Abstract
Cell 151 6: 1185–1199 doi:; DOI: 10.1016/j.cell.2012.10.047; published online December 07 2012
Many cancer cells produce energy by increasing aerobic glycolysis instead of oxidative metabolism. SIRT6, a member of the sirtuins NAD+-dependent deacetylase family, has been shown to be involved in multiple cellular processes including metabolism and ageing. In a recent study, Sebastian et al (2012) demonstrate a role of SIRT6 as a tumour suppressor that modulates aerobic glycolysis and inhibits ribosome biogenesis by repressing MYC transcriptional activity.
Cancer cells, in contrast to normal cells, generate the energy needed for their cellular processes by increasing aerobic glycolysis, a phenomenon termed ‘the Warburg effect’ (Vander Heiden et al, 2009).
Sirtuins, a family of NAD+-dependent deacetylases, have been linked to longevity in a variety of model organisms. These enzymes modulate a large set of biological processes, including cell survival, development, chromatin dynamics, DNA repair, metabolism and other phenomena (Houtkooper et al, 2012). Of particular interest is one member of this family, SIRT6, which is involved in regulation of mammalian ageing and metabolism (Kanfi et al, 2012).
SIRT6 regulates many cancer-related pathways, including genome stability, by modulating DNA double-strand break, base excision repair (BER) and inflammation via repression of NFkB and telomere maintenance (Kawahara et al, 2009; Jia et al, 2012). It was thus suggested that SIRT6 may play a major role in tumour development. In their recent study, Sebastián et al (2012) provide the first direct demonstration of SIRT6 function in tumorigenesis. The authors demonstrate that SIRT6 acts as tumour suppressor by modulating the metabolism of cancer cells. An initial indication of the relationship between SIRT6 and glucose metabolism was provided by Mostoslavsky et al (2006), who showed that SIRT6-deficient mice display severe metabolic abnormalities, including loss of subcutaneous fat, lymphopenia and acute hypoglycemia, which leads to death of the mice before they reach 1 month of age.
The severe hypoglycemia of SIRT6−/− mice was later explained by Zhong et al (2010), who demonstrated that SIRT6 regulates glucose homoeostasis via inhibiting multiple glycolytic genes, including the glucose transporter Glut1, by promoter binding and -deacetylation. This allows mitochondrial oxidative phosphorylation—but not glycolysis—to generate efficient ATP production. Accordingly, loss of SIRT6 increases glycolysis and diminishes mitochondrial respiration.
To demonstrate the involvement of SIRT6 in skewing cancer metabolism, the authors performed several key experiments. Injection of wild-type (WT) MEFs into immunodeficient mice, in the absence of oncogene activation, does not generate tumours. However, Sebastian et al showed that SIRT6-knockout (KO) MEFs formed tumours (even without oncogene activation). Moreover, re-expression of SIRT6 in KO MEFs completely abolished tumour formation. These results suggested that SIRT6 is a bona fide tumour suppressor. However, the mechanism(s) by which SIRT6 mediates its tumour suppressor activity remain elusive. Interestingly, immortalized SIRT6 KO MEFs exhibit increased glucose uptake and lactate production as well as higher levels of glycolytic enzymes. Based on these observations, the authors suggested that SIRT6 blocks the transition towards aerobic glycolysis. They further provide in vivo support for such a model by reporting increased colorectal adenomatosis in Apc−/− mice carrying a specific intestinal deletion of SIRT6.
Like other landmark research, this study paves the way for addressing additional interesting questions.
First, it is not clear whether the increased Warburg effect due to SIRT6 deficiency precedes or follows the initiation of tumour development. The authors provide intriguing evidence that the SIRT6-deficient cells form tumours regardless of oncogene activation. They also described a reduction in SIRT6 expression in various tumours, mostly in pancreatic and colorectal cancers. However, whether a mutation or deletion in SIRT6 is the primary and direct cause of tumour onset remains unknown.
Second, the transcription factor MYC is a key regulator of ribosome biogenesis. Interestingly, Sebastian et al, demonstrate that SIRT6 functions as a regulator of ribosome metabolism by co-repressing MYC transcriptional activity. In line with this observation, a previous study (Kanfi et al, 2008) showed that SIRT6 levels increased upon nutrient deprivation in cultured cells and in rats fed with a calorie-restricted diet. Therefore, it would be highly informative to establish a role for SIRT6 in repressing translation under conditions of limited energy sources. Further, it would be interesting to follow the tumour-suppressive function of SIRT6 in the context of reduced tumorigenesis as described during calorie restriction (Weindruch, 1992).
Third, SIRT6 regulates many pathways that can contribute to its tumour-suppressive effect. Loss of function mutation in SIRT6 is expected to result in increased chromosomal translocations and decreased genome stability; both mechanisms have been linked to tumour formation. In addition, mutation in SIRT6 might contribute to tumorigenesis via its anti-inflammatory role. In addition, a recent study by Bauer et al (2012) indicates that SIRT6 promotes cell migration in pancreatic cancer cells by enhancing Ca+2 responses. Therefore, mutation in SIRT6 might also have antitumour effects. It would thus be interesting to dissect the contribution of each of these mechanisms to the role of SIRT6 as tumour suppressor.
Finally, cancer is an age-related disease; therefore, one of the most puzzling questions is the relationship between the tumour-suppressive role of SIRT6 and its promotion of longevity. Male SIRT6-overexpressing mice (MOSES mice) have increased life span and improved metabolic parameters (Kanfi et al, 2012). The findings of Sebastian et al showing the tumour suppressor potential of SIRT6 together with those of Min et al (2012) who showed that increasing the level of SIRT6 markedly impairs cancer development suggest that the increased longevity may be (in part) due to reduced cancer incidence. Although Kanfi et al did not find a significant change in the number of tumours between WT and MOSES mice, it did appear that tumour onset was delayed in MOSES mice and that reduced cancer incidence might contribute to the lifespan extension. Thus, further research is required to establish the contribution of SIRT6 tumour suppressor activity to longevity.
The study of Sebastian et al provides solid evidence for the role of SIRT6 as a tumour suppressor. This adds to the other known functions of SIRT6 that might also operate in the regulation of tumorigenesis (Figure 1). However, to date, no known mutation in SIRT6 has been linked to enhanced cancer development. Therefore, as seen in other tumour suppressors like p53, the activity or the protein levels of SIRT6 might be downregulated during the process of tumorigenesis. The identity of such potential regulators of SIRT6 levels and activity seems an interesting road for future exploration.
Figure 1.
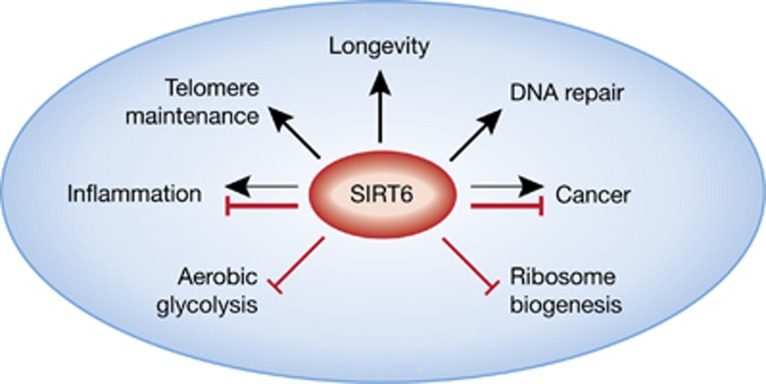
Regulation of multiple pathways by SIRT6.
References
- Bauer I, Grozio A, Lasiglie D, Basile G, Sturla L, Magnone M, Sociali G, Soncini D, Caffa I, Poggi A, Zoppoli G, Cea M, Feldmann G, Mostoslavsky R, Ballestrero A, Patrone F, Bruzzone S, Nencioni A (2012) The NAD+-dependent histone deacetylase SIRT6 promotes cytokine production and migration in pancreatic cancer cells by regulating Ca2+ responses. J Biol Chem 288: 40924–40937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Pirinen E, Auwerx J (2012) Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13: 225–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G, Su L, Singhal S, Liu X (2012) Emerging roles of SIRT6 on telomere maintenance, DNA repair, metabolism and mammalian aging. Mol Cell Biochem 364: 345–350 [DOI] [PubMed] [Google Scholar]
- Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY (2012) The sirtuin SIRT6 regulates lifespan in male mice. Nature 483: 218–221 [DOI] [PubMed] [Google Scholar]
- Kanfi Y, Shalman R, Peshti V, Pilosof SN, Gozlan YM, Pearson KJ, Lerrer B, Moazed D, Marine JC, de Cabo R, Cohen HY (2008) Regulation of SIRT6 protein levels by nutrient availability. FEBS Lett 582: 543–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang HY, Chua KF (2009) SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell 136: 62–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min L, Ji Y, Bakiri L, Qiu Z, Cen J, Chen X, Chen L, Scheuch H, Zheng H, Qin L, Zatloukal K, Hui L, Wagner EF (2012) Liver cancer initiation is controlled by AP-1 through SIRT6-dependent inhibition of survivin. Nat Cell Biol 14: 1203–1211 [DOI] [PubMed] [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D et al. (2006) Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124: 315–329 [DOI] [PubMed] [Google Scholar]
- Sebastián C, Zwaans BMM, Silberman DM, Gymrek M, Goren A, Zhong L, Ram O, Truelove J, Guimaraes AR, Toiber D, Cosentino C, Greenson JK, Mac Donald A, McGlynn L, Maxwell F, Edwards J, Giacosa S, Guccione E, Weissleder R, Bernstein BE et al. (2012) The histone deacetylase sirt6 is a novel tumor suppressor that controls cancer metabolism. Cell 151: 1185–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R (1992) Effect of caloric restriction on age-associated cancers. Exp Gerontol 27: 575–581 [DOI] [PubMed] [Google Scholar]
- Zhong L, D'Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T, Clish CB, Vaitheesvaran B, Iliopoulos O, Kurland I, Dor Y, Weissleder R, Shirihai OS, Ellisen LW, Espinosa JM, Mostoslavsky R (2010) The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 140: 280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]


