Abstract
HangAmDan-B (HAD-B) is a powdered mixture of eight ethnopharmacologically characterized folk medicines that is prescribed for solid masses and cancers in Korea. In view of the finding that macrophage-mediated inflammation is a pathophysiologically important phenomenon, we investigated whether HAD-B modulates inflammatory responses and explored the associated molecular mechanisms. The immunomodulatory activity of HAD-B in toll-like receptor-activated macrophages induced by lipopolysaccharide (LPS) was assessed by measuring nitric oxide (NO) and prostaglandin E2 (PGE2) levels. To identify the specific transcription factors (such as nuclear factor [NF]–κB and signaling enzymes) targeted by HAD-B, biochemical approaches, including kinase assays and immunoblot analysis, were additionally employed. HAD-B suppressed the production of PGE2 and NO in LPS-activated macrophages in a dose-dependent manner. Furthermore, the extract ameliorated HCl/EtOH-induced gastritis symptoms. Moreover, HAD-B significantly inhibited LPS-induced mRNA expression of inducible NO synthase and cyclooxygenase (COX)-2. Interestingly, marked inhibition of NF-κB and activating transcription factor was observed in the presence of HAD-B. Data from direct kinase assays and immunoblot analysis showed that HAD-B suppresses activation of the upstream signaling cascade involving spleen tyrosine kinase, Src, p38, c-Jun N-terminal kinase, and transforming growth factor β–activated kinase 1. Finally, kaempferol, but not quercetin or resveratrol was identified as a bioactive compound in HAD-B. Therefore, our results suggest that HAD-B possesses anti-inflammatory activity that contributes to its anticancer property.
Key Words: ATF-2, HangAmDan-B, inflammation, macrophages, NF-κB, p38, Syk
Introduction
Inflammation is a natural mode of defense against foreign materials in the human body. However, inflammatory responses occasionally trigger a serious condition leading to diseases, such as cancer, diabetes, atherosclerosis, and arthritis.1 Such inflammatory events are mostly managed by macrophages. These cells overproduce numerous soluble factors, including pro-inflammatory cytokines (tumor necrosis factor [TNF]-α and interleukin [IL]-1] and inflammatory molecules (nitric oxide [NO] and prostaglandins [PG]), that contribute to the onset of inflammatory responses and disease.2 Considerable efforts to date have facilitated our understanding of the molecular inflammatory events by which bacterial or viral products trigger molecular interactions between immunogens, pattern recognition receptors (e.g., toll-like receptor [TLR]) and their adaptor molecules (e.g., TIR-domain–containing adapter-inducing interferon-β [TRIF] and myeloid differentiation primary response gene (88) [MyD88]). For instance, lipopolysaccharide (LPS)/TLR4 interactions activate protein kinases, such as Src, spleen tyrosine kinase (Syk), phosphoinositide 3-kinase (PI3K), and Akt (protein kinase B), as well as mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38. The signaling cascades are eventually linked to the activation of inflammation-regulating transcription factors, such as nuclear factor (NF)–κB, activating transcription factor (ATF)–2, and activator protein (AP)–1.3,4 Targeting of specific signaling events or steps in the inflammatory response pathway is an important strategy for the development of anti-inflammatory drugs and treatment of inflammation-mediated diseases, such as cancer and atherosclerosis.5
HangAmDan-B (HAD-B), consisting of Radix Panax notoginseng, Cordyceps militaris, Cremastra appendiculata, Radix Panax ginseng, calculus bovis, Ipomoea batatas ‘Margarita’, Boswellia carteri, and Commiphora myrrha, is an upgraded version of HAD used traditionally for solid masses, as discussed in the classical texts “Xi Huang Wan” and “Wai Ke Zheng Zhi Quan Sheng Ji.”6–11 A mixture of these plants has been shown to exert strong anticancer activity against solid tumors, including pancreatic, lung, colorectal, and stomach cancers.12 Additionally, antiangiogenesis effects and inhibition of cancer cell proliferation and metastasis have been reported.13 In particular, case reports observed with HAD have been selected as part of the National Cancer Institute's Best Case Series Program.14 HAD-B has shown efficacy in inhibiting migration and proliferation of human umbilical vein endothelial cells and limiting the formation of capillary tube structures.15 Furthermore, safety evaluations of HAD-B have revealed no side-effects in either healthy subjects or cancer patients.16
In view of the finding that inflammation is a basic pathophysiological phenomenon in tumorigenic responses to specific cancer cells and tissues, an anti-inflammatory approach has been suggested for cancer therapy.17,18 In particular, pro-inflammatory roles of macrophages are critical in cancer cell survival, proliferation, migration, and metastasis, and thus, regulation of macrophage functions has been considered as a therapeutic strategy.19 In this respect, it is important to determine whether HAD-B displays modulatory activity against macrophage-mediated inflammatory responses. To address this issue, we investigated the anti-inflammatory effect of HAD-B on activated macrophages induced by the TLR4 ligand, LPS, by measuring the levels of NO and PGE2, and examining the molecular events involved in LPS-mediated inflammatory responses.
Materials and Methods
Materials
Ranitidine, sodium carboxylmethylcellulose (CMC), and LPS was obtained from Sigma Chemical Co. (St. Louis, MO, USA). SB203580, SP600125, BAY61-3606, and piceatannol were purchased from Calbiochem (La Jolla, CA, USA), and RAW264.7 cells from ATCC (Rockville, MD, USA). All other chemicals were of reagent grade. Anti-phospho or total antibodies against Src, Syk, ERK, p38, JNK, transforming growth factor β–activated kinase 1 (TAK1), mitogen-activated protein (MAP) kinase kinase (MKK)3/6, MKK4, p65 and p50 (NF-κB), ATF-2, p85/PI3K, inhibitors of κBα (IκBα), IκBα kinase (IKK), AP-1 (c-Jun, c-Fos, and Fra-1), β-actin, and γ-tubulin were obtained from Cell Signaling (Beverly, MA, USA).
Preparation of HAD-B
HAD-B (Dunsan Oriental Hospital, Daejeon, Korea), an herbal formula consisting of Radix P. notoginseng, C. militaris, C. appendiculata, Radix P. ginseng, calculus bovis, I. batatas ‘Margarita’, B. carteri, and C. myrrha in powder form, was prepared as reported previously12,13,16 and stored at −20°C until use.
Mice
Six-week-old male ICR mice were purchased from B&K (Fremont, CA, USA). Mice were given food pellets (Samyang, Daejeon, Korea) and water ad libitum under a 12-h light–12-h dark cycle. Studies were performed in accordance with guidelines established by the Sungkyunkwan University Institutional Animal Care and Use Committee.
Cell culture
RAW264.7 cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (Gibco BRL, Grand Island, NY, USA), glutamine, and antibiotics (penicillin and streptomycin) at 37°C with 5% CO2. For each experiment, cells were detached with a cell scraper. Experiments were performed with a cell density of 2×106 cells/mL. At this density, more than 99% of cells were viable, as evidenced by Trypan blue staining.20
Treatment with HAD-B
The stock solution (100 mg/mL) of HAD-B was suspended in 1 mL of dimethyl sulfoxide (DMSO) and sonicated for 6 h. After centrifugation, the DMSO soluble layer was prepared. Based on previous studies, noncytotoxic concentrations (0–200 μg/mL) of HAD-B were prepared by dilution with RPMI 1640 for in vitro experiments. For gastritis experiments, 200 mg/kg HAD-B suspended with 1% CMC solution was used for oral administration.
NO and PGE2 production
After RAW264.7 cells (1×106 cells/mL) were incubated for 18 h, cells were pretreated with HAD-B (0–200 μg/mL) for 30 min. Next, cells were stimulated with LPS and incubated for a further 24 h. The inhibitory effects of HAD-B on NO and PGE2 production were determined by analyzing their levels using Griess reagent, and enzyme immunoassay kits, respectively, as described previously.21,22
MTT assay
Cell proliferation was measured with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, as described in a previous report.23,24
Semiquantitative reverse transcription–polymerase chain reaction for mRNA detection
Total RNA from LPS-treated-RAW264.7 cells (5×106 cells/mL) was prepared using TRIzol Reagent (Gibco BRL), according to the manufacturer's protocol.25,26 Total RNA was stored at −70°C until use. Semiquantitative reverse transcription reactions were conducted using MuLV reverse transcriptase. Specifically, total RNA (1 μg) was incubated with oligo-dT15 for 5 min at 70°C and mixed with 5×first-strand buffer, 10 mM dNTPs, and 0.1 M DTT. The reaction mixture was further incubated for 5 min at 37°C and 60 min after the addition of MuLV reverse transcriptase (2 U). Reactions were terminated by heating for 10 min at 70°C. Total RNA was depleted by adding RNase H. Polymerase chain reaction (PCR) were conducted using the following conditions: 2 μL cDNA, 4 μM 5′ and 3′ primers, 10× buffer (10 mM Tris-HCl, pH 8.3, 50 mM KCl, and 0.1% Triton X-100), 250 μM dNTPs, 25 mM MgCl2, and 1 unit of Taq polymerase (Promega, Madison, WI, USA). The following conditions were used for amplification: 30 sec denaturation at 94°C, 30 sec annealing between 55 and 60°C, 45 sec extension at 72°C, and a final extension step of 5 min at 72°C. The primers (Bioneer, Daejeon, Korea) utilized in this experiment (Table 1) were used as reported previously.21,23
Table 1.
Reverse Transcription–Polymerase Chain Reaction Primers Used in This Experiment
| Name | Sequence (5′ to 3′) |
|---|---|
| iNOS | F: GGA GCC TTT AGA CCT CAA CAG A |
| R: TGA ACG AGG AGG GTG GTG | |
| TNF-α | F: TGC CTA TGT CTC AGC CTC TTC |
| R: GAG GCC ATT TGG GAA CTT CT | |
| COX-2 | F: CACTACATCCTGACCCACTT |
| R: ATGCTCCTGCTTGAGTATGT | |
| GAPDH | F: CAA TGA ATA CGG CTA CAG CAA C |
| R: AGG GAG ATG CTC AGT GTT GG |
iNOS, inducible nitric oxide synthase; TNF, tumor necrosis factor; COX, cyclooxygenase.
Total lysate and nuclear extract preparation and immunoblot analysis
Total lysates and nuclear extracts from LPS-treated RAW264.7 cells pretreated with HAD-B were prepared according to a previously published method.24,27 Immunoblot analyses of phospho- or total levels of transcription factors (p65, p50, c-Jun, c-Fos, Fra-1, and ATF-2), MAPK (ERK, p38, and JNK), MAP ERK kinase (MEK) 1/2, MKK3/6, MKK4, TAK1, IL-1 receptor-associated kinase (IRAK) 1, IκBα, IKKβ, p85/PI3K, γ-tubulin, and nonreceptor tyrosine kinases (Src and Syk) were performed according to previously published methods.25
Syk and Src kinase assays
To evaluate Syk and Src kinase inhibitory activities with purified enzymes, a kinase profiler service from Millipore was employed. Human Src or Syk (1–5 mU) was incubated with reaction buffer in a final reaction volume of 25 μL. Reactions were initiated by the addition of MgATP. After 40-min incubation at room temperature, reactions were terminated by adding 5 mL of 3% phosphoric acid solution. Reaction products (10 μL) were spotted onto a P30 filtermat, which was washed three times in 75 mM phosphoric acid and once in methanol for 5 min each before drying and scintillation counting.
EtOH/HCl-induced gastritis
Stomach inflammation was induced with EtOH/HCl, according to a published method.28,29 Fasted ICR mice were orally treated with HAD-B (200 mg/kg) or ranitidine (40 mg/kg) for 3 times (twice/day). Thirty minutes later after the last administration, 400 μL of 60% ethanol in 150 mM HCl was administered orally. Each animal was anesthetized with an overdose of urethane 1 h after the administration of necrotizing agents. The stomach was then excised and gently rinsed under running tap water. After opening the stomach along the greater curvature and spreading it out on a board, the area (in mm2) of mucosal erosive lesions was measured with a ruler and photographed. The gastric lesion after inducer alone was considered 100%.
High-performance liquid chromatography analysis
Phytochemical characteristics of HAD-B and the standard compounds resveratrol, kaempferol, and quercetin were identified by high-performance liquid chromatography (HPLC) analysis.30 The system was equipped with a model K-1001 HPLC pump, model K-2600 fast scanning spectrophotometer, and a model K-500 4-channel degasser (all from KNAUER Wellchrom, Berlin, Germany). Elution solvents were buffer A (0.1% trifluoroacetic acid [TFA] in H2O) and buffer B (0.08% TFA in 95% acetonitrile (can) + 5% H2O). The gradient step of the solvent was buffer A to buffer B per minute, and a Phenomenex Gemini C18 ODS (5 μm) column was used as reported previously.31
Statistical analysis
For statistical comparison, results were assessed using analysis of variance/Scheffe's post hoc test and Kruskal–Wallis/Mann–Whitney tests. P values<.05 were considered statistically significant. All statistical tests were carried out using the SPSS program (SPSS Inc., Chicago, IL, USA). Data were obtained from at least three independent experiments performed in triplicate, and presented as means±standard error of the mean. The other data are representative of three different experiments yielding similar results.
Results and Discussion
In view of the pathophysiological role of macrophages in tumorigenic responses and the utility of HAD-B as an ethnopharmacological medicine for cancer,14,17 we further investigated whether the formulation decreases macrophage-mediated inflammatory responses. Accordingly, the anti-inflammatory activity of HAD-B was examined using TLR-activated macrophages and molecular targets.
HAD-B blocked the production of NO (Fig. 1A) and PGE2 (Fig. 1B) from RAW264.7 cells under LPS stimulation in a dose-dependent manner. This inhibition by HAD-B does not appear to be due to its nonspecific suppressive effect, since no dramatic decrease in cell viability was observed upon treatment with HAD-B up to a concentration of 200 μg/mL (Fig. 1C). Analysis of mRNA levels (Fig. 1D) of inducible NO synthase (iNOS), a NO-producing enzyme, and cyclooxygenase (COX)-2, a PGE2-producing enzyme, revealed HAD-B-mediated inhibition of NO and PGE2 production at the transcriptional level. The inhibitory activity of HAD-B was also confirmed in vivo using a gastritis model. Thus, this extract (200 mg/kg) strongly ameliorated EtOH/HCl-induced gastric lesions similarly to the standard compound ranitidine (Fig. 1E), confirming HAD-B effectiveness both in vitro and in vivo.
FIG. 1.
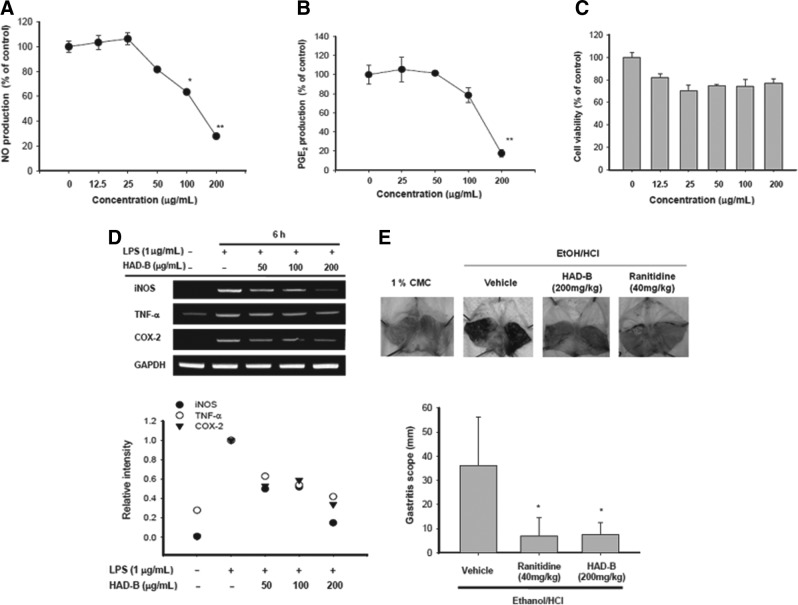
Effect of HAD-B on inflammatory responses in vitro. (A, B) To examine the anti-inflammatory effects in vitro, NO and PGE2 levels in culture supernatants prepared from LPS-activated RAW264.7 cells pretreated with HAD-B were determined with Griess reagent and EIA. (C) The viability of RAW264.7 cells pretreated with HAD-B was determined with the MTT assay. (D) iNOS, TNF-α, and COX-2 mRNA levels from LPS-activated RAW264.7 cells pretreated with HAD-B were determined using semiquantitative RT–polymerase chain reaction. (E) Mice orally administered with HAD-B for 3 times (twice/day) were orally treated with EtOH/HCl. After 1 h of fourth treatment, gastric lesions in the stomach were measured with a ruler (bottom) and photos of these were taken by a camera (top). The gastric lesion after inducer alone is represented by 100%. Relative intensity of bands was measured using densitometry scanning. *P<.05 and **P<.01, compared to the control group. HAD-B, HangAmDan-B; NO, nitric oxide; PGE2, prostaglandin E2; LPS, lipopolysaccharide; iNOS, inducible NO synthase; TNF, tumor necrosis factor; COX, cyclooxygenase; EIA, enzyme immunoassay.
Since LPS-induced macrophage activation is mainly regulated by inflammatory responses controlled by NF-κB, AP-1, and ATF-2,32 we initially examined whether nuclear translocation of these transcription factors is blocked upon HAD-B exposure by measuring their nuclear levels. Interestingly, the extract strongly suppressed the nuclear level of p65, a subunit of NF-κB,33 but not that of p50, at 15 and 30 min (Fig. 2A), implying that blockade of NF-κB activation is one of the major inhibitory pathways. Furthermore, HAD-B inhibited phosphorylation of ATF-2, but not AP-1 (c-Jun, Fra-1, and c-Fos), indicating that ATF-2 is another target transcription factor. HAD-B-mediated suppression of LPS-induced inflammatory responses with IC50 values of 115 (NO) and 156 (PGE2) μg/mL was not strong, but comparable to those of other medicinal plants, such as Phaseolus calcaratus, Sorbus commixta, Sanguisorba officinalis, Acer tegmentosum, Hibiscus cannabinus, and Cinnamomum camphora, with IC50 values ranging from 100 to 300 μg/mL.34–38 These results indicate that the inhibitory activity of HAD-B is derived from its ability to block LPS/TLR4-mediated inflammatory responses at the transcriptional level regulated by NF-κB and ATF-2.
FIG. 2.
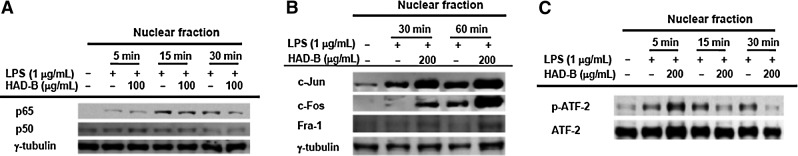
Effects of HAD-B on transcription factor activation. (A–C) The protein levels of NF-κB, AP-1 family, ATF-2, and γ-tubulin in nuclear fractions from LPS-activated RAW264.7 cells pretreated with HAD-B (200 μg/mL) were determined using immunoblot analysis. Levels of phospho- or total p65, p50, c-Jun, c-Fos, ATF-2, and Fra-1 after immunoblotting. NF, nuclear factor; ATF, activating transcription factor; AP, activator protein.
The upstream signaling events for activation of NF-κB and ATF-2 were recently determined. We further analyzed the upstream events using immunoblotting approaches. HAD-B strongly suppressed phosphorylation of IκBα, a negative regulator of NF-κB,39 which is a critical step in NF-κB activation,40 at 5 min (Fig. 3A). Furthermore, phosphorylation of upstream enzymes responsible for IκBα phosphorylation, such as IKK and p85/PI3K, was suppressed by HAD-B at the 5-min time point (Fig. 3A), implying that the real target is located upstream of PI3K. Interestingly, our data strongly suggest that non receptor-type protein tyrosine kinases (Syk and Src) associated with TLRs and their adaptor molecules, such as MyD88 and TRIF,41 are targets of HAD-B (Fig. 3B). The extract significantly suppressed activation at 2 and 3 min, as assessed from tyrosine phosphorylation levels of the kinases.42 The issue of whether the inhibition of tyrosine phosphorylation of both Src and Syk by HAD-B is linked to direct suppression of kinase activity was examined with a kinase assay using purified Syk or Src. Intriguingly, HAD-B suppressed the kinase activity of Syk, but not that of Src, suggesting that some of the components in HAD-B act as direct Syk kinase inhibitor(s), while HAD-B-mediated inhibition of Src phosphorylation is indirectly triggered. Recent studies have shown that several ethnomedicinal herbs, such as C. militaris, Kaempferia parviflora, S. commixta, include unidentified compounds that act directly as Syk inhibitors.36,43 Critical roles of Syk and Src in LPS/TLR4-mediated inflammatory pathways have been established using specific inhibitors, such as BAY61-3606, piceatannol, and PP2. For example, piceatannol is able to block the production of pro-inflammatory cytokines.42 PP2 has been also reported to suppress the release of inflammatory mediators, such as NO and PGE244 Coincidently, TLR ligand treatment with LPS, poly(I:C), and pam3CSK enhances the kinase activity of Syk.45,46 Moreover, Syk is able to associate with TLR4.47 Under our conditions, two Syk kinase inhibitors strongly suppressed NO and PGE2 production (Fig. 3D). Furthermore, HAD-B markedly inhibited IκBα phosphorylation at 5 min (Fig. 3A), which was predominantly mediated by Syk kinase,42 strongly supporting a role of Syk as an anti-inflammatory target of HAD-B.
FIG. 3.
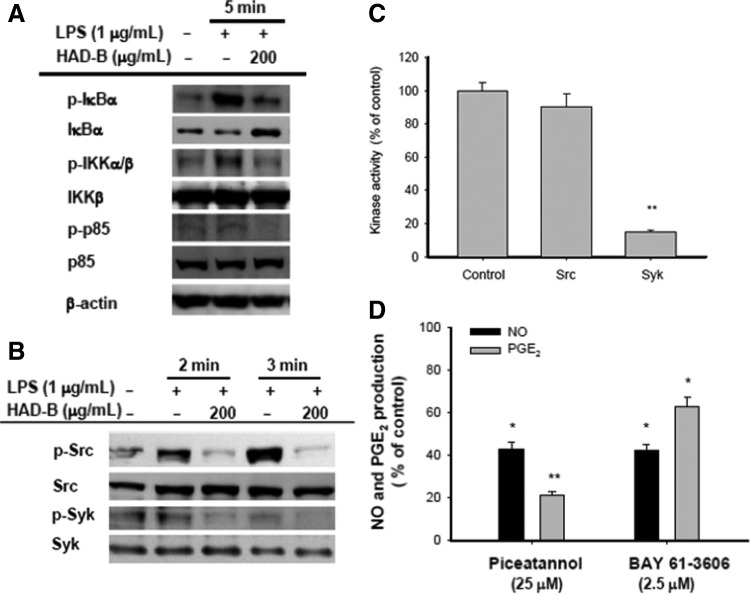
Effects of HAD-B on upstream signaling for NF-κB activation. (A, B) The phospho- or total protein levels of IκBα, IKK, p85, Src, Syk, and β-actin in whole lysates from LPS-activated RAW264.7 cells pretreated with HAD-B (200 μg/mL) were determined using immunoblot analysis. (C) Syk and Src kinase activities were determined with a direct kinase assay using purified enzymes. The control was set as 100% for each enzyme (Src or Syk) as the activity obtained with vehicle-only treatment. (D) Inhibitory effects of piceatannol and BAY 61-3606 on NO and PGE2 production were examined with the Griess assay and EIA. *P<.05 and **P<.01, compared to the control group. Syk, spleen tyrosine kinase; IκBα, inhibitors of κBα; IKK, IκBα kinase.
AP-1 and ATF-2 are other critical transcription factors that regulate LPS-mediated inflammatory gene expression.48,49 Several AP-1 family and CRE-binding proteins translocate to the nucleus during LPS stimulation.48 However, under our experimental conditions, effective inhibition of AP-1 activation by HAD-B treatment was not observed, rather, the nuclear levels of c-Jun, Fra-1, and c-Fos were enhanced (Fig. 2B). In contrast, HAD-B suppressed phosphorylation of ATF-2 to a significant extent at 15–30 min (Fig. 2C). The upstream signaling enzymes regulating ATF-2 phosphorylation targeted by HAD-B were examined by measuring their phosphorylation levels. Interestingly, phosphorylation levels of p38 and JNK at 5–15 min were dramatically decreased by HAD-B (Fig. 4A), suggesting that these pathways are relevant in the inhibitory mechanism. Conversely, the upregulation of AP-1 seems to be regulated by enhanced levels of ERK and MEK1/2 phosphorylation at 5 min, according to Figure 4. Nevertheless, the biological significance of increased levels of c-Jun, Fra-1, and c-Fos in HAD-B-treated cells was not determined yet. In terms of gene expression modulated by the AP-1 family, however, we will verify the expression levels of such genes in the following experiments. Since the phosphorylation levels of p38 and JNK are promoted by upstream kinases, MKK3/6 and MKK4/7, we further investigated the effects of HAD-B on these enzymes. As shown in Figure 4B, phosphorylation levels of MKK3/6, MKK4/7, as well as TAK1 were clearly inhibited at 5 and 15 min following HAD-B exposure (Fig. 4B). Degradation of IRAK, signifying activation of the upstream enzyme, TAK1,50 was not abrogated upon HAD-B exposure, suggesting that IRAK is not a target of HAD-B for the ATF-2 inhibitory route. Meanwhile, according to the inhibitory patterns of SB203580, a p38-specific inhibitor, and SP600125, a JNK inhibitor, we propose that the NO inhibitory effect of HAD-B (Fig. 1A) is not due to suppression of the MAPK/ATF-2 pathway, since NO production was not affected by the inhibitors (Fig. 4C). SB203580 and SP600125 strongly suppressed PGE2 release, whereas NO production was not reduced (Fig. 4C), suggesting that p38 and JNK play critical roles in COX-2 and PGE2 production, as reported previously.37,42
FIG. 4.
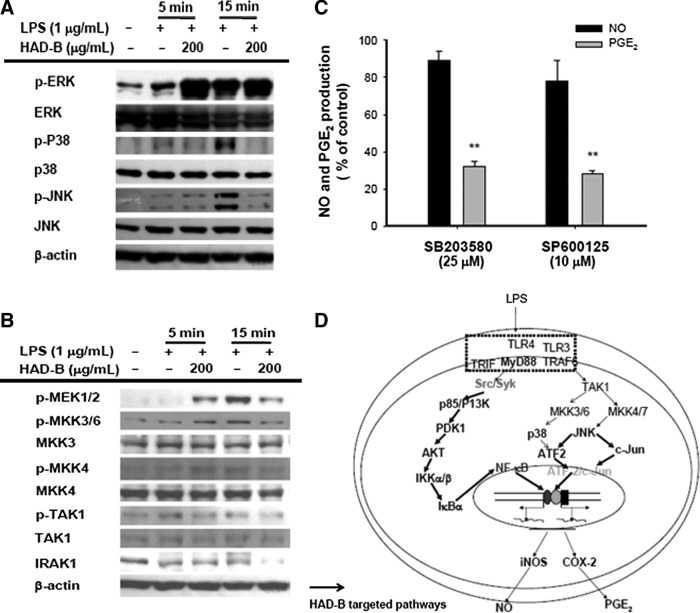
Effects of HAD-B on upstream signaling for ATF-2 activation. (A, B) Phospho- or total protein levels of p38, ERK, JNK, MEK1/2, MKK3/6, MKK4, TAK1, IRAK1, and β-actin in whole lysates from LPS-activated RAW264.7 cells pretreated with HAD-B (200 μg/mL) were determined using immunoblot analysis. (C) Inhibitory effects of SB203580 and SP600125 on NO and PGE2 production were examined with the Griess assay and EIA. (D) Schematic diagram of potential inhibitory pathways of HAD-B to modulate macrophage inflammatory responses. **P<.01, compared to the control group. ERK, extracellular signal-regulated kinase; MKK, mitogen-activated protein (MAP) kinase kinase; JNK, c-Jun N-terminal kinase; TAK1, transforming growth factor β–activated kinase 1; IRAK, IL-1 receptor-associated kinase; MEK, MAP ERK kinase.
Although HAD-B is a mixture of ethnopharmacologically valuable herbal extracts, identification of active components is essential to understand its anti-inflammatory pharmacology. Therefore, we evaluated which components are included in this extract using HPLC analysis with standard compounds, resveratrol, quercetin, and kaempferol, which are known anti-inflammatory compounds.41,51,52 As seen in Figure 5, kaempferol, but not quercetin and resveratrol was identified from this extract. Based on the level of peak area of standard kaempferol, the content of kaempferol was calculated as 0.001987%. Whether kaempferol is able to suppress the release of NO and PGE2 in LPS-treated RAW264.7 cells was finally investigated using standard kaempferol. As we expected, this compound strongly diminished the production of NO and PGE2 at 20 μM, suggesting that kaempferol could be the active component acting as a major anti-inflammatory compound in this extract. Nonetheless, the expected content of kaempferol by HPLC analysis of HAD-B seems not to be enough to exert significant inhibitory activity, implying that there are other unidentified active compounds in this extract. Indeed, several peaks with structural similarity to kaempferol were detected between 40 and 50 min (Fig. 5B). Considering that kaempferol displayed strong anti-inflammatory properties (Fig. 5C), and major peaks were similarly seen with kaempferol at 340 nm between 40 to 50 or even 50 to 60 min (Fig. 5C), flavonoid-type compounds could act as major principles contributing to the pharmacological activity of HAD-B. So far, unfortunately, we do not have direct evidence to explain what those peaks are and how strongly these components inhibit inflammatory responses. In terms of these questions, therefore, the answers will be addressed in subsequent experiments.
FIG. 5.
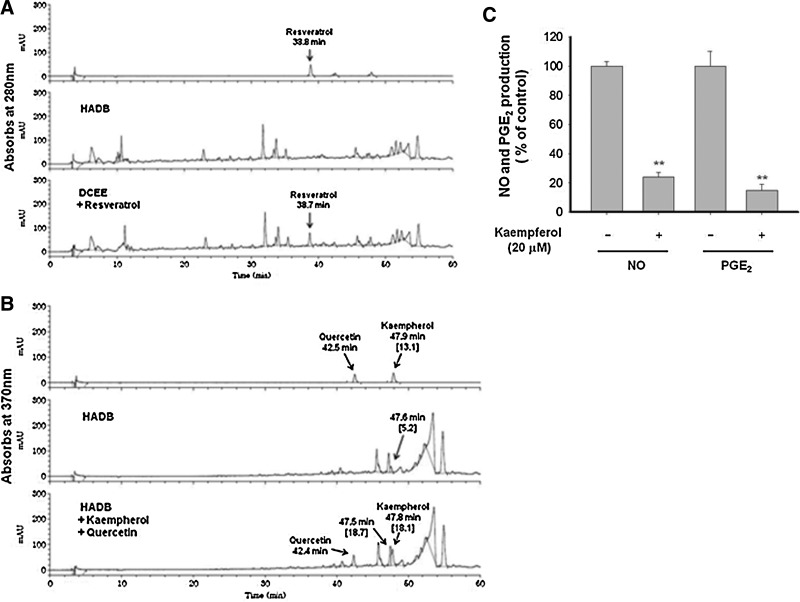
Analysis of the phytochemical profile of HAD-B and the inhibitory activity of kaempferol on NO and PGE2 production in LPS-treated RAW264.7 cells. (A, B) Phytochemical characteristics of HAD-B and standard compounds resveratrol, quercetin, and kaempferol were analyzed by high-performance liquid chromatography. (C) The inhibitory effect of kaempferol on NO and PGE2 production in LPS-treated RAW264.7 cells was examined by Griess assay and EIA. **P<.01 compared to the control group.
In 2004, “The Times” highlighted the pathophysiological significance of chronic inflammation by describing it as the “secret killer.” Since then, a number of studies have demonstrated that inflammation causes a range of serious diseases, such as cancer, vascular disorders, and diabetes.53,54 Although the molecular mechanisms by which inflammation triggers various diseases are not fully understood at present, toxic radicals and pro- and anti-inflammatory cytokines, such as TNF-α, TGF-β, IL-6, and IL-10, have been identified as critical factors in tumorigenic responses of cancer cells, including invasion, metastasis, migration, angiogenesis, and infiltration.17,55,56 Recent studies have additionally highlighted the cellular function of tissue-associated macrophages in cancerous conditions. These cells infiltrate tumor tissues and positively manage the release of various cytokines to facilitate tumor cell survival and metastasis.57 HAD-B has shown efficacy in inhibiting migration and proliferation of human umbilical vein endothelial cells and limiting the formation of capillary tube structures.13 Furthermore, HAD has been used for the treatment of solid tumors, including pancreatic, lung, colorectal, and stomach cancers, since its development in 1996.58 Accordingly, it is proposed that the anti-inflammatory activity of HAD-B strongly contributes to its anti-cancer property through functional suppression of tumor-associated macrophages, in addition to its direct anti-cancer activity.
In conclusion, HAD-B inhibits the production of NO and PGE2 at the transcriptional level. Suppression of NF-κB activation by both Syk and Src and TAK1/ATF-2 linked to MKK3/6/p38 and MKK4/7/JNK (summarized in Fig. 4D) is a putative inhibitory pathway target of HAD-B action. Since chronic inflammatory conditions contribute to tumorigenic responses and cancer metastasis, the anti-inflammatory activity of HAD-B may additionally be responsible for its anti-cancer activity. The therapeutic efficacy of HAD-B as a potent anti-inflammatory remedy will be further examined using detailed animal arthritis and gastritis models, and the active components identified.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No.:0004975), and Research Grant (1010050) from the National Cancer Center, Korea.
Author Disclosure Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- 1.Aggarwal BB. Shishodia S. Takada Y. Jackson-Bernitsas D. Ahn KS. Sethi G. Ichikawa H. TNF blockade: an inflammatory issue. Ernst Schering Res Found Workshop. 2006:161–186. doi: 10.1007/3-540-37673-9_10. [DOI] [PubMed] [Google Scholar]
- 2.Allam R. Anders HJ. The role of innate immunity in autoimmune tissue injury. Curr Opin Rheumatol. 2008;20:538–544. doi: 10.1097/BOR.0b013e3283025ed4. [DOI] [PubMed] [Google Scholar]
- 3.O'Neill LA. Fitzgerald KA. Bowie AG. The toll-IL-1 receptor adaptor family grows to five members. Trends Immunol. 2003;24:286–290. doi: 10.1016/s1471-4906(03)00115-7. [DOI] [PubMed] [Google Scholar]
- 4.O'Neill LA. The role of MyD88-like adapters in toll-like receptor signal transduction. Biochem Soc Trans. 2003;31:643–647. doi: 10.1042/bst0310643. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Lafuente A. Guillamon E. Villares A. Rostagno MA. Martinez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res. 2009;58:537–552. doi: 10.1007/s00011-009-0037-3. [DOI] [PubMed] [Google Scholar]
- 6.Konoshima T. Takasaki M. Tokuda H. Anti-carcinogenic activity of the roots of Panax notoginseng. II. Biol Pharm Bull. 1999;22:1150–1152. doi: 10.1248/bpb.22.1150. [DOI] [PubMed] [Google Scholar]
- 7.Yoo HS. Yoon J. Lee GH. Lee YW. Cho CK. Best case series program supportive cases of Cordyceps militaris- and panax notoginseng-based anticancer herbal formula. Integr Cancer Ther. 2011;10:NP1–3. doi: 10.1177/1534735411423921. [DOI] [PubMed] [Google Scholar]
- 8.Xia WB. Xue Z. Li S. Wang SJ. Yang YC. He DX. Ran GL. Kong LZ. Shi JG. Chemical constituents from tuber of Cremastra appendiculata. Zhongguo Zhong Yao Za Zhi. 2005;30:1827–1830. [PubMed] [Google Scholar]
- 9.Sohn J. Lee CH. Chung DJ. Park SH. Kim I. Hwang WI. Effect of petroleum ether extract of Panax ginseng roots on proliferation and cell cycle progression of human renal cell carcinoma cells. Exp Mol Med. 1998;30:47–51. doi: 10.1038/emm.1998.7. [DOI] [PubMed] [Google Scholar]
- 10.Frank MB. Yang Q. Osban J. Azzarello JT. Saban MR. Saban R. Ashley RA. Welter JC. Fung KM. Lin HK. Frankincense oil derived from Boswellia carteri induces tumor cell specific cytotoxicity. BMC Complement Altern Med. 2009;9:6. doi: 10.1186/1472-6882-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoemaker M. Hamilton B. Dairkee SH. Cohen I. Campbell MJ. In vitro anticancer activity of twelve Chinese medicinal herbs. Phytother Res. 2005;19:649–651. doi: 10.1002/ptr.1702. [DOI] [PubMed] [Google Scholar]
- 12.Choi YJ. Shin DY. Lee YW. Cho CK. Kim GY. Kim WJ. Yoo HS. Choi YH. Inhibition of cell motility and invasion by HangAmDan-B in NCI-H460 human non-small cell lung cancer cells. Oncol Rep. 2011;26:1601–1608. doi: 10.3892/or.2011.1440. [DOI] [PubMed] [Google Scholar]
- 13.Bang JY. Kim KS. Kim EY. Yoo HS. Lee YW. Cho CK. Choi Y. Jeong HJ. Kang IC. Anti-angiogenic effects of the water extract of HangAmDan (WEHAD), a Korean traditional medicine. Sci China Life Sci. 2011;54:248–254. doi: 10.1007/s11427-011-4144-3. [DOI] [PubMed] [Google Scholar]
- 14.Yoo HS. Cho CK. Hong MS. Review of the best case series methodology: best case series results of East-West Cancer Center. Integr Cancer Ther. 2008;7:182–188. doi: 10.1177/1534735408322844. [DOI] [PubMed] [Google Scholar]
- 15.Jeong HJ. Lee JY. Kim JB. Go H. Ko SG. Seo YW. Jeong S. Park J. Na HJ. Um JY. Kim HM. Hong SH. Induction of apoptosis by KI0477959 through activation of caspase-3 in human leukemia cell line, HL-60 cells. Int J Neurosci. 2008;118:1384–1399. doi: 10.1080/00207450701242859. [DOI] [PubMed] [Google Scholar]
- 16.Yoo HS. Lee HJ. Kim JS. Yoon J. Lee GH. Lee YW. Cho CK. A toxicological study of HangAmDan-B in mice. J Acupunct Meridian Stud. 2011;4:54–60. doi: 10.1016/S2005-2901(11)60007-1. [DOI] [PubMed] [Google Scholar]
- 17.Raz A. Levine G. Khomiak Y. Acute local inflammation potentiates tumor growth in mice. Cancer Lett. 2000;148:115–120. doi: 10.1016/s0304-3835(99)00329-8. [DOI] [PubMed] [Google Scholar]
- 18.Correa M. Machado J., Jr. Carneiro CR. Pesquero JB. Bader M. Travassos LR. Chammas R. Jasiulionis MG. Transient inflammatory response induced by apoptotic cells is an important mediator of melanoma cell engraftment and growth. Int J Cancer. 2005;114:356–363. doi: 10.1002/ijc.20673. [DOI] [PubMed] [Google Scholar]
- 19.Bauer AK. Dwyer-Nield LD. Keil K. Koski K. Malkinson AM. Butylated hydroxytoluene (BHT) induction of pulmonary inflammation: a role in tumor promotion. Exp Lung Res. 2001;27:197–216. doi: 10.1080/019021401300053948. [DOI] [PubMed] [Google Scholar]
- 20.Yang H. Lee SE. Jeong SI. Park CS. Jin YH. Park YS. Up-regulation of heme oxygenase-1 by Korean red ginseng water extract as a cytoprotective effect in human endothelial cells. J Ginseng Res. 2011;35:352–359. doi: 10.5142/jgr.2011.35.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho JY. Baik KU. Jung JH. Park MH. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, from Saussurea lappa. Eur J Pharmacol. 2000;398:399–407. doi: 10.1016/s0014-2999(00)00337-x. [DOI] [PubMed] [Google Scholar]
- 22.Jang SA. Kang SC. Sohn EH. Phagocytic effects of beta-glucans from the mushroom Coriolus versicolor are related to dectin-1, NOS, TNF-alpha signaling in macrophages. Biomol Ther. 2011;19:438–444. [Google Scholar]
- 23.Shen T. Lee J. Lee E. Kim SH. Kim TW. Cho JY. Cafestol, a coffee-specific diterpene, is a novel extracellular signal-regulated kinase inhibitor with AP-1-targeted inhibition of prostaglandin E2 production in lipopolysaccharide-activated macrophages. Biol Pharm Bull. 2010;33:128–132. doi: 10.1248/bpb.33.128. [DOI] [PubMed] [Google Scholar]
- 24.Yayeh T. Jung KH. Jeong HY. Park JH. Song YB. Kwak YS. Kang HS. Cho JY. Oh JW. Kim SK. Rhee MH. Korean red ginseng saponin fraction downregulates proinflammatory mediators in LPS stimulated RAW264.7 cells and protects mice against endotoxic shock. J Ginseng Res. 2012;36:263–269. doi: 10.5142/jgr.2012.36.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee YG. Lee WM. Kim JY. Lee JY. Lee IK. Yun BS. Rhee MH. Cho JY. Src kinase-targeted anti-inflammatory activity of davallialactone from Inonotus xeranticus in lipopolysaccharide-activated RAW264.7 cells. Br J Pharmacol. 2008;154:852–863. doi: 10.1038/bjp.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H. Kim J. Lee SY. Park JH. Hwang GS. Processed Panax ginseng, sun ginseng, decreases oxidative damage induced by tert-butyl hydroperoxide via regulation of antioxidant enzyme and anti-apoptotic molecules in HepG2 cells. J Ginseng Res. 2012;36:248–255. doi: 10.5142/jgr.2012.36.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoo DS. Rho HS. Lee YG. Yeom MH. Kim DH. Lee SJ. Hong S. Lee J. Cho JY. Ginsenoside F1 modulates cellular responses of skin melanoma cells. J Ginseng Res. 2011;35:86–91. [Google Scholar]
- 28.Okabe S. Miyake H. Awane Y. Cytoprotective effects of NC-1300 and omeprazole on HCl/ethanol-induced gastric lesions in rats. Jpn J Pharmacol. 1986;42:123–133. doi: 10.1254/jjp.42.123. [DOI] [PubMed] [Google Scholar]
- 29.Kim P. Jeong CS. Effects of Chenopodium album Linne on gastritis and gastric cancer cell growth. Biomol Ther. 2011;19:487–492. [Google Scholar]
- 30.Aguirre-Hernandez E. Gonzalez-Trujano ME. Martinez AL. Moreno J. Kite G. Terrazas T. Soto-Hernandez M. HPLC/MS analysis and anxiolytic-like effect of quercetin and kaempferol flavonoids from Tilia americana var. mexicana. J Ethnopharmacol. 2010;127:91–97. doi: 10.1016/j.jep.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 31.Lee DY. Cho JG. Lee MK. Lee JW. Lee YH. Yang DC. Baek NI. Discrimination of Panax ginseng roots cultivated in different areas in Korea using HPLC-ELSD and principal component analysis. J Ginseng Res. 2011;35:31–38. [Google Scholar]
- 32.Chang LC. Tsao LT. Chang CS. Chen CJ. Huang LJ. Kuo SC. Lin RH. Wang JP. Inhibition of nitric oxide production by the carbazole compound LCY-2-CHO via blockade of activator protein-1 and CCAAT/enhancer-binding protein activation in microglia. Biochem Pharmacol. 2008;76:507–519. doi: 10.1016/j.bcp.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Vermeulen L. De Wilde G. Notebaert S. Vanden Berghe W. Haegeman G. Regulation of the transcriptional activity of the nuclear factor-kappaB p65 subunit. Biochem Pharmacol. 2002;64:963–970. doi: 10.1016/s0006-2952(02)01161-9. [DOI] [PubMed] [Google Scholar]
- 34.Lee YG. Byeon SE. Kim JY. Lee JY. Rhee MH. Hong S. Wu JC. Lee HS. Kim MJ. Cho DH. Cho JY. Immunomodulatory effect of Hibiscus cannabinus extract on macrophage functions. J Ethnopharmacol. 2007;113:62–71. doi: 10.1016/j.jep.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 35.Lee HJ. Hyun EA. Yoon WJ. Kim BH. Rhee MH. Kang HK. Cho JY. Yoo ES. In vitro anti-inflammatory and anti-oxidative effects of Cinnamomum camphora extracts. J Ethnopharmacol. 2006;103:208–216. doi: 10.1016/j.jep.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Yu T. Lee YJ. Jang HJ. Kim AR. Hong S. Kim TW. Kim MY. Lee J. Lee YG. Cho JY. Anti-inflammatory activity of Sorbus commixta water extract and its molecular inhibitory mechanism. J Ethnopharmacol. 2011;134:493–500. doi: 10.1016/j.jep.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 37.Yu T. Lee YJ. Yang HM. Han S. Kim JH. Lee Y. Kim C. Han MH. Kim MY. Lee J. Cho JY. Inhibitory effect of Sanguisorba officinalis ethanol extract on NO and PGE production is mediated by suppression of NF-kappaB and AP-1 activation signaling cascade. J Ethnopharmacol. 2011;134:11–17. doi: 10.1016/j.jep.2010.08.060. [DOI] [PubMed] [Google Scholar]
- 38.Yu T. Lee J. Lee YG. Byeon SE. Kim MH. Sohn EH. Lee YJ. Lee SG. Cho JY. In vitro and in vivo anti-inflammatory effects of ethanol extract from Acer tegmentosum. J Ethnopharmacol. 2010;128:139–147. doi: 10.1016/j.jep.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 39.Bustamante J. Boisson-Dupuis S. Jouanguy E. Picard C. Puel A. Abel L. Casanova JL. Novel primary immunodeficiencies revealed by the investigation of paediatric infectious diseases. Curr Opin Immunol. 2008;20:39–48. doi: 10.1016/j.coi.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 40.von Bernuth H. Puel A. Ku CL. Yang K. Bustamante J. Chang HH. Picard C. Casanova JL. Septicemia without sepsis: inherited disorders of nuclear factor-kappa B-mediated inflammation. Clin Infect Dis. 2005;41(Suppl 7):S436–S439. doi: 10.1086/431994. [DOI] [PubMed] [Google Scholar]
- 41.Kim MH. Yoo DS. Lee SY. Byeon SE. Lee YG. Min T. Rho HS. Rhee MH. Lee J. Cho JY. The TRIF/TBK1/IRF-3 activation pathway is the primary inhibitory target of resveratrol, contributing to its broad-spectrum anti-inflammatory effects. Pharmazie. 2011;66:293–300. [PubMed] [Google Scholar]
- 42.Lee YG. Chain BM. Cho JY. Distinct role of spleen tyrosine kinase in the early phosphorylation of inhibitor of kappaB alpha via activation of the phosphoinositide-3-kinase and Akt pathways. Int J Biochem Cell Biol. 2009;41:811–821. doi: 10.1016/j.biocel.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 43.Oh JY. Choi WS. Lee CH. Park HJ. The ethyl acetate extract of Cordyceps militaris inhibits IgE-mediated allergic responses in mast cells and passive cutaneous anaphylaxis reaction in mice. J Ethnopharmacol. 2011;135:422–429. doi: 10.1016/j.jep.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 44.Yu T. Lee S. Yang WS. Jang HJ. Lee YJ. Kim TW. Kim SY. Lee J. Cho JY. The ability of an ethanol extract of Cinnamomum cassia to inhibit Src and spleen tyrosine kinase activity contributes to its anti-inflammatory action. J Ethnopharmacol. 2012;139:566–573. doi: 10.1016/j.jep.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 45.Wang L. Gordon RA. Huynh L. Su X. Park Min KH. Han J. Arthur JS. Kalliolias GD. Ivashkiv LB. Indirect inhibition of Toll-like receptor and type I interferon responses by ITAM-coupled receptors and integrins. Immunity. 2010;32:518–530. doi: 10.1016/j.immuni.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ulanova M. Asfaha S. Stenton G. Lint A. Gilbertson D. Schreiber A. Befus D. Involvement of Syk protein tyrosine kinase in LPS-induced responses in macrophages. J Endotoxin Res. 2007;13:117–125. doi: 10.1177/0968051907079125. [DOI] [PubMed] [Google Scholar]
- 47.Chaudhary A. Fresquez TM. Naranjo MJ. Tyrosine kinase Syk associates with toll-like receptor 4 and regulates signaling in human monocytic cells. Immunol Cell Biol. 2007;85:249–256. doi: 10.1038/sj.icb7100030. [DOI] [PubMed] [Google Scholar]
- 48.Casals-Casas C. Alvarez E. Serra M. de la Torre C. Farrera C. Sanchez-Tillo E. Caelles C. Lloberas J. Celada A. CREB and AP-1 activation regulates MKP-1 induction by LPS or M-CSF and their kinetics correlate with macrophage activation versus proliferation. Eur J Immunol. 2009;39:1902–1913. doi: 10.1002/eji.200839037. [DOI] [PubMed] [Google Scholar]
- 49.Proffitt J. Crabtree G. Grove M. Daubersies P. Bailleul B. Wright E. Plumb M. An ATF/CREB-binding site is essential for cell-specific and inducible transcription of the murine MIP-1 beta cytokine gene. Gene. 1995;152:173–179. doi: 10.1016/0378-1119(94)00701-s. [DOI] [PubMed] [Google Scholar]
- 50.Xiong Y. Qiu F. Piao W. Song C. Wahl LM. Medvedev AE. Endotoxin tolerance impairs IL-1 receptor-associated kinase (IRAK) 4 and TGF-beta-activated kinase 1 activation, K63-linked polyubiquitination and assembly of IRAK1, TNF receptor-associated factor 6, and IκB kinase gamma and increases A20 expression. J Biol Chem. 2011;286:7905–7916. doi: 10.1074/jbc.M110.182873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bischoff SC. Quercetin: potentials in the prevention and therapy of disease. Curr Opin Clin Nutr Metab Care. 2008;11:733–740. doi: 10.1097/MCO.0b013e32831394b8. [DOI] [PubMed] [Google Scholar]
- 52.Kwon JH. Kim JH. Choi SE. Park KH. Lee MW. Inhibitory effects of phenolic compounds from needles of Pinus densiflora on nitric oxide and PGE2 production. Arch Pharm Res. 2010;33:2011–2016. doi: 10.1007/s12272-010-1217-y. [DOI] [PubMed] [Google Scholar]
- 53.Schetter AJ. Heegaard NH. Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khatami M. ‘Yin and Yang’ in inflammation: duality in innate immune cell function and tumorigenesis. Expert Opin Biol Ther. 2008;8:1461–1472. doi: 10.1517/14712598.8.10.1461. [DOI] [PubMed] [Google Scholar]
- 55.Karin M. The IkappaB kinase—a bridge between inflammation and cancer. Cell Res. 2008;18:334–342. doi: 10.1038/cr.2008.30. [DOI] [PubMed] [Google Scholar]
- 56.Okamoto M. Kawai K. Reznikoff CA. Oyasu R. Transformation in vitro of a nontumorigenic rat urothelial cell line by hydrogen peroxide. Cancer Res. 1996;56:4649–4653. [PubMed] [Google Scholar]
- 57.Ohno S. Inagawa H. Dhar DK. Fujii T. Ueda S. Tachibana M. Ohno Y. Suzuki N. Inoue M. Soma G. Nagasue N. Role of tumor-associated macrophages (TAM) in advanced gastric carcinoma: the impact on FasL-mediated counterattack. Anticancer Res. 2005;25:463–470. [PubMed] [Google Scholar]
- 58.Jeong TY. Park BK. Lee YW. Cho CK. Yoo HS. Prospective analysis on survival outcomes of nonsmall cell lung cancer stages over IIIb treated with HangAm-Dan. Zhongguo Fei Ai Za Zhi. 2010;13:1009–1015. doi: 10.3779/j.issn.1009-3419.2010.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]


