Abstract
Objectives:
To determine the optimal medication for the treatment of renal colic using evidence based medicine (EBM) parameters (RR, ARR, NNT, NNH, ARI, RRI).
Sample and Methodology:
During 2010, an ITT study was conducted on 400 outpatients of the Sarajevo University Clinical Center Urology Clinic in order to investigate renal colic pain relief drugs. Each group consisting of 100 patients was administered either Metamizol amp. i.v., or Diclofenac amp. i.m., or Butylscopolamine amp. i.v., while 100 patients belonged to the placebo group that was given distilled water (aqua redestilata). All patients completed visual analogue pain scale (VAPS) from 0 to 10 prior to and after the treatment.
Results:
Using EBM parameters Diclofenac Na and Metamizol were shown to be the most efficient in the treatment of renal colic. In these two groups, relative risk (RR) was 21 and 8,5% respectively; Absolute Risk Reduction (ARR) was 74 and 86% respectively, and Number Needed to Treat (NNT) was 1 for both groups, while chi-squared (X2) test has shown that there is no statistically significant difference between these two drugs when it comes to their effect. In the Butylscopolamine group, RR was 81; ARR 18%, while NNT was 5. With respect to side effects, only in his group it was shown that Relative Risk Increase (RRI) was 84, ARI 83%, while Number Needed to Harm (NNH) was 2.
Conclusion:
The most optimal medication for the treatment of renal colic according to EBM parameters is Diclofenac Na, followed by Metamizol. Butylscopolamine is not recommended for the treatment of renal colic.
Key words: EBM, RR, ARI, NNT, renal colic, management.
1. INTRODUCTION
Renal colic represents a complex of acute symptoms characterized by very intense, agonizing pain, which requires fast diagnosis and precise and speedy treatment (1, 2, 3, 4, 5). Most often, patients constantly try to find unusual positions in order to ease the pain (6, 7, 8). These movements are opposite to the restricted movements of bed-ridden patients with peritoneal signs (1). A vast majority of urinary stones with acute pain attack are a consequence of acute obstruction and distension of the upper urinary tract. Intervention in renal colic has to be based on the awareness of the origin of pain, renal damage suffered, and it has to protect the kidney from any damage caused by prolonged obstruction. Many drugs have been proposed for the treatment of renal colic pain of which most commonly used are antiholinergic drugs and antimuscarinics. Given that all authors do not agree that muscarinic receptors are involved in urethral mobility, their use is disputable, particularly since ureteric peristalsis is necessary for stone passage. (2,3). Considering the contradictory results of studies related to the efficacy and side effects of tested drugs, the question arises as to how the effect and safety of the tested drugs can be scientifically quantified and qualified. The answer lies in the use of evidence based medicine and meta-analyses related to the treatment of renal colic.
2. SUBJECTS AND METHODOLOGY
During 2009-2010, a prospective open, comparative and randomized ITT (Intention To Treat) study on a sample of 400 adult patients, divided into 4 groups. Group A (100 patients) was administered Metamizol 2 g i.v., group B (100 patients) was administered Diclofenac 75 mg. i.m., group C (100 patients) Butylscopolamin amp. i.v., while placebo group (100 patients), received distilled water (aqua redestilata) i.v., as a control group. All the patients completed pain scale from 1 to 10 (VAPS, Visual Analogue Pain Scale) (4-7), with 0 representing no pain and 10 representing the worst imaginable pain. The patients were evaluated before the drug administration and half an hour after the therapy. In case the pain was not relieved, within 30 minutes an additional dose of the drug was administered or Tramal amp. 50 mg. i.v. (ITT), and if the patient did not respond to either drug, a more invasive urological treatment was applied (urethral catheterization, stenting or ESWL treatment). The patients were alternately administered the therapy from the groups 1, 2, 3 and 4, and then the cycle was repeated until all 400 patients were treated. Also, possible side effects of the tested drugs were monitored.
3. RESULTS
The testing was statistically processed based on the principle of Evidence Based Medicine (EBM). All calculations were made via the internet http://moosenose.com/EBCalculator.htm (free version). All results were compared with values for p<0.0001. A comparison was made of the pain intensity on admission and 30 minutes after the drug administration, among all the groups. The group having worse results was taken as a control group. The model of chi-squared (X2) test was used – a non-parametric twosample homogeneity test with frequencies distributed in 2 x 2 tables and EBM-based calculations.
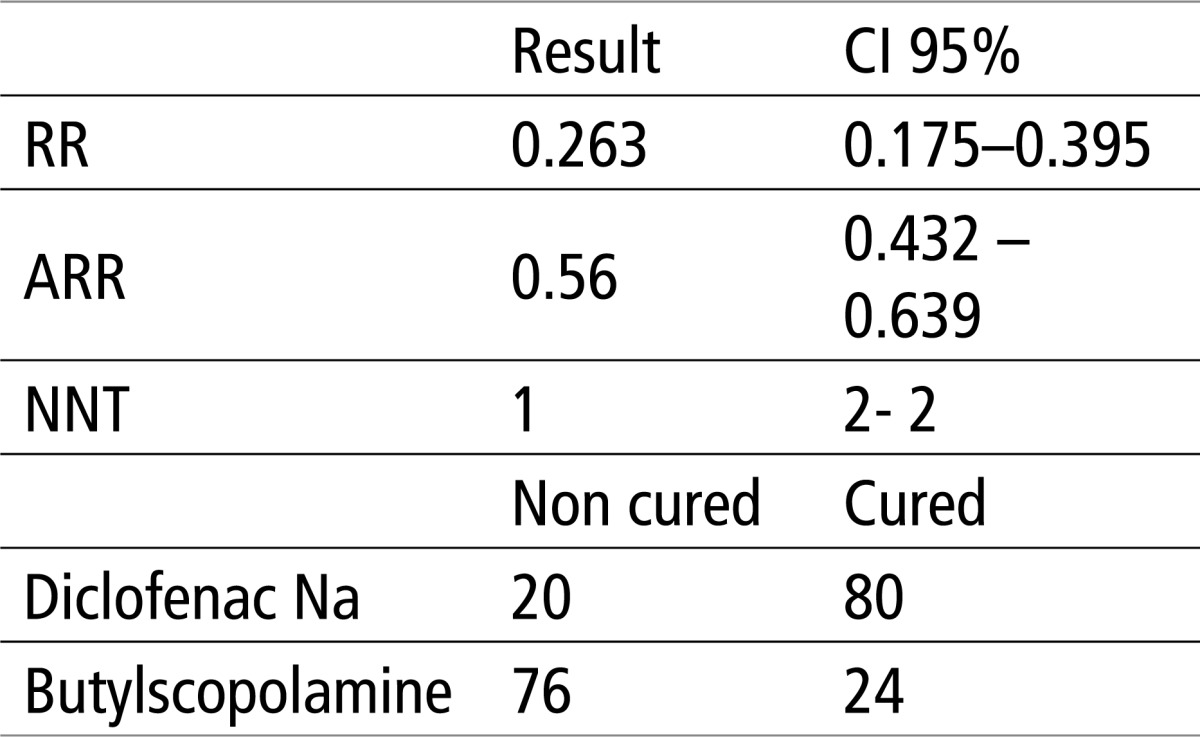
Table 1 shows that there is a significant difference of X2 test, for p<0.0001. The relative risk (RR, in our case the risk that in Diclofenac Na group there will be a weak response to therapy) is 0.263 or 26%, absolute risk reduction (ARR, in our case an absolute number whereby the treatment with Diclofenac Na reduces the risk of) is 0.56 or 56%, while the number needed to treat (NNT, in our case the number of patients in Diclofenac Na group needed to be treated with the drug in order to have a 100% success) is 1.
Table 1.
Testing the efficacy of Butylscopolamine and Diclofenac using X2 test and EBM parameters. X2 = 60.59 P< 0.0001
 |
The results in table 2 show that there is a significant difference of X2 test, for p<0.0001. The relative risk (RR) is 0.105 or 10.5%, the absolute risk reduction (ARR) is 0.68 or 68%, while the number of patients needed to treat for a favorable outcome in the experimental group (NNT) is 1.
Table 2.
Testing the efficacy of Butylscopolamine and Metamizol using X2 test and EBM parameters. X2 = 92.139, P<0.0001
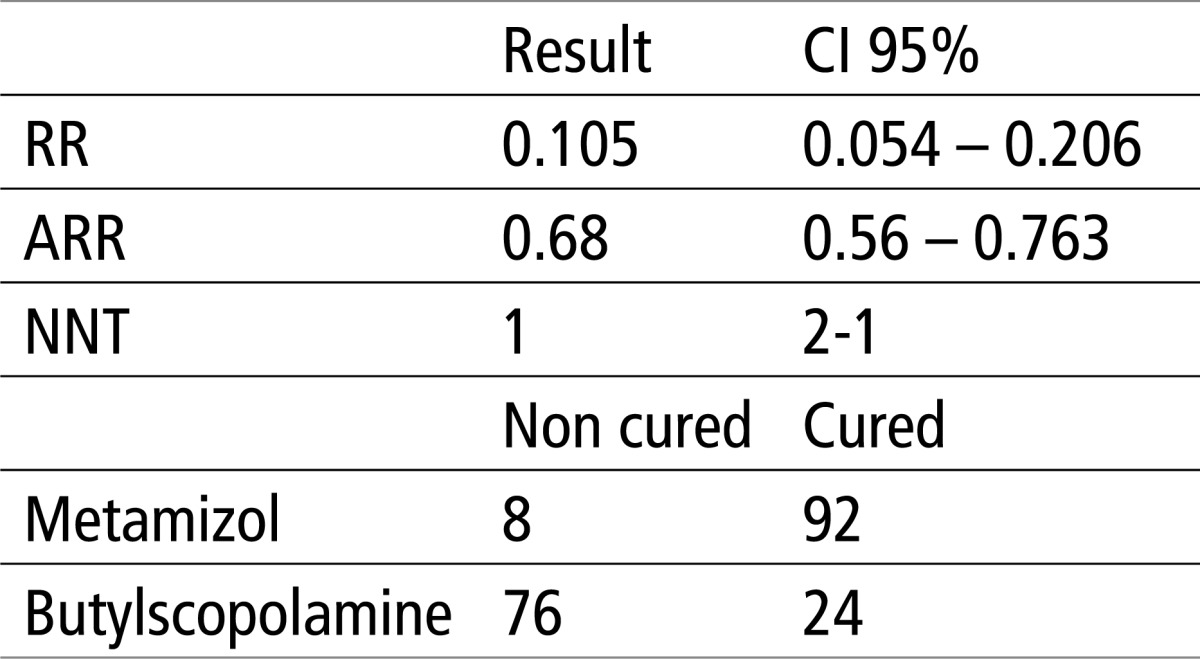 |
Table 3 shows that that there is a significant difference of X2 test, for p<0.0001. The relative risk (RR) is 0.085 or 8.5%, the absolute risk reduction (ARR) is 0.86 or 86%, while the number of patients needed to treat in order to achieve a favorable outcome in the experimental group (NNT) is 1 in the interval of 1 patient; therefore an ideal value, or meaning that each patient in the experimental group would have a favorable outcome, and each patient in the control group would have a weak response to therapy.
Table 3.
Testing the efficacy of aqua and Metamizol using EBM parameters and X2 test. X2 = 144.5, P<0.0001
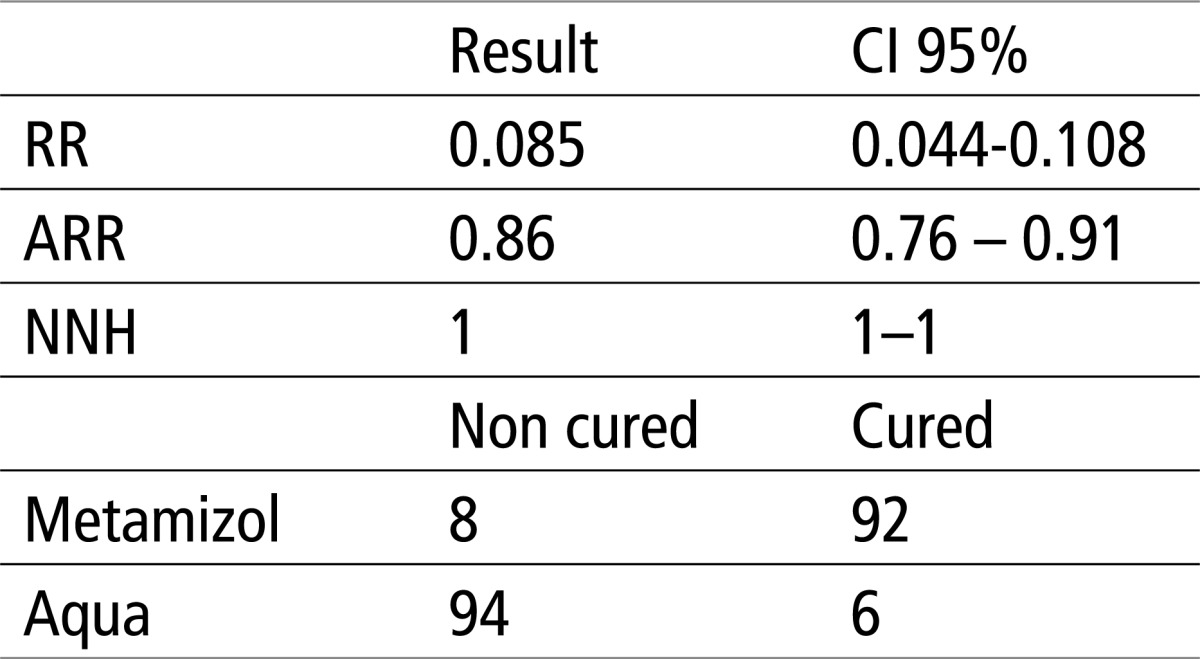 |
Table 4 shows that there is no statistical difference in X2 regarding the effect of Metamizol and Diclofenac Na, p>0.0001. The relative risk (RR) is 1.15, absolute risk reduction (ARR) is 0.12 or 12%, while the number of patients needed to treat in order to achieve a favorable outcome in the experimental group (NNT) is 9 in the interval of 5 to 43, where -5 NNH.
Table 4.
Testing the efficacy of Diclofenac and Metamizol using EBM parameters and X2 test. X2 = 5.025, P>0.0001 (0.15)
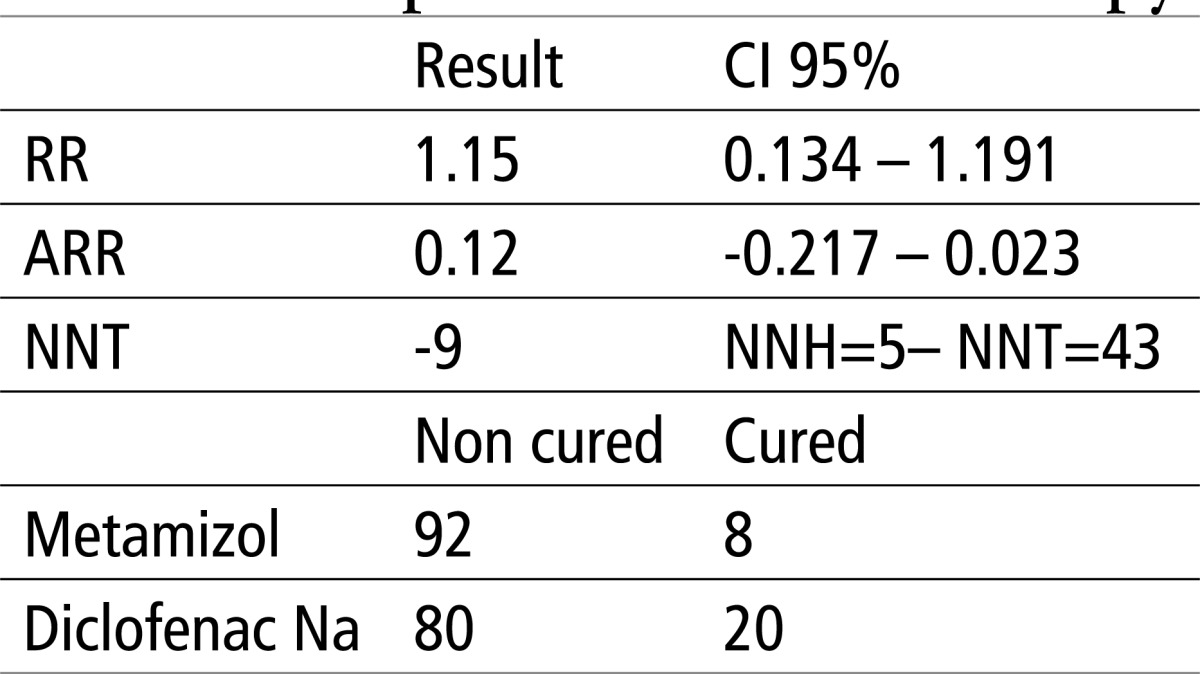 |
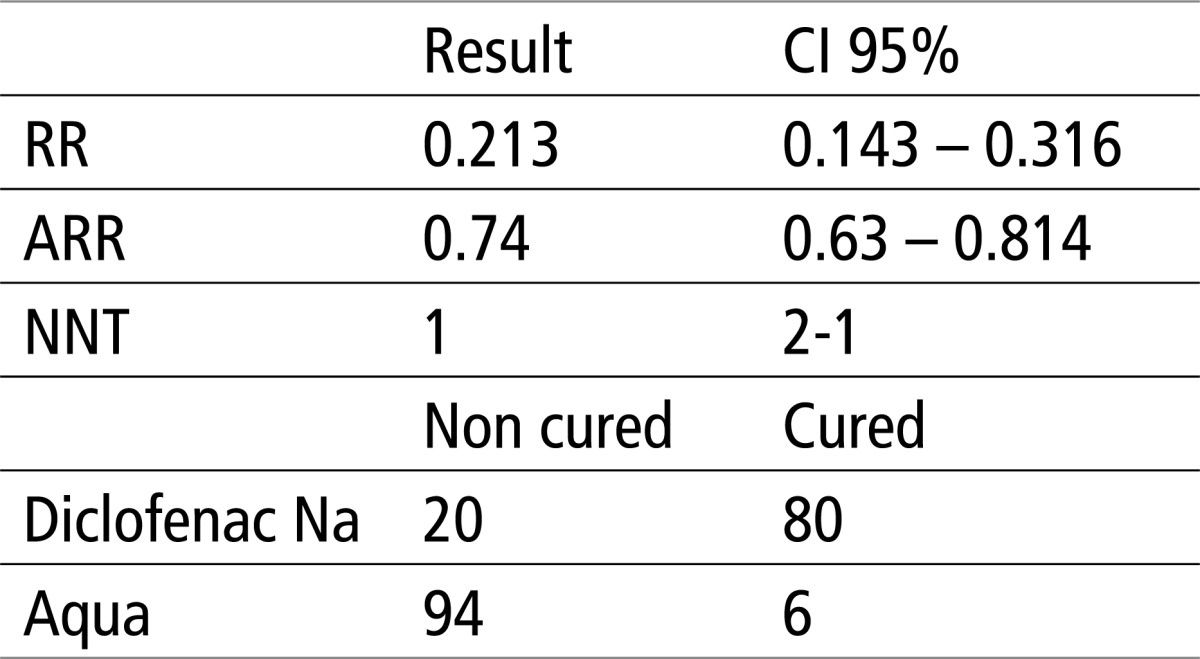
Table 5 shows that there is significant difference in X2 test, for p<0.0001. The relative risk (RR) is 0.21 or 21%, absolute risk reduction (ARR) is 0.74 or 74%, while the number of patients needed to treat in order to achieve a favorable outcome in the experimental group (NNT) is 1 in the range of 2-1 patients.
Table 5.
Testing the efficacy of aqua and Diclofenac using EBM parameters and X2 test. X2 = 108.711, P<0.0001
 |
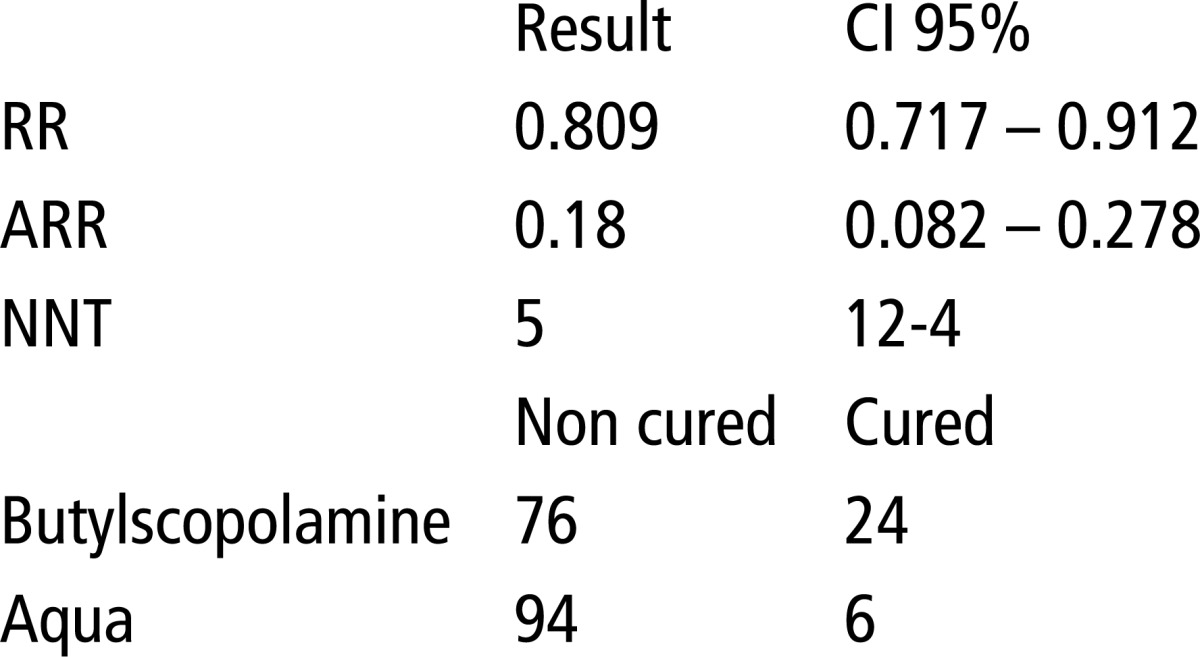
In Table 6, the results suggest that there is no statistical difference in X2 regarding the effect of Butylscopolamine and aqua, p>0.0001. The relative (RR) is 0.809 or 80%, absolute risk reduction (ARR) is 0.18 or 18%, while the number of patients needed to treat in order to achieve a favorable outcome in the experimental group (NNT) is 5 in the range of 12-4 patients.
Table 6.
Testing the efficacy of aqua and Butylscopolamine using EBM parameters and X2 test. X2 = 11.33, P>0.0001
 |
Should we statistically process side effects using EBM parameters, the following results are obtained:
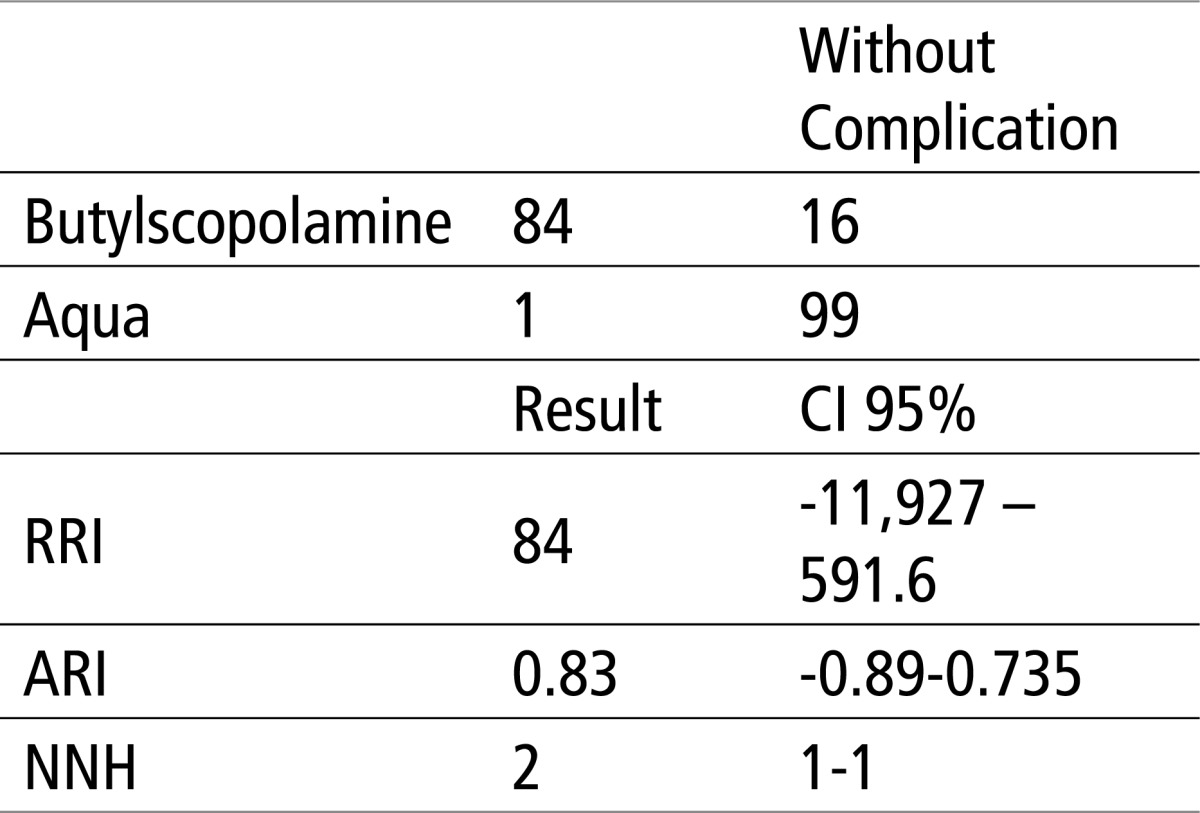
It is evident from the above that, in the comparison of Butylscopolamine, which was the only one showing side effects, there appeared RRI (relative risk increase) or the probability of complications arising in the Butylscopolamine group, for 84%, while ARI (absolute risk increase ), namely, absolute arithmetic difference of unfavorable response is 82%. The number of patients in this group that need to receive Butylscopolamine in order for side effects to show (NNH) is 2. For other groups, RRI is 0, ARI is 0, while NNH is infinite, i.e. 0.
4. DISCUSSION
Evidence-based medicine (EBM) is a comparatively young discipline of medical research that represents a process of systematic review, study, evaluation and conscientious and proper use of clinical results in order to treat individuals and population. During their work, cliniciansresearchers have dilemmas and differences regarding the results obtained from various sources of evidence; they are not sure which results are the most optimal ones for a given study. It is by using current best evidence for the treatment of a disease and acquiring practical and critical judgment, as well as developing IT skills that a more rational and practical approach to the unknown will be developed, while the explicit use of incorporated evidence, as well as patients’ state and preferences in making medical decisions will make a clinician “an evidence expert”. At the same time, it involves continuous tracking down the evidence, based on which medical decisions are made, and further develops the ability to look for and select the best evaluations from primary and secondary sources of available evidence in order to achieve the optimal treatment for the patient. (8, 9)
EBM came about based on the idea that the decision about the care of patients should involve the conscientious and equitable use of current best evidence related to the health of both individual patients and population. (10, 11). From ethical perspective, the strongest arguments supporting the EBM are that it identifies and provides for the best evaluated methods of health care (including those useless, and those causing detrimental effect) while enabling both patients and doctors to make proper decisions. Medical informatics and EBM are closely linked (12). Based on EBM principles, some parameters evolved that practically define the efficacy of a certain medical treatment. These parameters have been applied in this paper in order to investigate the efficacy of medications from the experimental group. For all parameters the CI (Confidence Interval, degree of probability) of 95% was used. Since all groups were monitored for up to 30 minutes following the administration of therapy, VAPS values prior to and after treatment were taken as a basis for the calculation of parameters. The parameters monitored are RR (Relative Risk), ARR (Absolute Risk Reduction) and NNT (Number Needed to Treat), NNH (Number Needed to Harm), ARI (Absolute Risk Increase), and RRI (Relative Risk Increase). For these calculations, the placebo group was used as a control group. The results obtained for X2 test suggest to a statistically significant difference between the Diclofenac/Butylscopolamine group, p<0.0001, Metamizol/B utylscopolamine p<0.0001, Diclofenac/Aqua (p<0.0001), Metamizol/Aqua (p<0.0001). Also, it was shown that there is no significant difference in terms of effect between the groups Metamizol/Diclofenac (p>0.0001) and Butylscopolamine / Aqua (p> 0.0001). In the group where Metamizol was tested, the relative risk of a weak therapeutic response (RR) is 8.5%, while the absolute risk reduction of a weak therapeutic response (ARR) in this group is 86%. The number of patients needed to treat with Metamizol in order to achieve a 100% successful outcome in the whole group (NNT) is 1. In the group of patients receiving Diclofenac, the RR is 23%, the ARR is 74%, while the NNT is 1. Therefore, there is a high degree of probability regarding the efficacy of these two medications and a low degree of probability that they fail to have effect. In the group of patients receiving Butylscopolamine, the RR is 81%, the ARR is 18%, while the NNT is very high–5. The comparison between the Butylscopolamine group as a control group and Diclofenac Na group as an experimental group, showed the following values: the RR–26%, the ARR–56%, and the NNT–1. The comparison between the Butylscopolamine group as a control group and Metamizol group as an experimental group, showed the following values: the RR – 10.5%, the ARR–68%, and the NNT–1. Also, in the Butylscopolamine group, a high degree of transitory side effects was shown (diplopia, dry mouth and dizziness, for which no medical treatment was indicated), and it was shown that the RRI was 84%, therefore, in 84% of the patients the relative risk of a side effect was increased, while the effect of the medication in terms of pain relief was equal to that in the placebo group. Having analyzed the whole paper and the literature (13) available, and taken into account pain relief statistical results and data on serious life-threatening side effects that may appear during the administration of Metamizol, which have not been verified during this study, one can say that Metamizol and Diclofenac are equal for the treatment of renal colic, however the medication of choice is Diclofenac Na, applied i.m. in the dosage of 75 mg.
5. CONCLUSION
The objective EBM is to use the evidence obtained using scientific methodology as an important element of medical practice. The EBM is about the quality of evidence relevant for a specific trial or treatment, including both the risk and benefit of the treatment, as well as the absence of a specific treatment. For the treatment of renal colic, meta-analyses evaluated 40 randomized control studies that fulfilled all the requirements for their results to comply with evidence-based medicine parameters. The results of this study are shown directly, through new statistical indicators, as a contribution to clinical decision-making on the optimal therapeutic approach.
Table 7.
Comparison in terms of complications between the Butylscopolamine group and control group using EBM parameters
 |
Conflict of interest
None declared.
REFERENCES
- 1.Marshall LS. Tanagho EA, McAninch JW. Smith’s General Urology. 16th. San Francisco: Lange Medical Book; 2004. Urinary Stone Disease. [Google Scholar]
- 2.Van Laecke E, Oosterlinck W. Physiopathology of renal colic and the therapeutic consequences. Acta Urologica Belgica. 1994;62:15–18. [PubMed] [Google Scholar]
- 3.Heid F, Jage J. The treatment of pain in urology. BJU International. 2002;90:481–488. doi: 10.1046/j.1464-410x.2002.02908.x. [DOI] [PubMed] [Google Scholar]
- 4.Jamison RN, Gracely RH, et al. Comparative study of electronic vs. paper VAS ratings: A randomized, crossover trial using healthy volunteers. Pain. 2002;99:341–347. doi: 10.1016/s0304-3959(02)00178-1. [DOI] [PubMed] [Google Scholar]
- 5.Lorig KR, Sobel DS, et al. Effect of a Self-Management Program on Patients with Chronic Disease. Eff. Clin. Pract. 2001;4:256–262. [PubMed] [Google Scholar]
- 6.Workman EA, Hubbard JR, et al. Comorbid Psychiatric Disorders and Predictors of Pain Management Program Success in Patients With Chronic Pain, Primary Care Companion. J Clin Psychiatry. 2002;4:137–140. doi: 10.4088/pcc.v04n0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marques AP, Rhoden L, et al. Pain Evaluation of Patients with Fibromyalgia, Osteoarthritis, and Low Back Pain. Rev Hosp Clin Fac Med. S. Paolo. 2001;56(1):5–10. doi: 10.1590/s0041-87812001000100002. [DOI] [PubMed] [Google Scholar]
- 8.Cohen AM, Stavri PZ. A categorisation and analysis of the criticisms of Evidence-Based Medicine. International Journal of Medical Informatics. 2004;73:35–43. doi: 10.1016/j.ijmedinf.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Adler RH, Uexkull T, et al. The two faces of medical evidence. Swiss Med Wkly. 2002;132:397–400. doi: 10.4414/smw.2002.10071. [DOI] [PubMed] [Google Scholar]
- 10.Sackett DL, Strauss SE, et al. second edition. London: Churchill Livingstone; 2000. Evidence Based Medicine, How to Practice and Teach EBM. [Google Scholar]
- 11.Sackett DL, Rosenberg WMC, et al. Evidence Based Medicine: what it is and what it isn’t. BMJ. 1996;312:71–72. doi: 10.1136/bmj.312.7023.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Druss B. Evidence based medicine: does it make a difference? BMJ. 2005;330:92. doi: 10.1136/bmj.330.7482.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holdgate A, Pollock T. Systematic review of the relative efficacy of non-steroidal anti-inflammatory drugs and opioids in the treatment of acute renal colic. BMJ. 2004 doi: 10.1136/bmj.38119.581991.55. doi:10.1136/bmj.38119.581991.55. [DOI] [PMC free article] [PubMed] [Google Scholar]


