytic activity of the recBC nuclease predominated. The effects of the single-strand nuclease activities seem to be modified from those observed for the purified enzyme in vitro (Karu et al., 1974). Inside the cell, the single-strand exonuclease activity is very weak and the single-strand endonuclease activity is abolished almost completely.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benzinger R., Kleber I., Huskey R. Transfection of Escherichia coli spheroplasts. I. General facilitation of double-stranded deoxyribonucleic acid infectivity by protamine sulfate. J Virol. 1971 May;7(5):646–650. doi: 10.1128/jvi.7.5.646-650.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzinger R., Kleber I. Transfection of Escherichia coli and Salmonella typhimurium spheroplasts: host-controlled restriction of infective bacteriophage P22 deoxyribonucleic acid. J Virol. 1971 Aug;8(2):197–202. doi: 10.1128/jvi.8.2.197-202.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzinger R., Scheible P. Transfection of Escherichia coli spheroplasts. IV. Transfection of rec+ and rec minus spheroplasts by native, denatured, and renatured T5 bacteriophage DNA after repair of single-strand breaks by polynucleotide ligase. J Virol. 1974 May;13(5):960–966. doi: 10.1128/jvi.13.5.960-966.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein H., Bernstein C. Circular and branched circular concatenates as possible intermediates in bacteriophage T4 DNA replication. J Mol Biol. 1973 Jul 5;77(3):355–361. doi: 10.1016/0022-2836(73)90443-9. [DOI] [PubMed] [Google Scholar]
- Boling M. E., Setlow J. K., Allison D. P. Bacteriophage of Haemophilus influenzae. I. Differences between infection by whole phage, extracted phage DNA and prophage DNA extracted from lysogenic cells. J Mol Biol. 1972 Feb 14;63(3):335–348. doi: 10.1016/0022-2836(72)90431-7. [DOI] [PubMed] [Google Scholar]
- Botstein D., Herskowitz I. Properties of hybrids between Salmonella phage P22 and coliphage lambda. Nature. 1974 Oct 18;251(5476):584–589. doi: 10.1038/251584a0. [DOI] [PubMed] [Google Scholar]
- Capaldo F. N., Ramsey G., Barbour S. D. Analysis of the growth of recombination-deficient strains of Escherichia coli K-12. J Bacteriol. 1974 Apr;118(1):242–249. doi: 10.1128/jb.118.1.242-249.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- Cohen G., Zimmer Z. Transfection of Escherichia coli by bacteriophage P1 DNA. Mol Gen Genet. 1974;128(2):183–186. doi: 10.1007/BF02654490. [DOI] [PubMed] [Google Scholar]
- Cosloy S. D., Oishi M. Genetic transformation in Escherichia coli K12. Proc Natl Acad Sci U S A. 1973 Jan;70(1):84–87. doi: 10.1073/pnas.70.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosloy S. D., Oishi M. The nature of the transformation process in Escherichia coli K12. Mol Gen Genet. 1973 Jul 31;124(1):1–10. doi: 10.1007/BF00267159. [DOI] [PubMed] [Google Scholar]
- Dove W. F., Weigle J. J. Intracellular state of the chromosome of bacteriophage lambda. I. The eclipse of infectivity of the bacteriophage DNA. J Mol Biol. 1965 Jul;12(3):620–629. doi: 10.1016/s0022-2836(65)80316-3. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Skalka A. Replication of bacteriophage lambda DNA dependent on the function of host and viral genes. I. Interaction of red, gam and rec. J Mol Biol. 1973 Apr 5;75(2):185–212. doi: 10.1016/0022-2836(73)90016-8. [DOI] [PubMed] [Google Scholar]
- GREEN D. M. INFECTIVITY OF DNA ISOLATED FROM BACILLUS SUBTILIS BACTERIOPHAGE, SP82. J Mol Biol. 1964 Dec;10:438–451. doi: 10.1016/s0022-2836(64)80065-6. [DOI] [PubMed] [Google Scholar]
- Goldmark P. J., Linn S. An endonuclease activity from Escherichia coli absent from certain rec- strains. Proc Natl Acad Sci U S A. 1970 Sep;67(1):434–441. doi: 10.1073/pnas.67.1.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmark P. J., Linn S. Purification and properties of the recBC DNase of Escherichia coli K-12. J Biol Chem. 1972 Mar 25;247(6):1849–1860. [PubMed] [Google Scholar]
- Goodgal S. H., Gromkova R. Separation of specific segments of transforming DNA after treatment with endodeoxyribonuclease. Proc Natl Acad Sci U S A. 1973 Feb;70(2):503–506. doi: 10.1073/pnas.70.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgpeth J., Goodman H. M., Boyer H. W. DNA nucleotide sequence restricted by the RI endonuclease. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3448–3452. doi: 10.1073/pnas.69.11.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henner W. D., Kleber I., Benzinger R. Transfection of Escherichia coli spheroplasts. 3. Facilitation of transfection and stabilization of spheroplasts by different basic polymers. J Virol. 1973 Oct;12(4):741–747. doi: 10.1128/jvi.12.4.741-747.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hout A., van de Putte P., Schuite A., Jonge A. J., Oosterbaan R. A. Interference between the rec A product and an ATP-dependent exonuclease in extracts of E. coli. Biochim Biophys Acta. 1970 Nov 12;224(1):285–287. doi: 10.1016/0005-2787(70)90649-0. [DOI] [PubMed] [Google Scholar]
- Karu A. E., MacKay V., Goldmark P. J., Linn S. The recBC deoxyribonuclease of Escherichia coli K-12. Substrate specificity and reaction intermediates. J Biol Chem. 1973 Jul 25;248(14):4874–4884. [PubMed] [Google Scholar]
- Kushner S. R. Differential thermolability of exonuclease and endonuclease activities of the recBC nuclease isolated from thermosensitive recB and recC mutants. J Bacteriol. 1974 Dec;120(3):1219–1222. doi: 10.1128/jb.120.3.1219-1222.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner S. R. In vivo studies of temperature-sensitive recB and recC mutants. J Bacteriol. 1974 Dec;120(3):1213–1218. doi: 10.1128/jb.120.3.1213-1218.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawhorne L., Kleber I., Mitchell C., Benzinger R. Transfection of Escherichia coli spheroplasts. II. Relative infectivity of native, denatured, and renatured lambda, T7, T5, T4, and P22 bacteriophage DNAs. J Virol. 1973 Oct;12(4):733–740. doi: 10.1128/jvi.12.4.733-740.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Nobrega F. G., Rola F. H., Pasetto-Nobrega M., Oishi M. Adenosine triphosphatase associated with adenosine triphosphate-dependent deoxyribonuclease (recB-recC enzyme-E. coli-ATP to phosphodiester hydrolysis ratio-DNA-dependent ATPase activity). Proc Natl Acad Sci U S A. 1972 Jan;69(1):15–19. doi: 10.1073/pnas.69.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M. An ATP-dependent deoxyribonuclease from Escherichia coli with a possible role in genetic recombination. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1292–1299. doi: 10.1073/pnas.64.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M., Cosloy S. D. The genetic and biochemical basis of the transformability of Escherichia coli K12. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1568–1572. doi: 10.1016/0006-291x(72)90520-7. [DOI] [PubMed] [Google Scholar]
- Pilarksi L. M., Egan J. B. Role of DNA topology in transcription of coliphage lambda in vivo. II. DNA topology protects the template from exonuclease attack. J Mol Biol. 1973 May 15;76(2):257–266. doi: 10.1016/0022-2836(73)90389-6. [DOI] [PubMed] [Google Scholar]
- Sakaki Y. Inactivation of the ATP-dependent DNase of Escherichia coli after infection with double-stranded DNA phages. J Virol. 1974 Dec;14(6):1611–1612. doi: 10.1128/jvi.14.6.1611-1612.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R. W., Ray D. S. Polynucleotide ligase and phiX174 single strand synthesis. Nat New Biol. 1971 Jun 9;231(23):170–173. doi: 10.1038/newbio231170a0. [DOI] [PubMed] [Google Scholar]
- Sigal N., Delius H., Kornberg T., Gefter M. L., Alberts B. A DNA-unwinding protein isolated from Escherichia coli: its interaction with DNA and with DNA polymerases. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3537–3541. doi: 10.1073/pnas.69.12.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmon V. F., Lederberg S. Degradation of bacteriophage lambda deoxyribonucleic acid after restriction by Escherichia coli K-12. J Bacteriol. 1972 Oct;112(1):161–169. doi: 10.1128/jb.112.1.161-169.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. A., Jr The genetic organization of chromosomes. Annu Rev Genet. 1971;5:237–256. doi: 10.1146/annurev.ge.05.120171.001321. [DOI] [PubMed] [Google Scholar]
- Unger R. C., Clark A. J. Interaction of the recombination pathways of bacteriophage lambda and its host Escherichia coli K12: effects on exonuclease V activity. J Mol Biol. 1972 Oct 14;70(3):539–548. doi: 10.1016/0022-2836(72)90558-x. [DOI] [PubMed] [Google Scholar]
- Unger R. C., Echols H., Clark A. J. Interaction of the recombination pathways of bacteriophage lambda and host Escherichia coli: effects on lambda recombination. J Mol Biol. 1972 Oct 14;70(3):531–537. doi: 10.1016/0022-2836(72)90557-8. [DOI] [PubMed] [Google Scholar]
- Wackernagel W. An improved spheroplast assay for lambda-DNA and the influence of the bacterial genotype on the transfection rate. Virology. 1972 Apr;48(1):94–103. doi: 10.1016/0042-6822(72)90117-1. [DOI] [PubMed] [Google Scholar]
- Wackernagel W. Genetic transformation in E. coli: the inhibitory role of the recBC DNase. Biochem Biophys Res Commun. 1973 Mar 17;51(2):306–311. doi: 10.1016/0006-291x(73)91257-6. [DOI] [PubMed] [Google Scholar]
- Wackernagel W., Hermanns U. Inhibition of exonuclease V after infection of E. coli by bacteriophage T7. Biochem Biophys Res Commun. 1974 Sep 23;60(2):521–527. doi: 10.1016/0006-291x(74)90271-x. [DOI] [PubMed] [Google Scholar]
- Wackernagel W., Radding C. M. Transfection by half molecules and inverted molecules of lambda DNA: requirement for exo and -promoted recombination. Virology. 1973 Apr;52(2):425–432. doi: 10.1016/0042-6822(73)90337-1. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Davidson N. Thermodynamic and kinetic studies on the interconversion between the linear and circular forms of phage lambda DNA. J Mol Biol. 1966 Jan;15(1):111–123. doi: 10.1016/s0022-2836(66)80213-9. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J. Characteristics of some multiply recombination-deficient strains of Escherichia coli. J Bacteriol. 1969 Oct;100(1):231–239. doi: 10.1128/jb.100.1.231-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J., Dressler D., Magazin M. Bacteriophage T7 DNA replication: a linear replicating intermediate (gradient centrifugation-electron microscopy-E. coli-DNA partial denaturation). Proc Natl Acad Sci U S A. 1972 Feb;69(2):499–504. doi: 10.1073/pnas.69.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M., Buttin G., Hurwitz J. The isolation and characterization from Escherichia coli of an adenosine triphosphate-dependent deoxyribonuclease directed by rec B, C genes. J Biol Chem. 1971 Nov;246(21):6543–6555. [PubMed] [Google Scholar]
- van Dorp B., Ceulen M. T., Heijneker H. L., Pouwels P. H. Properties of an ATP-dependent deoxyribonuclease from Micrococcus luteus. Evidence for a stable DNA-enzyme complex. Biochim Biophys Acta. 1973 Feb 23;299(1):65–81. doi: 10.1016/0005-2787(73)90398-5. [DOI] [PubMed] [Google Scholar]



