Introduction
In African countries highly affected by human immunodeficiency virus-1 (HIV-1), women are more likely to be tested for HIV-1 than men, due to scale-up of antenatal testing programs to prevent mother-to-child transmission (PMTCT) of HIV-1.1 As part of standard post-test counseling, HIV-1–seropositive women are advised to disclose their HIV-1 status to their partners and refer them for testing. Disclosure to male partners antenatally has been associated with improved adherence to prevention of mother-to-child transmission (PMTCT) regimens,2 better infant feeding practices,3 safer sex practices, and increased male partner testing.4 Conversely, women who have not disclosed their HIV-1 status to their partner have been observed to be more likely to have suboptimal adherence to PMTCT regimens,5–7 higher rates of noncompletion of PMTCT regimens,8 and fewer infants tested postnatally for HIV-1.9
In addition to disclosure's implications for infants, there are important health implications of disclosure for male partners themselves, whether the members of the couple are both affected by HIV-1 or not. Serodiscordant couples are common in sub-Saharan Africa,4,10,11 and effective prevention methods including male circumcision, pre-exposure prophylaxis, treatment as prevention/antiretroviral therapy (ART), and condom use can prevent female-to-male HIV-1 transmission if antenatal disclosure can bring men to testing.12 Similarly, disclosure to male partners in HIV-1–concordant-positive relationships creates opportunities to help men access cotrimoxazole and ART, which improve health and extend life expectancy.13 Testing male partners may also have implications outside the couple, as male partners may have other sexual partners.
Despite these findings, promotion of male partner disclosure is not a central component of most PMTCT programs. In the antenatal setting, opt-out testing has resulted in nearly all women testing routinely, but rates of partner disclosure by HIV-1–seropositive women are lower than when adults are self-initiating testing in other settings.14–16 While barriers to female-to-male disclosure are well-described,17,18 and include socio-economic vulnerability, fear of violence or abandonment, and family stigma, little has been published describing how to promote disclosure to male partners after antenatal testing.
We followed Kenyan HIV-1–seropositive women enrolled in a randomized clinical trial of herpes suppression during pregnancy until 1 year postpartum to determine disclosure rates, and to assess timing and correlates of disclosure. Our hypothesis was that women who were counseled and supported to disclose their HIV-1 status as part of a longitudinal research study would, over time, be more likely to disclose to partners.
Methods
Subjects/Setting
As part of a clinical trial using valacyclovir to reduce maternal HIV-1 RNA levels,19 we followed pregnant women from their first antenatal HIV-1–seropositive test, enrolled them in the study if eligible at 34 weeks gestation, and continued following women until 1 year postpartum. We collected data on disclosure from women at study entry and again at study exit. Women were recruited from public maternity clinics near the Mathare slum.
Procedures
Women were referred to the study at their first antenatal visit when HIV-1 infection was diagnosed. Although some women knew their HIV-1 status already, most were newly diagnosed. Informed consent for screening was obtained, and women's eligibility for the study was determined. Enrolled women were antibody-positive for both HIV-1 and herpes simplex virus-2 (HSV-2) and had a CD4 count >250 cells/μL. All 148 women were asked at enrollment whether they had disclosed their HIV-1 status to their partner. When this question was asked, women had known their HIV-1 diagnosis for at least 14 days and usually longer. After December 2009, a subset of 100 women were asked again at study exit if they had disclosed, and whether they were planning to disclose in the future.
Women met with community health workers trained in peer counseling at their monthly visits. Counseling topics included infant feeding practices, HIV-1 support systems, and testing of older children and partners; counseling sessions were unscripted, but consistently unhurried, private, and supportive, with an emphasis on empathy for each woman's situation. Community health workers were aware of the disclosure status of each woman. If a woman had not disclosed to her partner, the community health workers raised the subject of disclosure regularly, encouraging the woman to bring her partner to a voluntary counseling and testing (VCT) center for couple counseling and testing, where disclosure would occur during the testing process. Couple counseling with VCT staff was thought to be a safe, supportive place for disclosure to occur, and enabled the woman to learn her partner's status as well.
Statistical methods
Baseline characteristics of disclosing women and nondisclosing women were compared using chi-square and t-tests for categorical and continuous variables, respectively. In a subset of 100 women re-surveyed at study exit at 12 months postpartum, disclosure status and timing of disclosure were compared to enrollment results.
Results
Of the 148 women recruited into this study, 94 (71%) had completed primary school, 117 (79%) identified themselves as married or partnered, and 22 (19%) of the married women reported polygamous unions. Only 17 women (12%) had been in their current relationship less than 1 year. The median age of women in the study was 25 years and 114 (77%) had been pregnant before. At study enrollment at 34 weeks gestation, 72 (49%) of 148 women reported having disclosed their HIV-1-seropositive status to their sexual partner. Age, parity, educational level, marriage type (monogamous vs. polygamous) and employment status were not correlated with disclosure (Table 1).
Table 1.
Characteristics of Pregnant Women
| Characteristics (n=148) | N(%) or Median (IQR) | |
|---|---|---|
| Age at enrollment (years) | 25 | (22–29) |
| Married or long-term partner | 117 | (79) |
| ≤8 years educationa | 105 | (71) |
| Unemployed | 91 | (61) |
| Number of people in home | 3 | (2–4) |
| Lifetime number of sexual partnersa | 3 | (2–4) |
| Years in relationshipb | 4 | (2–7) |
| Primigravida | 34 | (23) |
| Age of sexual debut (years)a | 16 | (15–18) |
| Disclosed prior to enrollment | ||
| Yes | 72 | (48.6) |
| No | 65 | (43.9) |
| No partner | 9 | (6.1) |
| Refused | 2 | (1.3) |
| Partner tested for HIV-1 | ||
| Yes | 40 | (27.0) |
| No | 40 | (27.0) |
| Don't know | 58 | (39.2) |
| No partner | 10 | (6.8) |
N=148, an=147, bn=112 (some women not in relationship).
Disclosing women were more likely to be married or partnered (94% of disclosing women vs. 75% of nondisclosers, p=0.002) and were in partnerships longer than nondisclosing women (mean 6 vs. 4 years, respectively, p=0.006) (Table 2). Disclosure was correlated with partner's testing status, when known: 35 of 60 (58%) disclosing women reported that their partner had been tested for HIV-1, compared to 5 of 19 (26%) nondisclosing women; this difference was statistically significant (p=0.02). Although 117 women reported being in a long-term marital or partnered relationship, 138 women in the study reported a sexual partner when asked about disclosure. A majority of these women (98/138 women, 66%) reported the partner had not tested or did not know whether he had been tested. Male partners who were tested did not necessarily disclose their test results; of the 40 women reporting that their partner had been tested for HIV-1, 5 women (13%) did not know their partner's test result. Of the 35 women who knew their partner's HIV-1 test result, 31 (88%) had disclosed: 9 out of 11 women (82%) with HIV-1–seronegative partners disclosed, and 22 out of 24 women (92%) with HIV-1-seropositive partners disclosed.
Table 2.
Correlates of HIV-1 Disclosure During Pregnancy
| Correlate | N | n (%) or Mean (CI) | p Value | |
|---|---|---|---|---|
| Disclosed n=72 | Did not disclose n=65 | |||
| Age (years) | 137 | 26 (25–27) | 26 (25–27) | 0.66 |
| Lifetime number of partners | 136 | 4 (3–4) | 4 (3–5)a | 0.39 |
| Education ≤8 years | 136 | 47 (66%)a | 38 (59%) | 0.41 |
| Unemployed | 137 | 44 (61%) | 42 (66%) | 0.67 |
| Primagravida | 137 | 18 (25%) | 13 (20%) | 0.49 |
| Gestational week AZT taken | 137 | 30 (29–30) | 30 (29–30) | 0.32 |
| Infant acquired HIV-1b | 137 | 6 (4%) | 4 (3%) | 0.24 |
| HIV-1 RNA log10 copies/mL | 137 | 3.8 (3.6–4.1) | 4.0 (3.8–4.2) | 0.42 |
| Baseline CD4 | 137 | 485 (444–526) | 487 (440–533) | 0.95 |
| Married/partneredc | 137 | 68 (94%) | 49 (75%) | 0.002 |
| Time with partner (years)d | 112 | 6 (5–7) | 4 (3–5) | 0.006 |
| Partner tested for HIV-1e | 79 | 35 (58%) | 5 (26%) | 0.02 |
1 missing value; bInfant HIV-1 infection ascertained from birth until 12 months of age; cWomen defined whether they considered themselves married or partnered; dn=112: 61 disclosing women, 51 nondisclosing women; other women not in relationships or refused to answer; en=79: 60 disclosing women and 19 nondisclosing women; other women did not answer one or both questions.
Almost all women (97%) had initiated zidovudine (ZDV) for PMTCT by study enrollment and there were no differences in ZDV start date by disclosure status. There were also no differences in maternal facility delivery, mother or baby receiving single-dose nevirapine, and infant postnatal ZDV use. The 1-year infant HIV-1 transmission risk was 7%: there were 10 infant infections out of 143 live births. Infant infection did not differ by initial disclosure status. There were 4 infant infections among nondisclosing women and 6 infant infections among disclosing women (p=0.7).
After 1 year, 136 of the original 148 women (92%) remained in the study, and 100 of the 136 women (74%) were re-surveyed about disclosure at 12 months postpartum. Of these 100 women, 52 women reported disclosing and 40 had not disclosed or refused to answer. When we looked specifically at the initial group of 65 nondisclosing women, 14 new disclosures were noted, and 40 women had still not disclosed their status, with 23 women reporting that they did not ever intend to disclose. Of 52 women who reported timing of disclosure, 31 (60%) had disclosed within 3 days of testing, and 79% of disclosures occurred in the first 30 days after a positive test (Fig. 1). Of the mothers of HIV-1-infected infants who were re-surveyed at 12 months postpartum, 7 mothers of HIV-1-infected infants had disclosed and 2 still had not (1 was not re-surveyed).
FIG. 1.
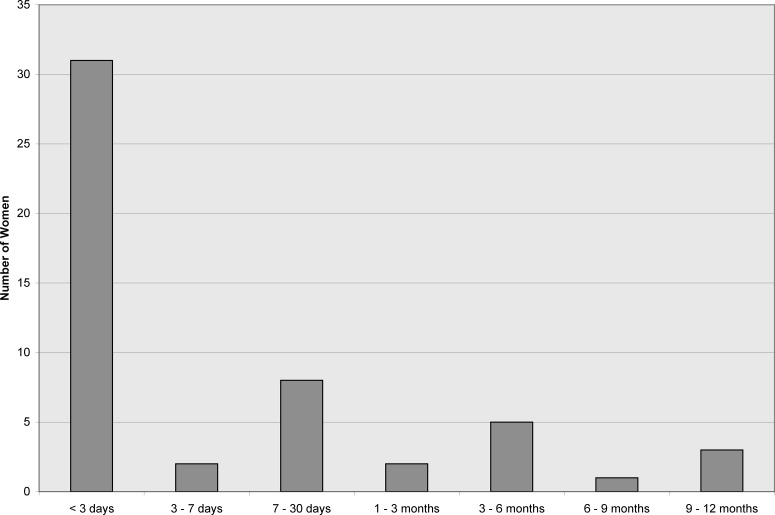
Timing of HIV-1 disclosure for 52 women with positive antenatal HIV test. Women answered this question at 12 months postpartum. This graph shows that 31 of 52 women (60%) disclosed within 3 days of their positive HIV-1 test.
Discussion
Disclosure to partners and partner testing remain key issues to achieving control of the heterosexual HIV-1 epidemic in sub-Saharan Africa. In our study population in urban Kenya, about 50% of childbearing HIV-1–seropositive women tested antenatally disclosed their status to their sexual partner during pregnancy. For the remaining women, counseling by community health workers and availability of couples counseling as a safer way to disclose resulted in few additional disclosures over 1 year's time. Most women did not know their partner's HIV-1 status. These gaps in partner disclosure highlight the need for more research and policy to promote disclosure among mothers diagnosed antenatally.
Partner factors remained key to understanding why women disclosed, with duration of partnership and partner's HIV-1 testing status significantly correlated with disclosure. Most women appeared to decide at the time of diagnosis whether to disclose, with the most common time of disclosure being within 3 days of testing. Although individual counseling encourages women that disclosure is important, suggests resources for disclosing more safely, and provides emotional support, it does not offer concrete strategies to address partner characteristics that may lead to women's reluctance to disclose.
Prior research has indicated that disclosure to male partners has important implications for infants born to HIV-1–seropositive women, but in our cohort, we did not observe differences in PMTCT outcomes between disclosing and nondisclosing women. Due to the small numbers of transmission events, we lacked statistical power to exclude anything but large differences.
There is clear evidence that promoting disclosure to partners in the antenatal setting can lead to both individual and public health benefits, but little is known about how to achieve this goal. Placing the burden of disclosure on women in our study resulted in only about 50% of women self-reporting disclosure. This is consistent with published rates of disclosure in a broad array of PMTCT settings,12,20–23 where only modest improvements in male partner disclosure have occurred during scale-up of universal testing of pregnant women. Our research indicates that rather than focusing on strategies that promote disclosure by women, such as counseling, we should emphasize strategies that make HIV-1 testing more appealing to men in this setting, such as shifts in infrastructure promoting ease and availability of testing, and reduction of stigma and barriers to male partner HIV-1 testing and treatment.14 This has been borne out in the case of Rwanda, where systematic couple counseling and community-based campaigns have been effective at increasing uptake of male partner testing. The government of Rwanda reported that of pregnant women who received an HIV-1 test in 2009, 84% of male partners were also tested for HIV-1.24 Further information about strategies used in Rwanda and adoption of couple counseling in the PMTCT infrastructure should be explored in more PMTCT settings as a mechanism to improve male partner disclosure.
Another benefit of including men in PMTCT testing efforts would be the ability to test male partners of HIV-1–seronegative expectant mothers, which could reduce HIV-1 transmission risk in serodiscordant couples and reduce maternal and infant infections through prevention of primary HIV-1 infection in pregnancy. Several studies have noted high rates of seroconversion among women in high prevalence settings who test negative in pregnancy.25,26 Bringing male partners of HIV-1–seronegative mothers for testing is still rare, but should be an important complementary activity in the PMTCT setting; this effort will be critical to success of efforts to eliminate pediatric HIV-1.
Our study was subject to limitations. It was not designed to assess disclosure as a primary outcome; information from women participating in a clinical trial may limit generalizability, and counseling to promote disclosure was general in nature and not uniform. It is possible some participants received different messages or different quantities of counseling compared to others. Systemic factors may also have limited the effectiveness of counseling messages, particularly since couple counseling centers were open during daytime hours, when men were often unable to attend. Another limitation was our ability to collect data on disclosure over time; partnerships were forming and dissolving throughout the study period, but we collected information on primary partners only, and we did not specify whether the partner was responsible for the pregnancy. Our re-survey at 12 months did not include all women and further limited our ability to track disclosure over time. Social desirability may have induced some women to report disclosing when it had not occurred, but since new disclosures were rare after study enrollment, this is unlikely to alter our results or conclusions.
In conclusion, our research shows that mothers diagnosed with HIV-1 antenatally face challenges in disclosing to their male partners. Reassuringly, many women did disclose in the immediate post-testing period; for those who did not disclose right away, counseling and support contributed to marginal improvements in disclosure over 1 year's time. Male partner testing was uncommon in our cohort, but it was significantly correlated with disclosure. More testing of couples in the antenatal setting and further research on appropriate methods to facilitate safe female-to-male HIV-1 disclosure are important paths forward to improve PMTCT outcomes, reduce HIV-1 transmission in couples, and recruit male partners to HIV-1 care and treatment.
Acknowledgments
We gratefully acknowledge the women who participated in this study, our study staff, and the staff of Mathare North Health Centre in Nairobi.
This study was supported by US National Institutes of Health (NIH) research grants [R03 HD 057773, R03 HD 057773-02S1, R01 AI076105, K24 AI087399, R01 AI06843-05 to CF, K24 HD054314 to GJS, K24 AI071113 and PO1 AI30731 to AW]; the University of Washington Center for AIDS Research (CFAR) (P30 AI027757), a Puget Sound Partners for Global Health Research and Technology Grant to BAR, and University of Washington Royalty Research Fund Grant #4027. GlaxoSmithKline donated study drug and matched placebo, but had no other role in the study. ALD was supported by NIH-funded University of Washington CFAR Training Grant (T32AI07140-32), ACR, FOO, and DNM were scholars in the International AIDS Research and Training Program, funded by the Fogarty International Center, NIH (D43-TW000007); ACR and FOO were Fogarty International Clinical Research Fellows funded by the Fogarty International Center, NIH (R24 TW007988).
Prior Presentation: This research was presented as abstract TUPE268 at the 6th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention, Rome, Italy, July 17–20, 2011.
Author Disclosure Statement
None of the authors has a commercial or other association, financial interest, activity, relationship or association that might pose a conflict of interest.
References
- 1.Kenya AIDS Indicator Survey. Nairobi, Kenya: National AIDS and STI Control Programme; 2007. 2008. Preliminary Report. [Google Scholar]
- 2.Aluisio A. Richardson BA. Bosire R. John-Stewart G. Mbori-Ngacha D. Farquhar C. Male antenatal attendance and HIV testing are associated with decreased infant HIV infection and increased HIV-free survival. J Acquir Immune Defic Syndr. 2010;56:76–82. doi: 10.1097/QAI.0b013e3181fdb4c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farquhar C. Mbori-Ngacha DA. Bosire RK. Nduati RW. Kreiss JK. John GC. Partner notification by HIV-1 seropositive pregnant women: Association with infant feeding decisions. AIDS. 2001;15:815–817. doi: 10.1097/00002030-200104130-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curran K. Baeten JM. Coates TJ. Kurth A. Mugo NR. Celum C. HIV-1 prevention for HIV-1 serodiscordant couples. Curr HIV/AIDS Rep. 2012;9:160–170. doi: 10.1007/s11904-012-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuonza LR. Tshuma CD. Shambira GN. Tshimanga M. Non-adherence to the single dose nevirapine regimen for the prevention of mother-to-child transmission of HIV in Bindura town, Zimbabwe: A cross-sectional analytic study. BMC Public Health. 2010;10:218. doi: 10.1186/1471-2458-10-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jasseron C. Mandelbrot L. Dollfus C, et al. Non-Disclosure of a pregnant woman's HIV status to her partner is associated with non-optimal prevention of mother-to-child transmission. AIDS Behav 2011. doi: 10.1007/s10461-011-0084-y. published online 2011/12/02. [DOI] [PubMed] [Google Scholar]
- 7.Duff P. Kipp W. Wild TC. Rubaale T. Okech-Ojony J. Barriers to accessing highly active antiretroviral therapy by HIV-positive women attending an antenatal clinic in a regional hospital in western Uganda. J Int AIDS Soc. 2010;13:37. doi: 10.1186/1758-2652-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirsten I. Sewangi J. Kunz A, et al. Adherence to combination prophylaxis for prevention of mother-to-child-transmission of HIV in Tanzania. PLoS One. 2011;6:e21020. doi: 10.1371/journal.pone.0021020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peltzer K. Mlambo G. Factors determining HIV viral testing of infants in the context of mother-to-child transmission. Acta Paediatr. 2010;99:590–596. doi: 10.1111/j.1651-2227.2009.01670.x. [DOI] [PubMed] [Google Scholar]
- 10.Guthrie BL. de Bruyn G. Farquhar C. HIV-1-discordant couples in sub-Saharan Africa: Explanations and implications for high rates of discordancy. Curr HIV Res. 2007;5:416–429. doi: 10.2174/157016207781023992. [DOI] [PubMed] [Google Scholar]
- 11.Were WA. Mermin JH. Wamai N, et al. Undiagnosed HIV infection and couple HIV discordance among household members of HIV-infected people receiving antiretroviral therapy in Uganda. J Acquir Immune Defic Syndr. 2006;43:91–95. doi: 10.1097/01.qai.0000225021.81384.28. [DOI] [PubMed] [Google Scholar]
- 12.Desgrees-du-Lou A. Brou H. Traore AT. Djohan G. Becquet R. Leroy V. From prenatal HIV testing of the mother to prevention of sexual HIV transmission within the couple. Soc Sci Med. 2009;69:892–899. doi: 10.1016/j.socscimed.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 13.Mermin J. Lule J. Ekwaru JP, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet. 2004;364:1428–1434. doi: 10.1016/S0140-6736(04)17225-5. [DOI] [PubMed] [Google Scholar]
- 14.Obermeyer CM. Baijal P. Pegurri E. Facilitating HIV disclosure across diverse settings: A review. Am J Public Health. 2011;101:1011–1023. doi: 10.2105/AJPH.2010.300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dillabaugh LL. Lewis Kulzer J. Owuor K, et al. Towards elimination of mother-to-child transmission of HIV: The impact of a rapid results initiative in Nyanza Province, Kenya. AIDS Res Treat. 2012;2012:602120. doi: 10.1155/2012/602120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anglewicz P. Chintsanya J. Disclosure of HIV status between spouses in rural Malawi. AIDS Care. 2011;23:998–1005. doi: 10.1080/09540121.2010.542130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makin JD. Forsyth BW. Visser MJ. Sikkema KJ. Neufeld S. Jeffery B. Factors affecting disclosure in South African HIV-positive pregnant women. AIDS Patient Care STDS. 2008;22:907–916. doi: 10.1089/apc.2007.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simbayi LC. Kalichman SC. Strebel A. Cloete A. Henda N. Mqeketo A. Disclosure of HIV status to sex partners and sexual risk behaviours among HIV-positive men and women, Cape Town, South Africa. Sex Transm Infect. 2007;83:29–34. doi: 10.1136/sti.2006.019893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drake AL. Roxby AC. Ongecha-Owuor F, et al. Valacyclovir suppressive therapy reduces plasma and breast milk HIV-1 RNA levels during pregnancy and postpartum: A randomized trial. J Infect Dis. 2012;205:366–375. doi: 10.1093/infdis/jir766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mucheto P. Chadambuka A. Shambira G. Tshimanga M. Gombe N. Nyamayaro W. Determinants of nondisclosure of HIV status among women attending the prevention of mother to child transmission programme, Makonde district, Zimbabwe, 2009. Pan Afr Med J. 2011;8:51. doi: 10.4314/pamj.v8i1.71169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardon A. Vernooij E. Bongololo-Mbera G, et al. Women's views on consent, counseling and confidentiality in PMTCT: A mixed-methods study in four African countries. BMC Public Health. 2012;12:26. doi: 10.1186/1471-2458-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olagbuji BN. Ezeanochie MC. Agholor KN. Olagbuji YW. Ande AB. Okonofua FE. Spousal disclosure of HIV serostatus among women attending antenatal care in urban Nigeria. J Obstet Gynaecol. 2011;31:486–488. doi: 10.3109/01443615.2011.563637. [DOI] [PubMed] [Google Scholar]
- 23.Medley A. Garcia-Moreno C. McGill S. Maman S. Rates, barriers and outcomes of HIV serostatus disclosure among women in developing countries: Implications for prevention of mother-to-child transmission programmes. Bull World Health Organ. 2004;82:299–307. [PMC free article] [PubMed] [Google Scholar]
- 24.UNGASS Country Progress Report Rwanda January 2008–December 2009. [Aug 1;2012 ]. http://data.unaids.org/pub/Report/2010/rwanda_2010_country_progress_report_en.pdf http://data.unaids.org/pub/Report/2010/rwanda_2010_country_progress_report_en.pdf
- 25.Kinuthia J. Kiarie JN. Farquhar C, et al. Cofactors for HIV-1 incidence during pregnancy and postpartum period. Curr HIV Res. 2010;8:510–514. doi: 10.2174/157016210793499213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moodley D. Esterhuizen T. Reddy L, et al. Incident HIV infection in pregnant and lactating women and its effect on mother-to-child transmission in South Africa. J Infect Dis. 2011;203:1231–1234. doi: 10.1093/infdis/jir017. [DOI] [PubMed] [Google Scholar]


