Abstract
To investigate the clinical efficacy of combining digital subtraction angiography-guided percutaneous vertebroplasty (PVP) and 125I seeds implantation for the treatment of spinal osteoplastic metastasis. A combination of PVP and 125I implantation was conducted for 50 patients with spinal osteoplastic metastasis, while the other 50 patients who received regular radiation therapy were used as a comparison. Visual analogue pain scale (VAS) and score of life quality (EORTCQLQ-30) were determined for all the patients. Surgery was successful in 89 spinal segments of vertebral body in 50 patients. Each segment of vertebral body was injected with 1–5 mL (2.8 mL for thoracic and 3.1 mL for lumbar vertebral body on average) of bone cement. Postoperative X-ray and CT examination showed that all the patients in the PVP group achieved spinal stability. During the follow-up examination from 6 months to 5 years, 49 patients (98.0%) had significantly relieved back pain, and only 1 case (2.0%) had no obvious improvement. Postoperative VAS score and Karnofsky performance score (KPS) were significantly different from the preoperative scores (p<0.05); and compared to the regular treatment group, PVP combined 125I seeds showed much better clinical efficacy (p<0.05). PVP is a minimally invasive treatment with easy operation and less complications. PVP can effectively relieve the pain, stabilize the spine, improve the life quality, and reduce the occurrence of paraplegia in patients with spinal osteoplastic metastasis. Utilization of 125I seeds with PVP can enhance the clinical efficacy.
Key words: 125I seeds, metastasis, percutaneous vertebroplasty, osteoblastic, spine
Introduction
Spinal metastasis is a common complication of cancer, and can be divided into osteoplastic, osteolytic, and mixed bone metastases.1 The key difference between osteoplastic and osteolytic metastasis lies in the different functions of cytokines released from the microenvironment of bone tissues. The activated cytokines during osteoplastic metastasis can facilitate osteogenesis. Most of the osteoplastic metastasis derives from prostate cancer. Some of them may also be derived from breast, lung, bladder, and nasopharyngeal cancers.2 In addition to severe spinal pain, osteoplastic metastasis can also cause pathological vertebral fractures, spinal cord compression, and paraplegia, which not only speeds up the patient's death, but also seriously decreases the patient's life quality. In recent years, with the development of a variety of comprehensive treatments for cancers, the survival time of patients was significantly increased and the negative attitude toward the treatment of spinal metastasis has been changed. Open surgery has large trauma, high incidence of complications, and needs long period of recovery, which interferes with the comprehensive chemotherapies for the primary tumors. For those damages across multiple segments of the spines, anterior and posterior surgeries are difficult to perform.3
125I is a synthetic radioisotope of iodine, which was often used for brachytherapy.4125I is a low-energy radioactive isotope that can emit γ ray and X-ray, which are of low enough energy to deliver a higher radiation dose to nearby tissues with a good curative effect and a little injury. The half-life of 125I is around 59 days. In the first few half-lives, a larger fraction of tumor cells are destroyed, which is apt to improve biological effects and clinical practice; the thickness of its' half-value layer in lead is 0.0025 cm, which makes it easier to protect operators. As a matter of fact, beyond the treatment target volumes, the dose drops rapidly with distance, leading to limited normal tissue damages.5,6
Percutaneous vertebroplasty (PVP) is a recently developed treatment for spinal metastases. It has been widely used in the treatment of osteolytic metastases and achieved satisfactory efficacy.7,8 Combinational utilization of 125I during surgery can improve the antitumor effect.9 Because the pressure in the diseased vertebral bodies of osteoplastic metastasis is high, it is difficult to inject bone cement. Thus, osteoplastic metastasis has been recognized as a contraindication of PVP. From May 2003 to June 2010, we treated 50 osteoplastic metastasis patients with the combination of PVP and 125I seeds implantation to compare with the effect of regular single radiotherapy. A satisfactory clinical efficacy was achieved.
Materials and Methods
Patient demographics and clinical data
The study was approved by the Advisory Committee of Tumor Hospital of Yunnan Province (China) and the hospital independent ethics committee. The information of patients is clarified in Table 1. The patients were randomly divided into the PVP combined 125I seeds implantation group and the regular radiotherapy group. Selection of the patients was based on the following five criteria. One, the patients did not have serious illness in the heart, lung, and brain, could lie on the back for 2 hours; two, the patients had pathological diagnosis for primary tumor and diseased vertebral bodies; three, back and lumbar pain were the main clinical symptoms; four, spinal osteoplastic impairment was confirmed by a CT scan; five, the expected survival period should be longer than 3 months. Regular chemotherapy based on the primary tumor and other comprehensive treatments were performed after PVP and radiation therapy, but radiotherapy was not performed for the PVP group. From Table 1, we could see there was no significant difference among patients between the two groups.
Table 1.
Baseline Patients and Tumor Data
| Parameters | PVP+125I group | Radiotherapy group | p-Value |
|---|---|---|---|
| Average age | 61.14±5.21 | 59.83±6.61 | 0.342 |
| Gender | |||
| Male | 20 | 19 | 0.653 |
| Female | 30 | 31 | |
| Primary tumor | |||
| Colon | 1 | 2 | |
| Prostate | 10 | 11 | |
| Breast | 19 | 18 | 0.732 |
| Lung | 20 | 19 | |
| Number of vertebral lesions | |||
| 1 | 31 | 29 | |
| 2 | 17 | 18 | 0.625 |
| 3 | 2 | 3 | |
| Number of metastatic foci | |||
| Thoracic vertebra | 38 | 39 | 0.563 |
| Lumbar vertebra | 33 | 35 | |
| Average VAS | 8.73±0.31 | 8.34±0.56 | 0.882 |
| Average EORTC score | 61.23±3.21 | 61.70±4.14 | 0.431 |
PVP, percutaneous vertebroplasty; VAS, visual analogue pain scale; EORTC, European Organization for Research and Treatment of Cancer.
Equipment for bone cement injection and drugs
Equipment for PVP, including needles, rotary syringe, and pressure apparatus were purchased from the Shangdong Guanlong Company. Isotope 125I was provided by the Atomic Energy Research Institute. The radioactive source is a cylindrical apparatus with a diameter of 0.8 mm and a length of 4.5 mm. The dose deposited by 125I seeds drops steeply with distance. A half-value layer is 0.025 mmPb. The surface was wrapped with the titanium and the source activity was from 0.3 to 1 mCi. They were sterilized by immersing in the bromogeramine solution for 30 minutes.
The treatment planning system was provided by the National Nuclear Corporation Beijing Kelin Zhong Medical Technology Institute. Before treatment, CT/MRI images were scanned into the system software for a three-dimensional stereoscopic digital-based image reconstruction. According to the lesion size, location, and the relationship with the surrounding normal tissues, three-dimensional icons, an isodose curve, and the absorbed dose instructions were precisely formulated (Fig. 1). Treatment plan was generated according to the initial dose of radioactive sources, coordinates of application device, and the depth of the needle.
FIG. 1.
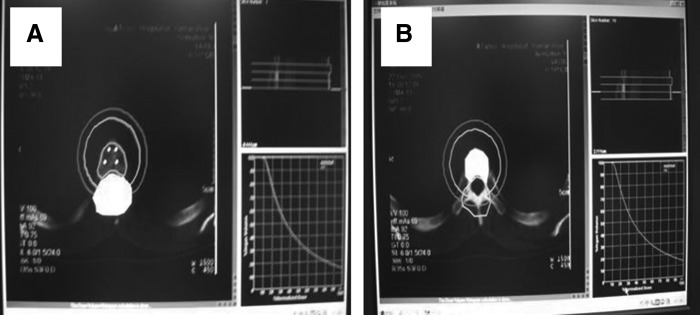
Therapy plan system and determination of the prescription dosage in the target areas. (A) Determination of the prescription dosage in the target areas; (B) Determination of maximum tolerant dosage of spinal cord.
Methods of surgery
X-ray, CT, and MRI examinations were performed before surgery to determine the location, number, and collapse degree of the diseased vertebral body. In addition, we also determined the degree of damage in the bone, integrity of the back wall of the vertebral body, pedicle infiltration, and pressures on the spinal cord. Routine cardiopulmonary function, blood sugar, prothrombin time, liver and kidney function and iodine allergy tests were performed. Fifteen (15) minutes before surgery, the analgesic treatment was conducted. Surgery was completed in the operating room under the guidance of the digital subtraction angiography (DSA) machine. Patients were in a prone position and the pedicle approach was adopted. The tilt angle of the pedicle, the lateral opening distance of the spinous process in the puncturing point and the depth from the puncture point to the pedicle were measured. The puncture point was located 2–3 cm beside the spinous process. With 1% lidocaine local anesthesia and orthotropic perspective, when the needle reached the depth of the cortical bone and the needle did not exceed the leading edge of the pedicle, the needle should be located within the pedicle shadow bull's eye sign. When the needle penetrated the bone cortex into the vertebral body, the needle core was removed and the needle was used as a channel to implant the 125I seeds. The distance between the every two 125I seeds injection sites in the target areas was 0.3 cm. The bilateral transpedicular approach should be used for insertion of the needle. For example, one side of the needle tip was in the mid-upper part of the vertebral body and the other side of the needle tip was in the mid-lower part of the vertebral body, so that 125I seeds were distributed in a three-dimensional manner in the vertebral body (Fig. 2). The needle with a bevel surface should be used because the direction of the needle can be adjusted once it was inserted and the locations where the 125I seeds were implanted were more satisfactory. After implantation of the 125I seeds, 5 mL of contrast agents were injected. Dispersion of the contrast agents and venous flow in the vertebral body were recorded by DSA. Residual contrast agent and blood in the vertebral body were absorbed with negative pressure to reduce the pressure in the vertebral body. Bone cement (PMMA with addition of nonion contrast agent) was sucked into the syringe after mixing. Bone cement was injected once it was in the tooth paste phase. Injection should be monitored by the lateral fluoroscopy (Fig. 3A). Leakage of the bone cement to the outside of the vertebral body was closely monitored. During injection, the needle direction was rotated continuously to avoid the hardening of the osteoplastic lesions. Good filling of the bone cement should be achieved to reduce the occurrence of leakage. After injection, the puncturing needle was pulled out to the cortical bone and the needle core was inserted. The puncturing needle was rotated to avoid the sticking by bone cement. The needle was pulled out before hardening of the bone cement (Fig. 3B). The amount of the bone cement was normally 1–5 mL (2.8 mL for thoracic and 3.1 mL for lumbar vertebral body on average). About the amount of bone cement injected, one thing needs to be noted, in the previous study, for the osteolytic lesions, generally average 4.5 mL for thoracic vertebral body and 6.2 mL for lumbar vertebral body were selected for PVP operation.9 Compared with osteolytic metastasis, osteoplastic metastasis caused a higher bone density in vertebra, thus left less space for bone cement to be injected, so in this present study, less amount of bone cement was injected; In addition, higher pressure was another reason for us to reduce the injected volume. The amount of implanted 125I seeds was 7–20 seeds/patient, with a mean of 10.5 seeds/patient. The initial dose rate was 0.4–1.3 cGy/h/seed at 1 cm distance, the 90% isodose line covered 90% of the tumor target volume, and matching peripheral dosage of 80–100 Gy. After 15–20 minutes of injection with bone cement (polymerization of bone cement was completed), the CT scan was performed (Figs. 4A, B and 5A, B).
FIG. 2.
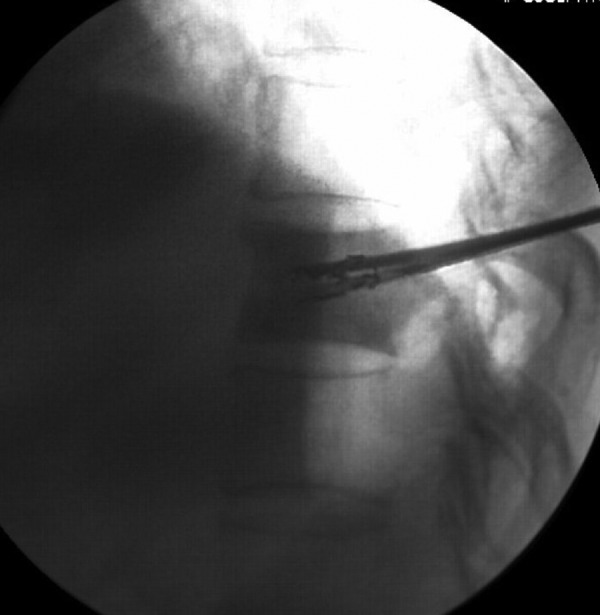
T11 osteoblastic metastasis from right lung cancer. During implantation of 125I seeds, one side of the needle was directed to the mid-upper part of the vertebral body, while the other side of the needle was directed to the mid-lower part of the vertebral body. The 125I seeds were distributed in the vertebral body in a 3D manner.
FIG. 3.
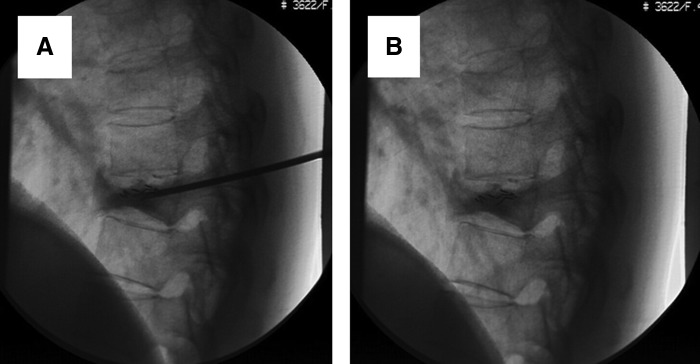
Treatment of T9 osteoblastic metastasis from left breast cancer with combination of PVP and implantation of 125I seeds during surgery (A) and after surgery (B). Digital subtraction angiography showed that 125I seeds were well distributed and bone cement was well filled. PVP, percutaneous vertebroplasty.
FIG. 4.
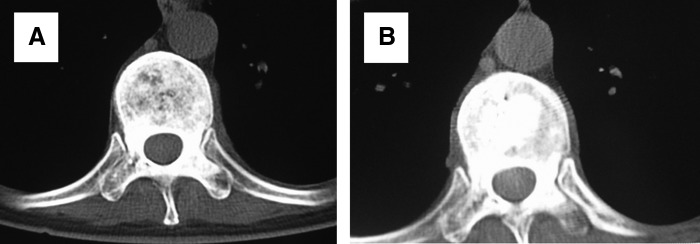
Treatment of T9 osteoblastic metastasis from left breast cancer with combination of PVP and implantation of 125I seeds during surgery (A) and after surgery (B). CT examination showed that bone cement was distributed in a diffusion manner.
FIG. 5.
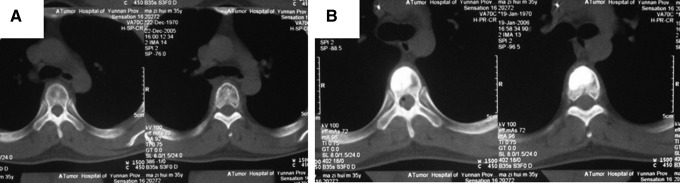
Treatment of T5 osteoblastic metastasis from right testicular seminoma with combination of PVP and implantation of 125I seeds during surgery (A) and after surgery (B). CT examination showed that bone cement was well filled.
Radiotherapy for regular treatment group
The treatment scheme consisted of irradiation of 6 MV X to thoracic and lumbar vertebrae in parallel opposed fields, including the diseased vertebrae and adjacent ones about 7–8 cm in diameter with a total dose of 3500–4600 cGy and a fractionated dose of 200–300 cGy, 5 times a week. The less serious patients would have a total dose of 2500∼3500 cGy and a fractionated dose of 200∼300 cGy, 2 times a week.
Assessment of treatment
The assessment about pain and life quality would be taken 1 week and 1 month after PVP or first radiotherapy. The follow-up data were collected 1 month, 6 months and 1 year after the treatment. The CT scan was supposed to be taken at intervals of 3 months aiming to observe the possibility of recurrence or a pathological fracture in the diseased region.
For the pain evaluation, patients were asked to confirm the pain degree according to the visual analogue pain scale (VAS), which used 0–10 to indicate different pain levels from mild to severe. Life quality assessment was represented by European Organization for Research and Treatment of Cancer (EORTC) score. Inquiry questionnaire of QLQ-C30 (Version 3.0) was developed by EORTC. It is composed of 30 questions covering 15 aspects, such as 10 motor functions, sensation, emotion, symptom, and other 5 questions. It is designed to evaluate life quality of cancer patients. The higher the score is the miserable the life would be. Meanwhile, vertebral CT/MRI was used to compare the changes of imaging before and after treatment, aimed to evaluate lesion condition filled with bone cement, leakage, vertebral compression, pathological fracture, and reoccurrence of diseased region.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 (SPSS Inc., Chicago, IL). All the data are recorded as average±standard deviation. The Student paired t-test was used for quantitative data and p<0.05was considered significant difference.
Results
Operative complications
We achieved a 100% success rate of the surgery. During the surgery, 2 patients reduced blood pressure, oxygen saturation, and oxygen pressure in arterial blood, and increased the level of thromboxane. After oxygen uptake and intravenous injection of dexamethasone, the parameters returned to the normal level. Infection and bleeding did not occur in the injected vertebral bodies and puncturing points. Leakage of PMMA to the anterior or lateral side of the vertebral body occurred in 42.0% (21/50) of the patients after PVP. However, no clinical symptoms were observed and no special treatments were applied. No spinal cord compression, nerve root compression, and other complications occurred. Vertebral body displacement, further vertebral body compression, spinal cord or nerve root compression, paralysis, and vertebral pain did not occur. The CT scan showed that bone cement was distributed in the surrounding or central areas of the tumor in a diffused manner (Fig. 5).
Imaging change
The follow-up CT results showed that all the diseased regions were filled with bone cement. Vertebral compression did not occur in PVP combined with radiotherapy group, but it happened in the single radiotherapy group with 2 cases in the first month, 7 cases in the 6th month, and 1 case after a year, totaling 17 cases or 20%, of which 3 patients suffered paraplegia. MRI showed that 6 cases in the single radiotherapy group have the reoccurrence tendency, but none in the combining group.
Pain alleviation
As Table 1 indicates, all patients suffered pain in various degrees, and the average VAS scores showed no difference in statistics (p>0.05) at beginning between the two treated groups (combined 8.73±0.31 vs. single 8.34±0.56). From Table 2, we found that the pain was relieved after treatment for both treated groups, however, the combined group reduced rapidly compared to the radiotherapy group one week after surgery; the VAS in the combined group dropped from 8.73±0.31 to 3.73±0.66, but the radiotherapy group did not show a significant improvement, from 8.34±0.56 to 8.43±0.34. In the other three later time points, 1 month, 6 months, and 1 year, all the VAS in the combined group was significantly lower than in the single radiotherapy group (p<0.01), after 1 month, 2.54±0.50 versus 5.46±0.72, after 6 months, 1.25±0.43 versus 3.54±0.35, 1 year later, 1.32±0.37 versus 4.73±0.28.
Table 2.
Comparison of NRS and EORTCQLQ-30 Scores Before and After Percutaneous Vertebroplasty
| |
VAS |
EORTCQLQ-30 |
||||
|---|---|---|---|---|---|---|
| Combined | Radiotherapy | p-Value | Combined | Radiotherapy | p-Value | |
| Before treatment | 8.73±0.31 | 8.34±0.56 | 0.882 | 61.23±3.21 | 60.73±4.03 | 0.536 |
| 1 week after treatment | 3.73±0.66 | 8.43±0.34 | 0.000 | 47.41±3.69 | 60.20±3.17 | 0.000 |
| 1 month after treatment | 2.54±0.50 | 5.46±0.72 | 0.000 | 24.23±2.45 | 32.57±4.32 | 0.000 |
| 6 months after treatment | 1.25±0.43 | 3.54±0.35 | 0.000 | 21.78±4.11 | 36.64±5.73 | 0.000 |
| 1 year after treatment | 1.32±0.37 | 4.73±0.28 | 0.000 | 18.96±4.79 | 40.19±3.32 | 0.000 |
Paired samples test is adopted before and after treatment.
Quality of life
Table 2 shows the scores of EORTCQLQ-C30 inquiry questionnaires from two groups decreased after 1 month treatment, which indicated that the quality of life had been improved for both. In PVP combined with 125I group, the score of EORTCQLQ-C30 improved rapidly compared to the radiotherapy group. One week after surgery, for the combined group, the life quality had significant improvement (61.23±3.21 vs. 47.41±3.69, p<0.01),for the single radiotherapy group, no obvious improvement was observed (60.73±4.03 vs. 60.20±3.17, p>0.05), In the other three later time points, 1 month, 6 months, and 1 year, same as VAS scores, all the EORTCQLQ-30 in the combined group was significantly lower than in the radiotherapy group (p<0.01), after 1 month, combined 24.23±2.45 versus single 32.57±4.32, after 6 months, 21.78±4.11 versus 36.64±5.73, 1 year later, 18.96±4.79 versus 40.19±3.32.
Discussion
The X-ray features of spinal osteoplastic metastases were characterized by the patchy, nodular, floccules high-density shadow located in the cancellous bones. The border of shadow could be clear or unclear and gradually migrating to the normal bone structure. Bone cortex is normally intact, the trabecular is thickened, and the trabecular gap is narrowed or even disappeared. Although the X-ray feature of osteoplastic metastasis is the enhancement of bone density, the hardness of the metastatic bones is decreased due to the disordered bone growth, which may also cause a pathological fracture. The purpose of surgery is to prevent a pathological fracture, reduce tumor burden, alleviate the symptoms, strengthen the skeletal system, and achieve weight-bearing as soon as possible. Common surgical methods include filling with tumor eliminating bone cement, internal fixation, and external fixation. Jónsson et al. thought that as long as surgical indications were understood and appropriate surgical approaches were selected, the pain could be alleviated, life quality also could be improved, and life span can be extended.10 However, high hardness in the spinal osteoplastic metastatic lesions blocked the puncturing pathways. In addition, high pressure within the vertebral bodies increased the risk of leakage. Therefore, most of the scholars regarded spinal osteoplastic metastasis as a contraindication for PVP.11,12
We speculate that reduced mechanical strength around the lesion is one of the reasons causing vertebral compression and pain. The surrounding areas of osteoma lesions should be the targets for PVP treatment of spinal osteoplastic metastasis. Through the injection of bone cement to the target regions, the mechanical strength can be increased,13 pain can be relieved, and further damage of vertebral body can be prevented. Operation for all the patients in this study was in accordance with this principle. Puncturing needle with a bevel surface was utilized, the direction of the needle was adjusted continuously under the monitor of DSA and injection of bone cement to the target regions was successfully completed with good distribution. Twenty-one cases (42.0%) had a small amount of PMMA leakage after PVP, compared with the previous study; this is a higher ratio. In the previous study, we had reported that minor paravertebral leakage of bone cement occurred in 6 patients among 40 patients (15.0%) who received same treatment for osteolytic metastasis between July 2004 and July 2006.9 We hypothesize the reason was contributed by the higher pressure and limited space in the vertebral body which osteoplastic metastasis caused. Compared with osteolytic lesions and other mixed bone metastasis, osteoplastic lesions increased the bone density which caused high pressure, thus it was easy to pump the bone cement out. However, significant clinical symptoms had not been observed and no special treatment was performed. That means, although bone cement leakage was considered the most common complication in this kind of operation, as matter of fact, in actual clinical treatment, it does not influence the efficacy much and does not cause serious trouble in patient treatment.
As a local treatment, PVP can be applied with other therapies to obtain a complementary and enhancing effect. Radiotherapy does not affect the mechanical properties of bone cement and efficacy itself.14125I seeds have lots of advantages, such as long half-life, low energy, sustainability, and precise positioning. 125I seeds can inhibit the uncontrolled proliferation of tumor cells to achieve antitumor effects.15–17 Based on the volume and density of the target cancer and the relationship with the adjacent organs, radioactive sources are appropriately implanted. Implantation of radioactive sources accurately to the target tissue can achieve direct blast effect to maximally kill the cancer cell and minimally damage the normal tissues.18,19 Forty-nine patients (98.0%) had postoperative pain relief. VAS score and EORTCQLQ-30 score were decreased significantly compared with preoperative scores. One case of T6 metastatic lung cancer with formation of soft tissue mass in the posterior side of vertebral body received combination of PVP and implantation of 125I seeds. Two months after surgery, MRI showed that the soft tissue mass in the posterior side of vertebral body completely disappeared. One year after surgery, MRI examination did not identify recurrence of local tumor, indicating that the local tumor was well controlled. The highlight of this study is to explore the possibility and potency of PVP implement in osteoplastic metastasis patients.
There is also limitation in this study. At present, 125I has been successfully used in treating prostatic cancers. Meanwhile, 125I was often used in other advanced cancer treatment. There is no unified dose standard for 125I being used in treating other tumors, so clinical experience is very important in practice. 125I seeds can cause myopathy if overdosed, compared with soft tissue, bone tissue has a higher density and better attenuation effect of radiation, so the implanted dose of 125I (80–100 Gy) in bone tissue was smaller compared with soft tissue at a distance. Because the dose of 125I decreased rapidly with the increasing implanted distance, the damage to the surrounding tissues could be reduced by cutting down the distance between the implanted 125I. Some research indicated that the attenuation factor of bone cement is 0.9951, which is smaller compared with stainless steel and titanium alloy,20 so using bone cement could avoid excessive radiation to local organization. In our clinical trials, there were no severe complications among 50 patients. PVP also has some disadvantages. Although the bone cement can solidify the target segments, the solid target segments can cause adjacent segment fractures, induce the new fractures; meanwhile, the most common risk is bone cement leakage, sometimes bone cement can leak into the spinal canal, cause spinal cord or nerve root compression symptoms. In this present study, although leakage happened, no serious consequence was observed. In case of safety, when PVP operation is performed, the whole process of injection must be monitored carefully to prevent leakage occurrence as much as possible.11
Conclusions
Osteoplastic spinal metastasis is not a contraindication of PVP. Based on the procedure which was mentioned in this study, during PVP surgery, bone cement can be successfully injected and distributed into peripheral bone lesions or central region. As the mechanical strength of vertebral body was increased, pain and local tumor progress was satisfactorily controlled. Combination of 125I seeds enhances the efficacy.
Acknowledgment
This study was supported by grants from the National Natural Science Foundation of China (No. 81260322/H1606), the Natural Science Foundation of Yunnan Province (No. 2012FB163), the Joint Specialized Research Fund from Yunnan Provincial Science and Technology Department and Kunming Medical University (No. 2011FB201), and Kunming Major Program of Science and Technology Development (No. 11S030003).
Disclosure Statement
No competing financial interests exist.
References
- 1.Shimazaki J. Higa T. Akimoto S, et al. Clinical course of bone metastasis from prostatic cancer following endocrine therapy: Examination with bone x-ray. Adv Exp Med Biol. 1992;324:269. doi: 10.1007/978-1-4615-3398-6_29. [DOI] [PubMed] [Google Scholar]
- 2.Scutellari PN. Antinolfi G. Galeotti R, et al. Metastatic bone disease. Strategies for imaging. Minerva Med. 2003;94:77. [PubMed] [Google Scholar]
- 3.Cybulski GM. Methods of surgical safe bilization for metastatic disease of the spine. Neurosurgery. 1989;23:240. doi: 10.1097/00006123-198908000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Wallace RE. Empirical dosimetric characterization of model I125-SL 125iodine brachytherapy source in phantom. Med Phys. 2000;27:2796. doi: 10.1118/1.1323980. [DOI] [PubMed] [Google Scholar]
- 5.Grimm PD. Blasko JC. Sylvester JE, et al. 10-year biochemical (prostate-specific antigen) control of prostate cancer with (125)I brachytherapy. Int J Radiat Oncol Biol Phys. 2001;51:31. doi: 10.1016/s0360-3016(01)01601-7. [DOI] [PubMed] [Google Scholar]
- 6.Corbett JF. Jezioranski JJ. Crook J, et al. The effect of seed orientation deviations on the quality of 125I prostate implants. Phys Med Biol. 2001;46:2785. doi: 10.1088/0031-9155/46/11/303. [DOI] [PubMed] [Google Scholar]
- 7.Yang ZZ. Xu JB. Yuan T, et al. Treating metastatic vertebral tumor with percutaneous vertebroplasty: A report of 28 cases. Chin J Cancer. 2005;24:194. [PubMed] [Google Scholar]
- 8.Sun G. Cong YJ. Xie ZG, et al. Percutaneous vertebroplasty using instruments and drugs made in China for vertebral metastases. Chin Med J (Engl) 2003;116:1207. [PubMed] [Google Scholar]
- 9.Yang ZZ. Yang DK. Xie L, et al. Treatment of metastatic spinal tumors by percutaneous vertebroplasty versus percutaneous vertebroplasty combined with interstitial implantation of 125I seeds. Acta Radiol. 2009;50:1142. doi: 10.3109/02841850903229133. [DOI] [PubMed] [Google Scholar]
- 10.Jónsson B. Sjöström L. Olerud C, et al. Outcome after limited posterior surgery for thoracic and lumbar spine metastases. Eur Spine J. 1996;5:36. doi: 10.1007/BF00307825. [DOI] [PubMed] [Google Scholar]
- 11.Cotton A. Dewatre F. Cortet B, et al. Percutaneous vertebroplasty for osteolytic metastases and myleoma: Effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology. 1996;200:525. doi: 10.1148/radiology.200.2.8685351. [DOI] [PubMed] [Google Scholar]
- 12.Cortet B. Cotton A. Boutry N, et al. Percutaneous vertebroplasty in patients with osteolytic metastases or multiple myeloma. Rev Rhum Engl Ed. 1997;64:177. [PubMed] [Google Scholar]
- 13.Murphy KJ. Nwankwo IJ. Gailloud P. Percutaneous vertebroplasty in the treatment ofblastic vertebral column metastasis from breast cancer. J Vasc Interv Radiol. 2007;18:321. doi: 10.1016/j.jvir.2006.12.725. [DOI] [PubMed] [Google Scholar]
- 14.Weill A. Chiras J. Simon JM, et al. Spinal metastases: Indicaion for and results of percutaneous injection of acylic surgical cement. Radiology. 1996;199:241. doi: 10.1148/radiology.199.1.8633152. [DOI] [PubMed] [Google Scholar]
- 15.Anglesio S. Calamia E. Fiandra C, et al. Prostate brachytherapy with iodine-125 seeds: Radiation protection issues. Tumor. 2005;91:335. doi: 10.1177/030089160509100410. [DOI] [PubMed] [Google Scholar]
- 16.Xue J. Waterman F. Handler J, et al. Localization of linked 125I seeds in postimplant TRUS images for prostate brachytherapy dosimetry. Int J Radiat Oncol BiolPhys. 2005;62:912. doi: 10.1016/j.ijrobp.2005.02.041. [DOI] [PubMed] [Google Scholar]
- 17.Xu J. Mei MH. Chen Q, et al. Interstitial 125I implants for liver malignancies. Chin J Exp Surg. 2005;22:368. [Google Scholar]
- 18.Jiang YL. Meng N. Wang JJ, et al. CT-guided iodine-125 seed permanent implantation for recurrent head and neck cancers. Radiat Oncol. 2010;5:68. doi: 10.1186/1748-717X-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gastaldi E. Chiono L. Gallo F, et al. Dosimetry doesn't seem to predict the control of organ-confined prostate cancer after I-125 brachytherapy. Evaluation in 150 patients. Arch Ital Urol Androl. 2009;81:215. [PubMed] [Google Scholar]
- 20.Song JM. Wang YP. Liu ZQ. The vitro study of effect of different implant to the radiotherapy. Ortho Biomech Mater Clin Stu. 2010;7:9. [Google Scholar]


