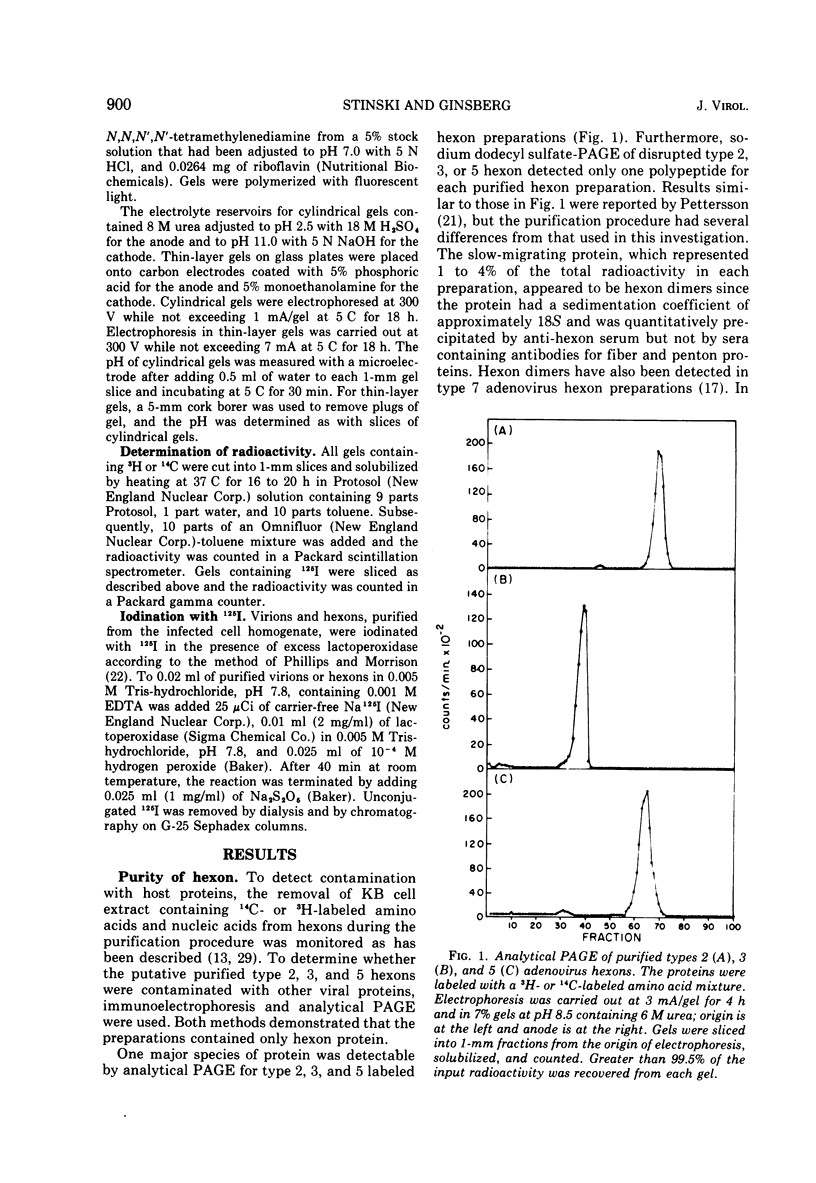
Abstract
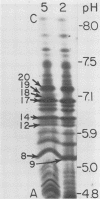
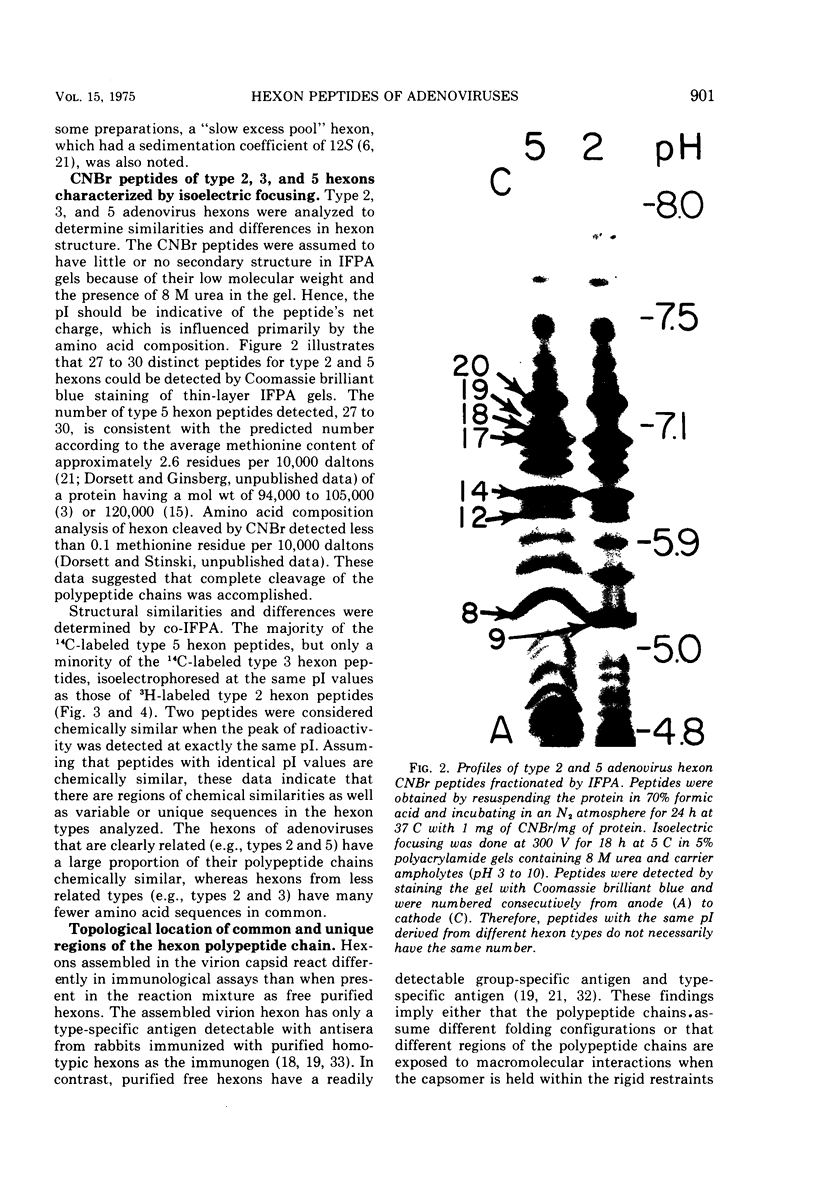
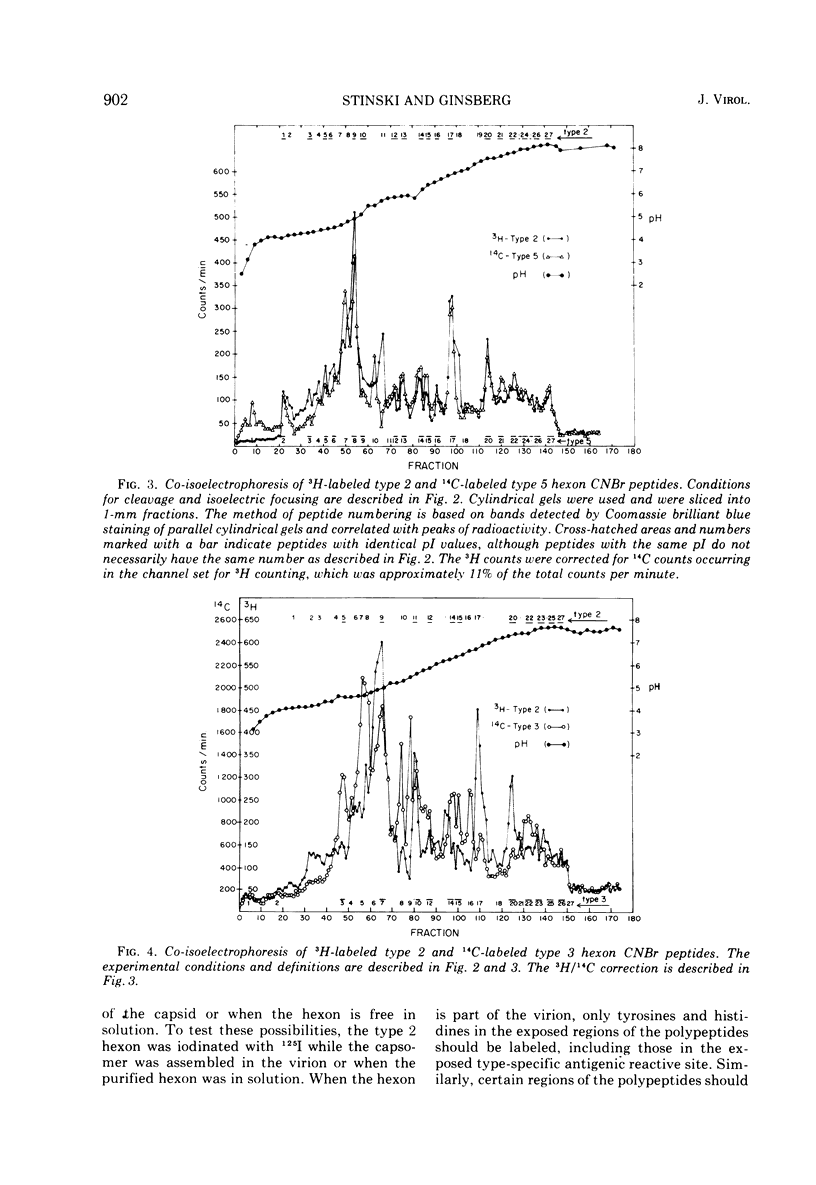
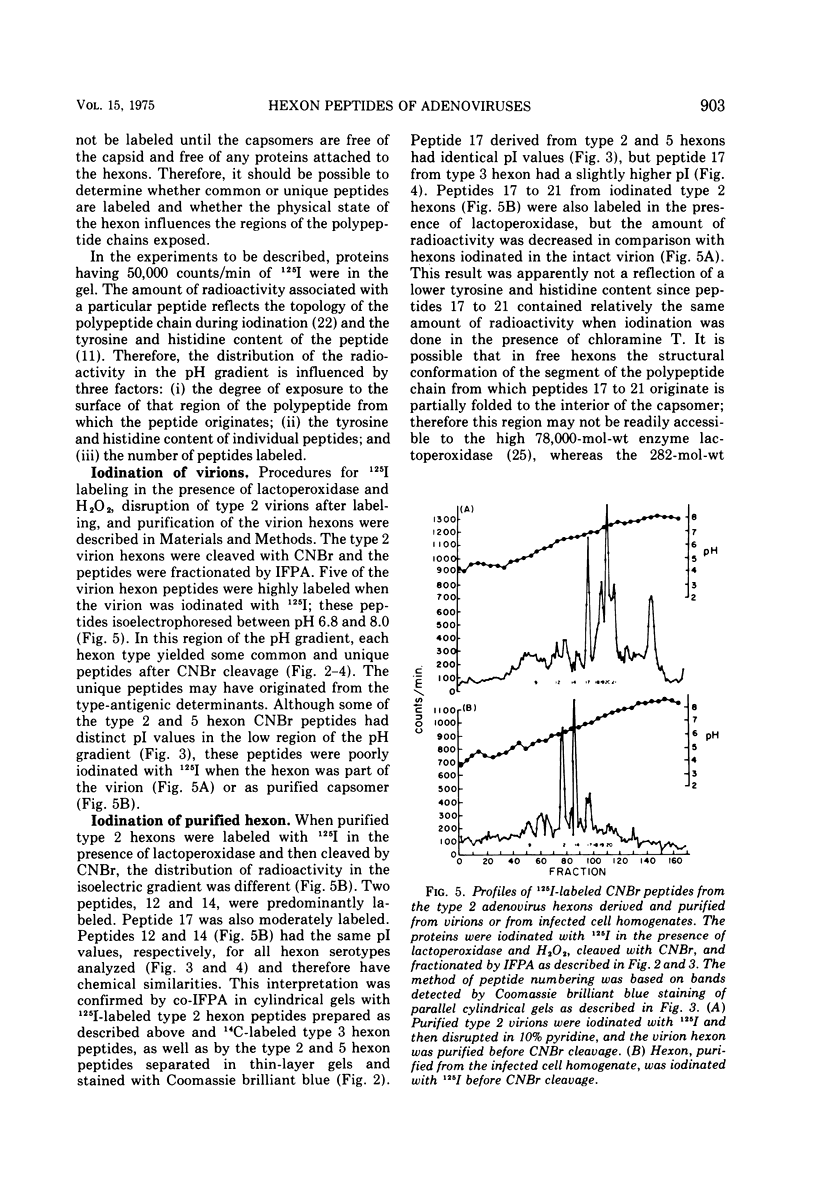
Peptides of hexons from type 2 and 5 (subgroup III) and type 3 (subgroup I) adenoviruses were produced by treatment with cyanogen bromide and were separated by isoelectric focusing in polyacrylamide gels containing 8 M urea. Peptides with identical isoelectric points, but from different hexon types, were considered to have structural similarities. According to this criterion for chemical relatedness, about two-thirds of the type 2 and 5 hexon peptides may be considered similar. In contrast, the majority of the type 3 hexon peptides differed chemically from peptides of type 2 and 5 hexons. Virions and free hexons were iodinated with 125-I in the presence of lactoperoxidase and H-2-O-2. When 125-I-labeled virions were disrupted and the hexon was purified, the highly labeled cyanogen bromide peptides were labeled. When purified hexons from the excess cellular pool were iodinated, peptides common to types 2, 3, and 5 (peptides 12 and 14) were most extensively labeled. Thus, hexons assembled in virions and those free in solution were iodinated differently. The data suggest that immunologically the hexons in viral capsids react differently from unassembled hexons because the polypeptide chains assume slightly different folding configurations in the two hexon forms and therefore expose different regions of the protein to antibodies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awdeh Z. L., Williamson A. R., Askonas B. A. Isoelectric focusing in polyacrylamide gel and its application to immunoglobulins. Nature. 1968 Jul 6;219(5149):66–67. doi: 10.1038/219066a0. [DOI] [PubMed] [Google Scholar]
- Bello L. J., Ginsberg H. S. Relationship between deoxyribonucleic acid-like ribonucleic acid synthesis and inhibition of host protein synthesis in type 5 adenovirus-infected KB cells. J Virol. 1969 Feb;3(2):106–113. doi: 10.1128/jvi.3.2.106-113.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornick G., Sigler P. B., Ginsberg H. S. Mass of protein in the asymmetric unit of hexon crystals--a new method. J Mol Biol. 1973 Feb 5;73(4):533–538. doi: 10.1016/0022-2836(73)90099-5. [DOI] [PubMed] [Google Scholar]
- Franklin R. M., Pettersson U., Akervall K., Strandberg B., Philipson L. Structural proteins of adenovirus. V. Size and structure of the adenovirus type 2 hexon. J Mol Biol. 1971 May 14;57(3):383–395. doi: 10.1016/0022-2836(71)90098-2. [DOI] [PubMed] [Google Scholar]
- Friedman M. P., Lyons M. J., Ginsberg H. S. Biochemical consequences of type 2 adenovirus and Simian virus 40 double infections of African green monkey kidney cells. J Virol. 1970 May;5(5):586–597. doi: 10.1128/jvi.5.5.586-597.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS E., WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [PubMed] [Google Scholar]
- Ginsberg H. S., Pereira H. G., Valentine R. C., Wilcox W. C. A proposed terminology for the adenovirus antigens and virion morphological subunits. Virology. 1966 Apr;28(4):782–783. doi: 10.1016/0042-6822(66)90271-6. [DOI] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R., Wensink P. C., MacHattie L. A., Thomas C. A., Jr Adenovirus DNA. I. Molecular weight and conformation. Proc Natl Acad Sci U S A. 1967 May;57(5):1302–1309. doi: 10.1073/pnas.57.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Lawrence W. C., Ginsberg H. S. Intracellular uncoating of type 5 adenovirus deoxyribonucleic acid. J Virol. 1967 Oct;1(5):851–867. doi: 10.1128/jvi.1.5.851-867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Ginsberg H. S. Mechanism by which fiber antigen inhibits multiplication of type 5 adenovirus. J Virol. 1967 Aug;1(4):747–757. doi: 10.1128/jvi.1.4.747-757.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. II. Soluble proteins, cores, top components and the structure of the virion. Virology. 1968 Sep;36(1):126–136. doi: 10.1016/0042-6822(68)90122-0. [DOI] [PubMed] [Google Scholar]
- Montgomery P. C., Williamson A. R. Molecular restriction of anti-hapten antibody elicited in neonatal rabbits. Nature. 1970 Dec 26;228(5278):1306–1308. doi: 10.1038/2281306a0. [DOI] [PubMed] [Google Scholar]
- Norrby E., Marusyk H., Hammarskjöld M. L. The relationship between the soluble antigens and the virion of adenovirus type 3. V. Identification of antigen specificities available at the surface of virions. Virology. 1969 Jul;38(3):477–482. doi: 10.1016/0042-6822(69)90161-5. [DOI] [PubMed] [Google Scholar]
- Nász I., Lengyel A., Cserba I. Comparative studies of adenovirus hexon antigens. Arch Gesamte Virusforsch. 1972;36(1):80–92. doi: 10.1007/BF01250298. [DOI] [PubMed] [Google Scholar]
- Pereira H. G., Laver W. G. Comparison of adenovirus types 2 and 5 hexons by immunological and biochemical techniques. J Gen Virol. 1970 Nov;9(2):163–167. doi: 10.1099/0022-1317-9-2-163. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Philipson L., Höglund S. Structural proteins of adenoviruses. I. Purification and characterization of the adenovirus type 2 hexon antigen. Virology. 1967 Dec;33(4):575–590. doi: 10.1016/0042-6822(67)90057-8. [DOI] [PubMed] [Google Scholar]
- Pettersson U. Structural proteins of adenoviruses. VI. On the antigenic determinants of the hexon. Virology. 1971 Jan;43(1):123–136. doi: 10.1016/0042-6822(71)90230-3. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. The arrangement of proteins in the human erythrocyte membrane. Biochem Biophys Res Commun. 1970 Jul 27;40(2):284–289. doi: 10.1016/0006-291x(70)91007-7. [DOI] [PubMed] [Google Scholar]
- Piña M., Green M. Biochemical studies on adenovirus multiplication. IX. Chemical and base composition analysis of 28 human adenoviruses. Proc Natl Acad Sci U S A. 1965 Aug;54(2):547–551. doi: 10.1073/pnas.54.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prage L., Pettersson U., Höglund S., Lonberg-Holm K., Philipson L. Structural proteins of adenoviruses. IV. Sequential degradation of the adenovirus type 2 virion. Virology. 1970 Oct;42(2):341–358. doi: 10.1016/0042-6822(70)90278-3. [DOI] [PubMed] [Google Scholar]
- ROSEN L. Hemagglutination by adenoviruses. Virology. 1958 Jun;5(3):574–577. doi: 10.1016/0042-6822(58)90050-3. [DOI] [PubMed] [Google Scholar]
- Rombauts W. A., Schroeder W. A., Morrison M. Bovine lactoperoxidase. Partial characterization of the further purified protein. Biochemistry. 1967 Oct;6(10):2965–2977. doi: 10.1021/bi00862a002. [DOI] [PubMed] [Google Scholar]
- Smith K. O., Trousdale M., Gehle W. Adenovirus-antibody agglutination reactions. J Immunol. 1965 Nov;95(5):810–817. [PubMed] [Google Scholar]
- Valentine R. C., Pereira H. G. Antigens and structure of the adenovirus. J Mol Biol. 1965 Aug;13(1):13–20. doi: 10.1016/s0022-2836(65)80076-6. [DOI] [PubMed] [Google Scholar]
- Velicer L. F., Ginsberg H. S. Cytoplasmic synthesis of type 5 adenovirus capsid proteins. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1264–1271. doi: 10.1073/pnas.61.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velicer L. F., Ginsberg H. S. Synthesis, transport, and morphogenesis of type adenovirus capsid proteins. J Virol. 1970 Mar;5(3):338–352. doi: 10.1128/jvi.5.3.338-352.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterberg O. Isoelectric focusing of proteins in polyacrylamide gels. Biochim Biophys Acta. 1972 Jan 26;257(1):11–19. doi: 10.1016/0005-2795(72)90248-6. [DOI] [PubMed] [Google Scholar]
- WILCOX W. C., GINSBERG H. S., ANDERSON T. F. STRUCTURE OF TYPE 5 ADENOVIRUS. II. FINE STRUCTURE OF VIRUS SUBUNITIS. MORPHOLOGIC RELATIONSHIP OF STRUCTURAL SUBUNITS TO VIRUS-SPECIFIC SOLUBLE ANTIGENS FROM INFECTED CELLS. J Exp Med. 1963 Aug 1;118:307–314. doi: 10.1084/jem.118.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILCOX W. C., GINSBERG H. S. PRODUCTION OF SPECIFIC NEUTRALIZING ANTIBODY WITH SOLUBLE ANTIGENS OF TYPE 5 ADENOVIRUS. Proc Soc Exp Biol Med. 1963 Oct;114:37–42. doi: 10.3181/00379727-114-28579. [DOI] [PubMed] [Google Scholar]
- WILCOX W. C., GINSBERG H. S. Purification and immunological characterization of types 4 and 5 adenovirus-soluble antigens. Proc Natl Acad Sci U S A. 1961 Apr 15;47:512–526. doi: 10.1073/pnas.47.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILCOX W. C., GINSBERG H. S. STRUCTURE OF TYPE 5 ADENOVIRUS. I. ANTIGENIC RELATIONSHIP OF VIRUS-STRUCTURAL PROTEINS TO VIRUS-SPECIFIC SOLUBLE ANTIGENS FROM INFECTED CELLS. J Exp Med. 1963 Aug 1;118:295–306. doi: 10.1084/jem.118.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]