Abstract
Conjugation of the 64Cu PET radioisotope (t1/2 = 12.7 hours) to long circulating liposomes enables long term liposome tracking. To evaluate the potential clinical utility of this radiotracer in diagnosis and therapeutic guidance, we compare image contrast, tumor volume, and biodistribution of 64Cu-liposomes to metrics obtained with the dominant clinical tracer, 18F-FDG. Twenty four female FVB mice with MET1 mammary carcinoma tumor grafts were examined. First, serial PET images were obtained with the 18F-FDG radiotracer at 0.5 hours after injection and with the 64Cu-liposome radiotracer at 6, 18, 24, and 48 hours after injection (n = 8). Next, paired imaging and histology were obtained at four time points: 0.5 hours after 18F-FDG injection and 6, 24, and 48 hours after 64Cu-liposome injection (n = 16). Tissue biodistribution was assessed with gamma counting following necropsy and tumors were paraffin embedded, sectioned, and stained with hematoxylin and eosin. The contrast ratio of images obtained using 18F-FDG was 0.88 ± 0.01 (0.5 hours after injection), whereas with the 64Cu-liposome radiotracer the contrast ratio was 0.78 ± 0.01, 0.89 ± 0.01, 0.88 ± 0.01, and 0.94 ± 0.01 at 6, 18, 24, and 48 hours, respectively. Estimates of tumor diameter were comparable between 64Cu-liposomes and 18F-FDG, 64Cu-liposomes and necropsy, and 64Cu-liposomes and ultrasound with Pearson’s r-squared values of 0.79, 0.79, and 0.80, respectively. Heterogeneity of tumor tracer uptake was observed with both tracers, correlating with regions of necrosis on histology. The average tumor volume of 0.41 ± 0.05 cc measured with 64Cu-liposomes was larger than that estimated with 18F-FDG (0.28 ± 0.04 cc), with this difference apparently resulting primarily from accumulation of the radiolabeled particles in the pro-angiogenic tumor rim. The imaging of radiolabeled nanoparticles can facilitate tumor detection, identification of tumor margins, therapeutic evaluation and interventional guidance.
Keywords: 64Cu, 18F-FDG, liposomes, mammary carcinoma, PET, preclinical, biodistribution
Introduction
Liposomes passively accumulate in tumors due to the enhanced permeability and retention effect of leaky vasculature and dysfunctional lymphatics [1]. This effect has been used to enhance drug delivery [2], but can also generate contrast for imaging and detection of tumors. While classic contrast agents are expected to readily clear from the body, pegylated liposomes have a long blood circulation half-life [3]. Early liposomal radiolabeling efforts made use of 18F (t1/2 = 109.8 minutes), which allowed liposomes to be tracked for a few hours [4,5]. Conjugation with 64Cu (t1/2 = 12.7 hours) allows liposomes to be tracked over several days [6]. Use of a radioisotope with a half-life comparable to liposome kinetics enhances the contrast generated from tumor accumulation and blood clearance of liposomes, while avoiding additional radioactive hazards associated with longer lived isotopes [6,7]. Enhanced contrast may improve high-sensitivity detection and tracking of tumors that display enhanced permeability and retention of nanoparticles, as well as the ability to use imaging to predict therapeutic response to liposomal drug formulations. In addition, the long lived radioisotope expands the practical window for diagnostic imaging as well as facilitating PET-guided interventions such as radiation therapy, where 18F-FDG images are used in treatment planning [8], or surgery, where 18F-FDG and handheld gamma probes are used to localize tumors [9,10].
Labeling of liposomes with 64Cu is enabled by advances in radioisotope metal chelation chemistry. Requirements include stable chelation in vitro and in vivo, fast, gentle labeling, and minimal purification of labeled nanoparticles. 6-[p-(bromoacetamido)benzyl]-1,4,8,11-tetraazacyclotetradecane-N,N’,N’’,N’’’-tetraacetic acid (BAT) -PEG1.2k-lipid (BAT-PEG-lipid) containing liposomes fulfill each of these requirements. BAT is a highly stable copper chelator, demonstrating excellent stability when conjugated to 67Cu in serum over five days [11]. We have previously demonstrated the stability of BAT in 64Cu-liposomes, where incubation of 64Cu-liposomes with mouse serum at 37°C revealed that 6 % of radioactivity transferred to serum after 20 hours, and 8 % transferred to serum after 48 hours, as measured by thin layer chromatography [6]. Attachment of BAT to a pegylated lipid enables production of liposomes that can be quickly and gently labeled and require minimal purification [6]. Gentle chelation conditions are important to liposome labeling, as high chelation temperatures can disrupt many liposome formulations. Although 4,11-bis(carboxymethyl)-1,4,8,11-tetraazabicyclo[6.6.2]hexadecane (CB-TE2A) has been shown to chelate 64Cu with great stability, in a liposomal labeling system, the high temperature required for chelation can affect liposomal stability. In addition, direct comparisons of the blood radioactivity using BAT and CB-TE2A to track liposomes have demonstrated that the blood circulation of the radiolabeled particle is very similar [12], consistent with previous studies of radiolabeled antibodies [13].
18F-FDG acts as a glucose analogue approximating cellular metabolism. It is ideal for imaging because it is not metabolized but rather phosphorylated and trapped within cells, and also rapidly clears from the bloodstream [14], leading to a stable imaging window and high image contrast. Furthermore, as high glucose utilization is typical of cancerous cells, it is broadly applicable in cancer imaging. However, inflammation and infection may also lead to high 18F-FDG uptake [15], potentially confounding cancer imaging. Further, not all tumors are metabolically active [16], and many normal tissues display high uptake of 18F-FDG [17], modulated by disease states such as diabetes [18] or physical exertion before and during scanning. These factors complicate interpretation of 18F-FDG PET imaging and help to motivate the development of novel imaging tracers.
There are many ways to measure tracer uptake and tumor volume in an 18F-FDG scan. Options to measure metabolic activity include nonlinear kinetic analysis [19], simplified kinetic models [20], and corrected standardized uptake value (SUV) [21]. Nonlinear kinetic analysis is the gold standard of metabolic activity measurement with PET. This method makes use of a three compartment pharmacokinetic model, driven by 18F-FDG region of interest (ROI) analysis of tumors and the aortic input function. Unfortunately, measurement of the aortic input function requires long dynamic scans or repeated arterial blood sampling, rendering the gold standard clinically impractical. The simplified kinetic model requires a single venous blood sample, using population average parameters and the venous blood sample to calculate the input function. SUV is the ratio of the tracer in the region of interest to the injected dose of tracer. This method is the simplest and most commonly clinically used. Various correction factors for patient physiology are used to allow for better approximation of metabolic uptake, including bodyweight, body surface area, and blood sugar. It will be important to choose the appropriate image quantitation metrics to compare the utility of 18F-FDG and 64Cu-liposomes.
The MET1 mammary carcinoma model is a syngeneic mouse model of breast cancer. These tumor cells express polyoma virus middle T, a molecular mimic of human Her2/neu, commonly overexpressed in human breast cancer [22]. Both genes induce oncogenesis through the Shc and PI-3 kinase pathways [23]. MET1 mammary carcinomas are histopathologically similar to high grade human breast cancer; they form solid tumors with central necrosis, are locally invasive, highly mitotic, with adenocarcinomatous gland formation [24]. MET1 mammary carcinoma serves as a good model of human primary breast cancer.
To further assess the utility of radiolabeled nanoparticles, we perform a comparison of images generated with 18F-FDG and 64Cu-liposomes. We hypothesize that 64Cu-liposomes can serve as effective tumor contrast agents in the murine MET1 mammary carcinoma model. We examine image quality, biodistribution, tumor delivery, tumor heterogeneity, and tumor size estimation.
Methods and materials
Solvents and other reagents were purchased from Sigma-Aldrich unless otherwise noted. Lipids and a liposome mini-extruder were purchased from Avanti Polar Lipids. BAT-PEG-lipid conjugate was synthesized by Fmoc solid phase synthesis as previously described [6]. 64CuCl2 was purchased from Washington University in St. Louis (MO) while 18F-FDG was purchased from PETNET Solutions (San Diego, CA) under a protocol controlled by the University of California, Davis. Dulbecco’s Phosphate Buffered Saline (DPBS) was purchased from Invitrogen Corporation (Carlsbad, CA).
Preparation of 64Cu-liposomes
The 64Cu-liposome labeling procedure followed a previously reported method [6]. In brief, lipids in chloroform (0.555/0.39/0.05/0.005 mol/mol/mol/mol, hydrogenated soy phosphatidylcholine (HSPC), cholesterol, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[metho xy(polyethylene glycol)-2000] (DSPE-PEG2k-OMe), BAT-PEG-lipid) were transferred to a test tube and solvent evaporated under nitrogen. After overnight lyophilization, lipids were suspended in 0.1 M ammonium citrate solution (400 μL, 300 mOsm, pH 5.5), and incubated in a warm bath (60°C) alternating with gentle vortexing for 10 minutes. The lipid mixture was extruded through a 100 nm membrane filter (Whatman, NJ) at 60°C. After cooling to room temperature, buffered 64CuCl2 (18.5 MBq/mg lipid) in 0.1 M ammonium citrate (pH 5.5, 0.1 ml) was added to the extruded lipid solution. The mixture was incubated at 30°C for 40 minutes, then 0.1 M ethylenediaminetetraacetic acid in deionized water (20 μL) was added and incubated for an additional 10 minutes. 64Cu-liposomes and unbound copper were separated by size exclusion chromatography with Sephadex G75 resin (GE Healthcare) in DPBS. A portion of 64Cu-liposomes were retained until the radioactivity decayed, nanoparticle size was then measured by Dynamic Light Scattering performed with a Nicomp 380 ZLS (Particle Sizing Systems, CA).
In vivo study design
All animal studies were approved by the University of California, Davis Animal Care and Use Committee (Figure 1). A total of 24 female FVB mice were examined (6-10 weeks old, 15-25 g, Charles River, MA). During procedures, mice were anesthetized, induced with 3.5 % isoflurane and maintained with 2.0 - 2.5 % isoflurane. Syngeneic MET1 mammary carcinomas [24] were transplanted into the mammary fat pad of the first cohort of eight mice 13 days prior to injection, and the second cohort of sixteen mice were transplanted 21 days prior. Mice in the first cohort underwent PET scanning 0.5 hours after injection with 18F-FDG, waited 4 hours, and then were injected with 64Cu-liposomes. Mice were then imaged at 6, 18, 24 and 48 hours, and sacrificed for necropsy and biodistribution. Mice in the second cohort underwent ultrasound imaging 12 hours prior to 18F-FDG injection, were imaged 0.5 hours after injection, and then after a wait period of 4 hours were injected with 64Cu-liposomes and imaged at 6, 24, and 48 hours following liposome injection. Subgroups of four mice were sacrificed for necropsy, biodistribution, and histological sections after each imaging time point.
Figure 1.
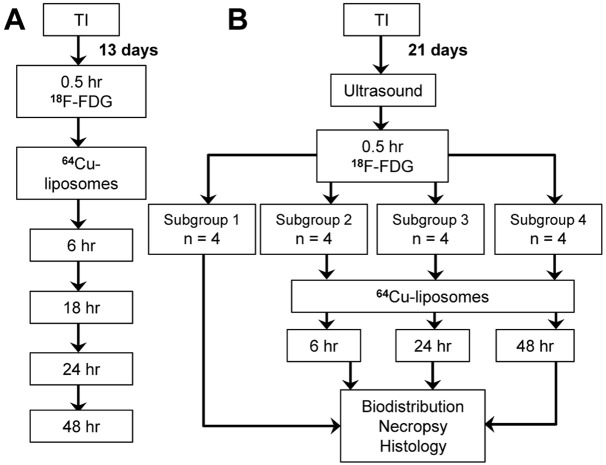
Study design diagram. A: The first cohort (n = 8 mice) was imaged 13 days after tumor implantation. Images were first acquired with 18F-FDG 0.5 hours after injection. Mice were then injected with 64Cu-liposomes and imaged at 6, 18, 24, and 48 hours after injection. B: The second cohort (n = 16 mice) was imaged 21 days after tumor implantation with ultrasound and then with 18F-FDG at 0.5 hours after injection. Subgroups of 4 mice were then sacrificed at each imaging time point: 0.5 hours after 18F-FDG injection (without PET imaging), 6, 24, and 48 hours after 64Cu-liposome injection (with 18F-FDG and 64Cu-liposome PET imaging). TI = Tumor Implantation.
Ultrasound imaging
Mice were depilated with hair clippers and Veet® cream. Tumors were imaged with a Siemens Sequoia ultrasound system (Siemens Preclinical Solutions). Tumors were sized by hand segmentation of elliptical regions of interest on transverse and sagittal images, and tumor volume was then estimated from the diameters of an ellipsoid assuming rotational symmetry about the major diameter.
PET imaging
Mice were injected with 500 MBq/kg 18F-FDG via tail vein and imaged after 0.5 hours of rest. All PET imaging was performed with two mice side by side for 0.5 hours, using a microPET Focus 120 scanner (Siemens Preclinical Solutions). Four hours after completion of 18F-FDG imaging, mice were injected with 1 mg of 64Cu-liposomes (300 and 600 MBq/kg), and imaged for 0.5 hours at each time point following injection. PET Images were reconstructed with maximum a posteriori algorithm and tumors manually segmented by a single blinded observer with ImageJ (National Institutes of Health) and ASIPro software (Siemens Preclinical Solutions). 64Cu-liposome PET tumor volumes were modeled by subtraction of a 0.8 mm spherical shell from ROI. Activity measured within 3D volumes was time-decay corrected and expressed as percent injected dose per cc of tissue (%ID/cc). Contrast ratios were calculated as in Equation 1 [25], with values expressed as %ID/cc and averaged across subjects. Heterogeneity was calculated as the sum of the absolute value of the difference between individual pixels and the ROI average value over the ROI average value in %ID/cc (Equation 2), and averaged across subjects. This metric captures the variability in tracer uptake within a tumor.
Equation 1:
Contrast Ratio = (tumormax-musclemean)/(tumormax+musclemean)
Equation 2
Heterogeneity = ∑ROI|(pixel-mean)/mean|
Necropsy and biodistribution
Following imaging, mice were euthanized by cervical dislocation, their organs removed, weighed, and radioactivity measured by Wizard 1470 Automatic Gamma Counter (Perkin Elmer). Tumors were formalin fixed overnight and then paraffin embedded, sectioned, and stained with hematoxylin and eosin. Tumor size was estimated by measuring the long axis and the longest perpendicular distance of a paraffin section and then modeling the tumor as a sphere with diameter given by the average of the two measured distances.
Results
Characterization of 64Cu-liposomes
Liposomes measured 120 ± 13.6 nm in diameter. Decay corrected labeling yield was greater than 95% by radioactive thin layer chromatography.
PET image analysis
PET images of a mouse obtained after injection with 18F-FDG or 64Cu-liposomes are displayed in Figure 2. 64Cu-liposomes label the blood pool, strongly enhancing the heart, jugular veins, and liver at 6 hours after injection. The outline of tumors is also visible. After 18 hours, radioactivity appears in the intestines and bladder, and tumors are more strongly labeled. At 24 hours after injection, the strength of the signal from the heart, jugular veins, and liver has fallen, although tumor activity is equivalent to that observed at 18 hours. At 48 hours after injection, the tumor accumulation remains high while other areas continue to lose signal, the blood activity is reduced, and intestines are no longer distinguishable from surrounding tissues. In the cohort of mice injected 13 days after tumor implantation, tumor accumulation peaks at 18 hours (Table 1). In the second cohort, injected with the radiotracer 21 days after implantation, extravasation increases over 48 hours while total tumor activity falls over 48 hours (Table 2) due to the decrease in blood activity. In both cohorts, 64Cu-liposome accumulation was greater than that of 18F-FDG, at later time points, where 64Cu-liposome accumulation exceeded 18F-FDG accumulation from 18 to 48 hours (Figure 3). Distribution of contrast within the tumor was heterogeneous, with regions of contrast hypointensity correlating with necrosis as demonstrated by hematoxylin and eosin (Figure 4). Evaluation of heterogeneity reveals that in the first cohort (with smaller tumors), heterogeneity is comparable amongst all categories except that it is less in 64Cu-liposome images at 6 hours. In the second cohort (with larger tumors), all 64Cu-liposome images demonstrate greater heterogeneity than those obtained with 18F-FDG (Figure 5).
Figure 2.

In a mouse from the cohort imaged 13 days after tumor implantation, PET images obtained 0.5 hours after injection with 18F-FDG are compared with images obtained 6, 18, 24, and 48 hours after injection with the 64Cu-liposome tracer. 64Cu-liposome accumulation in tumors (arrows) increased from 6 to 18 hours and then remained consistent through 48 hours while background activity decreased.
Table 1.
In Vivo Tumor Accumulation and Contrast Ratio, 18F-FDG and 64Cu-liposomes, cohort imaged 13 days after tumor implantation. All values are reported as mean ± standard error of the mean (SEM) (n = 16 tumors)
| Tracer | Time point | PET Tumor Volume (cc) | Contrast Ratio | Average Tumor Accumulation (%ID/cc) | Total Tumor Accumulation (%ID) |
|---|---|---|---|---|---|
| 18F-FDG | 0.5 hr | 0.12 ± 0.01 | 0.88 ± 0.01 | 5.7 ± 0.3 | 0.7 ± 0.1 |
| 64Cu-liposomes | 6 hr | 0.04 ± 0.01 | 0.78 ± 0.01 | 3.7 ± 0.1 | 0.4 ± 0.1 |
| 64Cu-liposomes | 18 hr | 0.09 ± 0.01 | 0.89 ± 0.01 | 7.6 ± 0.3 | 1.5 ± 0.1 |
| 64Cu-liposomes | 24 hr | 0.08 ± 0.01 | 0.88 ± 0.01 | 6.0 ± 0.2 | 1.1 ± 0.1 |
| 64Cu-liposomes | 48 hr | 0.09 ± 0.01 | 0.94 ± 0.01 | 5.9 ± 0.2 | 1.1 ± 0.1 |
Table 2.
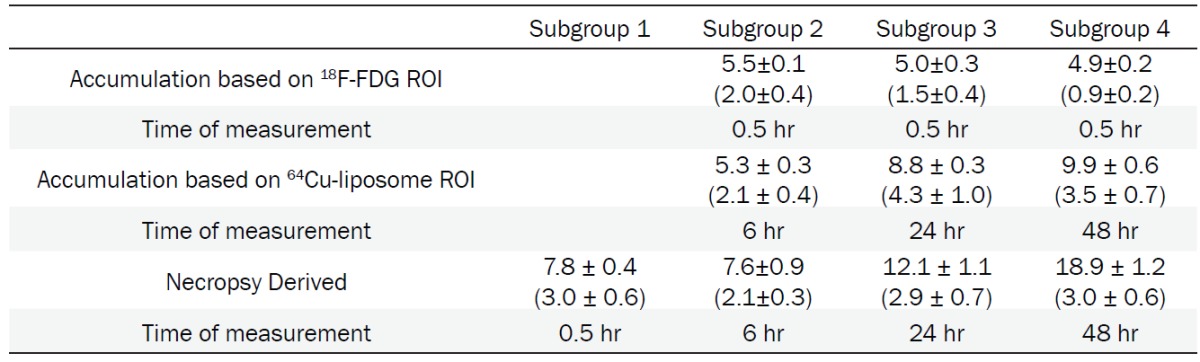
Ex Vivo and In Vivo Tumor Accumulation, 18F-FDG and 64Cu-liposomes, cohort imaged 21 days after tumor implantation. Subgroup 1 did not undergo PET imaging as mice were sacrificed 0.5 hour after 18F-FDG injection. All values are reported as mean ± SEM (n = 8 tumors). Values are average %ID/cc, with total tumor %ID in parenthesis
|
Figure 3.
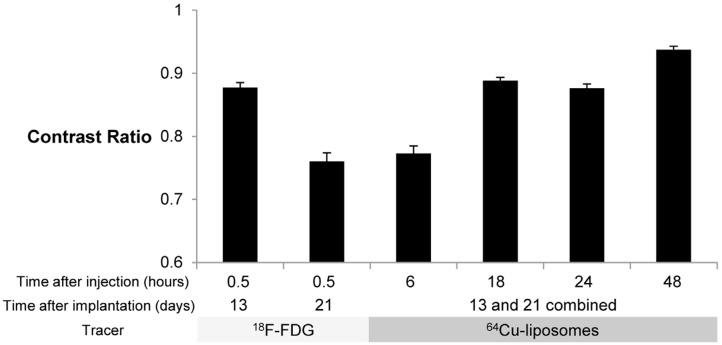
Contrast Ratio comparison with 18F-FDG and 64Cu-liposome tracers. Contrast ratio was defined as (TumorMax – MuscleMean) / (TumorMax + MuscleMean) in %ID/cc. Error bars represent SEM. For mice imaged with 18F-FDG 13 days after implantation and mice imaged 18 hours after injection with 64Cu-liposomes, n = 16 tumors, for all other groups, n = 24 tumors. Cohort imaged 13 days after implantation was maintained under anesthesia for the period between injection of 18F-FDG and imaging, while mice imaged 21 days after implantation were awakened between injection of 18F-FDG and imaging, resulting in a reduction in the contrast ratio. For the mice that remained under anesthesia during 18F-FDG image acquisition (13 days after implantation), at the earliest time point, the contrast ratio is higher for images obtained with 18F-FDG than 64Cu-liposomes, but is comparable at the 18 and 24 hour time points. At 48 hours after injection of 64Cu-liposomes, tumor contrast ratio was in all cases superior to 18F-FDG images.
Figure 4.
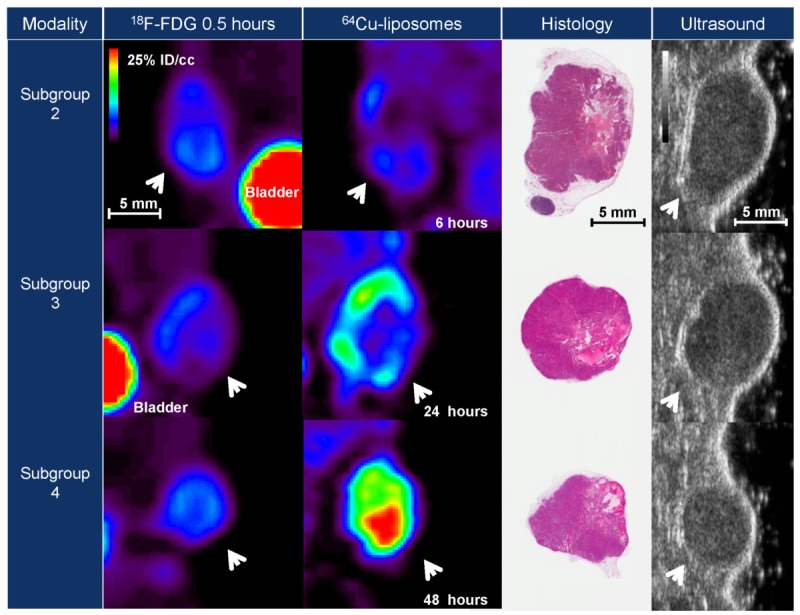
Typical images resulting from 18F-FDG and 64Cu-liposome PET tracers, histopathology and ultrasound.Images are representative of subgroups 2-4 (as described in Figure 1) acquired 21 days after tumor implantation.Tumor volume is comparable with 18F-FDG and 64Cu-liposome tracers. In addition, hypointense regions observedwith both PET tracers correlate to regions of necrosis as determined by hematoxylin and eosin staining.
Figure 5.

Tumor Heterogeneity, as observed with 18F-FDG and 64Cu-liposome PET tracers, for cohorts imaged 13 and21 days after tumor implantation. Heterogeneity was defined as sum of absolute value in %ID/cc of (pixel - ROImean)/ ROImean (Equation 2) with error bars representing SEM. N = 16 tumors for the cohort of mice imaged 13 days afterimplantation, n = 8 tumors for the cohort of mice imaged 21 days after implantation. Heterogeneity of imagesobtained with 64Cu-liposomes is less than that obtained with 18F-FDG at 6 hours in the cohort imaged 13 days afterimplantation, but is greater in all other groups.
Biodistribution
Ex vivo gamma counting of organs demonstrates that liposomes remain in circulation over 48 hours, with 6.1 ± 0.3 %ID/g still present within the blood pool after 48 hours (Table 4). Tumor radioactivity assessed ex vivo confirmed image derived trends, although absolute values assessed by ex vivo methods are larger likely due to attenuation and differing ROI selection as compared with surgical margins. Selection of surrounding, lower intensity tissue depresses tumor average intensity relative to dissection values. At all time points liposomes demonstrated lower accumulation in heart, muscle, and brain compared to 18F-FDG. The brain received 6.9 ± 0.6 %ID/g of 18F-FDG versus 0.8 ± 0.0 %ID/g of 64Cu-liposomes. Murine ex vivo FDG distribution after 1 hour demonstrated high uptake in the urine and heart, with 98.5 ± 2.2 and 51.2 ± 6 %ID/g respectively. High uptake of both tracers was evident in tumor as well with 7.8 ± 0.4 %ID/g of 18F-FDG compared to 12.1 ± 1.1 and 18.9 ± 1.2 %ID/g of 64Cu-liposomes detected in the tumor after 24 and 48 hours, respectively.
Table 4.
Ex Vivo Biodistribution, 18F-FDG and 64Cu-liposomes, cohort imaged 21 days after tumor implantation. All values are reported as mean ± SEM (n = 8 for tumors and kidneys, n = 4 all other organs)
| %ID/g | %ID | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Group | Subgroup 2 | Subgroup 3 | Subgroup 3 | Subgroup 4 | Subgroup 1 | Subgroup 2 | Subgroup 3 | Subgroup 4 |
|
| ||||||||
| Tracer | 18F-FDG | 64Cu-liposomes | 18F-FDG | 64Cu-liposomes | ||||
|
| ||||||||
| Time point | 0.5 hr | 6 hr | 24 hr | 48 hr | 0.5 hr | 6 hr | 24 hr | 48 hr |
| Blood | 1.8 ± 0.3 | 41.9 ± 1.3 | 19.1 ± 1.7 | 6.1 ± 0.3 | - | - | - | - |
| Urine | 98.5 ± 2.2 | 1.4 ± 0.3 | 5.3 ± 0.8 | 1.9 ± 0.4 | - | - | - | - |
| Spleen | 3.1 ± 0.4 | 18.2 ± 2.3 | 19.2 ± 1.1 | 12.5 ± 0.7 | 0.4 ± 0.1 | 1.8 ± 0.2 | 1.6 ± 0.2 | 1.0 ± 0.0 |
| Lungs | 4.5 ± 0.3 | 8.5 ± 0.6 | 5.1 ± 0.4 | 3.4 ± 0.1 | 0.7 ± 0.0 | 1.2 ± 0.1 | 0.9 ± 0.2 | 0.5 ± 0.0 |
| Diaphragm | 5.7 ± 1.6 | 4.8 ± 0.7 | 3.4 ± 0.6 | 1.6 ± 0.1 | 0.4 ± 0.0 | 0.3 ± 0.1 | 0.2 ± 0.0 | 0.1 ± 0.0 |
| Heart | 51.2 ± 6.1 | 4.8 ± 0.5 | 4.1 ± 0.3 | 2.5 ± 0.3 | 5.9 ± 0.6 | 0.5 ± 0.1 | 0.5 ± 0.0 | 0.3 ± 0.0 |
| Liver | 2.0 ± 0.3 | 9.4 ± 3.1 | 13.8 ± 0.5 | 7.2 ± 0.4 | 2.4 ± 0.3 | 10.6 ± 3.6 | 14.4 ± 0.3 | 7.6 ± 0.2 |
| Kidneys | 4.7 ± 0.3 | 8.6 ± 0.6 | 7.4 ± 0.2 | 6.1 ± 0.2 | 0.7 ± 0.0 | 1.2 ± 0.1 | 1.1 ± 0.1 | 0.9 ± 0.0 |
| Duodenum | 2.7 ± 0.3 | 3.9 ± 0.3 | 6.7 ± 0.3 | 5.3 ± 0.2 | - | - | - | - |
| Jejunum | 3.0 ± 0.4 | 3.3 ± 0.2 | 5.4 ± 0.1 | 4.9 ± 0.5 | - | - | - | - |
| Quadriceps | 2.2 ± 0.3 | 0.8 ± 0.1 | 1.2 ± 0.2 | 0.6 ± 0.1 | - | - | - | - |
| Fat pad | 1.5 ± 0.2 | 2.0 ± 0.3 | 1.5 ± 0.3 | 1.0 ± 0.1 | - | - | - | - |
| Brain | 6.9 ± 0.6 | 0.8 ± 0.0 | 0.4 ± 0.0 | 0.2 ± 0.0 | 2.8 ± 0.3 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 |
| Bone | 2.0 ± 0.2 | 2.4 ± 0.3 | 2.6 ± 0.3 | 1.7 ± 0.1 | - | - | - | - |
| Tumors | 7.8 ± 0.4 | 7.6 ± 0.9 | 12.1 ± 1.1 | 18.9 ± 1.2 | 3.0 ± 0.6 | 2.1 ± 0.3 | 2.9 ± 0.7 | 3.0 ± 0.6 |
Tumor size estimation
Tumor size was evaluated with ultrasound, necropsy weight, and histological sections as well as 18F-FDG and 64Cu-liposome PET ROI analysis (Table 3). Images from three tumors from different time points imaged by multiple methods are presented in Figure 4. Images from each agent and modality display gross morphological similarities. Tumor volume correlated strongly with each modality, and the slopes of linear regression analysis were near 1 (Figure 6).
Table 3.
Tumor Volume, cohort imaged 21 days after tumor implantation. Tumor volume is reported as mean ± SEM, with tumor volume measured in cc (n = 8 tumors). 64Cu-liposome volume includes correction by subtraction of spherical shell
| Method | Subgroup 2 | Subgroup 3 | Subgroup 4 |
|---|---|---|---|
| 64Cu-liposomes | 0.25 ± 0.06 | 0.29 ± 0.07 | 0.18 ± 0.04 |
| Necropsy | 0.32 ± 0.06 | 0.26 ± 0.06 | 0.16 ± 0.03 |
| Ultrasound | 0.22 ± 0.05 | 0.15 ± 0.04 | 0.08 ± 0.02 |
| 18F-FDG | 0.37 ± 0.08 | 0.29 ± 0.07 | 0.18 ± 0.03 |
| Histology | 0.16 ± 0.05 | 0.17 ± 0.06 | 0.06 ± 0.02 |
Figure 6.

Correlation between tumor volumes determined with 64Cu-liposome PET images, necropsy, 18F-FDG PET images, and ultrasound in cohort of mice imaged 21 days after tumor implantation (n = 24 tumors). Each technique demonstrates strong correlation and comparable slope.
Discussion
There are many methods to measure tracer uptake and tumor volume in an 18F-FDG scan. Functional measures of permeability cannot be directly compared against functional measures of metabolic activity. Here, we chose to use an uncorrected SUV, expressed as percentage of initial dose per mass of tissue (%ID/g), as a simple measure of uptake which is directly applicable to both tracers. For comparison of image quality, however, a simple comparison of SUV ignores the normal background distribution of 18F-FDG [17] and liposomes. Calculation of the contrast ratio includes correction for background, facilitating comparison of local uptake. We found that the contrast ratio for the 64Cu-liposome radiotracer was equivalent to or greater than that obtained with 18F-FDG at most time points.
Upon administration, 64Cu-liposomes distribute within the blood pool, and are slowly trapped by tumors and the reticuloendothelial system. The liver and spleen process and excrete the nanoparticles, clearing the blood pool over 48 hours. When mice are imaged at 6 hours, a high blood concentration of 64Cu-liposomes, 41.9 ± 1.3 %ID/cc, reduces the tumor contrast ratio. However, at later time points, hepatic excretion of liposomes reduces blood concentration, improving background. In addition, tumors continue to accumulate the tracer over 48 hours, with serial images demonstrating increases from 0.7 ± 0.1 to 1.1 ± 0.1 %ID/cc. This effect could not be observed with previous radiolabeling methods using 18F, however, the longer half-life of 64Cu enables tumor visualization with a high tumor/background contrast ratio over 48 hours. This extended tumor image contrast could be used for tumor detection, but also for direct measurement of nanoparticle accumulation, individual prediction of therapeutic efficacy, or in guidance of therapeutic intervention.
There are many clinical methods of quantitating tumor size with PET, including tumor volume directly measured from region of interest segmentation, the product of the greatest perpendicular dimensions of tumors (WHO) or the longest tumor dimension on transaxial images (RECIST) [26]. Comparing imaging studies obtained with 18F-FDG and 64Cu-liposomes in a preclinical model of mammary carcinoma allows for the measurement of tumor volume by necropsy and weight in addition to PET, ultrasound, and histologic approaches. Then, comparison to the gold standard of weight on necropsy is most easily accomplished with tumor volume as opposed to one or two dimensional metrics. The quantitative image metrics of SUV, contrast ratio, and tumor volume facilitate comparison between 18F-FDG and 64Cu-liposomes.
As nanoparticle accumulation has a different physiological basis than 18F-FDG accumulation, it is important to examine the comparative estimates of tumor volume. Five methods were used to estimate tumor size, with strong correlation between all five methods. As the raw average tumor diameter measured with 64Cu-liposomes was ~1.3 mm larger than that estimated by 18F-FDG, 64Cu-liposome based size estimates were modified to include the subtraction of a 0.8 mm spherical shell from the tumor, bringing 64Cu-liposome image derived size estimates in line with 18F-FDG image derived size estimates. Comparison of PET images suggests that 64Cu-liposomes are accumulating in a rim about the tumor (as compared with 18F-FDG which did not concentrate in this region), most likely due to angiogenesis and tumor expansion. With this accumulation near the tumor margin in combination with the positron range effect, the tumor diameter is expected to be larger with the 64Cu-liposome tracer. The correction resulted in close equivalence of linear regression for necropsy weight, 18F-FDG, and 64Cu-liposomes. Growth of tumor over the 48 hour study period, as well as the hypothesis that enhanced permeability extends beyond the metabolically active part of the tumor may also contribute to this difference in diameter. Tumor growth is not sufficient to explain this effect, as the average tumor size of the subgroup sacrificed at 48 hours was less than that of the previous subgroups. The ability to easily identify the angiogenic rim with 64Cu-liposomes is intriguing for surgical resection and external beam radiation, where it may serve as a rational, imaging driven margin for excision.
We also utilized these radiotracers to image tumor heterogeneity. MET1 tumors demonstrate central necrosis and cystic regions which appear on 18F-FDG PET as hypointense regions which may be caused by necrosis or inhibition of tumor metabolism. 64Cu-liposome imaging produces similar patterns of hypointensity (Figure 4), which may be due to loss of vascular supply to cystic and necrotic regions, visible in histological sections. While 18F-FDG, a small molecule, may easily diffuse through the extracellular space, 64Cu-liposomes, with an average diameter of 120 nm, extravasate at a slower rate than small molecules. This correlation may be useful for assessment of therapeutic response, in which 64Cu-liposomes would be administered as a physiologic imaging tracer of necrosis. In addition, 64Cu-liposome images demonstrate a higher degree of heterogeneity as compared to 18F-FDG (Figure 5). This heterogeneity results from the long circulating half-life of 64Cu-liposomes (Table 4) and preferential accumulation of nanoparticles in regions of high vascular flow and permeability. In the tumors imaged 13 days after tumor implantation, at 6 hours after injection of the tracer, the difference between hypointense and hyperintense regions of the tumor is less than that of 18F-FDG, but as nanoparticles continue to gather in the tumor, the contrast between regions of high and low uptake is greater than that of metabolic differences shown with 18F-FDG. In the more nanoparticle-avid tumors from the cohort of mice imaged 21 days after implantation, 64Cu-liposomes demonstrate greater heterogeneity at all time points. Thus, 64Cu-liposomes are potential contrast agents for the assessment of nanoparticle delivery and vascular heterogeneity, with contrast advantages relative to 18F-FDG.
Conclusion
This study compared tumor images generated by 64Cu-liposomes with images generated by the dominant clinical tracer, 18F-FDG. Inclusion of this standard provides a method to compare our proposed contrast agent against the clinical standard in our animal model of mammary carcinoma. A multiplicity of methods for calculating tumor dose and size were used and corroborated, including image derived tumor accumulation and size, ultrasound and histological size estimates, as well as tumor radioactivity and tumor weight following necropsy. A limitation of the study was that the tumors had to be imaged sequentially with two tracers as opposed to simultaneously and therefore variations in growth patterns can influence results. Another limitation is that the MET1 tumor model involves implantation of a tumor into a known location and therefore observers were aware of the tumor location. This study demonstrates that in the mouse tumor model, 64Cu-liposomes generate images with contrast and volume estimates comparable to 18F-FDG. 64Cu labeling facilitates liposomal tracking, has a mechanism of tumor uptake unique from that of 18F-FDG, and we find that 64Cu-liposomes may serve as a useful clinical contrast agent for detection of tumors, delineation of margins, prediction of therapeutic response, determination of therapeutic efficacy, and interventional guidance.
Acknowledgments
This work was supported by the National Cancer Institute, NIH RO1 CA 103828 NIH RO1 CA134659, and Radiological Society of North America Resident Research Grant RR0811.
Conflict of interest statement
The authors declare that they have no conflict of interest.
References
- 1.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 2.Northfelt DW, Dezube BJ, Thommes JA, Miller BJ, Fischl MA, Friedman-Kien A, Kaplan LD, Du Mond C, Mamelok RD, Henry DH. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: results of a randomized phase III clinical trial. J Clinical Oncol. 1998;16:2445–2451. doi: 10.1200/JCO.1998.16.7.2445. [DOI] [PubMed] [Google Scholar]
- 3.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268:235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 4.Oku N, Tokudome Y, Tsukada H, Okada S. Real-time analysis of liposomal trafficking in tumor-bearing mice by use of positron emission tomography. Biochim Biophys Acta. 1995;1238:86–90. doi: 10.1016/0005-2736(95)00106-d. [DOI] [PubMed] [Google Scholar]
- 5.Marik J, Tartis MS, Zhang H, Fung JY, Kheirolomoom A, Sutcliffe JL, Ferrara KW. Long-circulating liposomes radiolabeled with [18F] fluorodipalmitin ([18F] FDP) Nucl Med Biol. 2007;34:165–171. doi: 10.1016/j.nucmedbio.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seo JW, Zhang H, Kukis DL, Meares CF, Ferrara KW. A Novel Method to Label Preformed Liposomes with 64Cu Positron Emission Tomography (PET) Imaging. Bioconjug Chem. 2008;19:2577–2584. doi: 10.1021/bc8002937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen AL, Binderup T, Rasmussen P, Henriksen JR, Elema DR, Kjær A, Andresen TL. 64Cu loaded liposomes as positron emission tomography imaging agents. Biomaterials. 2011;32:2334–2341. doi: 10.1016/j.biomaterials.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 8.Ford EC, Herman J, Yorke E, Wahl RL. 18F-FDG PET/CT for Image-Guided and Intensity-Modulated Radiotherapy. J Nucl Med. 2009;50:1655–1665. doi: 10.2967/jnumed.108.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piert M, Burian M, Meisetschlager G, Stein HJ, Ziegler S, Nahrig J, Picchio M, Buck A, Siewert JR, Schwaiger M. Positron detection for the intraoperative localisation of cancer deposits. Eur J Nucl Med Mol Imaging. 2007;34:1534–1544. doi: 10.1007/s00259-007-0430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim WW, Kim JS, Hur SM, Kim SH, Lee SK, Choi JH, Kim S, Choi JY, Lee JE, Kim JH, Nam SJ, Yang JH, Choe JH. Radioguided surgery using an intraoperative PET probe for tumor localization and verification of complete resection in differentiated thyroid cancer: a pilot study. Surgery. 2011;149:416–424. doi: 10.1016/j.surg.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Moi MK, Meares CF, Mccall MJ, Cole WC, Denardo SJ. Copper-Chelates as Probes of Biological-Systems - Stable Copper-Complexes with a Macrocyclic Bifunctional Chelating Agent. Anal Biochem. 1985;148:249–253. doi: 10.1016/0003-2697(85)90653-0. [DOI] [PubMed] [Google Scholar]
- 12.Seo JW, Mahakian LM, Kheirolomoom A, Zhang H, Meares CF, Ferdani R, Anderson CJ, Ferrara KW. Liposomal Cu-64 Labeling Method Using Bifunctional Chelators: Poly(ethylene glycol) Spacer and Chelator Effects. Bioconjug Chem. 2010;21:1206–1215. doi: 10.1021/bc100018n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kukis DL, Li M, Meares CF. Selectivity of Antibody-Chelate Conjugates for Binding Copper in the Presence of Competing Metals. Inorg Chem. 1993;32:3981–3982. [Google Scholar]
- 14.Gallagher BM, Ansari A, Atkins H, Casella V, Christman DR, Fowler JS, Ido T, MacGregor RR, Som P, Wan CN, Wolf AP, Kuhl DE, Reivich M. Radiopharmaceuticals XXVII. 18F-labeled 2-deoxy-2-fluoro-d-glucose as a radiopharmaceutical for measuring regional myocardial glucose metabolism in vivo: tissue distribution and imaging studies in animals. J Nucl Med. 1977;18:990–996. [PubMed] [Google Scholar]
- 15.Mochizuki T, Tsukamoto E, Kuge Y, Kanegae K, Zhao S, Hikosaka K, Hosokawa M, Kohanawa M, Tamaki N. FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models. J Nucl Med. 2001;42:1551–1555. [PubMed] [Google Scholar]
- 16.Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I. Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clinical Oncol. 1998;16:3375–3379. doi: 10.1200/JCO.1998.16.10.3375. [DOI] [PubMed] [Google Scholar]
- 17.Nakamoto Y, Tatsumi M, Hammoud D, Cohade C, Osman MM, Wahl RL. Normal FDG distribution patterns in the head and neck: PET/CT evaluation. Radiology. 2005;234:879–885. doi: 10.1148/radiol.2343030301. [DOI] [PubMed] [Google Scholar]
- 18.Diederichs CG, Staib L, Glatting G, Beger HG, Reske SN. FDG PET: elevated plasma glucose reduces both uptake and detection rate of pancreatic malignancies. J Nucl Med. 1998;39:1030–1033. [PubMed] [Google Scholar]
- 19.Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE. Tomographic Measurement of Local Cerebral Glucose Metabolic-Rate in Humans with (F-18)2-Fluoro-2-Deoxy-D-Glucose - Validation of Method. Ann Neurol. 1979;6:371–388. doi: 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]
- 20.Hunter GJ, Hamberg LM, Alpert NM, Choi NC, Fischman AJ. Simplified measurement of deoxyglucose utilization rate. J Nucl Med. 1996;37:950–955. [PubMed] [Google Scholar]
- 21.Huang SC. Anatomy of SUV. Nucl Med Biol. 2000;27:643–646. doi: 10.1016/s0969-8051(00)00155-4. [DOI] [PubMed] [Google Scholar]
- 22.Ross J, Fletcher J. The HER-2/neu Oncogene in Breast Cancer: Prognostic Factor, Predictive Factor, and Target for Therapy. Oncologist. 1998;3:237–252. [PubMed] [Google Scholar]
- 23.Webster MA, Hutchinson JN, Rauh MJ, Muthuswamy SK, Anton M, Tortorice CG, Cardiff RD, Graham FL, Hassell JA, Muller WJ. Requirement for both Shc and phosphatidylinositol 3’ kinase signaling pathways in polyomavirus middle T-mediated mammary tumorigenesis. Mol Cell Biol. 1998;18:2344–2359. doi: 10.1128/mcb.18.4.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borowsky AD, Namba R, Young LJ, Hunter KW, Hodgson JG, Tepper CG, McGoldrick ET, Muller WJ, Cardiff RD, Gregg JP. Syngeneic mouse mammary carcinoma cell lines: two closely related cell lines with divergent metastatic behavior. Clin Exp Metastasis. 2005;22:47–59. doi: 10.1007/s10585-005-2908-5. [DOI] [PubMed] [Google Scholar]
- 25.Nomori H, Watanabe K, Ohtsuka T, Naruke T, Suemasu K, Uno K. Evaluation of F-18 fluorodeoxyglucose (FDG) PET scanning for pulmonary nodules less than 3 cm in diameter, with special reference to the CT images. Lung Cancer. 2004;45:19–27. doi: 10.1016/j.lungcan.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]


