Abstract
Tumor microenvironment plays important roles in tumor development and metastasis. Features of the tumor microenvironment that are significantly different from normal tissues include acidity, hypoxia, overexpressed proteases and so on. Therefore, these features can serve as not only biomarkers for tumor diagnosis but also theraputic targets for tumor treatment. Imaging modalities such as optical, positron emission tomography (PET) and magnetic resonance imaging (MRI) have been intensively applied to investigate tumor microenvironment. Various imaging probes targeting pH, hypoxia and proteases in tumor microenvironment were thus well developed. In this review, we will focus on recent examples on fluorescent probes for optical imaging of tumor microenvironment. Construction of these fluorescent probes were based on characteristic feature of pH, hypoxia and proteases in tumor microenvironment. Strategies for development of these fluorescent probes and applications of these probes in optical imaging of tumor cells or tissues will be discussed in this review paper.
Keywords: Optical imaging, tumor microenvironment, pH, hypoxia, protease
Introduction
Tumor microenvironment acts as “soil” in the formation, growth and metastasis of tumors [1]. The difference between tumor microenvironment and normal tissue microenvironment is also critical for the development of tumor diagnosis and chemotherapy targeting tumor tissues [2]. Imaging of tumor microenvironment is an emerging area with the development of new imaging techniques as well as molecularly specific probes. For example, novel cryo-imaging of the glioma tumor microenvironment has been used to reveal migration and dispersal pathways in vivid three-dimensional detail [3]. The optical frequency domain imaging enables three-dimensional microscopy of the tumor microenvironment in vivo [4]. Noninvasive assessment of tumor microenvironment was realized using dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and positron emission tomography imaging (PET) in neck nodal metastases [5]. Using multiphoton microscopy, it is also possible to do in vivo real-time imaging of chemotherapy response on the liver metastatic tumor microenvironment [6]. A multi-modal optical diagnostic approach for in vivo imaging of tumor vascular network and blood microcirculation has been developed recently utilizing a combined use of fluorescence intravital microscopy (FIM), dynamic light scattering (DLS) and spectrally enhanced microscopy (SEM) modalities [7].
The construction of probes targeting tumor microenvironment which allows non-invasive imaging of tumor microenvironment is an important research field in molecular imaging. A wide variety of nanoparticle platforms are being developed for the construction of molecular imaging probes targeting tumor microenvironment. Metabolically directed nanoparticles such as the pH-titratable superparamagnetic iron oxide were found to have improved nanoparticle accumulation in acidic tumor [8]. Magnetic nanoparticle clusters encapsulated inside a liposome, under the influence of an external magnet, can target tumor microenvironment and give high contrast magnetic resonance imaging (MRI) [9]. Receptor-ligand interactions are frequently used for nanoprobes to target tumor microenvironment [10-12]. In addition to probes for MRI and PET imaging, fluorescent probes with response to the special features of tumor microenvironment represent the “smart” probe in the optical imaging of tumor microenvironment [13]. We will focus on recent progress on the construction of “smart” fluorescent probes for optical imaging of tumor microenvironment in this review paper.
Fluorescent probes with sensitive response to pH, hypoxia and proteases overexpressed in tumor microenvironment could find applications in optical imaging of tumor microenvironment. We will summarize the principle and strategies used for the construction of fluorescent probes which can respond to these important features of tumor microenvironment. Applications of these “smart” fluorescent probes in optical imaging of tumor will also be reviewed with an emphasis on latest examples reported in the past three years. Multimodality imaging with fluorescence as one modality will also be covered in this review paper. Ratiometric probes whose excitation or emission spectra could shift with pH change, hypoxia or tumor microenvironment related proteases have the potential to quantify the imaging target. They will also be introduced in this review.
Fluorescent Imaging of acidic tumor microenvironment
The change of pH in tumor microenvironment compared to normal tissue has been well recognized and the slightly acidic pH in tumor microenvironment has become an important issue in the design of anti-tumor therapy [13]. The pH of the tumor extracellular space is in the range of pH 6.2–6.9, which is lower than that of normal tissues (pH 7.4). Therefore fluorescent probes which could be activated specifically in this pH range are useful for optical imaging of the acidic tumor microenvironment. Small molecular probe and nanoprobe have both been constructed to fluoresce in the acidic tumor microenvironment but not in normal tissues.
Fluorescent pH probes based on small molecules
Activatable fluorescent probes which have sufficient specificity and sensitivity in tumor tissue could minimize the background signal from nontarget tissues in optical imaging. Therefore great efforts have been devoted to the synthesis of organic dye modified small molecules whose fluorescence could be turned on by protonation within the narrow pH range corresponding to tumor microenvironment. The dye molecule in the activatable pH fluorescence probes with practical use in optical imaging includes boron-dipyrromethene (BODIPY) and the near-infrared (NIR) fluorescent dye cyanine (Cy).
Based on the photon-induced electron transfer (PeT) theory, amino groups such as those in N,N-dialkylated anilines have highest occupied molecular orbital energy levels which is sufficient to cause PeT toward the BODIPY fluorophore and make it non-fluorescent. Upon protonation of the amino group to interrupt the PeT, the emission of BODIPY restores to give bright fluorescent signal over 500 nm. Urano et al. developed a series of acidic pH-sensitive fluorescence probes based on the integration of different N,N-dialkylated aniline functionality with 2,6-dicarboxyethyl-1,3,5,7-tetramethyl-BODIPY [14]. These compounds were reported to be almost nonfluorescent in the nonprotonated form due to PeT from the aniline moiety to the fluorophore, but become highly fluorescent in the protonated form with fluorescent quantum yield around 0.55–0.60. Thus an increase greater than 300-fold in fluorescent emission was achieved upon turn-on of the fluorescent probes under specific pH.
NIR pH-activatable probe has been developed using pH-sensitive Cy dye (Figure 1A) [15]. Cy dye is a series of NIR fluorescent dyes with high extinction coefficients. When Cy dye molecules were modified with amines or other N-containing moieties such as terpyridine (Tpy), they became pH-sensitive Cy dye because PeT between Cy and the N-containing group could be interrupted upon protonation of the N atoms. For example, Tpy-Cy in which Cy was linked with Tpy by an aminomethylphenyl group has been reported to respond linearly and rapidly to minor pH fluctuations within the range of 6.7-7.9 due to fast PeT process [16]. The aminocyanine bearing a diamine moiety were reported to have a 46- to 83-nm red shift of the absorption maximum under acidic conditions, thus were used as ratiometric pH probes [17]. Tumor selective NIR pH-activatable probe was developed by conjugating pH-sensitive Cy dye to a cyclic arginine-glycine-aspartic acid (cRGD) peptide targeting αvβ3 (Figure 1B) [15].
Figure 1.
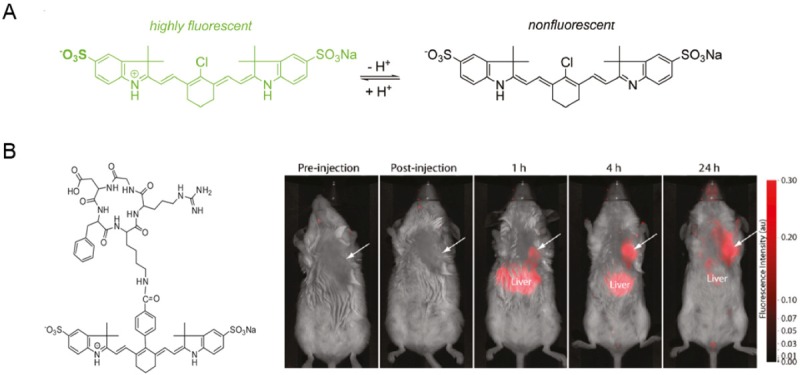
A. Mechanism for activation of pH sensitive Cy dyes. B. Structure of cRGD linked pH activatable Cy dye and its application in optical imaging of orthotopic 4T1/luc tumor, arrows indicated the tumor region. Adapted from reference [15].
Small molecules based activatable fluorescent pH probes may be widely applied in imaging of tumor microenvironment due to the flexibility of small molecules. With the modification of different targeting groups, these probes could specifically image various interest tumors. However, only few dyes could be protonated to produce ‘turn-on’ fluorescent signals and these dyes could only response to a specific narrow pH range, which will limit applications of these probes in tumor imaging. Development of other small molecule based fluorescent pH probes is thus of great importance.
pH nanoprobes for optical imaging
Construction of nanoprobes for imaging of tumor microenvironment is of great interest currently [8,15,18,19]. Various nanoparticles have been developed for optical imaging or multi-modality imaging [20]. Fluorescent nanorods and nanospheres have also been used for real-time in vivo probing of nanoparticle shape-dependent tumor penetration [21]. Compared to small molecular probes, fluorescence nanoprobes have advantages such as tunable circulation lifetime, up-regulated accumulation in tumor and enhanced sensitivity by labeling multiple imaging reporters on a single nanoparticle. The mechanisms of pH responsive fluorescence of the nanoprobes include fluorescent resonance energy transfer (FRET) effect or self-aggregation associated energy transfer effect (SAET) which can be referred to recent review [22].
Quantum dots (QD) have been used as FRET donor in pH nanoprobes. A charge-transfer coupled pH probe has been developed using QD-dopamine-peptide bioconjugates [23]. The response of the QD conjugates to pH was based on the change of intrinsic redox properties of the coated dopamine which upon oxidation at basic pH could turn into a quencher of QD. Ratiometric pH probes based on FRET pairs with QD as donor have also been developed. Organic dyes which have response to pH change such as fluorescein isothiocyanate (FITC) and the pH dye SNARF have been coated to QD surface to get QD-based ratiometric pH probes [24,25]. Fluorescent diblock copolymer with pH response has also been coated on QD surface to get color distinctive, ratiometric pH probe [26]. QD coated with pH-sensitive fluorescent proteins has been reported recently, which showed dramatically improved sensitivity and photostability compared to the widely used fluorescent dyes for pH imaging [27]. FRET between the quantum dot and multiple fluorescent proteins were found to be able to modulate the emission ratio from fluorescent proteins and quantum dots and a change more than 12 fold could be achieved between pH 6 and 8. Most of these QD-based pH probes have just been tested in live cells and their applications for in vivo imaging are yet to be explored. One example using dextran based pH-sensitive NIR nanoprobe has been demonstrated on the in vivo differential-absorption dual-wavelength photoacoustic imaging of tumors [28].
Construction of the other type of pH nanoprobe based on self-quenching dye or SAET is emerging after the FRET-type pH probes. The carbocyanine derivative IR783 was linked to the dextran-based nanoparticle via an acid-sensitive hydrozone bond which could be cleaved under the acidic tumor microenvironment [29]. The self-quenching between the spatially neighboring IR783 fluorophores could be diminished upon the cleavage of the dye molecule off the nanoparticle and give high tumor-to-normal tissue (T/N) signal ratio and a prolonged time-window for tumor visualization in vivo. Gao et al. reported a set of tunable, pH-activatable micellar nanoparticles based on the supramolecular self-assembly of ionizable block copolymer micelles as a SAET type pH nanoprobe (Figure 2A) [30]. Using amine groups with different pKa in the polymer backbone as shown in Figure 2B, a series of SAET probes could be obtained which can be activated at different pH because only the protonated ammonium groups could lead to micelle dissociation into unimers with a dramatic increase in fluorescence emission. Specific activation of nanoprobes in acidic tumor cells was observed (Figure 2C). Multicolored, pH tunable fluorescent nanoplatform has been developed using the same principle through the use of commonly available pH-insensitive dyes [31]. The fluorescence wavelengths can be fine-tuned from green to NIR emission range (500-820 nm) on the nanoplatform and their fluorescence ON/OFF activation can be achieved within 0.25 pH units.
Figure 2.
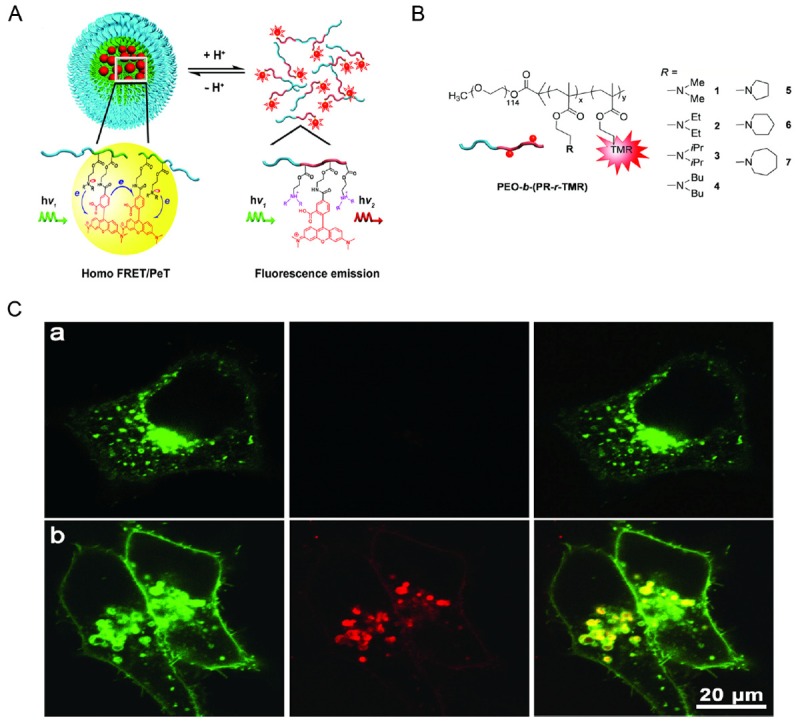
A. Design of pH-activatable micellar nanoprobes. B. Different amine groups linked to the micelle backbone to make the nanoprobes activatable at different pH upon protonation of the amine group. C. Fluorescent images of cells treated of pH-activatable nanoprobe with (top panel) or without (bottom panel) the inhibition of lysosomal acidification. Nanoprobe activation was indicated by the red fluorescence signals. Adapted from reference [30].
Other than quantum dots and polymeric nanoparticles, newly emerging nanomaterial has also been used to construct pH nanoprobes. Ma et al. reported a carbon nanodots (CDs) based ratiometric pH probe which could measure the intracellular pH in the whole cell quantitatively [32]. As shown in Figure 3A, the amino-coated CDs were coated with different molar ratios of pH-sensitive FITC to pH insensitive rhodamine B isothiocyanate (RBITC). The dual-labeled CDs allowed facile adjustment of pH response range and showed good biocompatibility and intracellular dispersibility (Figure 3B). It could be a promising pH nanoprobe for ratiometric imaging of tumor microenvironment in vivo.
Figure 3.
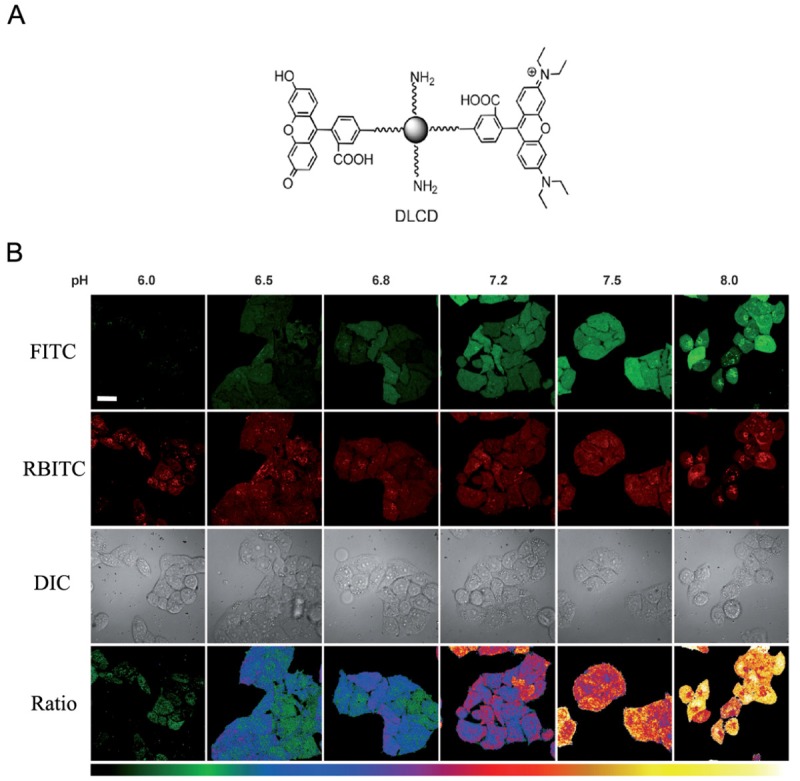
A. Structure of the dual-labeled carbon nanodot as ratiometric pH nanoprobe. B. Fluorescent images of HeLa cells at pH 6.0, 6.5, 6.8, 7.2, 7.5 and 8.0, respectively. Adapted from reference [32].
Conjugation with nanoparticles could improve the biocompatibility and delivery efficiency of small fluorescent probes and adjust the emissions of these fluorescent probes to interest range. Therefore, development of pH nanoprobes greatly broadens the applications of pH sensitive small molecule dye in optical imaging of tumor microenvironment. The specificity may be realized by attaching targeting groups onto the surface of nanoprobes. While, these nanoprobes also could only response to a specific narrow pH range. And the big size of the probes will cause unexpected high uptake of liver and kidney when performing in vivo imaging, which is also a common problem of nanoparticles. Development of more biocompatible nanocarriers and other pH nanoprobes may help to solve these problems.
Fluorescent imaging of tumor hypoxia
Hypoxia refers to the situation where tumor tissues lack of oxygen as a result of the rapid tumor growth. The imbalance between the supply and consumption of oxygen leads to significantly lower oxygen concentration in tumor region than in normal tissues, making hypoxia a characteristic feature of locally advanced tumors [33]. It has also been suggested that hypoxia could promote tumor invasion and metastasis and resistance of tumor cells to therapy as well [33,34]. Therefore, fluorescent imaging of hypoxia in tumor microenvironment is of great significance. Both oxygen-sensitive fluorescent probes and bioreductive fluorescent probes have been constructed to image tumor hypoxia in vitro and in vivo.
Oxygen-sensitive fluorescent probes
Since oxygen is a powerful quencher for phosphors [35], phosphorescent probes including metalloporphyrin complexes and ruthenium and iridium complexes were developed for imaging of hypoxia. Lack of oxygen in tumor regions can significantly increase the brightness of these probes, making them good candidates for imaging purpose.
Phosphor oxyphor G2, a palladium porphyrin complex, was used to evaluate the oxygen distribution in murine tumor tissues [36]. Three tumor types with different oxygen levels were examined, showing characteristic and consistent oxygen profiles. These results demonstrated that phosphorescence quenching is capable of providing real-time determination of oxygen concentrations within tumors. A red light-emitting iridium complex, BTP, was reported by Tobita et al. as a hypoxia-sensing probe [37,38]. This probe exhibited hypoxia-dependent light emission in cultured cell lines and in vivo imaging results also revealed that BTP had the ability to image hypoxia in tumors.
To quantify oxygen levels in cells and tissues, ratiometric imaging with the help of reference dye can be used. A small molecule oxygen sensor which consisted of coumarin 343 as an oxygen-insensitive fluorophore and iridium complex BTP as an oxygen-sensitive phosphore was further developed by Tobita et al. and applied to monitor oxygen levels in tumor cells (Figure 4) [39]. An oxygen-sensitive palladium meso-tetraphenylporphyrin and an inert reference dye were loaded onto polystyrene nanoparticles for ratiometric imaging of tumor hypoxia [40]. The ratio fluorescence of this nanoparticle-based probe also exhibited dose dependence on the oxygen concentrations. With the targeting group, monoclonal antibody Herceptin, on the surface of nanoparticles, targeted in vitro and in vivo imaging were realized. These results showed good correlation between ratiometric response and oxygen levels. Another ratiometirc probe for hypoxia imaging, which is just a single-component, was developed by Fraser et al. [41]. This probe utilized a dual-emissive boron biomaterial with weak fluorescence as reference and strong phosphorescence as ‘turn-on’ signal. The signal ratio of this simple probe was found well consistent with oxygen concentrations in vitro and in vivo. Fluorescent probes discussed above exhibited oxygen dependent signals, which made them quite suitable to image hypoxia with high specificity. Especially when phosphorescence was combined with fluorescence, quantitative information about oxygen within tumor regions could be obtained. However, toxicity of these heavy metal based probes is a major concern when applying them in the future. Strategies to avoid the toxicity of these fluorescent probes or discovery of other potential fluorescent probes will promote the development of oxygen-sensitive fluorescent probes for imaging of tumor hypoxia.
Figure 4.
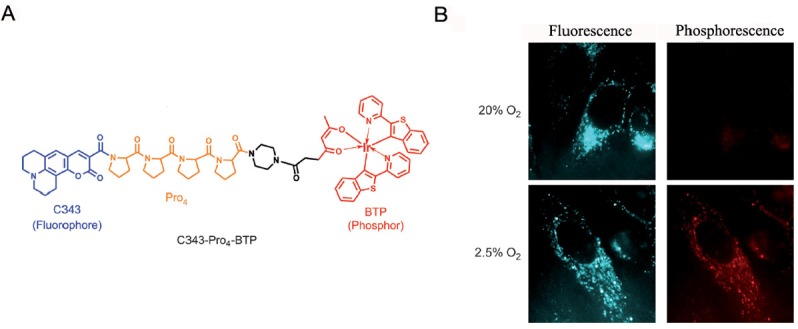
A. Chemical structure of ratiometric probe C343-Pro4-BTP. B. Luminescent images of HeLa cells incubated under aerobic and hypoxic conditions. Adapted from reference [39].
Bioreductive fluorescent probes
Hypoxic environment within tumors could cause bioreductive reactions of small molecules such as 2-nitroimidazole and indolequinone. This feature has also been well used to construct fluorescent probes for hypoxia imaging. 2-Nitroimidazole, also known as hypoxia marker, could be selectively reduced and bound in hypoxic tissues. Therefore, it has been employed in various probes as targeting moiety for imaging of hypoxia in tumors [42]. Novel fluorescent markers containing naphthalimides and two 2-nitroimidazole chains were developed by Zhang et al. for imaging of hypoxic cells [43]. NIR fluorescent dye Cy was linked with two 2-nitroimidazoles as a fluorescent probe for in vitro and in vivo imaging of tumor hypoxia (Figure 5A) [44]. In vitro imaging showed higher levels of fluorescence in hypoxic cells than in normoxic cells after treatment with this probe. And in vivo and ex vivo fluorescence imaging also revealed specific accumulation of this probe in tumors, suggesting it is a promising in vivo optical imaging probe for tumor hypoxia (Figure 5B).
Figure 5.
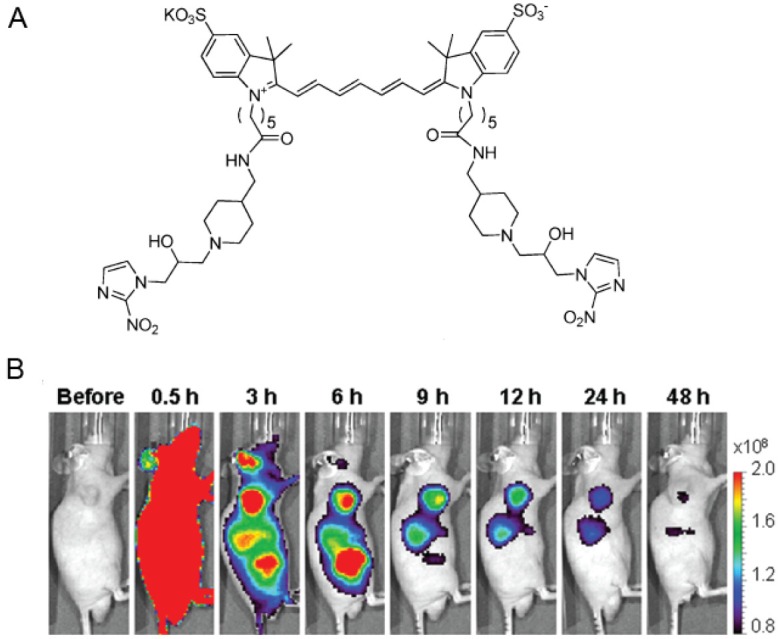
A. Chemical structure of 2-nitroimidazoles modified NIR dye Cye. B. In vivo optical imaging of subcutaneous tumors. Adapted from reference [44].
Except using bioreductive products to target tumors, bioreductive reactions have also been used to construct ‘turn-on’ fluorescent imaging probes for hypoxia. As substituents of indolequinone derivatives could be removed by reduction under hypoxic conditions, Nishimoto et al. developed a new class of fluorescent probes for investigating hypoxia [45]. Nagano et al. developed hypoxia-sensitive NIR fluorescent probes QCys based on the findings that azo functional group could be cleaved under hypoxic conditions [46]. QCys consisted of NIR dyes Cy and quencher BHQ which were linked together by azo functional groups. Potential applications of these probes for in vivo real-time fluorescence imaging were also evaluated, showing good imaging quality. These ‘turn-on’ fluorescent probes represent a novel type of probes for optical imaging of tumor microenvironment.
Besides, ratiometric fluorescent probes based on bioreductive reactions have also been developed. A new prodrug derivative containing a p-nitrobenzyl moiety which is a hypoxia-selective leaving group activated by nitro reduction and a selective ratiometric fluorescent sensor (RHP) was synthesized by Xiao et al. [47]. Cell imaging results showed good ability of this probe to distinguish aerobic and hypoxic tumor cells. A dual-response fluorescent probe, UTX-12, was designed by Hori et al., which consisted of a p-nitro benzyl moiety and SNARF [48]. UTX-12 could image not only tumor hypoxia but also tumor acidic environment. Clear differences in fluorescence between hypoxic and aerobic conditions in tumor cells were observed with UTX-12 as probe.
Fluorescent probes utilizing bioreductive feature of tumor microenvironment allowed efficient imaging of tumor hypoxia. However, instability of these probes in vivo may lead to high non-specific background signal. Efficient tumor imaging can be realized with further development of these bioreductive fluorescent probes.
Fluorescent imaging of proteases in tumor microenvironment
Development of tumor is often associated with overexpression of proteases in tumor microenvironment in comparison with that in normal tissues [49]. Recent evidence has suggested that proteases such as matrix metalloproteinases (MMPs), cathepsins and fibroblast activation protein alpha (FAPα) could promote tumor growth, invasion and metastasis [50-52]. Therefore, these proteases could act as not only biomarkers for diagnosis but also potential targets for therapy [53-55]. Imaging of proteases in tumor microenvironment is thus of great research interest. The abilities of proteases to covalently bind with target molecules or cleave specific substrates have been widely used to construct fluorescent probes for imaging purpose.
Activity-based fluorescent probes
As some proteases are capable of covalently modifying enzyme targets in an activity dependent manner, covalent reaction between proteases and reactive functional groups that are linked with fluorescent tags was then used to develop a type of activity-based fluorescent probes. The covalent binding between fluorphore and target proteases allows direct imaging of interest protease from others.
Cathepsins, which belong to the lysosomal cysteine protease family, have been shown to play important roles in various cancers [56]. A number of activity-based fluorescent probes have been developed toward cathepsins. Epoxide reactive electrophiles were conjugated with fluorphore Cy5 to image cathepsin X [57]. With these probes, cathepsin X was labeled and visualized in complex lysates, intact cells and living mice. As the unknown function of cathepsin X, these probes represent a type of valuable research tools. A highly reactive group of cathepsin, acyloxymethyl ketone (AMOK), was used to generate another type of activity-based fluorescent probes for cathepsins. NIR dye Cy5 and quencher dye QSY21 were linked together through AMOK linker. Reaction between AMOK and cathepsin dissociates the fluorphore and quencher, producing fluorescent labeling of cathepsin. Quenched activity-based fluorescent probes which are constructed by peptide backbones were developed by Bogyo et al. to image cysteine cathepsin in vivo [58]. Cathepsin activity was directly monitored by these probes. Later, an optimized nonpeptidic probe was further developed to investigate cathepsin S [59]. This probe provided high tumor-specific fluorescence signals in vivo by targeting tumor-associated macrophages.
Besides cathepsins, some other proteases have also been labeled by activity-based fluorescent probes. Integral membrane seine hydrolase KIAA1363, which is highly expressed in aggressive human cancer cell lines and primary tumors, was successfully labeled by its inhibitor linked fluorphore BODIPY [60]. This covalent labeling allowed determination of the localization and half-life for KIAA1363 in cancer cells. Legumain, which is also a lysosomal cysteine protease, was also fluorescently labeled by activity-based probe LP-1 (Figure 6A) [61]. This probe employed aza-peptidyl Asn epoxide that is highly selective inhibitor as targeting group and NIR fluorphore as labeling tag. Imaging results by using this probe showed specific labeling of legumain in various normal tissues as well as in solid tumors when applied in vivo (Figure 6B).
Figure 6.
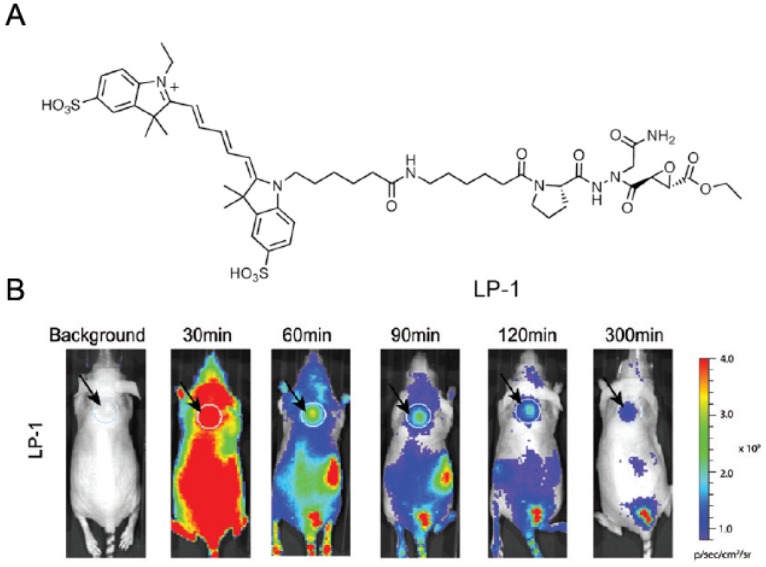
A. Chemical structure of activity-based fluorescent probe LP-1. B. In vivo optical imaging of active legumain using LP-1. Adapted from reference [61].
Activity-based fluorescent probes discussed above had the ability to form permanent covalent bonds with target proteases, allowing direct imaging of these proteases. These labeled proteases could be further subject to biochemical analysis, enabling discovery of more detailed information about target proteases. While, these probes usually lack of cell permeability and rapid diffusion, which limits their use. And most of these activity-based fluorescent probes usually generate a background fluorescence signal regardless of whether they were bound to target proteases or not. Further development to improve the efficiency of these probes is needed.
Activatable fluorescent probes
Unique catalytic activities of proteases that can cleave specific substrate enable design of smart fluorescent probes that can be turned on upon meeting with the enzyme target. These activatable fluorescent probes hold great potential for imaging of proteases due to the enhanced tumor to background signal ratio. Various proteases such as MMPs, caspase and thrombin have been successfully imaged by using activatable fluorescent porbes [62].
Favorite peptide substrates of MMPs were used to construct a series of activatable fluorescent probes for imaging of MMPs. NIR dye IRDye 800CW was linked with quencher BHQ-3 through MMPs substrate [63]. Expected cleavage fragments were demonstrated in in vitro assay and extensive metabolism of this probe was also found in vivo. To increase the stability of these activatable fluorescent probes, poly(ethylene) glycol groups were attached to the end of these probes, which use NIR dye Cy5.5 as the fluorphore and BHQ-3 as the quencher [64]. These activatable probes allowed real- and long- time imaging of MMPs in vivo. With RGD group in the activatable fluorescent probes, improved in vivo imaging of MMP with minimized nonspecific accumulation in irrelevant tissues has also been realized [65]. High cell permeability of activatable fluorescent probes for MMPs was realized using BODIPY as the fluorphore [66]. Comparing to using sulfoCy5 as fluorphore, probe BODIPY-MMP exhibited an increment of fluorescence inside cells and longer visualization of MMP activity in vivo due to better cell permeability of BODIPY. Other than using dyes as quencher, nanoparticles also could serve as quenchers to generate activatable fluorescent probes for MMPs. By using Fe3O4 nanoparticles [67] and flower-like Au-Fe3O4 nanoparticles [68] to quench the fluorescence of NIR dye Cy5.5, in vivo multimodality imaging of MMP-2 has also been realized.
Recently, Li et al. reported the first activatable fluorescent probe for in vivo imaging of FAPα, which is overexpressed in various cancers. This probe ANPFAP (Figure 7A) consisted of a NIR dye Cy5.5 and a quencher QSY21, which were linked by the peptide substrate (KGPGPNQC) specific for FAPα [69]. In vitro assay demonstrated high specificity and sensitivity of ANPFAP to FAPα. In vivo imaging also showed high accumulation of ANPFAP in U87MG tumor models with FAPα expression, while no signals were observed in C6 tumor models without FAPα expression (Figure 7B).
Figure 7.
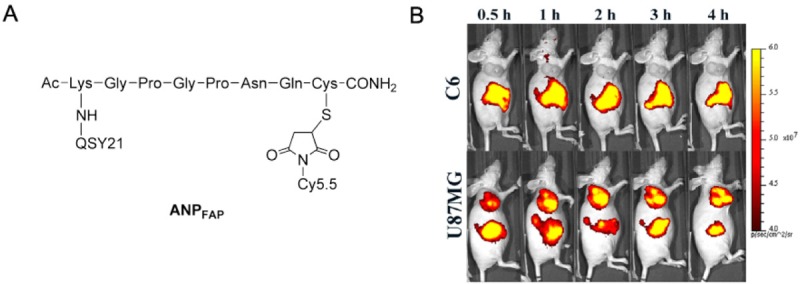
A. Chemical structure of ANPFAP. B. In vivo imaging of FAPα in C6 and U87MG tumor-bearing mice. Adapted from reference [69].
Except using FRET pair between dye and quencher, another type of activatable fluorescent probes which utilize two fluorphores for ratiometric imaging of proteases have also been developed. FRET pair of coumarin343 and TAMRA were linked together by peptide sequence (QPMAVVQSVP) specific for neutrophil elastase (NE) [70]. Enzyme cleavage of these probes showed good response toward NE and cell imaging also exhibited good correlation between fluorescence ratio and NE expression. NIR dyes Cy5 and Cy7 were connected by peptide sequence LFSRPP, which is specific substrate of thrombin [71]. With the fluorescence ratio signal between Cy5 and Cy7, rapid in vivo readout of thrombin activation was provided, demonstrating great potential of ratiometric probes in protease imaging.
Activatable fluorescent probes using specific substrate of interest enzyme could enhance the tumor-to-background signal and allow noninvasive monitoring of enzyme activity. However, the existed large number and diversity of proteases hinders the development of highly specific substrates. These designed substrates usually suffer from unexpected cleavage by other enzymes. Also, there are various enzymes present in serum, causing extremely instability of these activatable fluorescent probes when applied in vivo. Development of activatable fluorescent probes with high specificity and stability is thus required.
Conclusion and perspective
Acidity, hypoxia and overexpressed proteases, significant features of tumor microenvironment, play important roles in tumor development and metastasis. Optical imaging of these features has attracted many scientists’ attention and various fluorescent probes targeting these features have been developed and widely applied in biomedical research. Imaging results from these fluorescent probes have provided useful information about tumor microenvironment, which is helpful for early diagnosis and theranostics of tumors. For example, an optical nanoprobe consisting of Cy5.5 labeled MMP-2 substrate and gold nanoparticles was reported by Ahn et al. for use in protease inhibitor drug screening and early diagnosis of cancer in vivo [72]. Apoptosis in mice treated with drugs was successfully monitored with caspase-specific activity-based fluorescent probes [73]. Except for features in tumor microenviroment mentioned in this review paper, others such as caspase, epidermal growth factor receptor (EGFR) and integrin αvβ3 are also potential targets for tumor imaging. Another promising imaging target in tumor microenviroment is microRNA, which is a type of short non-conding RNAs and capable of silencing genes. Recent evidence has suggested that microRNAs are closely related with cancer development [74] and could function as biomarkers and therapeutic targets of cancers [75-77]. Optical imaging of microRNAs is thus of great potential and under current research interest [78,79].
Optical imaging is limited by the depth of penetration in tissue and lack of quantitative information. Integration with other imaging modalities such as PET and MRI will help to solve this problem. For example, more detailed information about MMPs has been obtained by combining PET imaging and MRI with fluorescence imaging [67,80,81]. Caspase-3 activity [82] and EGFR status [83] were determined by coupling MRI with fluorescence imaging. Fluorescence imaging has also been combined with PET and MRI for multimodality imaging of integrin αvβ3 [84]. Efficient and specific delivery of imaging agents to interest tissues is also critical for imaging of tumor microenvironment. To address this issue, nanocarriers may be used [85]. With the development of multimodality or multifunctional imaging probes targeting different features in tumor microenvironment, more comprehensive information will be collected for biomedical research.
Acknowledgements
Financial support the National Basic Research Program of China (No. 2011CB935800) and the Natural Science Foundation of Jiangsu Province (BK2012012) was acknowledged.
References
- 1.Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7:139–147. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 3.Burden-Gulley SM, Qutaish MQ, Sullivant KE, Lu H, Wang J, Craig SE, Basilion JP, Wilson DL, Brady-Kalnay SM. Novel cryo-imaging of the glioma tumor microenvironment reveals migration and dispersal pathways in vivid three-dimensional detail. Cancer Res. 2011;71:5932–5940. doi: 10.1158/0008-5472.CAN-11-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vakoc BJ, Lanning RM, Tyrrell JA, Padera TP, Bartlett LA, Stylianopoulos T, Munn LL, Tearney GJ, Fukumura D, Jain RK, Bouma BE. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat Med. 2009;15:1219–1223. doi: 10.1038/nm.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jansen JF, Schoder H, Lee NY, Wang Y, Pfister DG, Fury MG, Stambuk HE, Humm JL, Koutcher JA, Shukla-Dave A. Noninvasive assessment of tumor microenvironment using dynamic contrast-enhanced magnetic resonance imaging and 18F-fluoromisonidazole positron emission tomography imaging in neck nodal metastases. Int J Radiat Oncol Biol Phys. 2010;77:1403–1410. doi: 10.1016/j.ijrobp.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka K, Okigami M, Toiyama Y, Morimoto Y, Matsushita K, Kawamura M, Hashimoto K, Saigusa S, Okugawa Y, Inoue Y, Uchida K, Araki T, Mohri Y, Mizoguchi A, Kusunoki M. In vivo real-time imaging of chemotherapy response on the liver metastatic tumor microenvironment using multiphoton microscopy. Oncol Rep. 2012;28:1822–1830. doi: 10.3892/or.2012.1983. [DOI] [PubMed] [Google Scholar]
- 7.Kalchenko V, Madar-Balakirski N, Meglinski I, Harmelin A. In vivo characterization of tumor and tumor vascular network using multimodal imaging approach. J Biophotonics. 2011;4:645–649. doi: 10.1002/jbio.201100033. [DOI] [PubMed] [Google Scholar]
- 8.Crayton SH, Tsourkas A. pH-titratable superparamagnetic iron oxide for improved nanoparticle accumulation in acidic tumor microenvironments. ACS Nano. 2011;5:9592–9601. doi: 10.1021/nn202863x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikhaylov G, Mikac U, Magaeva AA, Itin VI, Naiden EP, Psakhye I, Babes L, Reinheckel T, Peters C, Zeiser R, Bogyo M, Turk V, Psakhye SG, Turk B, Vasiljeva O. Ferri-liposomes as an MRI-visible drug-delivery system for targeting tumours and their microenvironment. Nat Nanotechnol. 2011;6:594–602. doi: 10.1038/nnano.2011.112. [DOI] [PubMed] [Google Scholar]
- 10.Saatchi K, Soema P, Gelder N, Misri R, McPhee K, Baker JH, Reinsberg SA, Brooks DE, Hafeli UO. Hyperbranched polyglycerols as trimodal imaging agents: design, biocompatibility, and tumor uptake. Bioconjug Chem. 2012;23:372–381. doi: 10.1021/bc200280g. [DOI] [PubMed] [Google Scholar]
- 11.Locke LW, Mayo MW, Yoo AD, Williams MB, Berr SS. PET imaging of tumor associated macrophages using mannose coated 64Cu liposomes. Biomaterials. 2012;33:7785–7793. doi: 10.1016/j.biomaterials.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanabe K, Zhang Z, Ito T, Hatta H, Nishimoto S. Current molecular design of intelligent drugs and imaging probes targeting tumor-specific microenvironments. Org Biomol Chem. 2007;5:3745–3757. doi: 10.1039/b711244k. [DOI] [PubMed] [Google Scholar]
- 13.Iessi E, Marino ML, Lozupone F, Fais S, Milito AD. Tumor acidity and malignancy: novel aspects in the design of anti-tumor therapy. Cancer Ther. 2008;6:55–66. [Google Scholar]
- 14.Urano Y, Asanuma D, Hama Y, Koyama Y, Barrett T, Kamiya M, Nagano T, Watanabe T, Hasegawa A, Choyke PL, Kobayashi H. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat Med. 2009;15:104–109. doi: 10.1038/nm.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H, Akers W, Bhushan K, Bloch S, Sudlow G, Tang R, Achilefu S. Near-infrared pH-activatable fluorescent probes for imaging primary and metastatic breast tumors. Bioconjug Chem. 2011;22:777–784. doi: 10.1021/bc100584d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang B, Yu F, Li P, Tong L, Duan X, Xie T, Wang X. A near-infrared neutral pH fluorescent probe for monitoring minor pH changes: imaging in living HepG2 and HL-7702 cells. J Am Chem Soc. 2009;131:3016–3023. doi: 10.1021/ja809149g. [DOI] [PubMed] [Google Scholar]
- 17.Myochin T, Kiyose K, Hanaoka K, Kojima H, Terai T, Nagano T. Rational design of ratiometric near-infrared fluorescent pH probes with various pKa values, based on aminocyanine. J Am Chem Soc. 2011;133:3401–3409. doi: 10.1021/ja1063058. [DOI] [PubMed] [Google Scholar]
- 18.Wong C, Stylianopoulos T, Cui J, Martin J, Chauhan VP, Jiang W, Popovic Z, Jain RK, Bawendi MG, Fukumura D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci U S A. 2011;108:2426–2431. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies A, Lewis DJ, Watson SP, Thomas SG, Pikramenou Z. pH-controlled delivery of luminescent europium coated nanoparticles into platelets. Proc Natl Acad Sci U S A. 2012;109:1862–1867. doi: 10.1073/pnas.1112132109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He X, Gao J, Gambhir SS, Cheng Z. Near-infrared fluorescent nanoprobes for cancer molecular imaging: status and challenges. Trends Mol Med. 2010;16:574–583. doi: 10.1016/j.molmed.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chauhan VP, Popovic Z, Chen O, Cui J, Fukumura D, Bawendi MG, Jain RK. Fluorescent nanorods and nanospheres for real-time in vivo probing of nanoparticle shape-dependent tumor penetration. Angew Chem Int Ed. 2011;50:11417–11420. doi: 10.1002/anie.201104449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Li C. pH responsive fluorescence nanoprobe imaging of tumors by sensing the acidic microenvironment. J Mater Chem. 2011;21:15862–15871. [Google Scholar]
- 23.Medintz IL, Stewart MH, Trammell SA, Susumu K, Delehanty JB, Mei BC, Melinger JS, Blanco-Canosa JB, Dawson PE, Mattoussi H. Quantum-dot/dopamine bioconjugates function as redox coupled assemblies for in vitro and intracellular pH sensing. Nat Mater. 2010;9:676–684. doi: 10.1038/nmat2811. [DOI] [PubMed] [Google Scholar]
- 24.Jin T, Sasaki A, Kinjo M, Miyazaki J. A quantum dot-based ratiometric pH sensor. Chem Commun. 2010;46:2408–2410. doi: 10.1039/b921602b. [DOI] [PubMed] [Google Scholar]
- 25.Somers RC, Lanning RM, Snee PT, Greytak AB, Jain RK, Bawendi MG, Nocera DG. A nanocrystal-based ratiometric pH sensor for natural pH ranges. Chem Sci. 2012;3:2980–2985. doi: 10.1039/C2SC20212C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paek K, Chung S, Cho CH, Kim BJ. Fluorescent and pH-responsive diblock copolymer-coated core-shell CdSe/ZnS particles for a color-displaying, ratiometric pH sensor. Chem Commun. 2011;47:10272–10274. doi: 10.1039/c1cc13848k. [DOI] [PubMed] [Google Scholar]
- 27.Dennis AM, Rhee WJ, Sotto D, Dublin SN, Bao G. Quantum dot-fluorescent protein FRET probes for sensing intracellular pH. ACS Nano. 2012;6:2917–2924. doi: 10.1021/nn2038077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang G, Si Z, Yang S, Li C, Xing D. Dextran based pH-sensitive near-infrared nanoprobe for in vivo differential-absorption dual-wavelength photoacoustic imaging of tumors. J Mater Chem. 2012;22:22575–22581. [Google Scholar]
- 29.Li C, Xia JA, Wei XB, Yan HH, Si Z, Ju SH. pH-Activated Near-Infrared Fluorescence Nanoprobe Imaging Tumors by Sensing the Acidic Microenvironment. Adv Funct Mater. 2010;20:2222–2230. [Google Scholar]
- 30.Zhou K, Wang Y, Huang X, Luby-Phelps K, Sumer BD, Gao J. Tunable, ultrasensitive pH-responsive nanoparticles targeting specific endocytic organelles in living cells. Angew Chem Int Ed. 2011;50:6109–6114. doi: 10.1002/anie.201100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou K, Liu H, Zhang S, Huang X, Wang Y, Huang G, Sumer BD, Gao J. Multicolored pH-tunable and activatable fluorescence nanoplatform responsive to physiologic pH stimuli. J Am Chem Soc. 2012;134:7803–7811. doi: 10.1021/ja300176w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi W, Li X, Ma H. A tunable ratiometric pH sensor based on carbon nanodots for the quantitative measurement of the intracellular pH of whole cells. Angew Chem Int Ed. 2012;51:6432–6435. doi: 10.1002/anie.201202533. [DOI] [PubMed] [Google Scholar]
- 33.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 34.Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol. 2004;14:198–206. doi: 10.1016/j.semradonc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Rumsey WL, Vanderkooi JM, Wilson DF. Imaging of phosphorescence: a novel method for measuring oxygen distribution in perfused tissue. Science. 1988;241:1649–1651. doi: 10.1126/science.241.4873.1649. [DOI] [PubMed] [Google Scholar]
- 36.Ziemer LS, Lee WM, Vinogradov SA, Sehgal C, Wilson DF. Oxygen distribution in murine tumors: characterization using oxygen-dependent quenching of phosphorescence. J Appl Physiol. 2005;98:1503–1510. doi: 10.1152/japplphysiol.01140.2004. [DOI] [PubMed] [Google Scholar]
- 37.Zhang SJ, Hosaka M, Yoshihara T, Negishi K, Iida Y, Tobita S, Takeuchi T. Phosphorescent Light-Emitting Iridium Complexes Serve as a Hypoxia-Sensing Probe for Tumor Imaging in Living Animals. Cancer Research. 2010;70:4490–4498. doi: 10.1158/0008-5472.CAN-09-3948. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi T, Zhang S, Negishi K, Yoshihara T, Hosaka M, Tobita S, Iida Y. Phosphorescent light-emitting iridium complexes serve as a hypoxia-sensing probe for tumor imaging in living animals. Cancer Res. 2010;70:4490–8. doi: 10.1158/0008-5472.CAN-09-3948. [DOI] [PubMed] [Google Scholar]
- 39.Yoshihara T, Yamaguchi Y, Hosaka M, Takeuchi T, Tobita S. Ratiometric molecular sensor for monitoring oxygen levels in living cells. Angew Chem Int Ed. 2012;51:4148–4151. doi: 10.1002/anie.201107557. [DOI] [PubMed] [Google Scholar]
- 40.Napp J, Behnke T, Fischer L, Wurth C, Wottawa M, Katschinski DM, Alves F, Resch-Genger U, Schaferling M. Targeted luminescent near-infrared polymer-nanoprobes for in vivo imaging of tumor hypoxia. Anal Chem. 2011;83:9039–9046. doi: 10.1021/ac201870b. [DOI] [PubMed] [Google Scholar]
- 41.Zhang G, Palmer GM, Dewhirst MW, Fraser CL. A dual-emissive-materials design concept enables tumour hypoxia imaging. Nat Mater. 2009;8:747–751. doi: 10.1038/nmat2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ballinger JR. Imaging hypoxia in tumors. Semin Nucl Med. 2001;31:321–329. doi: 10.1053/snuc.2001.26191. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Xu Y, Qian X, Liu J, Shen L, Li J, Zhang Y. Novel fluorescent markers for hypoxic cells of naphthalimides with two heterocyclic side chains for bioreductive binding. Bioorg Med Chem. 2006;14:2935–2941. doi: 10.1016/j.bmc.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Okuda K, Okabe Y, Kadonosono T, Ueno T, Youssif BG, Kizaka-Kondoh S, Nagasawa H. 2-Nitroimidazole-tricarbocyanine conjugate as a near-infrared fluorescent probe for in vivo imaging of tumor hypoxia. Bioconjug Chem. 2012;23:324–329. doi: 10.1021/bc2004704. [DOI] [PubMed] [Google Scholar]
- 45.Tanabe K, Hirata N, Harada H, Hiraoka M, Nishimoto S. Emission under hypoxia: one-electron reduction and fluorescence characteristics of an indolequinone-coumarin conjugate. Chembiochem. 2008;9:426–432. doi: 10.1002/cbic.200700458. [DOI] [PubMed] [Google Scholar]
- 46.Kiyose K, Hanaoka K, Oushiki D, Nakamura T, Kajimura M, Suematsu M, Nishimatsu H, Yamane T, Terai T, Hirata Y, Nagano T. Hypoxia-sensitive fluorescent probes for in vivo real-time fluorescence imaging of acute ischemia. J Am Chem Soc. 2010;132:15846–15848. doi: 10.1021/ja105937q. [DOI] [PubMed] [Google Scholar]
- 47.Cui L, Zhong Y, Zhu W, Xu Y, Du Q, Wang X, Qian X, Xiao Y. A new prodrug-derived ratiometric fluorescent probe for hypoxia: high selectivity of nitroreductase and imaging in tumor cell. Org Lett. 2011;13:928–931. doi: 10.1021/ol102975t. [DOI] [PubMed] [Google Scholar]
- 48.Nakata E, Yukimachi Y, Kariyazono H, Im S, Abe C, Uto Y, Maezawa H, Hashimoto T, Okamoto Y, Hori H. Design of a bioreductively-activated fluorescent pH probe for tumor hypoxia imaging. Bioorg Med Chem. 2009;17:6952–6958. doi: 10.1016/j.bmc.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 49.Choi KY, Swierczewska M, Lee S, Chen X. Protease-activated drug development. Theranostics. 2012;2:156–178. doi: 10.7150/thno.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mason SD, Joyce JA. Proteolytic networks in cancer. Trends Cell Biol. 2011;21:228–237. doi: 10.1016/j.tcb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 52.Lee HO, Mullins SR, Franco-Barraza J, Valianou M, Cukierman E, Cheng JD. FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer. 2011;11:245. doi: 10.1186/1471-2407-11-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 54.Overall CM, Kleifeld O. Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 55.Brennen WN, Rosen DM, Wang H, Isaacs JT, Denmeade SR. Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug. J Natl Cancer Inst. 2012;104:1320–1334. doi: 10.1093/jnci/djs336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gocheva V, Joyce JA. Cysteine cathepsins and the cutting edge of cancer invasion. Cell Cycle. 2007;6:60–64. doi: 10.4161/cc.6.1.3669. [DOI] [PubMed] [Google Scholar]
- 57.Paulick MG, Bogyo M. Development of activity-based probes for cathepsin X. ACS Chem Biol. 2011;6:563–572. doi: 10.1021/cb100392r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blum G, von Degenfeld G, Merchant MJ, Blau HM, Bogyo M. Noninvasive optical imaging of cysteine protease activity using fluorescently quenched activity-based probes. Nat Chem Biol. 2007;3:668–677. doi: 10.1038/nchembio.2007.26. [DOI] [PubMed] [Google Scholar]
- 59.Verdoes M, Edgington LE, Scheeren FA, Leyva M, Blum G, Weiskopf K, Bachmann MH, Ellman JA, Bogyo M. A nonpeptidic cathepsin S activity-based probe for noninvasive optical imaging of tumor-associated macrophages. Chem Biol. 2012;19:619–628. doi: 10.1016/j.chembiol.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang JW, Moellering RE, Cravatt BF. An activity-based imaging probe for the integral membrane hydrolase KIAA1363. Angew Chem Int Ed. 2012;51:966–970. doi: 10.1002/anie.201107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee J, Bogyo M. Development of near-infrared fluorophore (NIRF)-labeled activity-based probes for in vivo imaging of legumain. ACS Chem Biol. 2010;5:233–243. doi: 10.1021/cb900232a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee S, Park K, Kim K, Choi K, Kwon IC. Activatable imaging probes with amplified fluorescent signals. Chem Commun. 2008:4250–4260. doi: 10.1039/b806854m. [DOI] [PubMed] [Google Scholar]
- 63.Linder KE, Metcalfe E, Nanjappan P, Arunachalam T, Ramos K, Skedzielewski TM, Marinelli ER, Tweedle MF, Nunn AD, Swenson RE. Synthesis, in vitro evaluation, and in vivo metabolism of fluor/quencher compounds containing IRDye 800CW and Black Hole Quencher-3 (BHQ-3) Bioconjug Chem. 2011;22:1287–1297. doi: 10.1021/bc100457s. [DOI] [PubMed] [Google Scholar]
- 64.Zhu L, Xie J, Swierczewska M, Zhang F, Quan Q, Ma Y, Fang X, Kim K, Lee S, Chen X. Real-time video imaging of protease expression in vivo. Theranostics. 2011;1:18–27. doi: 10.7150/thno/v01p0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu L, Xie J, Swierczewska M, Zhang F, Lin X, Fang X, Niu G, Lee S, Chen X. Dual-functional, receptor-targeted fluorogenic probe for in vivo imaging of extracellular protease expressions. Bioconjug Chem. 2011;22:1001–1005. doi: 10.1021/bc200005w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Myochin T, Hanaoka K, Komatsu T, Terai T, Nagano T. Design strategy for a near-infrared fluorescence probe for matrix metalloproteinase utilizing highly cell permeable boron dipyrromethene. J Am Chem Soc. 2012;134:13730–13737. doi: 10.1021/ja303931b. [DOI] [PubMed] [Google Scholar]
- 67.Cha EJ, Jang ES, Sun IC, Lee IJ, Ko JH, Kim YI, Kwon IC, Kim K, Ahn CH. Development of MRI/NIRF ‘activatable’ multimodal imaging probe based on iron oxide nanoparticles. J Control Release. 2011;155:152–158. doi: 10.1016/j.jconrel.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 68.Xie J, Zhang F, Aronova M, Zhu L, Lin X, Quan Q, Liu G, Zhang G, Choi KY, Kim K, Sun X, Lee S, Sun S, Leapman R, Chen X. Manipulating the power of an additional phase: a flower-like Au-Fe3O4 optical nanosensor for imaging protease expressions in vivo. ACS Nano. 2011;5:3043–3051. doi: 10.1021/nn200161v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li J, Chen K, Liu H, Cheng K, Yang M, Zhang J, Cheng JD, Zhang Y, Cheng Z. Activatable near-infrared fluorescent probe for in vivo imaging of fibroblast activation protein-alpha. Bioconjug Chem. 2012;23:1704–1711. doi: 10.1021/bc300278r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gehrig S, Mall MA, Schultz C. Spatially resolved monitoring of neutrophil elastase activity with ratiometric fluorescent reporters. Angew Chem Int Ed. 2012;51:6258–6261. doi: 10.1002/anie.201109226. [DOI] [PubMed] [Google Scholar]
- 71.Whitney M, Savariar EN, Friedman B, Levin RA, Crisp JL, Glasgow HL, Lefkowitz R, Adams SR, Steinbach P, Nashi N, Nguyen QT, Tsien RY. Ratiometric Activatable Cell-Penetrating Peptides Provide Rapid In Vivo Readout of Thrombin Activation. Angew Chem Int Ed. 2012 doi: 10.1002/anie.201205721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee S, Cha EJ, Park K, Lee SY, Hong JK, Sun IC, Kim SY, Choi K, Kwon IC, Kim K, Ahn CH. A near-infrared-fluorescence-quenched gold-nanoparticle imaging probe for in vivo drug screening and protease activity determination. Angew Chem Int Ed. 2008;47:2804–2807. doi: 10.1002/anie.200705240. [DOI] [PubMed] [Google Scholar]
- 73.Edgington LE, Berger AB, Blum G, Albrow VE, Paulick MG, Lineberry N, Bogyo M. Noninvasive optical imaging of apoptosis by caspase-targeted activity-based probes. Nat Med. 2009;15:967–973. doi: 10.1038/nm.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 75.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, Sun F, Lu J, Yin Y, Cai X, Sun Q, Wang K, Ba Y, Wang Q, Wang D, Yang J, Liu P, Xu T, Yan Q, Zhang J, Zen K, Zhang CY. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133–144. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 77.Li LM, Hu ZB, Zhou ZX, Chen X, Liu FY, Zhang JF, Shen HB, Zhang CY, Zen K. Serum microRNA profiles serve as novel biomarkers for HBV infection and diagnosis of HBV-positive hepatocarcinoma. Cancer Res. 2010;70:9798–9807. doi: 10.1158/0008-5472.CAN-10-1001. [DOI] [PubMed] [Google Scholar]
- 78.Kang WJ, Cho YL, Chae JR, Lee JD, Ali BA, Al-Khedhairy AA, Lee CH, Kim S. Dual optical biosensors for imaging microRNA-1 during myogenesis. Biomaterials. 2012;33:6430–6437. doi: 10.1016/j.biomaterials.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 79.Zhang J, Fu Y, Mei Y, Jiang F, Lakowicz JR. Fluorescent metal nanoshell probe to detect single miRNA in lung cancer cell. Anal Chem. 2010;82:4464–4471. doi: 10.1021/ac100241f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang CW, Li Z, Conti PS. Radioactive Smart Probe for Potential Corrected Matrix Metalloproteinase Imaging. Bioconjug Chem. 2012;23:2159–67. doi: 10.1021/bc3001968. [DOI] [PubMed] [Google Scholar]
- 81.Park J, Yang J, Lim EK, Kim E, Choi J, Ryu JK, Kim NH, Suh JS, Yook JI, Huh YM, Haam S. Anchored proteinase-targetable optomagnetic nanoprobes for molecular imaging of invasive cancer cells. Angew Chem Int Ed. 2012;51:945–948. doi: 10.1002/anie.201106758. [DOI] [PubMed] [Google Scholar]
- 82.Mizukami S, Takikawa R, Sugihara F, Shirakawa M, Kikuchi K. Dual-function probe to detect protease activity for fluorescence measurement and 19F MRI. Angew Chem Int Ed. 2009;48:3641–3643. doi: 10.1002/anie.200806328. [DOI] [PubMed] [Google Scholar]
- 83.Davis SC, Samkoe KS, O’Hara JA, Gibbs-Strauss SL, Payne HL, Hoopes PJ, Paulsen KD, Pogue BW. MRI-coupled fluorescence tomography quantifies EGFR activity in brain tumors. Acad Radiol. 2010;17:271–276. doi: 10.1016/j.acra.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Y, Yang Y, Cai W. Multimodality Imaging of Integrin alpha(v)beta(3) Expression. Theranostics. 2011;1:135–148. doi: 10.7150/thno/v01p0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu L, Kate P, Torchilin VP. Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting. ACS Nano. 2012;6:3491–3498. doi: 10.1021/nn300524f. [DOI] [PMC free article] [PubMed] [Google Scholar]


