Abstract
A clickable alkyne-modified arylborimidine is rapidly converted in 15 minutes to a highly polar 18F-aryltrifluoroborate anion (18F-ArBF3 -) at high specific activity. Following labeling, the alkyne-18F-ArBF3 - was conjugated to the peptide bombesin (BBN) within 25 minutes in a second step without need for prior work-up making this one-pot-two-step method easy, user-friendly, and generally applicable. Bombesin was chosen to provide functional PET images of prostate cancer xenografts in mice of which there are few. Whereas BBN is labeled to provide some of the first in vivo tumor images based on this technique, click-labeling is recognized for its generality and broad substrate scope. Hence these results are likely to be useful for click labeling most peptides and other biomolecules.
Keywords: 18F-labeling, PET imaging, click chemistry, bombesin imaging
Introduction
PET imaging provides high resolution and dynamic images of target distribution and tracer clearance. Although many β+-emitting isotopes have proven potentially useful, PET is most commonly associated with the use of 18F. Indeed, the excellent nuclear properties of 18F-fluorine which make it an ideal isotope for PET imaging include: i) a low β+-emission energy for high resolution; ii) a near single decay path (> 97% β+) such that nearly all decay events contribute to imaging signal; and iii) a moderate half-life (109.8 min) which is long enough for physiological perfusion yet short enough to minimize patient radiation exposure. Moreover the facile and on-demand ability to produce hundreds of milliCuries of 18F-fluoride by 18O(p,n)18F in hospital cyclotrons account for its widespread use in clinical imaging [1].
Nevertheless, the poor reactivity of 18F-fluoride ion in water along with a relatively short half-life makes aqueous 18F-labeling of biomolecules difficult. To overcome the lack of reactivity in water, prosthetics are often synthesized under anhydrous conditions at high temperature prior to bioconjugation. Arylboronates capture 18F-fluoride ion directly under aqueous conditions to afford a water-soluble, non-coordinating 18F-labeled aryltrifluoroborate anion (18F-ArBF3 -) that is highly polar (log P < -4 for the 18F-ArBF3) [2-4]. Labeling proceeds rapidly at moderate temperature (20-40 °C) and at moderately acidic pH 2-3, making this method unique in its radiosynthetic attributes. Once the reaction is quenched to pH 7.5, the only stably isolable, radiolabeled product that remains is the 18F-ArBF3 - [2]. Preliminary PET images of a biotinylated 18F-ArBF3 - verified the in vivo stability of an 18F-ArBF3 - as there was little if any apparent signal in bone [3]. The clinically trialed drug Marimastat was conjugated to a boronate and directly labeled at low specific activity ~0.1 Ci/μmol, to reveal tumor associated matrix metalloprotease activity in breast cancer xenografts [5,6]. Furthermore, LymphoseekTM was labeled with pendant fluorescent 18F-ArBF3 - groups to provide sentinel lymph node images both with PET imaging and correlated fluorescence [7]. Most recently, we labeled RGD with a pendant 18F-ArBF3 -, albeit at low specific activity (Li et al., Am J Nucl Med Mol Imaging 2013;3(1):(in press)).
Recently, one-pot-two-step click reactions (Cu+-catalyzed and strain-promoted [2+3] cycloaddition reactions) are proving generalizable for labeling myriad peptides and other biomolecules [8-15]. Yet most click procedures still suffer from the drawback of needing dry conditions to produce a clickable 18F-labeled prosthetic. In addition, the radioprosthetic can be quite hydrophobic, a characteristic that often results in blood retention and lower image quality. The unique and potential advantages of using an 18F-ArBF3 - for labeling are: i) rapid radiosynthesis in aqueous conditions and ii) the production of a polar (anionic) 18F-prosthetic whose hydrophilicity favors clearance. These advantages, coupled with the ability to use click chemistry for general peptide labeling would represent an important and broadly enabling advance.
Compared to other labeling methods that have been extensively explored, 18F-ArBF3 - labeling is a relatively unexplored method that awaits validation in terms of producing tumor-specific images. Recently, we developed an alkyne-modified 18F-ArBF3 - for a one-pot-two-step 18F-labeling of RGD whereby no work-up is required between steps (Li et al. Am J Nucl Med Mol Imaging 2013;3(1):(in press)). Labeling proceeds in as little as 20 minutes while click conjugation requires another 20 minutes. Therefore the overall radiosynthesis time compares extremely favorably with other, recently reported click-type labeling that take up to 100 minutes or more [16,17].
To further investigate the potential for labeling other peptides beyond RGD, here we have labeled bombesin, (BBN), at high specific activity (>1 Ci/μmol) for functional tumor imaging. BBN is a tetradecapeptide with high affinity (Kd ~ 1 nM) for the gastrin-releasing peptide receptor (GRPR) [18,19], which is overexpressed on the cell surface of prostate [20], breast [21] lung [22], and some neuroendocrine tumors (NETs) [23]. Moreover, GRPR overexpression correlates with tumor differentiation [23] and enhanced mitogenic activity making it a useful target for diagnosing and grading specific neoplasms [24,25]. Hence, BBN analogs represent important ligands for imaging GRPR-positive tumors in vivo in order to improve cancer diagnosis and prognosis [7,26-28].
The N-terminal positions 1-3 of BBN tolerate bioconjugation to prosthetics, as evidenced by early reports on BBN-chelator conjugates labeled with 99mTc for use in SPECT [29-31]. Similar constructs chelate β+-emitting metal ions [32] such as 64Cu [33-36], and 68Ga [37,38], for PET imaging. Whereas chelation of β+-radiometals offers great promise [39], demetallation has been observed with certain chelators. More stable chelates designed against demetallation often require much higher temperatures for labeling (80-90 °C) [40]. In terms of 18F-labeling, BBN had been labeled via acylation of Lys-3 by 18F-benzoate-NHS [41,42], and more recently by direct nucleophilic aromatic displacement on an activated N-terminal benzamide at 130 °C [13,18]. A recent report described strain-promoted two-step click 18F-labeling using a para-18F-benzylazide that reacts with a cyclooctyne-BBN conjugate yet no images were presented [43]. Recently, an elegant approach for direct 18F-labeling employs a pendant 19F-fluoro-di-tert-butylphenylsilane (SiFA) group that undergoes 19F-18F isotope exchange [13,44-46]. Yet, when a 19F-SiFA-BBN analog was labeled accordingly, no images were reported as uptake in tumor was lower than in blood [42]. A further challenge in GRPR imaging is that BBN agonists may also elicit mitogenic effects that would contraindicate their use in human patients. Hence there is great interest in imaging BBN antagonists that do not induce receptor signaling [27,47,48].
Here we develop an alkyne that is rapidly radio-synthesized in good yield, and which is condensed with an azido-BBN antagonist via a Cu+-catalyzed [2+3] cycloaddition reaction. Three radiosyntheses are explicitly detailed in this work that delivered 18F-labeled 2 at varying specific activities of 0.08 Ci/μmol, 0.24 Ci/μmol, and 1.9 Ci/μmol at EOS, (end of synthesis). Biodistribution and corroborating PET images at 1 Ci/μmol, with blocking controls showed tumor-specific uptake thereby validating this labeling method of which the critical components are identified in Figure 1.
Figure 1.
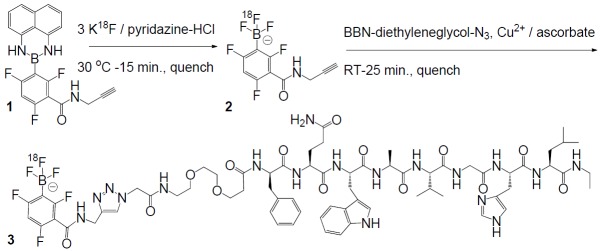
Radiosynthetic scheme for rapid one-pot-two-step 18F-labeling of BBN following conversion of borimidine 1 to alkyne-modified 18F-ArBF3 -.
Experimental section
Materials
Commercially available chemicals were purchased from Novabiochem, Sigma-Aldrich, Acros Organics, Oakwood or Alfa Aesar. Solvents were obtained from Fisher Scientific and used without further purification unless otherwise noted. The 18F Trap & Release column (HCO3 - form, ~ 10 mg) was purchased from ORTG, Inc., and C18 Sep-Pak cartridge (Vac 1cc, 50 mg) was obtained from Waters. TLC analysis was performed on aluminum-backed silica gel-60 plates from EMD Chemicals. Flash chromatography was carried out on SiliaFlash F60 (230-400 mesh) from SiliCycle. ESI-LRMS was performed on a Waters ZQ with a single quadrupole detector, attached to a Waters 2695 HPLC. ESI-HRMS were obtained on a Waters-Micromass LCT with a time-of-flight (TOF) detector. Alkyne-modified arylborimidine precursors were prepared as described previously (Li et al. Am J Nucl Med Mol Imaging 2013;3(1):(in press)).
Solid phase synthesis of BBN-N3
2-Azidoacetyl-diethyleneglycol-[D-Phe6]-BBN(6-13)NHEt was synthesized using an AAPPTEC Endeavor 90 peptide synthesizer via the Nα-Fmoc protocol starting with (3-[(ethyl-Fmoc-amino)methyl]indol-1-yl)acetyl AM resin. The Fmoc protecting group was removed using 20% piperidine in DMF. Couplings of the following protected amino acids, Fmoc-Leu-OH, Fmoc-His(Trt)-OH, Fmoc-Gly-OH, Fmoc-Val-OH, Fmoc-Ala-OH, Fmoc-Trp(Boc)-OH, Fmoc-Gln(Trt)-OH, Fmoc-D-Phe-OH, and Fmoc-PEG2-OH, were carried out in NMP with 2.5 eq. of the noted amino acids activated in situ using HBTU in the presence of DIPEA (amino acid: HBTU: DIPEA 1:1:2). The last coupling was performed in DCM with 40 eq. of bromoacetic acid activated in situ using DIC (bromoacetic acid:DIC 2:1). The coupling reactions were monitored by Kaiser test, and repeated if required. After coupling, the resin was treated with 10 eq. of sodium azide in DMSO overnight to generate the azide functional group for use in click reaction. The peptide was then deprotected and cleaved from the resin using trifluoroacetic acid/tri-isopropylsilane/H2O/phenol/thioanisole/ethanedithiol (81.5:1:5:5:5:2.5). The crude peptide was precipitated with cold Et2O and collected by filtration and then purified by HPLC system via an isocratic condition (31.5% H2O containing 0.1% TFA and 68.5 % CH3CN containing 0.1% TFA) over the course of 25 min at a flow rate of 4.5 mL/min on a Phenomenex Luna C-18 semi-preparative column (250 mm × 10 mm, 5 mm) monitored on-line for UV absorption at 220 nm. The fractions of product with a retention time of 18.8 min were collected, pooled and followed by lyophilization. Overall yield: 41% (> 97% purity). ESI-MS: [M+1]+: 1227.0, [M+Na]+: 1249.0.
BBN-ArBF3 -
(3) BBN-N3 (5.4 mg, 4.4 μmol), alkyne-ArBF3 (2) (5.5 mg, 17.2 μmol), and DIPEA (10 μL, 57.4 μmol) in DMF (200 μL) and H2O (25 μL) was added with 400 mM sodium ascorbate (470 μL) and 100 mM CuSO4 (270 μL). The reaction was shaken at room temperature for 2 hr. and then injected to HPLC for purification to give the desired product. Yield: 1.4 mg, 22%. HRMS: calcd. for C68H88BN18O14F6 -: 1505.6725, found: 1505.6752. HPLC chromatography was performance on the Agilent 1100 HPLC system equipped with an auto-injector, a fraction collector and a diode array detector. For preparation (tR= 14.5 min): column: Agilent Eclipse XDB-C18 5 mm 9.4 x 250 mm column, flow rate: 3 mL/min, column temperature: 50 °C, HPLC gradient: solvent A: 0.04 M NH4HCO2, solvent B: CH3CN, 0 min to 3 min, 0% B to 5% B, 3 min to 6 min, 5% B to 20% B, 6 min to 12 min, 20% B to 50% B, 12 min to 15 min, 50% B to 100% B, 15 min to 15.5 min, 100% B to 95% B, 15.5 min to 16 min, 95% B to 5% B, 16 min to 17 min, 5% B. For analysis (tR = 20.7 min), column: Phenomenex Jupiter 10μ C18 300A 4.6 × 250 mm column, flow rate: 1 mL/min, column temperature: 50 °C. HPLC gradient: solvent A: 0.04 M NH4HCO2, solvent B: CH3CN, 0 min to 5 min, 0% B to 5% B, 5 min to 10 min, 5% B to 20% B, 10 min to 20 min, 20% B to 50% B, 20 min to 25 min, 50% B to 100% B, 25 min to 28 min, 100% B to 95% B, 28 min to 30 min, 95% B to 5% B, 30 min to 32 min, 5% B. Following collection, the sample was lyophilized.
General radiosynthetic methods
18F-fluoride ion was obtained from the bombardment of 18O-H2O with 12.5 MeV protons in a niobium target and transferred to the hot cell through a pre-activated anion exchange column (HCO3 - or Cl- form). Following quench, the crude reaction was injected onto an analytical RP C18 HPLC column connected to an Agilent 1200 series with a viable wavelength detector and Bioscan radioactivity NaI detector. In all cases, a Phenomenex Jupiter 10m C18 300Å 4.6 mm × 250 mm column was used with a flow rate: 1 mL/min at room temperature. The HPLC gradient used was: solvent A: 0.04 M NH4HCO2, solvent B: CH3CN, 0 min to 5 min, 0% B to 5% B, 5 min to 10 min, 5% B to 20% B, 10 min to 20 min, 20% B to 50% B, 20 min to 25 min, 50% B to 100% B, 25 min to 28 min, 100% B to 95% B, 28 min to 30 min, 95% B to 5% B, 30 min to 32 min, 5% B to 0.5% B.
Radiosyntheis 1 (for ex vivo biodistribution study)
18F-Fluoride ion was trapped on an anion exchange column (ORTG, HCO3 - form) and eluted with 4 mg/mL NaClO4 solution (0.3 mL) into a glass V-vial containing CH3CN (0.5 mL). The 18F-fluoride ion solution (136.9 mCi at EOB) was concentrated under He flow at 110 °C. The dry 18F-fluoride ion was then resuspended in 0.127 M KHF2 (5 μL). The freshly prepared 18F-fluoride ion solution (2 μL, 23.7 mCi at 23 min at a specific activity of 0.047 Ci/μmol), was added to the mixture of 1 (100 nmol) in THF (4 μL) and conc. HCl (0.5 μL). The reaction was incubated at room temperature for ~ 20 min and then quenched with 5% NH4OH in 50% aqueous EtOH (10 μL) at 45 min The entire quenched reaction (14.7 mCi at 49 min) was transferred to a mixture of BBN-N3 (100 nmol) in THF (5 μL) and freshly prepared 0.75 M sodium ascorbate (4 μL). Then freshly prepared 0.25 M CuSO4 solution (2 μL) was added and the click reaction was then left at room temperature for ~ 30 min The reaction (8.88 mCi at 84 min) was diluted with 5% NH4OH in 50% aqueous EtOH (70 μL) and HPLC purified. The fraction containing the desired product (2.99 mCi at 104 min) was collected manually, diluted with H2O (15 mL), and loaded to a preactivated Waters C18 Sep-Pak cartridge (100 mg). The cartridge was further washed with H2O (10 mL) and eluted with EtOH fractions (50 μL/fraction). The desalted product (2.17 mCi at 118 min) was diluted with saline buffer (2 mL) and expedited for biodistribution studies (specific activity calculated to be ~ 0.077 Ci/μmol).
Radiosynthesis 2 (for animal PET imaging)
18F-Fluoride ion (6 μL, 33.1 mCi at EOB) was added to an eppendorf tube containing 1 (30 nmol) and 19F-fluoride ion (30 nmol, in the form of KHF2) in DMF (5 μL) and 0.55 M solution of pyridazine HCl buffer (pH 1.8) in aqueous DMF (2 μL). The reaction was then dried in a desiccator under a vacuum of 0.1 mm Hg connected to a glass column 2 cm × 10 cm filled with 3Å molecular sieves to trap any evolved 18F-HF. After 20 min, the vacuum was released and the reaction was dissolved in 5% NH4OH in 50% aq. EtOH (5 μL) containing BBN-N3 (100 nmol), followed by the addition of freshly made 0.6 M sodium ascorbate (4 μL) and 0.2 M CuSO4 (2 μL). After 25 min at room temperature, the reaction was quenched with 5% NH4OH in 50% aqueous EtOH (100 μL) and the entire reaction (8.4 mCi) was HPLC purified. The desired product (2.87 mCi at 80 min) was collected manually in 3 fractions which were diluted with H2O (20 mL) and loaded onto a preactivated Waters C18 Sep-Pak cartridge (50 mg, 1 cc). The cartridge was washed with H2O (10 mL). The radiotracer was then released with EtOH into two major fractions (50 μL per fraction). The two major fractions were combined and diluted with saline buffer (1.9 mL) for delivery (2.2 mCi, specific activity calculated to be ~1.9 Ci/μmol at time of packaging) signifying an isolated radiochemical yield of 6% (not corrected for decay). Independent measurement of the specific activity by HPLC based on reinjection of radiolabeled 3 when recorded at 220 nm gave less reliable data including the appearance of another non-radiolabeled peak, likely representing conjugation to the unlabeled arylboronates, which could reduce effective specific activity to 0.5-1 Ci/μmol.
Radiosynthesis 3 (for plasma stability test)
18F-Fluoride ion (106.5 mCi at EOB) in an eppendorf tube was concentrated in the desiccator under vacuum ~ 22 min to give a white pellet. KHF2 (2 μL, 0.127 M, 254 nmol) was added to (82.5 mCi at 35 min) to bring the SA of the 18F-fluoride ion to 0.162 Ci/μmol and this solution was added to 1 (100 nmol) in THF (5 μL). To initiate the labeling, conc. HCl (0.5 μL) was added to the reaction. The reaction mixture was incubated at room temperature for ~ 20 min and then quenched with 5% NH4OH in 50% aqueous EtOH (10 μL) containing BBN-N3 (100 nmol). To the quenched reaction (64.2 mCi at 59 min) was added with freshly prepared 0.6 M sodium ascorbate (6 μL) and 0.2 M CuSO4 (3 μL). After 25 min, the reaction was quenched with 5% NH4OH in 50% aqueous EtOH (200 μL). Approximately 80 μL of the diluted crude (21 mCi at 107 min) was HPLC purified. The desired product (5 mCi at 138 min) was collected and desalted by solid phase extraction described above. The two EtOH fractions (total 4.27 mCi at 150 min in 100 μL) were combined and directly used for the plasma stability test (SA 0.24 Ci/μmol).
Plasma stability test
BBN-18F-ArBF3 - (4.27 mCi) in EtOH (100 μL) was diluted in saline buffer (2 mL). For each assay, the saline solution (200 μL) was mixed with plasma (200 μL), incubated at 30 °C for 0, 15, 30, 60 and 120, and quenched by the addition of 75% aqueous CH3CN (400 μL). The resulting mixture was vortexed and centrifuged at 13 krpm for 20 min. The supernatant was isolated, filtered, and analyzed by HPLC for further analysis shown below the percent converted to other products was plotted on the graph below and is similar to serum stabilities seen for bombesin.
Biodistribution studies and PET imaging
The animal protocol used in the animal studies was approved by the Institutional Animal Care Committee of the University of British Columbia and was performed in compliance with the Canadian Council on Animal Care Guidelines. 6-8 weeks old male nude mice purchased from Simonsen laboratories were used for animal studies. 18F-Labeled 3, at low specific activity, was imaged in two healthy mice without tumors and a biodistribution study was conducted on these mice. For induction of tumor xenografts, male nude mice were inoculated subcutaneously with 5 x 106 PC-3 tumor cells on each shoulder. PC-3 tumor cells were freshly expanded in sterilized PBS/matrigel mixture prior to inoculation. The tumors were allowed to grow 3-4 weeks to reach a suitable size (5-7mm in diameter) for biodistribution studies and PET/CT imaging. For biodistribution studies in mice with tumors, once anesthetized, 10-20 μCi of tracer was injected via the tail vain. To determine the specificity of the in vivo uptake in receptor positive tissues, 100 μg of unlabeled BBN as a blocking agent was pre-injected (10 min) to an additional group of mice. Mice were humanely euthanized by carbon dioxide and dissected 1 hour post-injection. Tissues of interest were collected, rinsed, dried and counted in a gamma counter (Cobra-II Auto Gamma, Canberra Packard Canada). The tissue weight and associated cpm (counts per min) were used to calculate the percentage of injected dose per gram of tissue (%ID/g). PET imaging was performed in the Siemens Inveon multimodality small animal PET/CT scanner. For Dynamic PET/CT imaging, tumor-bearing animals were anaesthetized using 1.5-2% isoflurane and the tail vein was catheterized. Mice were placed onto the imaging bed while anaesthetized. A 10 minute CT attenuation scan followed by a 60 minute Dynamic PET scan was carried out. 100 μCi of radiotracer was injected via catheter 30 seconds after PET acquisition started. For blocking studies, 100 μg of unlabeled BBN was injected prior to the CT attenuation. Mice were euthanized after scanning. The list-mode data was histogrammed at various time intervals, and reconstructed by an iterative reconstruction algorithm (3D OSEM/MAP) using the Inveon Acquisition Workplace Software (Siemens), applying normalization, dead time, random and attenuation correction. The attenuation correction map was obtained from the CT scan data.
Results
Briefly, carboxytrifluorophenylboronic acid, the precursor of an in-vivo stable ArBF3 -, was protected with diaminonaphthalene (dan) [49,50] and then converted to the propargylamide providing the required alkyne functionality (Li et al., Am J Nucl Med Mol Imaging 2013;3(1):(in press)). In contrast to previous reports where tetraphenylpinacol (tpp) was used to protect the boron, we chose the more acid-labile (dan), which is released more rapidly to provide higher chemical/radiochemical yields of the ArBF3 -. Compound 1 (Figure 1) therefore represents a shelf-stable “radiosynthon” precursor that can be readily labeled under aqueous conditions as an 18F-ArBF3 - 2, which then undergoes Cu+-mediated conjugation, all in one pot, in less than 1 hour.
Here we feature click labeling of a truncated octapeptide bombesin analog [D-Phe6]-BBN(6-13)NHEt (BBN) that was chosen because it is an antagonist for the GRPR and does not elicit any mitogenic response upon binding [47,51]. An azide was affixed to the N-terminal diethyleneglycol (deg) carboxamide linker. Unlabeled alkyne-ArBF3 - 2 was prepared according to standard protocols [52-54]. With significant quantities of unlabeled 2 in hand, conditions for Cu+-catalyzed conjugation were optimized in terms of reaction time, Cu+-concentration, and the minimum amount of excess N3-BBN needed to convert 2 to 3 in high yield (>95%). Competition binding assays were performed using GRPR-expressing PC3 cells to verify that 3 exhibited a Ki of ~3 nM (Figure 2), consistent with other BBN derivatives.
Figure 2.
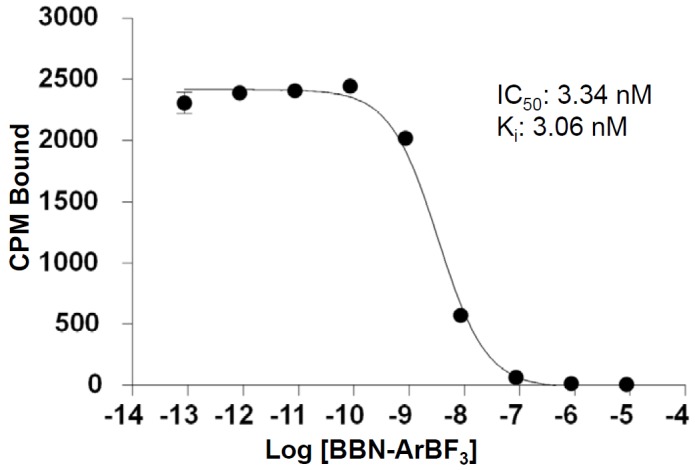
The binding constant (Ki) of BBN-ArBF3 - was determined by performing a competitive binding assay using 125I-Tyr-BBN (Perkin Elmer) on PC-3 human prostate adenocarcinoma cells.
Three different radiosyntheses detailed herein involved relatively low levels of 18F-activity (~ 50 mCi) and gave reproducibly quantitative (> 95%) conversion of 2 to produce 3 with an overall isolated radiochemical yield of 20±10% (n=3, Figure 3, not decay corrected). In each case, the crude reaction was applied to an analytical HPLC column and the crude radiotraces are provided in Figure 3, which demonstrates reproducibly excellent radiochemical purity that elutes in essentially a single, easily collected, radiolabeled peak corresponding to the labeled bombesin (note that incorporated 18F-fluoride ion elutes at 2-4 minutes). A small peak eluting ~1 min prior to the desired peak in Figure 3C was attributed to a minor unidentified labeled product. In each case, the HPLC-purified radiolabeled material was checked for radiochemical purity (see supporting information for radiotraces).
Figure 3.
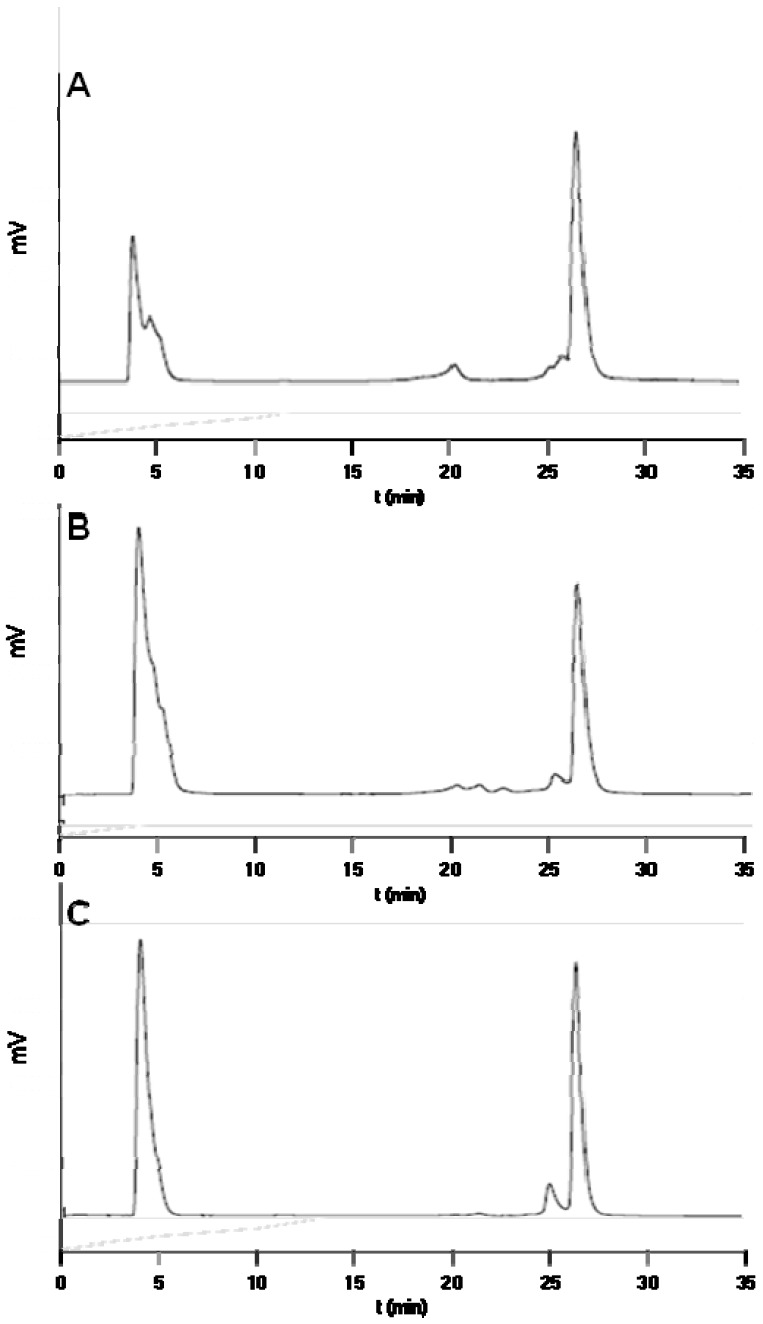
HPLC radiotraces of the crude reactions from three independent preparations of 18F-labeled 3 eluting at ~26.5 min: A: radiotrace for tumor imaging, B: for serum stability assays, and C: for imaging clearance in non-tumor mice. The fraction eluting at ~26 min was collected for further study.
Plasma stability assays on radiochemically pure material showed high stability with ~ 10% degradation and/or solvolytic defluoridation observed after 2 hours (Figure 4). This is compares slightly less favorably to other plasma stability studies of labeled bombesin [18,55] For in vivo stability studies, ~100 μCi of 3 in 200 μL PBS was injected into two healthy mice. As expected, the tracer cleared rapidly from the lungs to the liver, intestine, pancreas, and predominantly the bladder (see supporting information for images and time activity curves). In order to investigate tumor-specific uptake, eight mice, of which four were pre-blocked with 100 μg BBN, were injected with 10-20 μCi of 3 and after 60 minutes were sacrificed to generate ex vivo biodistribution data that showed in the unblocked specific tumor uptake of 1.27 ± 0.35 %ID/g and a tumor:blood ratio of ~1.5 mice while in the blocked mice, tumor uptake of 0.88 ± 0.26 %ID/g (P-value = 0.009) and a tumor:blood ratio of ~0.9. These data are summarized in Figure 5. As expected, blocking also reduced uptake in the pancreas. To corroborate these data, 18F-labeled 3 was prepared at higher specific activity (Radiosynthesis 2) and injected into two mice which presented two separate human prostate cancer xenograft tumors (PC3), of which one had been pre-blocked via tail vein injection with 100 μg BBN, were injected with 100 μCi of 3. Mice were imaged by dynamic PET-CT for 90 minutes. Specific tumor uptake was observed in both tumors with maximal uptake observed approximately 60 minutes post injection. The pre-blocked mouse showed much less tumor uptake (Figure 6). Taken together, these in vivo images and biodistribution data demonstrate GRPR-specific tumor uptake of 3.
Figure 4.
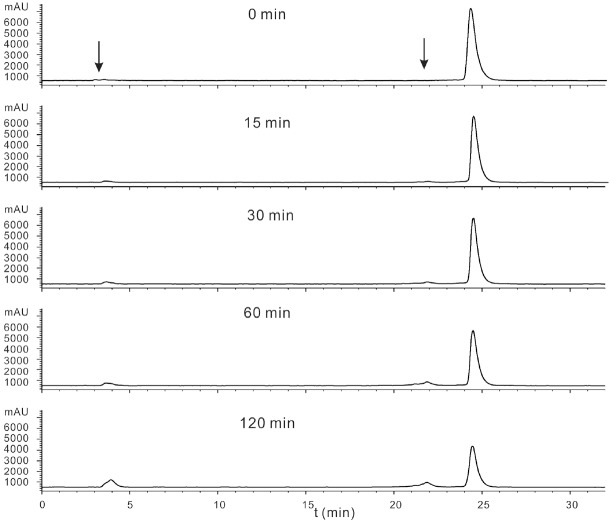
Purified BBN-18F-ArBF3 - was diluted in saline buffer (2 mL). For each assay, the saline solution (200 μL) was mixed with plasma (200 μL), incubated at 30°C for 0, 15, 30, 60 and 120, and quenched by the addition of 75% aqueous CH3CN (400 μL). Following centrifugation, the supernatant was isolated, filtered, and analyzed by HPLC for further analysis. The red arrows indicate peaks that represent time dependent degradation products and free 18F-fluoride ion or other high polar material.
Figure 5.
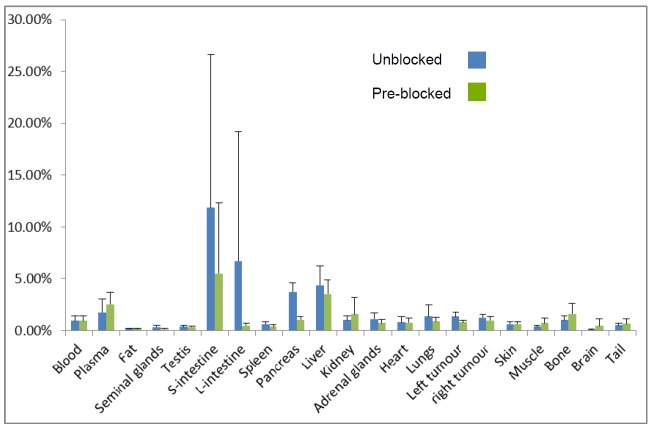
Ex-vivo Biodistribution data; blue bars show the average %ID/g from the unblocked animals (n=4) whilegreen bars show the average %ID/g from blocked animals (n=4).
Figure 6.
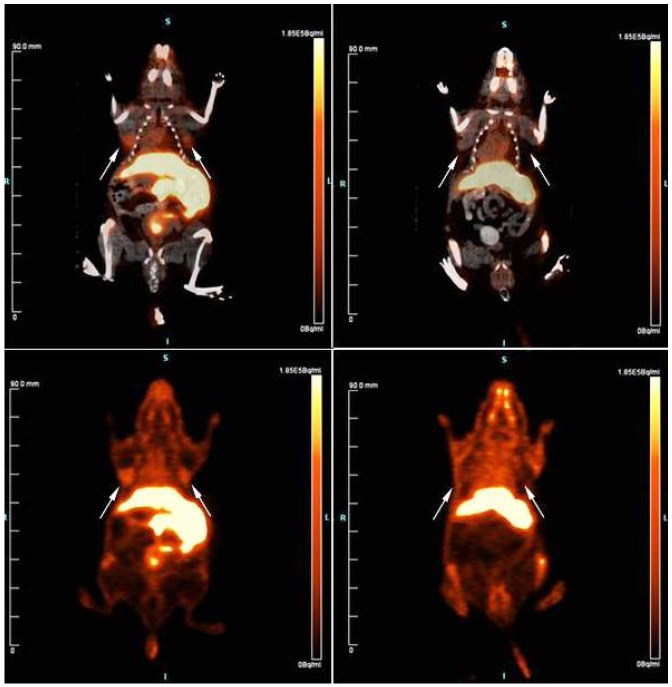
PET-CT and PET images at 60 minutes: top panels are a combined PET-CT while bottom panels are thepure PET image; unblocked mouse is shown in the left while the blocked mouse (pre-injected with 100 μg BBN(6-14)) is shown on the right; white arrows indicate position of xenograft tumors that have been derived from PC3human prostate adenocarcinoma cells.
Discussion
Herein, we have radiosyntheisized an alkyne-18F-ArBF3 - under aqueous conditions that is cleanly conjugated to a bombesin antagonist in a one-pot-two-step procedure, providing functional images of GRPR-positive tumors. Notably, this method uses nanomole (microgram) quantities of precursor borimidine and azidopeptide while high specific activity >1 Ci/μmol was achieved using just 30 mCi of 18F-activity. The use of relatively low levels of radioactive material provides reasonable yields of radiotracer at high specific activities while minimizing safety concerns. Nevertheless, use of 800 mCi of 18F-activity as commonly used in production labs, will provide considerably higher specific activities as well as higher yields, a proposition that we have belabored elsewhere at length [3,56].
While we expect that specific activities can be increased if needed, the values herein are on par with those previously reported for 64Cu-labeled BBN derivatives [57] leading to the conclusion that the somewhat inferior image quality herein can be a complex manifestation that is only partially ensured by appropriately high specific activity and may be highly sensitive to several factors including prosthetic hydrophobicity and/or radiotracer clearance. An example of such complications is seen with the very hydrophobic 18F-SiFA-BBN-derivative that was labeled at a specific activity in excess of 6 Ci/μmol, yet tumor uptake was lower than in blood suggesting that tracer lipophilicity could adversely impede imaging and result in blood-pool retention [42]. A notable advantage of 18F-ArBF3 - prosthetics is that they are inherently polar, non-coordinating anions and therefore expedite blood clearance. More detailed work will be needed to examine the effect of specific activity on image quality in the context of this and other bombesin agonists and antagonists.
In terms of image quality, the %ID/g and tumor-to-muscle and tumor-to-blood ratios were somewhat lower than desired. This is in stark contrast to other BBN analogs labeled with 68Ga and 64Cu that provided extraordinarily high contrast images [25]. While more work will be needed to verify the cause of these lower uptake values, it is likely that imaging with this particular antagonist gives lower quality images irrespective of what means are used for radiolabeling. Nevertheless, side-by-side comparisons of this and other antagonists, where each is labeled with the 18F-ArBF3 -, as well as comparison of images obtained when this antagonist is labeled with different labeling modalities, will provide a more definitive assessment as to the cause of low apparent tumor uptake herein. Finally, clearance is very rapid with >50% ID/g clearing to the bladder (data not shown). This along with moderate serum stability would also account for lower tumor uptake. Typically, the development of a clinically used tracer will require various chemical modifications to the peptide and/or the linker to modulate tumor clearance and/or increase affinity.
In these images, bone uptake (0.67 – 1.2 %ID/g) was also observed. Although this may be of some concern, this value is low and is consistent with what has been previously observed with 64Cu- labeled BBN analogs [33,34] as well as with 18F-SiFA-labeled BBN that also exhibited solvolytic fluoride ion loss [42,58]. Herein, apparent bone uptake might be due to a) trace amounts of free 18F-fluoride ion i.e. 1% that were not removed, b) specific bone uptake of BBN-18F-ArBF3 - or its metabolites and c) solvolytic/metabolic defluoridation of the 18F-ArBF3 -. Notably previous reports on the in vivo imaging of tracers linked to the same 18F-ArBF3 - did not reveal bone uptake, which would suggest that solvolytic defluoridation may not be the cause of bone revelation here. Serum stability assays however showed a new radiolabeled product eluting at 22 min to suggest proteolytic degradation while a very polar species which eluted at 4 min is consistent with fluoride liberation. In terms of fluoride solvolysis, the ArBF3 - is known to solvolyze slowly, (kobs ~ 0.3±.2 · 10-3 min-1, as measured by 19F-NMR spectroscopy) however given this rate constant, the release of 10% fluoride would likely take four hours, not two. Nevertheless, inasmuch as bone signal will need to be further minimized, use of other 18F-labeled ArBF3 - conjugates known to defluoridate even more slowly than the one used herein [59,60] will shed light on whether bone uptake arises from solvolytic/metabolic defluoridation of the 18F-ArBF3 - or accumulation of the 18F-ArBF3 --BBN conjugate itself.
Conclusion
The use of boron to capture 18F-fluoride ion represents a relatively new labeling platform that provides a distinct advantage of working in aqueous conditions and mild temperatures to rapidly afford a highly polar, rapidly clearing 18F-ArBF3 - anion that enhances the clearance of ligands to which it is attached. Here we have extended a rapid one-pot-two-step method that uses low amounts of 18F-activity and only microgram quantities of precursors to labeling bombesin, which revealed tumor-specific uptake. As rapid labeling times are essential for working with a short-lived isotope such as 18F-fluorine, this work represents an attractive means of labeling peptides with a uniquely polar radiosython, in what represents one of the more rapid one-pot-two-step labeling methods reported to date. In summary the salient advantages embodied in this labeling method are: i) use of low levels of radioactivity (< 50 mCi), ii) rapid synthesis time: ~40 minutes with reasonable overall isolated radiochemical yields, iii) the use of aqueous conditions at room temperature, iv) the production of a highly polar, rapidly clearing 18F-labeled anion, v) a one pot reaction that does not require workup between steps, and vi) a Cu+-catalyzed [2+3] cycloaddition which, based on the well-known generality of click chemistry should be extendable to any peptide, oligonucleotide, and antibody worthy of labeling.
Acknowledgements
This work was supported by a grant from the Canadian Cancer Society #20071.
Supporting Information
References
- 1.Okarvi SM. Recent progress in fluorine-18 labelled peptide radiopharmaceuticals. Eur J Nucl Med. 2001;28:929–938. doi: 10.1007/s002590100508. [DOI] [PubMed] [Google Scholar]
- 2.Ting R, Harwig C, Lo J, Li Y, Adam MJ, Ruth TJ, Perrin DM. Substituent Effects on Aryltrifluoroborate Solvolysis in Water: Implications for Suzuki-Miyaura Coupling and the Design of Stable 18F-Labeled Aryltrifluoroborates for Use in PET Imaging. J Org Chem. 2008;73:4662–4670. doi: 10.1021/jo800681d. [DOI] [PubMed] [Google Scholar]
- 3.Ting R, Harwig CW, auf dem Keller U, McCormick S, Austin P, Overall CM, Adam MJ, Ruth TJ, Perrin DM. Towards [18F] -Labeled Aryltrifluoroborate Radiotracers - In Vivo PET Imaging of Stable Aryltrifluoroborate Clearance in Mice. J Am Chem Soc. 2008;130:12045–12055. doi: 10.1021/ja802734t. [DOI] [PubMed] [Google Scholar]
- 4.Ting R, Lo J, Adam MJ, Ruth TJ, Perrin DM. Capturing aqueous (18)F -fluoride with an arylboronic ester for PET: Synthesis and aqueous stability of a fluorescent (18)F -labeled aryltrifluoroborate. J Fluor Chem. 2008;129:349–358. [Google Scholar]
- 5.Keller UAD, Bellac CL, Li Y, Lou YM, Lange PF, Ting R, Harwig C, Kappelhoff R, Dedhar S, Adam MJ, Ruth TJ, Benard F, Perrin DM, Overall CM. Novel Matrix Metalloproteinase Inhibitor F-18 Marimastat-Aryltrifluoroborate as a Probe for In vivo Positron Emission Tomography Imaging in Cancer. Cancer Res. 2010;70:7562–7569. doi: 10.1158/0008-5472.CAN-10-1584. [DOI] [PubMed] [Google Scholar]
- 6.Abiraj K, Mansi R, Tamma ML, Fani M, Forrer F, Nicolas G, Cescato R, Reubi JC, Maecke HR. Bombesin Antagonist-Based Radioligands for Translational Nuclear Imaging of Gastrin-Releasing Peptide Receptor-Positive Tumors. J Nucl Med. 2011;52:1970–1978. doi: 10.2967/jnumed.111.094375. [DOI] [PubMed] [Google Scholar]
- 7.Ambrosini V, Tomassetti P, Franchi R, Fanti S. Imaging of NETs with PET radiopharmaceuticals. Quar J Nucl Med and Mol Imag. 2010;54:16–23. [PubMed] [Google Scholar]
- 8.Aigner A. Applications of RNA interference: current state and prospects for siRNA-based strategies in vivo. App Microbiol and Biotech. 2007;76:9–21. doi: 10.1007/s00253-007-0984-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moses JE, Moorhouse AD. The growing applications of click chemistry. Chem Soc Rev. 2007;36:1249–1262. doi: 10.1039/b613014n. [DOI] [PubMed] [Google Scholar]
- 10.Glaser M, Arstad E. “Click labeling” with 2-F-18 fluoroethylazide for positron emission tomography. Bioconjug Chem. 2007;18:989–993. doi: 10.1021/bc060301j. [DOI] [PubMed] [Google Scholar]
- 11.Thonon D, Kech C, Paris J, Lemaire C, Luxen A. New Strategy for the Preparation of Clickable Peptides and Labeling with 1-(Azidomethyl)-4- F-18 -fluorobenzene for PET. Bioconjug Chem. 2009;20:817–823. doi: 10.1021/bc800544p. [DOI] [PubMed] [Google Scholar]
- 12.Mercier F, Paris J, Kaisin G, Thonon D, Flagothier J, Teller N, Lemaire C, Luxen A. General Method for Labeling siRNA by Click Chemistry with Fluorine-18 for the Purpose of PET Imaging. Bioconjug Chem. 2011;22:108–114. doi: 10.1021/bc100263y. [DOI] [PubMed] [Google Scholar]
- 13.Kostikov AP, Iovkova L, Chin J, Schirrmacher E, Wangler B, Wangler C, Jurkschat K, Cosa G, Schirrmacher R. N-(4-(di-tert-butyl F-18 fluorosilyl)benzyl)-2-hydroxy-N, N-dimethylethylammonium bromide ( F-18 SiFAN(+)Br(-)): A novel lead compound for the development of hydrophilic SiFA-based prosthetic groups for F-18-labeling. J Fluor Chem. 2011;132:27–34. [Google Scholar]
- 14.Carpenter RD, Hausner SH, Sutcliffe JL. Copper-Free Click for PET: Rapid 1,3-Dipolar Cycloadditions with a Fluorine-18 Cyclooctyne. Acs Med Chem Lett. 2011;2:885–889. doi: 10.1021/ml200187j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Priem T, Bouteiller C, Camporese D, Romieu A, Renard PY. Synthesis and reactivity of a bis-sultone cross-linker for peptide conjugation and F-18 -radiolabelling via unusual “double click” approach. Org Biomol Chem. 2012;10:1068–1078. doi: 10.1039/c1ob06600e. [DOI] [PubMed] [Google Scholar]
- 16.Olberg DE, Cuthbertson A, Solbakken M, Arukwe JM, Qu H, Kristian A, Bruheim S, Hjelstuen OK. Radiosynthesis and Biodistribution of a Prosthetic Group ((18)F-FENMA) Conjugated to Cyclic RGD Peptides. Bioconjug Chem. 2010;21:2297–2304. doi: 10.1021/bc1003229. [DOI] [PubMed] [Google Scholar]
- 17.Jeon J, Shen B, Xiong L, Miao Z, Lee KH, Rao J, Chin FT. Efficient Method for Site-Specific 18F-Labeling of Biomolecules Using the Rapid Condensation Reaction between 2-Cyanobenzothiazole and Cysteine. Bioconjug Chem. 2012;23:1902–1908. doi: 10.1021/bc300273m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honer M, Mu LJ, Stellfeld T, Graham K, Martic M, Fischer CR, Lehmann L, Schubiger PA, Ametamey SM, Dinkelborg L, Srinivasan A, Borkowski S. F-18-Labeled Bombesin Analog for Specific and Effective Targeting of Prostate Tumors Expressing Gastrin-Releasing Peptide Receptors. J Nucl Med. 2011;52:270–278. doi: 10.2967/jnumed.110.081620. [DOI] [PubMed] [Google Scholar]
- 19.Butters M, Harvey JN, Jover J, Lennox AJJ, Lloyd-Jones GC, Murray PM. Aryl Trifluoroborates in Suzuki-Miyaura Coupling: The Roles of Endogenous Aryl Boronic Acid and Fluoride. Angew Chem Int Ed. 2010;49:5156–5160. doi: 10.1002/anie.201001522. [DOI] [PubMed] [Google Scholar]
- 20.Markwalder R, Reubi JC. Gastrin-releasing peptide receptors in the human prostate: Relation to neoplastic transformation. Cancer Res. 1999;59:1152–1159. [PubMed] [Google Scholar]
- 21.Halmos G, Wittliff JL, Schally AV. Characterization of bombesin/gastrin-releasing peptide receptors in human breast cancer and their relationship to steroid receptor expression. Cancer Res. 1995;55:280–287. [PubMed] [Google Scholar]
- 22.ToiScott M, Jones CLA, Kane MA. Clinical correlates of bombesin-like peptide receptor subtype expression in human lung cancer cells. Lung Cancer. 1996;15:341–354. doi: 10.1016/0169-5002(95)00597-8. [DOI] [PubMed] [Google Scholar]
- 23.Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocrine Rev. 2004;25:458–511. doi: 10.1210/er.2003-0014. [DOI] [PubMed] [Google Scholar]
- 24.Cuttitta F, Carney DN, Mulshine J, Moody TW, Fedorko J, Fischler A, Minna JD. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. Nature. 1985;316:823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- 25.Cornelio DB, Roesler R, Schwartsmann G. Gastrin-releasing peptide receptor as a molecular target in experimental anticancer therapy. Ann Oncol. 2007;18:1457–1466. doi: 10.1093/annonc/mdm058. [DOI] [PubMed] [Google Scholar]
- 26.Banerjee SR, Pullambhatla M, Byun Y, Nimmagadda S, Green G, Fox JJ, Horti A, Mease RC, Pomper MG. 68Ga-Labeled Inhibitors of Prostate-Specific Membrane Antigen (PSMA) for Imaging Prostate Cancer. J Med Chem. 2010;53:5333–5341. doi: 10.1021/jm100623e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansi R, Wang XJ, Forrer F, Waser B, Cescato R, Graham K, Borkowski S, Reubi JC, Maecke HR. Development of a potent DOTA-conjugated bombesin antagonist for targeting GRPr-positive tumours. Eur J Nucl Med and Mol Imag. 2011;38:97–107. doi: 10.1007/s00259-010-1596-9. [DOI] [PubMed] [Google Scholar]
- 28.Min K, Jo H, Song K, Cho M, Chun YS, Jon S, Kim WJ, Ban C. Dual-aptamer-based delivery vehicle of doxorubicin to both PSMA (+) and PSMA (-) prostate cancers. Biomaterials. 2011;32:2124–2132. doi: 10.1016/j.biomaterials.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 29.Baidoo KE, Lin KS, Zhan YG, Finley P, Scheffel U, Wagner HN. Design, synthesis, and initial evaluation of high-affinity technetium bombesin analogues. Bioconjug Chem. 1998;9:218–225. doi: 10.1021/bc9701959. [DOI] [PubMed] [Google Scholar]
- 30.Smith CJ, Volkert WA, Hoffman TJ. Radiolabeled peptide conjugates for targeting of the bombesin receptor superfamily subtypes. Nucl Med Biol. 2005;32:733–740. doi: 10.1016/j.nucmedbio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Kunstler JU, Veerendra B, Figueroa SD, Sieckman GL, Rold TL, Hoffman TJ, Smith CJ, Pietzsch HJ. Organometallic Tc-99m(III) ‘4+1’ bombesin(7-14) conjugates: Synthesis, radiolabeling, and in vitro/in vivo studies. Bioconjug Chem. 2007;18:1651–1661. doi: 10.1021/bc700197m. [DOI] [PubMed] [Google Scholar]
- 32.Zeglis BM, Lewis JS. A practical guide to the construction of radiometallated bioconjugates for positron emission tomography. Dalt Trans. 2011;40:6168–6195. doi: 10.1039/c0dt01595d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogers BE, Bigott HM, McCarthy DW, Della Manna D, Kim J, Sharp TL, Welch MJ. MicroPET imaging of a gastrin-releasing peptide receptor-positive tumor in a mouse model of human prostate cancer using a Cu-64-labeled bombesin analogue. Bioconjug Chem. 2003;14:756–763. doi: 10.1021/bc034018l. [DOI] [PubMed] [Google Scholar]
- 34.Garrison JC, Rold TL, Sieckman GL, Figueroa SD, Volkert WA, Jurisson SS, Hoffman TJ. In vivo evaluation and small-animal PET/CT of a prostate cancer mouse model using Cu-64 bombesin analogs: Side-by-side comparison of the CB-TE2A and DOTA chelation systems. J Nucl Med. 2007;48:1327–1337. doi: 10.2967/jnumed.107.039487. [DOI] [PubMed] [Google Scholar]
- 35.Parry JJ, Andrews R, Rogers BE. MicroPET imaging of breast cancer using radiolabeled bombesin analogs targeting the gastrin-releasing peptide receptor. Breast Cancer Res and Treatment. 2007;101:175–183. doi: 10.1007/s10549-006-9287-8. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman TJ, Smith CJ. True radiotracers: Cu-64 targeting vectors based upon bombesin peptide. Nucl Med Biol. 2009;36:579–585. doi: 10.1016/j.nucmedbio.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Dimitrakopoulou-Strauss A, Hohenberger P, Haberkorn U, Macke HR, Eisenhut M, Strauss LG. Ga-68-Labeled bombesin studies in patients with gastrointestinal stromal tumors: Comparison with F-18-FDG. J Nucl Med. 2007;48:1245–1250. doi: 10.2967/jnumed.106.038091. [DOI] [PubMed] [Google Scholar]
- 38.Dimitrakopoulou-Strauss A, Seiz M, Tuettenberg J, Schmieder K, Eisenhut M, Haberkorn U, Strauss LG. Pharmacokinetic Studies of Ga-68-Labeled Bombesin (Ga-68-BZH(3)) and F-18 FDG PET in Patients With Recurrent Gliomas and Comparison to Grading Preliminary Results. Clin Nucl Med. 2011;36:101–108. doi: 10.1097/RLU.0b013e318203bb24. [DOI] [PubMed] [Google Scholar]
- 39.Pandya DN, Dale AV, Kim JY, Lee H, Ha YS, An GI, Yoo J. New Macrobicyclic Chelator for the Development of Ultrastable Cu-64-Radiolabeled Bioconjugate. Bioconjug Chem. 2012;23:330–335. doi: 10.1021/bc200539t. [DOI] [PubMed] [Google Scholar]
- 40.Ait-Mohand S, Fournier P, Dumulon-Perreault V, Kiefer GE, Jurek P, Ferreira CL, Benard F, Guerin B. Evaluation of Cu-64-Labeled Bifunctional Chelate-Bombesin Conjugates. Bioconjug Chem. 2011;22:1729–1735. doi: 10.1021/bc2002665. [DOI] [PubMed] [Google Scholar]
- 41.Zhang XZ, Cai WB, Cao F, Schreibmann E, Wu Y, Wu JC, Xing L, Chen XY. F-18-labeled bombesin analogs for targeting GRP receptor-expressing prostate cancer. J Nucl Med. 2006;47:492–501. [PubMed] [Google Scholar]
- 42.Hoehne A, Mu L, Honer M, Schubiger PA, Ametamey SM, Graham K, Stellfeld T, Borkowski S, Berndorff D, Klar U, Voigtmann U, Cyr JE, Friebe M, Dinkelborg L, Srinivasan A. Synthesis, F-18-labeling, and in vitro and in vivo studies of bombesin peptides modified with silicon-based building blocks. Bioconjug Chem. 2008;19:1871–1879. doi: 10.1021/bc800157h. [DOI] [PubMed] [Google Scholar]
- 43.Campbell-Verduyn LS, Mirfeizi L, Schoonen AK, Dierckx RA, Elsinga PH, Feringa BL. Strain-Promoted Copper-Free “Click” Chemistry for 18F Radiolabeling of Bombesin. Angew Chem Int Ed. 2011;50:11117–11120. doi: 10.1002/anie.201105547. [DOI] [PubMed] [Google Scholar]
- 44.Schirrmacher E, Wangler B, Cypryk M, Bradtmoller G, Schafer M, Eisenhut M, Jurkschat K, Schirrmacher R. Synthesis of p-(Di-tertbutyl (18)F fluorosilyl)benzaldehyde ( F-18 Si-FA-A) with high specific activity by isotopic exchange: A convenient Labeling synthon for the F-18-labeling of n-amino-oxy derivatized peptides. Bioconjug Chem. 2007;18:2085–2089. doi: 10.1021/bc700195y. [DOI] [PubMed] [Google Scholar]
- 45.Schirrmacher R, Bradtmoller G, Schirrmacher E, Thews O, Tillmanns J, Siessmeier T, Buchholz HG, Bartenstein P, Waengler B, Niemeyer CM, Jurkschat K. F-18-labeling of peptides by means of an organosilicon-based fluoride acceptor. Angew Chem Int Ed. 2006;45:6047–6050. doi: 10.1002/anie.200600795. [DOI] [PubMed] [Google Scholar]
- 46.Kostikov AP, Chin J, Orchowski K, Niedermoser S, Kovacevic MM, Aliaga A, Jurkschat K, Wangler B, Wangler C, Wester HJ, Schirrmacher R. Oxalic Acid Supported Si-F-18-Radiofluorination: One-Step Radiosynthesis of N-Succinimidyl 3-(Di-tert-butyl F-18 fluorosilyl) benzoate ( F-18 SiFB) for Protein Labeling. Bioconjug Chem. 2012;23:106–114. doi: 10.1021/bc200525x. [DOI] [PubMed] [Google Scholar]
- 47.Cescato R, Maina T, Nock B, Nikolopoulou A, Charalambidis D, Piccand V, Reubi JC. Bombesin receptor antagonists may be preferable to agonists for tumor targeting. J Nucl Med. 2008;49:318–326. doi: 10.2967/jnumed.107.045054. [DOI] [PubMed] [Google Scholar]
- 48.Nanda PK, Pandey U, Bottenus BN, Rold TL, Sieckman GL, Szczodroski AF, Hoffman TJ, Smith CJ. Bombesin analogues for gastrin-releasing peptide receptor imaging. Nucl Med Biol. 2012;39:461–471. doi: 10.1016/j.nucmedbio.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 49.Iwadate N, Suginome M. Synthesis of masked haloareneboronic acids via iridium-catalyzed aromatic C-H borylation with 1,8-naphthalenediaminatoborane (danBH) Journal of Organomet Chem. 2009;694:1713–1717. [Google Scholar]
- 50.Noguchi H, Shioda T, Chou CM, Suginome M. Differentially protected, benzenediboronic acids: Divalent cross-coupling modules for the efficient synthesis of boron-substituted oligoarenes. Org Lett. 2008;10:377–380. doi: 10.1021/ol702420x. [DOI] [PubMed] [Google Scholar]
- 51.Nock B, Nikolopoulou A, Chiotellis E, Loudos G, Maintas D, Reubi JC, Maina T. Tc-99m Demobesin 1, a novel potent bombesin analogue for GRP receptor-targeted tumour imaging. Eur J Nucl Med and Mol Imag. 2003;30:247–258. doi: 10.1007/s00259-002-1040-x. [DOI] [PubMed] [Google Scholar]
- 52.Vedejs E, Chapman RW, Fields SC, Lin S, Schrimpf MR. Conversion of Arylboronic Acids into Potassium Aryltrifluoroborates - Convenient Precursors of Arylboron Difluoride Lewis-Acids. J Org Chem. 1995;60:3020–3027. [Google Scholar]
- 53.Quach TD, Batey RA. Ligand- and base-free copper(II)-catalyzed C-N bond formation: Cross-coupling reactions of organoboron compounds with aliphatic amines and anilines. Org Lett. 2003;5:4397–4400. doi: 10.1021/ol035681s. [DOI] [PubMed] [Google Scholar]
- 54.Molander GA, Ham J. Synthesis of functionalized organotrifluoroborates via the 1,3-dipolar cycloaddition of azides. Org Lett. 2006;8:2767–2770. doi: 10.1021/ol060826r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang HW, Chen JH, Waldherr C, Hinni K, Waser B, Reubi JC, Maecke HR. Synthesis and evaluation of bombesin derivatives on the basis of pan-bombesin peptides labeled with indium-111, lutetium-177, and yttrium-90 for targeting bombesin receptor-expressing tumors. Cancer Res. 2004;64:6707–6715. doi: 10.1158/0008-5472.CAN-03-3845. [DOI] [PubMed] [Google Scholar]
- 56.Li Y, Ting R, Harwig CW, Keller UAD, Bellac CL, Lange PF, Inkster JAH, Schaffer P, Adam MJ, Ruth TJ, Overall CM, Perrin DM. Towards kit-like F-18-labeling of marimastat, a noncovalent inhibitor drug for in vivo PET imaging cancer associated matrix metalloproteases. Med Chem Commun. 2011;2:942–949. [Google Scholar]
- 57.Lears KA, Ferdani R, Liang KX, Zheleznyak A, Andrews R, Sherman CD, Achilefu S, Anderson CJ, Rogers BE. In Vitro and In Vivo Evaluation of Cu-64-Labeled SarAr-Bombesin Analogs in Gastrin-Releasing Peptide Receptor-Expressing Prostate Cancer. J Nucl Med. 2011;52:470–477. doi: 10.2967/jnumed.110.082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balentova E, Collet C, Lamande-Langle S, Chretien F, Thonon D, Aerts J, Lemaire C, Luxen A, Chapleur Y. Synthesis and hydrolytic stability of novel 3- F-18 fluoroethoxybis (1-methylethyl)silyl propanamine-based prosthetic groups. J Fluor Chem. 2011;132:250–257. [Google Scholar]
- 59.Li Y, Asadi A, Perrin DM. Hydrolytic stability of nitrogenous-heteroaryltrifluoroborates under aqueous conditions at near neutral pH. J Fluor Chem. 2009;130:377–382. [Google Scholar]
- 60.Wade CR, Zhao H, Gabbai FP. Stabilization of zwitterionic aryltrifluoroborates against hydrolysis. Chem Commun. 2010;46:6380–6381. doi: 10.1039/c0cc02117b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


