Abstract
In embryonic stem cell culture, small molecules can be used to alter key signaling pathways to promote self-renewal and inhibit differentiation. In mice, small-molecule inhibition of both the FGF/MEK/Erk and the GSK3β pathways during preimplantation development suppresses hypoblast formation, and this results in more pluripotent cells of the inner cell mass (ICM). In this study, we evaluated the effects of different small-molecule inhibitors of the FGF/MEK/Erk and GSK3β pathway on embryo preimplantation development, early lineage segregation, and subsequent embryonic stem cell derivation in the humans. We did not observe any effect on blastocyst formation, but small-molecule inhibition did affect the number of OCT3/4- and NANOG-positive cells in the human ICM. We found that combined inhibition of the FGF/MEK/Erk and GSK3β pathways by PD0325901 and CHIR99021, respectively, resulted in ICMs containing significantly more OCT3/4-positive cells. Inhibition of FGF/MEK/Erk alone as well as in combination with inhibition of GSK3β significantly increased the number of NANOG-positive cells in blastocysts possessing good-quality ICMs. Secondly, we verified the influence of this increased pluripotency after 2i culture on the efficiency of stem cell derivation. Similar human embryonic stem cell (hESC) derivation rates were observed after 2i compared to control conditions, resulting in 2 control hESC lines and 1 hESC line from an embryo cultured in 2i conditions. In conclusion, we demonstrated that FGF/MEK/Erk and GSK3β signaling increases the number of OCT3/4- and NANOG-positive cells in the human ICM, but does not improve stem cell derivation.
Introduction
In the mouse, blastocyst formation is the result of 2 successive differentiation events that are driven by the action of the transcription factors OCT3/4, CDX2, NANOG, and GATA6 [1,2]. The first segregation event starts at the compacted morula stage and results in 2 distinct lineages, the trophectoderm (TE) and the inner cell mass (ICM). Subsequently, there is a loss of CDX2 in the ICM, resulting in its segregation from the TE [3–6]. The ICM produces the embryonic cells, extraembryonic mesoderm, and primitive endoderm (PE), whereas the TE becomes a part of the chorion and the placenta [3,5].
The second segregation event allocates the ICM into epiblast (EPI) and hypoblast or PE. Initially, the precursor cells of EPI and PE are mixed within the ICM and express NANOG and GATA6, respectively [1]. In cow, pig, and human, OCT3/4 expression is not exclusively restricted to the ICM, but is first coexpressed with CDX2 in the TE [3,7,8]. Berg et al. underlined interspecies diversity in the segregation of the first lineages, which is based on evolutionary-conserved divergence of OCT3/4 regulation [8]. Therefore, it is clear that the order of temporal and spatial use of the genetic modules and possibly signaling networks is distinct in different animals. Importantly, the mechanism and timing of lineage specification in humans are largely unknown, and few studies have evaluated the expression patterns of known key regulators [9–12].
The first successful mouse embryonic stem cell (mESC) lines were derived from the ICM in 1981 [13,14]. Initially, mESC derivation was only possible from the 129 strain using standard optimized culture conditions, while it was less efficient and mostly unsuccessful in other strains of mice [13,14]. By using small molecules to inhibit specific signaling pathways involved in pluripotency, the derivation of naïve mESC from strains other than the 129 strain became feasible as well. Two specific inhibitors, PD0325901 and CHIR99021 (also known as the 2i condition), permitted the derivation of ESC lines, even from nonpermissive strains like the nonobese diabetic strain [15] and from rat embryos, resulting in the first rat ESC lines [16,17]. PD0325901 acts on the fibroblast growth factor (FGF) pathway by targeting mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK kinase or MEK), which causes blockade of differentiation [18]. CHIR99021 inhibits glycogen synthase kinase (GSK) 3β, thereby activating the Wnt pathway, which plays an important role in self-renewal. Other small molecules have also shown to affect stem cell derivation and support embryonic stem cell self-renewal [19]. For example, a combination of 3 inhibitors (SU5402 for FGFR, PD184352 for ERK, and CHIR99021 for GSK3β) supports stem cell derivation and culture of the mouse strain C57BL/6N, whereas the combination of LIF, PD98059, and BIO increased the mESC derivation efficiency by 5-fold [20–23].
Interestingly, similar small molecules have recently been reported to manipulate cell fate during preimplantation development. In vitro culture of mouse embryos in the 2i condition directs the ICM of mouse embryos toward a state in which all cells are NANOG positive and GATA4 negative, thus inhibiting hypoblast formation [24]. Yamanaka et al. confirmed the role of FGF signaling in the segregation of the PE and EPI in the mouse blastocyst, and stated that the FGF/MAPK signaling influences the specification of the PE and EPI lineages in a stochastic and progressive way [25]. Recently, Roode et al. showed that inhibiting FGF and GSK3β signaling in the 2i condition does not prevent hypoblast formation in humans [11]; however, only one combination of small molecules was tested, and the effect on subsequent stem cell derivation was not investigated.
We have explored the use of both single and combined inhibitors on blastocyst development and early lineage restriction in the human. Secondly, we verified the effect of manipulating cell fate during early embryonic development on its capacity to derive human embryonic stem cells (hESCs). These studies in human are necessary to clarify if the molecular signaling pathways that are involved during first- and second-lineage commitment in mice are conserved in human. By studying the differences in timing and underlying molecular mechanisms, we could obtain some crucial information about the lineage commitment during early human embryonic development and the origin of pluripotent cells in human.
Materials and Methods
Ethical aspects
The Institutional Review Board approval was obtained from the Ethics Committee, Ghent University Hospital (2009/281), and the Belgian Federal Ethics Committee on Embryo Research (Adv-030). All patients donating embryos for the present study signed informed consents before their IVF/ICSI cycle.
Embryo source
Fresh, spare embryos that did not meet the IVF laboratory's cryopreservation criteria after embryo transfer due to high fragmentation (≥25%) on day 2 or day 3 of development (day of oocyte retrieval being day 0), multinucleated blastomeres on day 2, delayed development (<5 blastomeres on day 3 or a ≤1 blastomere increase from day 2 to 3), and/or abnormal fertilization (0, 1, or ≥3PN) on day 1 were used in this study. For inclusion into the study, embryos must have contained at least 4 blastomeres on day 3 and could not have had more than 50% fragmentation.
Thawing of 1,2-propanediol-frozen embryos
In addition to these fresh embryos, embryos that had previously been frozen in 1,2-propanediol (PrOH) were used as well. These embryos were thawed by exposure to sequential thawing solutions: solution 1 contained 1M PrOH and 0.2M sucrose in Dulbecco's Phosphate-Buffered Saline (DPBS), supplemented with 0.5% human serum albumin (HSA); solution 2 contained 0.5 M PrOH and 0.2 M sucrose in DPBS/HSA; solution 3 contained 0.2 M sucrose in DPBS/HSA; and solution 4 contained DPBS/HSA. Straws were removed from liquid nitrogen and kept at room temperature for 40 s, followed by 30 s on a 30°C warm plate. The embryos were expelled from the straw and quickly moved to solution 1 for 5 min at room temperature, followed by solutions 2, 3, and 4 each for 5 min at room temperature. Finally, the embryos were placed in a fresh drop of solution 4 at 37°C for 5 min before they were cultured at 6% CO2 and 5% O2.
Embryo scoring and culture
In total, over 1,000 fresh, day-3 spare embryos and 131 frozen embryos cultured from the zygote stage in a Cook Cleavage medium (Cook Ireland LTD., Limerick, Ireland, www.cookmedical.com) at 37°C, 5% O2, and 6% CO2 were collected from the IVF lab. After inclusion into the study, embryos were randomized to 5 different small-molecule treatment groups based on their number of blastomeres on day 3 of development. Day-3 embryos were then cultured for 3 more days in the Cook Blastocyst medium (Cook Ireland LTD., Limerick, Ireland, www.cookmedical.com) supplemented with the studied small molecules. Small molecules used in this study were Parke Davis 0325901 (PD0325901; Cayman, Ann Arbor, MI; 1 μM), 6-bromoindirubin-3′-oxime (BIO; Cayman, 3 μM), and CHIR99021 (CHIR, Axon Medchem, 3 μM). PD0325901 is an inhibitor of the MEK pathway, and BIO and CHIR99021 are both inhibitors of the GSK3β pathway (activation of Wnt pathway), with CHIR99021 being a more specific GSK3β inhibitor than BIO. Used concentrations were based on recent publications in mice [21,32]. Embryos were randomized to the 5 following study groups: (i) 3 μM BIO, (ii) 1 μM PD0325901, (iii) 3 μM BIO and 1 μM PD0325901, (iv) 3 μM CHIR99021 and 1 μM PD0325901 (2i), and (v) control. Embryos were randomized over the different study groups based on the blastomere number on day 3 of development, by using QuickCalc's random number generator. Embryos were evaluated morphologically on day 4, 5, and 6 of their development. On day 6 in the afternoon, all blastocysts were scored at a fixed time interval using the grading system taken from Stephenson et al. [26]. Under this scoring system, ICM grades A and B are reserved for large and distinct ICMs, with grade A being more compact and B having larger, less-compact cells. Grade C scoring is used for small ICMs, grade D for degenerating ICMs, and grade E when no apparent ICM is visible. For this study, grades A and B were considered good-quality ICMs, while C, D, and E were considered poor-quality ICMs. TE grades of A, B, or C correspond to good, moderate, or poor quality, respectively. Blastocyst expansion was graded from 1 (no expansion of overall size) to 6 (fully hatched from zona pellucida). After scoring, the blastocysts were either used for immunostaining or hESC derivation purposes.
Additional 30 embryos were cultured in the Cook Blastocyst medium from day 3 until day 7 of embryonic development, to determine the differences in NANOG and GATA6 expression between day-6 and day-7 blastocysts.
Immunocytochemistry
Blastocysts were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min (room temperature) or overnight (4°C). They were rinsed in PBS and stored in PBS (4°C) until staining. At that point, blastocysts were permeabilized in 1×PBS+0.1% Triton X-100 for 10 min, moved to PBS+0.5% Triton X-100 for 20 min, rinsed in PBS shortly, and eventually blocked overnight in PBS+0.05% Tween-20+1% bovine serum albumin (blocking solution). Primary antibodies applied were OCT3/4 (1:200, SC-8628; Santa Cruz Biotechnology Inc., Heidelberg, Germany), NANOG (1:200, AF1997; R&D Systems, Oxon, United Kingdom), and GATA6 (1:200, SC-9055; Santa Cruz Biotechnology Inc.). Blastocysts were treated with primary antibodies in the blocking solution for 48 h at 4°C. After this incubation, embryos were rinsed in 1×PBS and treated with secondary antibodies in the blocking solution for 48 h at 4°C. Secondary antibodies used were donkey anti-goat-FITC (1:200, 705-095-147; Bioconnect, Huissen, The Netherlands) and donkey anti-rabbit-CY3 (1:2000; 711-165-152; Bioconnect). Blastocysts were rinsed again and mounted on glass slides in small drops of Vectashield containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories Ltd., Peterborough, United Kingdom). Positive ICM cell numbers were counted under a fluorescence microscope (Olympus 1×71; Olympus, Aartselaar, Belgium) equipped with filters for DAPI (377 nm), FITC (485 nm), and Cy3 (560 nm). Cells that were not positive for OCT3/4, NANOG, or GATA6 were considered TE. Stem cells were used as positive controls, whereas blastocysts incubated with an antibody diluent, without the primary antibody, were used as negative controls.
hESC derivation
The protocol used for embryonic stem cell derivation in our lab was recently reported by O'Leary et al. [27,28]. Briefly, day-6 (D6) blastocysts with good- and poor-quality ICMs were exposed to warmed Acidic Tyrode's (Sigma, Bornem, Belgium, T1788) to remove their zona pellucida. After washing, the blastocyst was transferred to individual culture dishes with a nearly confluent feeder layer of mitomycin-C- (Sigma, M4287) treated CD1 mouse embryonic fibroblasts (MEFs). The culture environment from the blastocyst stage to hESCs consisted of 37°C, 6% CO2, and 5% O2 in a standard hESC culture medium consisting of the knockout Dulbecco's modified Eagle medium (KO-DMEM; Invitrogen, Merelbeke, Belgium, 10829-018), supplemented with 20% knockout serum replacement (KO-SR; Invitrogen, 10828-028), 1% non-essential amino acids (NEAA; Invitrogen, 11140-035), 0.1 mM l-glutamine (Invitrogen, 25030-024), 1% penicillin/streptomycin (Invitrogen, 15140-122), 0.1 mM beta-mercaptoethanol (Sigma, M3148), and 4 ng/mL basic fibroblast growth factor (bFGF; Invitrogen, 13256-029). Observation and refreshment were done daily to observe areas of presumed ICM-derived cells (post-ICM intermediates or PICMIs [28]) or emerging colonies. Once hESC outgrowths emerged, they were mechanically passaged until numerous stable colonies could safely be transferred to a tissue culture flask. The hESC-populated flasks were expanded with 1 mg/mL collagenase type IV (Invitrogen, 17104-019) every 5 to 6 days with daily medium refreshment.
Alkaline phosphatase live staining
The alkaline phosphatase (AP) live staining was performed using 1×AP Live Stain working solution, by diluting the 500× stock solution (Invitrogen, A14353) in DMEM/F-12 (Invitrogen, 10565-018). Stem cells were first rinsed with prewarmed DMEM/F-12 and then incubated with the 1× AP working solution for 30 min. At this point, the AP live stain was removed, and cells were washed again twice with DMEM/F-12 for 5 min per wash. After the fresh DMEM/F-12 medium was added, pictures could be captured within 30–90 min of staining. After visualization, DMEM/F-12 could be replaced with a fresh hESC medium, and the cells could be maintained in culture.
Karyotyping of hESC lines
Nearly confluent culture flasks of hESCs at early passages were induced to metaphase by colcemide (1:100 in salt solution; Karyomax, Invitrogen, 15210-057), harvested with 0.25% trypsin–EDTA (Invitrogen, 25200-056), treated with a hypotonic solution of HCL (Sigma, T-3038), and fixed in a 1:3 methanol/acetic acid solution (Merck, Overijse, Belgium, 1.06009.1000; Sigma, A 6283). The metaphase spreads were then prepared and analyzed as described previously using the G-banding technique [29]. For karyotyping, 10 numerical metaphase spreads and 3 complete karyograms were analyzed.
Cryopreservation of hESC lines
Before freezing, colonies of hESCs were treated with 10 μM Y27632 (ROCK inhibitor, ROCKi) for 1 h, after which enzymatic passaging with 1 mg/mL collagenase type IV (Invitrogen, 17104-019) was carried out. When the cell pellet was collected, 2 mL precooled freezing medium (90% hESC medium with ROCKi and 10% DMSO) was added, and the cells were stored in a cryofreezing container (Nalgene, Roskilde, Denmark, 5100-0001) at −80°C. Later on, cryovials were thawed in a 37°C warm water bath, and the cells were added to 10 mL hESC medium plus ROCKi. After centrifugation, the supernatants were removed, and the pellet was resuspended in 1 mL medium with ROCKi. This cell suspension was then cultured in a tissue flask covered with MEFs, and 2 days later, after the first culture refreshment with standard hESC medium, cells were refreshed daily.
Statistical analysis
Developmental data were analyzed by contingency table analysis followed by Chi-square or Fisher's exact tests where appropriate. P-values of ≤0.05 were considered to be significant. Parameters of blastocyst quality were compared using one-way analysis of variance followed by Tukey post-test when the level of significance reached P<0.05.
Results
Impact on blastocyst formation and quality
Blastocyst rates of embryos cultured in the presence of PD0325901 and CHIR99021, PD0325901 and BIO, and PD0325901 tended to be higher than the control group, but not statistically different. Embryos cultured in the presence of BIO alone showed similar blastocyst formation (31.1%) as the control embryos (Table 1, 38.9%, 36.8%, and 36.7% vs. 29.8%, respectively; P=0.18, P=0.14, and P=0.11, respectively; and 31.1% vs. 29.8%, respectively). A and B ICMs were considered as good-quality ICMs, whereas C, D, and E ICMs were considered as poor-quality ICMs. The control group and most of the different small-molecule groups contained equal amounts of good- and poor-quality ICMs (Table 1). In the BIO group, 63.5% of the blastocysts had a good-quality ICM (compared to the control group, P=0.06). After scoring the TE, no significant differences in formation and quality were observed between the different small-molecule groups and the control.
Table 1.
Overall, A/B-, and C/D/E-Quality Inner Cell Mass Blastocyst Formation Efficiency in Different Small-Molecule Groups
| Experimental group | Development to blastocysts (%) | Type A/B (%) | Type C/D/E (%) |
|---|---|---|---|
| Control (n=469) | 29.8 (n=140) | 52.9 (n=74) | 47.1 (n=66) |
| BIO (n=167) | 31.1 (n=52) | 63.5 (n=33) | 36.5 (n=19) |
| PD0325901 (n=177) | 36.7 (n=65) | 48.0 (n=31) | 52.0 (n=34) |
| BIO+PD0325901 (n=133) | 36.8 (n=49) | 44.9 (n=22) | 55.1 (n=27) |
| CHIR+PD0325901 (n=59) | 38.9 (n=23) | 52.2 (n=12) | 47.8 (n=11) |
2i stimulates the number of OCT3/4 single-positive cells in human blastocysts
To compare the influence of the MEK and GSK3β inhibitors on lineage segregation, embryos were cultured from day 3 to day 6 in presence of different inhibitors of these pathways, alone and in combination. Blastocysts were immunostained to enquire GATA6 and OCT3/4 expression. Both single and double expression of these markers was observed.
As we observed a predominant expression of the lineage markers OCT3/4 and GATA6 in the presumptive ICM clump, only these positive ICM cells were counted for analyzing the effect of the small molecules on second lineage segregation in the following experiments. Both single- and double-positive cells were observed in the ICM, but only the single-positive cells were included in our analysis. Combined expression of both GATA6 and OCT3/4 is suggesting a future hypoblast fate.
In blastocysts with both good- (grades A and B) and poor-quality ICMs (grades C and D), there was a significant increase in OCT3/4 single-positive cells in the 2i group compared to the control and all other treatment groups (aside from PD0325901 in good-quality blastocysts). There was no difference in the number of GATA6 single- or double-positive cells. Embryos cultured in the presence of BIO and PD0325901, alone or in combination, did not show more OCT3/4- or GATA6 single-positive cells compared to the control group. Blastocysts with poor-quality ICMs in the BIO & PD0325901 group showed significantly fewer OCT3/4 single-positive cells compared to their control (Table 2, Fig. 1a).
Table 2.
Mean Numbers of OCT3/4-, GATA6-, and Double-Positive Inner Cell Mass Cells in Blastocysts with A/B- and C/D-Quality Inner Cell Mass in Different Small-Molecule Groups
| OCT3/4 alone | GATA6 alone | Dp | ICM | |
|---|---|---|---|---|
| A- & B-quality ICM | ||||
| Control (n=10) | 5.10±4.48 | 6.10±4.82 | 6.20±10.79 | 17.40±10.36 |
| BIO (n=14) | 6.43±5.80 | 5.71±4.94 | 2.21±4.06 | 14.36±6.15 |
| PD0325901 (n=7) | 9.43±6.83 | 3.29±3.77 | 4.57±5.16 | 17.29±8.83 |
| BIO & PD0325901 (n=5) | 6.40±4.62 | 6.00±5.70 | 1.80±4.02 | 14.20±7.01 |
| CHIR & PD0325901 (n=6) | 14.17±5.78a | 7.83±6.37 | 1.00±2.00 | 23.00±13.10 |
| C- & D-quality ICM | ||||
| Control (n=9) | 4.11±2.98 | 4.22±4.44 | 4.56±8.76 | 12.89±5.51 |
| BIO (n=3) | 1.67±1.53 | 8.67±8.33 | 3.00±2.56 | 13.33±6.81 |
| PD0325901 (n=4) | 3.75±2.87 | 7.25±3.69 | 0.50±1.00 | 11.50±3.32 |
| BIO & PD0325901 (n=7) | 0.86±2.27b | 2.29±3.30 | 7.67±3.69 | 10.71±5.50 |
| CHIR & PD0325901 (n=6) | 13.00±7.72a | 5.50±5.21 | 2.50±3.73 | 21.00±7.16b |
Statistic significances compared to control: aP<0.01; bP<0.05.
Mean±standard deviation.
Dp, number of double-positive cells for OCT3/4 and GATA6; ICM, number of inner cell mass cells.
FIG. 1.
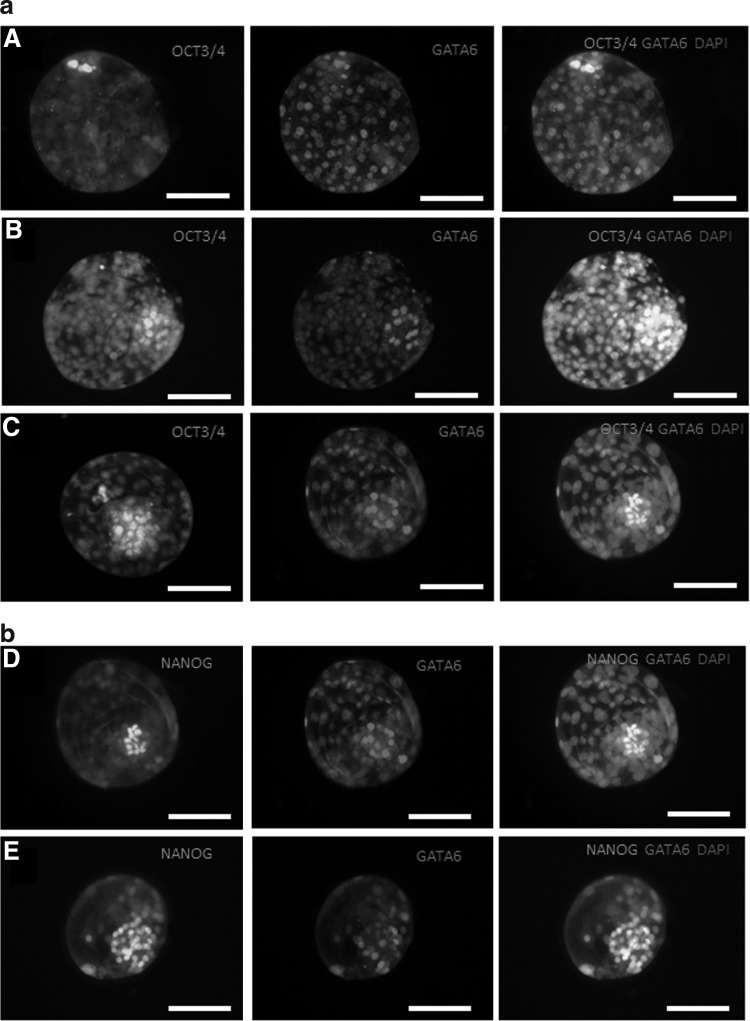
(a) Immunofluorescent staining for OCT3/4 and GATA6 and a merged image with 4′,6-diamidino-2-phenylindole (DAPI) of day-6 blastocyst in the control (A), PD0325901 (B), and 2i (C) group. Note the difference in number of OCT3/4-positive inner cell mass (ICM) cells in the PD0325901 and 2i blastocysts compared to the control. Scale bar, 100 μm. (b) Immunofluorescent staining for NANOG and GATA6 and a merged image with DAPI of day-6 blastocyst in the control (D) and 2i (E) group. Note the difference in number of NANOG-positive ICM, cells in the 2i blastocyst compared to the control. Scale bar, 100 μm.
Nanog expression increases after FGF/MEK/Erk inhibition
As the initial blastocyst stainings showed that the number of OCT3/4 single-positive cells was significantly higher in the 2i group, we wanted to check if there were also more NANOG-positive cells in those blastocysts. NANOG is, together with OCT3/4, one of the most important pluripotency markers for subsequent stem cell derivation purposes. In addition, it is accepted to be a more specific marker for the EPI, whereas OCT3/4 is a more general ICM marker [10,30]. By performing these additional stainings in the PD0325901 and 2i group, we wanted to check if the observed increase in OCT3/4-positive cells is accompanied with a shift toward more EPI cells in the ICM during the second lineage segregation. Again, double-positive cells were not included for analysis.
Overall, we observed that NANOG and GATA6 were expressed in a mutually exclusive way in the cells of the ICM, with cells being either positive for NANOG or for GATA6, with few exceptions in the control group, where some cells were double positive. In the blastocysts with good-quality ICMs, we observed significantly more NANOG-positive cells after culturing in the presence of PD0325901 alone and also more NANOG-positive cells in the 2i group. As we observed before, no effect on the number of GATA6-positive cells could be found. In blastocysts with poor-quality ICMs, the number of NANOG-positive cells did not increase after MEK inhibition, neither alone nor in combination with GSK3β inhibition. In these blastocysts, a trend toward less GATA6-positive cells could be observed in the 2i group, but this was not significant (P=0.06) (Table 3 and Fig. 1b).
Table 3.
Mean Numbers of NANOG-, GATA6-, and Double-Positive Inner Cell Mass Cells in Blastocysts with A/B- and C/D-Quality Inner Cell Mass in Different Small-Molecule Groups
| NANOG alone | GATA6 alone | Dp | ICM | |
|---|---|---|---|---|
| A- & B-quality ICM | ||||
| control (n=6) | 7.67±4.59 | 4.50±3.67 | 3.33±3.44 | 15.5±8.96 |
| PD0325901 (n=14) | 15.00±7.30a | 7.00±4.74 | 0.00 | 22.00±10.36 |
| CHIR & PD0325901 (n=11) | 14.27±8.66a | 5.55±5.26 | 0.00 | 19.82±11.75 |
| C- & D-quality ICM | ||||
| control (n=8) | 5.75±3.54 | 9.00±5.18 | 0.63±0.92 | 13.67±7.13 |
| PD0325901 (n=11) | 6.27±2.90 | 5.00±5.83 | 0.00 | 11.27±5.22 |
| CHIR & PD0325901 (n=4) | 5.75±1.71 | 3.25±2.22 | 0.00 | 9.00±3.56 |
Statistic significances compared to control: aP<0.05.
Mean±standard deviation.
Dp, number of double-positive cells for NANOG and GATA6s.
ICM cell number tends to increase after 2i treatment
During the immunofluorescent analysis of the blastocysts, we also estimated the number of ICM cells based on cell location. This showed an increase in the ICM cell number in the 2i group compared to the control group, when looking at the OCT3/4-stained blastocysts. This increase was significant in the poor-quality blastocysts (21.00±7.16 vs. 12.89±5.51, P<0.05), but not in the good-quality blastocyst group (23.00±13.10 vs. 17.40±10.36) (see Tables 2 and 3).
Blastocyst culture until day 7
To check whether the expression of NANOG and GATA6 changes in blastocysts from day 6 to day 7, we cultured some additional blastocysts until day 7 of embryonic development, and stained these for the EPI marker NANOG and the hypoblast marker GATA6. We observed a significant decrease in the number of NANOG-positive cells (P<0.05) and a trend toward less GATA6-positive cells (P=0.07) compared to day-6 blastocysts (Table 4).
Table 4.
Mean Numbers of NANOG-Alone and GATA6-Alone Positive Inner Cell Mass Cells
| NANOG alone | GATA6 alone | |
|---|---|---|
| Day 6 (n=14) | 8.36±4.98 | 8.50±5.37 |
| Day 7 (n=7) | 3.57±3.60a | 4.00±4.20 |
Statistic significances compared to control: aP<0.05.
Mean±standard deviation.
Stem cell derivation
As we observed a higher number of pluripotent OCT3/4- and NANOG-positive cells after embryo culture in the presence of the small-molecule inhibitors PD0325901 and CHIR99021, we verified if subsequent derivation of hESCs could be performed.
After initial embryo culture in presence or absence of the 2 inhibitors, day-6 blastocysts with both good- and poor-quality ICMs were plated in a standard hESC medium on MEFs (13 control blastocysts: 7 with good-quality ICMs and 6 with poor-quality ICMs; 20 2i blastocysts: 10 with good-quality ICMs and 10 with poor-quality ICMs). Of the plated control blastocysts, 23% formed PICMIs [34], and 15.4% formed embryonic stem cell colonies. This resulted in the derivation of 2 new hESC lines in standard conditions (UGent11-1 and UGent11-2; Table 5 and Fig. 2). Of the 20 plated 2i blastocysts, 1 (5%) initial outgrowth was formed, which resulted in a new embryonic stem cell line (UGent11-3; Table 5 and Fig. 2). From these results, no differences in the derivation efficiency between the groups can be deduced. All 3 established lines originated from blastocysts with an A/B quality ICM. Their pluripotent status was confirmed by immunostaining for OCT3/4 and NANOG, and they were cultured for at least 30 passages. Similar to undifferentiated pluripotent cultures of previously established hESC lines, all our cell lines possessed high levels of AP activity. Karyotype analysis revealed that all lines had a normal karyotype (Table 6 and Fig. 3). The cells could be cryopreserved without any effect on their ability to re-establish pluripotent hESC colonies. We must remark that the hESC line originating from the 2i blastocyst showed lower survival after passaging and lower self-renewal capacity. This problem could however be solved by passaging this line in the presence of the ROCK inhibitor Y27632 (10 μM), which has shown to have a positive effect on cell survival [31]. Interestingly, this line is a sibling from one of the control lines, which means that both lines are derived from sibling embryos that were cultured in different conditions (control and 2i). Additional experiments, plating 2i embryos in a 2i-supplemented medium, elucidated the absence of successful hESC derivation in these conditions (Table 5).
Table 5.
Overview of the Postinner Cell Mass-Intermediate Formation and Human Embryonic Stem Cell Derivation Rates of Control and 2i Blastocysts in Standard or 2i-Supplemented Human Embryonic Stem Cell Medium
| PICMI formation | hESC derivation (% of attached blastocysts) | hESC derivation (% of PICMIs cultured) | |
|---|---|---|---|
| Control in standard hESC medium | 23.0% (3/13) | 15.3% (2/13) | 66.6% (2/3) |
| 2i in standard hESC medium | 5.0% (1/20) | 5.0% (1/20) | 100% (1/1) |
| 2i in 2i supplemented hESC medium | 0% (0/16) | 0% (0/16) | 0% (0/0) |
PICMI, post-ICM-intermediate; hESC, human embryonic stem cell.
FIG. 2.
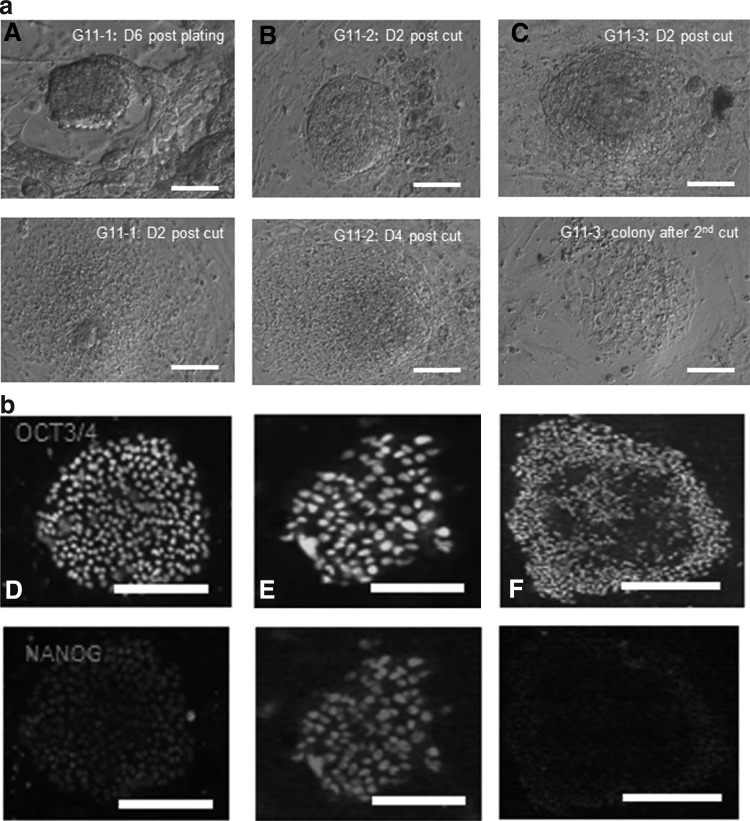
(a) Bright-field pictures of the early stages in human embryonic stem cell (hESC) derivation. (A) Control hESC line G11-1: day 6 postplating the blastocyst and day 2 postcutting the area of interest; (B) Control hESC line G11-2: day 2 and 4 postcutting the area of interest after plating the day-6 control blastocyst; (C) hESC line G11-3 (embryo culture in 2i): day 2 postcutting the area of interest after plating the day-6 control blastocyst and established hESC colony after 2nd cutting. Scale bar, 100 μm. (b) Immunofluorescent staining of all derived hESC lines for OCT3/4 and NANOG. (D) Control hESC line G11-1; (E) control hESC line G11-2; (F) hESC line G11-3 (embryo culture in 2i). Scale bar, 50 μm.
Table 6.
Overview of the Number of Counted, Analyzed, and Normal Metaphases of Each Human Embryonic Stem Cell Line, Using the G-Banding Technique
| Cell line | Number of counted metaphases | Number of analyzed metaphases | Percentage of normal metaphases |
|---|---|---|---|
| Control line G11-1 | 10 | 3 | 100% |
| Control line G11-2 | 10 | 3 | 100% |
| 2i line G11-3 | 10 | 3 | 100% |
FIG. 3.
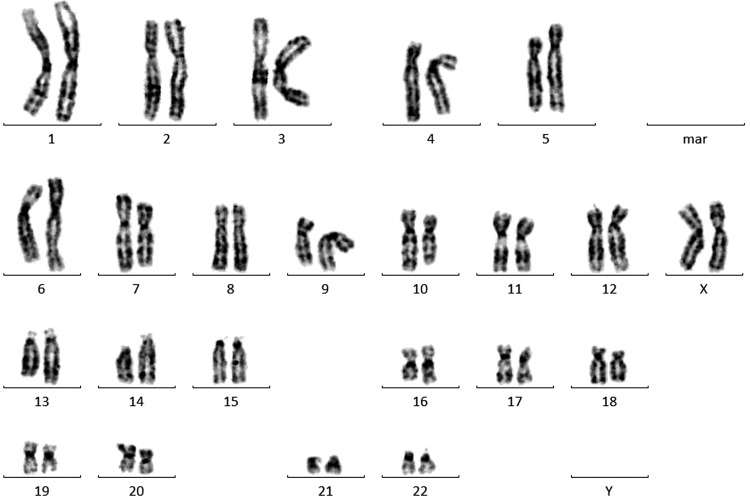
Example of G-banding, showing a normal karyogram in all lines.
Discussion
In the past, differences in lineage segregation between different species have been reported, based on evolutionary divergence of OCT3/4 regulation [3,7,8]. More specifically, changes in Cdx2:Oct4 ratios and the relative timing of TE commitment are observed. However, the mechanism of lineage segregation in humans has only been studied by a few groups [9,10,12,32].
In the present study, early lineage segregation was evaluated at one fixed time point on day 6 of development. The exact timing of OCT3/4 restriction to the ICM is however still not clear. We observed the human ICM to have cells expressing OCT3/4, NANOG, and GATA6. Only cells without coexpression of these genes were taken into account for analyzing the effect of the small molecules on second lineage segregation. Some blastocysts showed expression of NANOG or OCT3/4 in a few TE cells as well, but this was only seen in a very small minority of the early blastocysts. As such, our results are in agreement with those of Chen et al., where OCT3/4 expression was also predominantly detected in the ICM, but not with the results of Cauffman et al. We did not see any differences in the number of OCT3/4-positive cells according to the expansion stage of the blastocyst (data not shown). This sustains the suggestion that the first segregation event in human takes place at day 6 of development.
In mice, the FGF-signaling pathway plays an important role in the segregation of the hypoblast and EPI from the ICM. It was demonstrated that by modulation of the FGF pathway, the fate of the ICM cells to become hypoblast or EPI can be altered [24,25]. Inhibition of the FGF/MAPK pathway led to a reallocation of the ICM cells toward EPI and an inhibition of hypoblast formation [25]. In contrast, addition of exogenous FGF shifted the balance toward more hypoblast cells [30]. It seems that FGF/ERK signaling is crucial in the formation of the hypoblast, and altered signaling can lead to defective hypoblast formation, as the lineage segregation of hypoblast and EPI requires the activation of the receptor tyrosine kinase (RTK)-Ras-MAPK signaling pathway [1]. Recently, Roode et al. published that human hypoblast formation is not dependent on FGF signaling [11]. They observed no reduction of GATA4-positive cells upon inhibition of FGF/Erk signaling, but there were also no effects on the NANOG-positive cell population. Our study confirms that FGF/MEK/Erk inhibition does not prevent the formation of GATA6-positive cells, but we did observe an increase in the number of OCT3/4- and NANOG-positive cells in the ICM upon 2i treatment. The increase in OCT3/4- or NANOG-positive cells may have occurred by 3 distinct mechanisms: (i) an increase of EPI cell proliferation; (ii) an upregulation of OCT4 and NANOG in the pre-EPI cells, or (iii) acceleration in the differentiation of the blastomeres to EPI. Comparison of the ICM cell numbers in the different treatment groups suggested that this increase is probably caused by an increase in EPI proliferation in the 2i culture condition.
In the article of Roode et al. [11], GATA6-positive cells were distributed throughout the whole blastocyst. We did not observe GATA6 expression in the TE cells (except for some early blastocysts, but due to its rarity, we only focused on the expression of GATA6 inside the ICM). The observed difference in GATA6 expression between the experiments of Roode et al. and the ones presented here could however be the result of a distinct antibody specificity, as both experiments applied different GATA6 antibodies. Our experiments differ from the previous, because we used over 1,000 human spare embryos to analyze the effect of both single and double inhibition of FGF/MEK/Erk and GSK3β on the ICM quality of D6 blastocysts, and we stained 125 blastocysts with early lineage segregation markers. The number of human embryos used by Roode et al. was not mentioned. Importantly, we also performed a separate analysis for blastocysts of both good- and poor-quality ICMs.
Our results show that also in humans, inhibition of the FGF/MEK/Erk and GSK3β pathway (2i condition) leads to a significant increase in OCT3/4- and NANOG-positive cells in the blastocysts of good quality, while the ones of poor quality showed more OCT3/4-positive cells and similar amounts of NANOG-positive cells. Interestingly, hypoblast formation was not prevented during second lineage segregation in human, which reveals still important differences between human and mouse. These differences might explain the inability of obtaining hESCs from the embryo level onward in human using the 2i condition. Another explanation for that might be the used culture medium, as the 2i condition is known to maintain the culture of naïve ESCs, a culture medium that is supplemented with LIF might be more supportive.
Since the availability of human spare embryos is much lower compared to animal models, we did not do any dose finding, but used small-molecule concentrations that were reported before in mouse studies. It is possible, however, that other doses are needed to obtain similar effects in humans as in mice. In this study, both BIO and CHIR99021 were used as GSK3β inhibitors, and it is known that CHIR99021 is a more specific GSK3β inhibitor [33]. From our results, we could not observe an increase in the number of pluripotent EPI cells in the ICM by the addition of BIO, alone or combined with PD0325901. This will probably result from the nonspecificity of BIO, as the combination CHIR99021-PD0325901 was more effective in increasing pluripotency.
Staining of human blastocysts at day 7 of development showed a decrease in the number of NANOG-positive cells (P<0.05) and a trend toward less GATA6-positive cells (P=0.07) compared to D6 blastocysts. As standard culture in the Cook Blastocyst medium is only reported until day 5/6 of development, it is possible that our culture environment was suboptimal for supporting embryonic development until day 7. The observation that the blastocyst quality decreased from day 6 to 7 substantiates this suggestion.
As we were able to increase the number of OCT3/4- and NANOG-positive cells by inhibition of FGF and GSK3β signaling, we wanted to verify if there was any effect on the capability of these embryos to derive hESCs. In mice, the derivation efficiency in standard culture systems is very low [34]. However, it has been shown that the addition of different factors to the culture medium (LIF, PD98059, and BIO) can lead to a 5-fold increase in mESC derivation rates [20]. In human, we observed an hESC derivation efficiency of 15.4% in the control group, and of 5% in the 2i condition. The low number of cell lines generated, however, did not allow any conclusion to be drawn regarding the efficiency of derivation. From literature, it is sometimes difficult to compare efficiencies on hESC derivation, since information about the origin, number, and quality of embryos that were used and the number and quality of plated blastocysts is not always described in reports. Ström et al. showed a derivation efficiency of 12.8% using fresh spare embryos (30 permanent lines/234 blastocysts) [35]. In a comparable study from our group, we obtained a similar efficiency of stem cell derivation using a higher number of embryos compared to our present study: 12.1%, 16 permanent lines on 132 plated blastocysts [27]. Interestingly, embryos resulting in a successful hESC line originated from a patient cycle in which a successful pregnancy was established. This reflects the overall health of a cohort of embryos within a patient, which has recently been shown to affect the hESC derivation efficiency [26].
All hESC lines were derived from blastocysts with good-quality ICMs, which contained more OCT3/4- and NANOG-positive cells. Previously, we showed that good-quality ICMs originating from embryos with multiple poor-quality traits are unable to generate hESC lines, in contrast to good-quality ICMs from embryos with only a single poor-quality trait [27]. In the current study, there was no difference in the number of poor-quality traits of day-3 embryos between the groups that could have influenced stem cell derivation efficiency. Also, the maternal age and blastomere number on day 3 did not differ between the groups, confirming the proper randomization of the embryos.
The initial slow growth of the created 2i line (UGent11-3) could be due to the presence of the FGF inhibitor PD0325901 in the culture medium, as it is known that hESCs rely on the FGF pathway for their sustained self-renewal and pluripotency. Also, it needs to be taken into account that the used culture conditions for hESC derivation were similar between the different groups, as this study was based on standard hESC culture. Aiming toward derivation of naïve hESCs would require other culture conditions, such as the use of the N2B27 medium supplemented with 2i and LIF lacking bFGF, but this was beyond the scope of the present article. Hanna et al. succeeded in transforming existing hESC into naïve hESCs by transfection of the cells with Oct4 and Klf4 or Klf4 and Klf2 in the presence of PD0325901, CHIR99021, LIF, and the Klf4/Flf2 regulator forskolin [37]. These inhibitors have also been used to derive naïve pig stem cells from the ICM, combined with transfection of KLF2 or KLF4 and OCT3/4 [38]. Therefore, additional experiments based on the use of 2i condition during derivation might lead toward naïve hESCs from the blastocyst stage onward.
Recently, we identified an intermediate stage between the ICM and eventual hESC colony, which we called post-ICM intermediate (PICMI), and which contains a unique signature compromising both early- and late-EPI markers [28]. This finding supports the evidence that existing hESCs are more similar to mouse EPI stem cells (mEpiSC) than to mESCs, which are derived from the ICM. By using a derivation medium that contains the perfect cocktail of small molecules and growth factors, it should be possible to increase the derivation rates in the human, and to push the cells toward a more naïve state. The derivation of naïve hESCs could open new windows for patient-specific clinical applications and disease-relevant research [37].
Conclusion
To our knowledge, this is the first report showing that the combination of inhibitors of FGF/MEK/Erk and GSK3B signaling increases the number of OCT3/4- and NANOG-positive cells in the human ICM, but does not improve stem cell derivation. We observed significantly more OCT3/4-positive cells in all blastocysts and also more NANOG-positive cells in blastocysts with good-quality ICMs after using the 2i condition. There were no significant differences in numbers of GATA6-positive cells. Also, we succeeded in the derivation of 3 different hESC lines from D6 blastocysts, with 1 line coming from an embryo that was cultured in the 2i condition. Interestingly, 2 of the derived stem cell lines are sibling lines, derived from sibling embryos that were cultured in different conditions. Our future research will focus on the use of different kinds of small-molecule-supplemented derivation media, which could hopefully lead to higher derivation rates of a new type of naïve hESCs.
Acknowledgments
M.V.d.J. is holder of a Ph.D. grant of the Agency for Innovation by Science and Technology (IWT, grant number SB093128), Belgium. G.D. and this research is supported by the FLemish Foundation for Scientific Research (FWO-Vlaanderen, grant number FWO-3G062910) and a Concerted Research Actions funding from BOF (Bijzonder Onderzoeksfonds University Ghent, grant number BOF GOA 01G01112). S.C.d.S.L. is supported by The Netherlands Organization for Scientific Research (grant number VENI 916.76.015). P.D.S. is holder of a fundamental clinical research mandate by the Flemish Foundation of Scientific Research (FWO-Vlaanderen), Belgium.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Chazaud C. Yamanaka T. Pawson T. Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2- MAPK pathway. Dev Cell. 2006;10:615–624. doi: 10.1016/j.devcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Plusa B. Piliszek A. Frankenberg S. Artus J. Hadjantonakis A. Distinct sequential cell behaviours direct primitive endoderm formation in the mouse blastocyst. Development. 2008;135:3081–3091. doi: 10.1242/dev.021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strumpf D. Mao CA. Yamanaka Y. Ralston A. Chawengsaksophak K. Beck F. Rossant J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132:2093–2102. doi: 10.1242/dev.01801. [DOI] [PubMed] [Google Scholar]
- 4.Dietrich JE. Hiiragi T. Stochastic patterning in the mouse pre-implantation embryo. Development. 2007;134:4219–4231. doi: 10.1242/dev.003798. [DOI] [PubMed] [Google Scholar]
- 5.Niwa H. Toyooka Y. Shimosato D. Strumpf D. Takahashi K. Yagi R. Rossant J. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell. 2005;123:917–929. doi: 10.1016/j.cell.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 6.Ralston A. Rossant J. Genetic regulation of stem cell origins in the mouse embryo. Clin Genet. 2005;68:106–112. doi: 10.1111/j.1399-0004.2005.00478.x. [DOI] [PubMed] [Google Scholar]
- 7.Rossant J. Stem cells: the magic brew. Nature. 2007;448:260–262. doi: 10.1038/448260a. [DOI] [PubMed] [Google Scholar]
- 8.Berg DK. Smith CS. Pearton DJ. Wells DN. Broadhurst R. Donnison M. Pfefferi PL. Trophectoderm lineage determination in cattle. Dev Cell. 2011;20:244–255. doi: 10.1016/j.devcel.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Cauffman G. Liebaers I. Van Steirteghem A. Van de Velde H. POU5F1 isoforms show different expression patterns in human embryonic stem cells and preimplantation embryos. Stem Cells. 2006;24:2685–2691. doi: 10.1634/stemcells.2005-0611. [DOI] [PubMed] [Google Scholar]
- 10.Cauffman G. De Rycke M. Sermon K. Liebaers I. Van de Velde H. Markers that define stemness in ESC are unable to identify the totipotent cells in human preimplantation embryos. Hum Repr. 2009;24:63–70. doi: 10.1093/humrep/den351. [DOI] [PubMed] [Google Scholar]
- 11.Roode M. Blair K. Snell P. Elder K. Marchant S. Smith A. Nichols J. Human hypoblast formation is not dependent on FGF signalling. Dev Biol. 2012;361:358–363. doi: 10.1016/j.ydbio.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen AE. Egli D. Niakan K. Deng J. Akutsu H. Yamaki M. Cowan C. Fitz-Gerald C. Zhang K. Melton DA. Eggan K. Optimal timing of inner cell mass isolation increases the effciency of human embryonic stem cell derivation and allows generation of sibling cell lines. Cell Stem Cell. 2009;4:103–106. doi: 10.1016/j.stem.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans MJ. Kaufman M. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 14.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Procl Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nichols J. Jones K. Phillips JM. Newland SA. Roode M. Mansfield W. Smith A. Cooke A. Validated germline-competent embryonic stem cell lines from nonobese diabetic mice. Nat Med. 2009;15:814–818. doi: 10.1038/nm.1996. [DOI] [PubMed] [Google Scholar]
- 16.Buehr M. Meek S. Blair K. Yiang J. Ure J. Silva J. McLay R. Hall J. Ying Q. Smith A. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Li P. Tong C. Mehrian-Shai R. Jia L. Wu N. Yan Y. Maxson RE. Schulze EN. Song H. Hsieh C. Pera MF. Ying Q. Germline competent embryonic stem cells derived from rat blastocysts. Cell. 2008;135:1299–1310. doi: 10.1016/j.cell.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vojtek AB. Der CJ. Increasing complexity of the Ras signalingpathway. J Biol Chem. 1998;273:19925–19928. doi: 10.1074/jbc.273.32.19925. [DOI] [PubMed] [Google Scholar]
- 19.Li W. Ding S. Small molecules that modulate embryonic stem cell fate and somatic cell reprogramming. Trends Pharmacol Sci. 2010;31:36–45. doi: 10.1016/j.tips.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Doungpunta J. Santhi A. Sathanawongs A. Jaraujindaa Y. Oranratnachaia A. Fivefold increase in derivation rates of mouse embryonic stem cells after supplementation of the media with multiple factors. Theriogenology. 2009;72:232–242. doi: 10.1016/j.theriogenology.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Kiyonari H. Kanek M. Abe S. Aizawa S. Three inhibitors of FGF receptor, ERK, and GSK3 establishes germline-competent embryonic stem cells of C57BL/6N mouse strain with high efficiency and stability. Genesis. 2010;48:317–327. doi: 10.1002/dvg.20614. [DOI] [PubMed] [Google Scholar]
- 22.Park J. Kim C. Tong Y. Amano T. Lin C. Tian XC. Reprogramming of mouse fibroblasts to an intermediate state of differentiation by chemical induction. Cell Reprogram. 2011;13:121–131. doi: 10.1089/cell.2010.0067. [DOI] [PubMed] [Google Scholar]
- 23.Yu J. Chau KF. Vodvanok MA. Jiang J. Jiang Y. Efficient feeder-free episomal reprogramming with small molecules. Plos One. 2011;6:e17557. doi: 10.1371/journal.pone.0017557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichols J. Silva J. Roode M. Smith A. Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development. 2009;136:3215–3222. doi: 10.1242/dev.038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamanaka Y. Lanner F. Rossant J. FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse embryo. Development. 2010;137:715–724. doi: 10.1242/dev.043471. [DOI] [PubMed] [Google Scholar]
- 26.Stephenson EL. Braude BR. Mason C. Proposal for a universal minimum information convention for the reporting on the derivation of human embryonic stem cell lines. Regen Med. 2006;1:739–50. doi: 10.2217/17460751.1.6.739. [DOI] [PubMed] [Google Scholar]
- 27.O'Leary T. Heindryckx B. Lierman S. Van der Jeught M. Menten B. Deforce D. Cornelissen R. Chuva de Sousa Lopes S. De Sutter P. The influence of early embryo traits on human embryonic stem cell derivation efficiency. Stem Cells Dev. 2011;20:785–793. doi: 10.1089/scd.2010.0338. [DOI] [PubMed] [Google Scholar]
- 28.O'Leary T. Heindryckx B. Lierman S. van Bruggen D. Goeman JJ. Vandewoestyne M. Deforce D. Chuva de Sousa Lopes S. De Sutter P. Tracking in vitro human inner cell mass progression during embryonic stem cell derivation. Nature Biotech. 2012;26:278–282. doi: 10.1038/nbt.2135. [DOI] [PubMed] [Google Scholar]
- 29.Meisner LF. Johnson JA. Protocols for cytogenetic studies of human embryonic stem cells. Methods. 2008;45:133–141. doi: 10.1016/j.ymeth.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Darr H. Mayshar Y. Benvenisty N. Overexpression of NANOG in human ES cells enables feeder-free growth while inducing primitive ectoderm features. Development. 2006;133:1193–1201. doi: 10.1242/dev.02286. [DOI] [PubMed] [Google Scholar]
- 31.Li X. Meng G. Krawetz R. Liu S. Rancourt DE. The ROCK inhibitor Y-27632 enhances the survival rate of human embryonic stem cells following cryopreservation. Stem Cells Dev. 2008;17:1079–1086. doi: 10.1089/scd.2007.0247. [DOI] [PubMed] [Google Scholar]
- 32.Hyslop L. Stojkovic M. L Armstrong L. Walter T. Stojkovic P. Przyborski S. Herbert M. Murdoch A. Strachan T. Lako M. Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells. 2005;23:1035–1043. doi: 10.1634/stemcells.2005-0080. [DOI] [PubMed] [Google Scholar]
- 33.Zhen Y. Sørensen V. Jin Y. Suo Z. Wiedłocha A. Indirubin-3′-monoxime inhibits autophosphorylation of FGFR1 and stimulates ERK1/2 activity via p38 MAPK. Oncogene. 2007;26:6372–6385. doi: 10.1038/sj.onc.1210473. [DOI] [PubMed] [Google Scholar]
- 34.Tielens S. Verhasselt B. Liu J. Dhont M. Van Der Elst J. Cornelissen M. Generation of embryonic stem cell lines from mouse blastocysts developed in vivo and in vitro: relation to Oct-4 expression. Reproduction. 2006;132:59–66. doi: 10.1530/rep.1.00887. [DOI] [PubMed] [Google Scholar]
- 35.Ström S. Rodriguez K. Holm F. Bergström R. Eklund L. Strömberg A. Hovatta O. No relationship between embryo morphology and successful derivation of human embryonic stem cell lines. Plos One. 2010;5:e15329. doi: 10.1371/journal.pone.0015329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Leary T. Duggal G. Lierman S. Van den Abbeel E. Heindryckx B. De Sutter P. The influence of patient and cohort parameters on the incidence and developmental potential of embryos with poor quality traits for use in human embryonic stem cell derivation. Hum Reprod. 2012;27:1581–1589. doi: 10.1093/humrep/des040. [DOI] [PubMed] [Google Scholar]
- 37.Hanna J. Cheng AW. Saha K. Jongpil K. Lengner CJ. Soldnera F. Cassadya JP. Muffata J. Careya BW. Jaenischa R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci U S A. 2010;107:9222–9227. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Telugu BPVL. Ezashi T. Sinha S. Alexenko AP. Spate L. Prather RS. Roberts RM. Leukemia inhibitory factor (LIF)-dependent, pluripotent stem cells established from inner cell mass of porcine embryos. J Biol Chem. 2011;286:28948–28953. doi: 10.1074/jbc.M111.229468. [DOI] [PMC free article] [PubMed] [Google Scholar]


