Abstract
Positron Emission Tomography (PET) has become a popular imaging technique widely used for diagnostic purposes. To date, much attention has been devoted to 18F-fluoride because of the characteristics of its nuclear decay, as well as its relative ease of preparation from 18O-water. However, with a half-life of 110 minutes, swift and efficient incorporation of 18F-fluorine into biomolecules is necessary to minimize loss of activity. Therefore, the discovery of rapid and reliable incorporation of 18F-fluorine atoms into biomolecules would be highly beneficial, especially if these protocols can be carried out directly in irradiated 18O-water. In the study published in the American Journal of Nuclear Medicine and Molecular Imaging, cyclo-RGD-18F-aryltrifluoroborate conjugates were prepared based on one-step and one-pot-two-step methods. This paper represents recent efforts on the design and development of novel PET tracers based on the “Kit like” 18F labeling method.
Keywords: Positron emission tomography (PET), 18F, RGD, integrin αvβ3, molecular imaging
Introduction
Positron emission tomography (PET) is a noninvasive, quantitative, repeatable and highly sensitive imaging modality that provides in vivo radiolabeled biomolecule distribution information [1]. Unlike anatomical imaging modalities, such as computed tomography (CT) and magnetic resonance imaging (MRI), the functional information provided by PET imaging allows an earlier diagnosis of the disease state, which is crucial to provide reliable prognosis and therapeutic intervention. For example, the success of 2-deoxy-2-18F-fluoro-D-glucose (18F-FDG) has made PET a routine clinical practice in cancer diagnosis, patient stratification, and monitoring the treatment of cancer patients [2]. Various positron-emitting radionuclides, including 11C, 18F, 64Cu, 68Ga, and 125I, have been employed in PET probe designs and syntheses. Among them, 18F-fluroide (t1/2 = 110 min; β+, 99%) is an ideal short-lived PET isotope produced in small biomedical cyclotrons. Its half-life is long enough to allow syntheses, transportation, and imaging procedures to be extended over the course of hours, yet with reduced radiation doses for patients in contrast to long-lived radioisotopes. Furthermore, the low positron energy of 18F decay results in a short positron range in tissue, leading to high resolution PET imaging.
18F labeling of RGD peptides using traditional methods
Direct incorporation of 18F into biomolecules, like proteins and peptides, at high specific activity is challenging due to the limited functional groups for nucleophilic 18F-fluorination. Moreover, harsh radiofluorination conditions (i.e., high temperature, strongly basic) cause denaturation and decomposition of sensitive biomolecules. In order to circumvent this obstacle, peptides and proteins are generally labeled with 18F using fluorinated prosthetic groups (also referred as bifunctional labeling agents) [3]. For example, various RGD peptides (with high affinity towards integrin αvβ3) have been labeled with 18F and extensively studied in animal and clinical research. The vitronectin receptor integrin αvβ3 has been the focus of intense research because of its major role in several distinct processes, particularly osteoclast mediated bone resorption, angiogenesis, pathological neovascularization, and tumor metastasis [4]. In 2001, Galacto-RGD was labeled with 4-nitrophenyl 2-18F-fluoropropionate (18F-NPFP), which successfully imaged integrin αvβ3 expression in vivo. The clinical PET studies of 18F-Galacto-RGD show highly favorable biodistribution in humans and visualization of αvβ3 expression with high contrast [5,6]. Although promising results were obtained, the overall radiochemical yield for 18F-Galacto-RGD was 29 ± 5% with a total reaction time of 200 ± 18 min, including final HPLC preparation [7]. N-succinimidyl 4-18F-fluorobenzoate (18F-SFB) was also used to label monomeric, dimeric and tetrameric RGD peptides through the sidechain ε-amino group of the lysine residue [8-10]. The radiotracers were evaluated in vivo and demonstrated favorable tumor targeting efficacy and pharmacokinetics. The radiolabeling was finished in approximately 2 h with 20-25% decay-corrected yields and specific activity of 230 GBq/μmol (6.2 Ci/μmol) at end of synthesis. Other reactions that have been used for 18F labeling of RGD peptide include “click” chemistry, tetrazine-trans-cyclooctene ligation, thiol/maleimide and oxime/aldehyde chemistry (18F-AH111585) [11-16].
“Kit like” 18F labeling of RGD peptides
Although encouraging results were obtained for 18F-labeled RGD probes, most of the above methods used the conventional formation of a C-18F bond. The main shortcomings of this approach include multistep synthetic pathways, time-consuming procedures, and most notably the need for specially trained radiochemist. To overcome these problems, “Kit Formulation” for 18F-labeling has been proposed. The basic idea is to introduce an atom to the target compound, which could form a stable bond with F-. In an ideal situation, the labeled compound could be obtained by simply mixing the 18F- with the modified precursor. The “Kit like” 18F labeling approach is appealing because time-consuming synthetic steps can be carried out before introduction of the shortlived 18F radionuclide. However, this field of research remains relatively underdeveloped with only a few types of captors investigated thus far, which include boron fluoride acceptor, silicon fluoride acceptor, phosphorous-18F compounds, and NOTA-Al-fluoride complex [17-24]. In this study published in the American Journal of Nuclear Medicine and Molecular Imaging, Perrin et al reported an elegant approach to synthesize 18F labeled RGD peptide based on arylboronic compound featuring electron-withdrawing substituents [25]. Because the arylboronic compounds quickly react with fluoride ions to form the corresponding arylfluoroborates, a simple one-step or one-reactor RGD labeling could be accomplished in aqueous media, which lead to two RGD probes: RGD-SuPi-18F-ArBF3 - and RGD-trAz-18F-ArBF3 - (Figure 1) [25].
Figure 1.
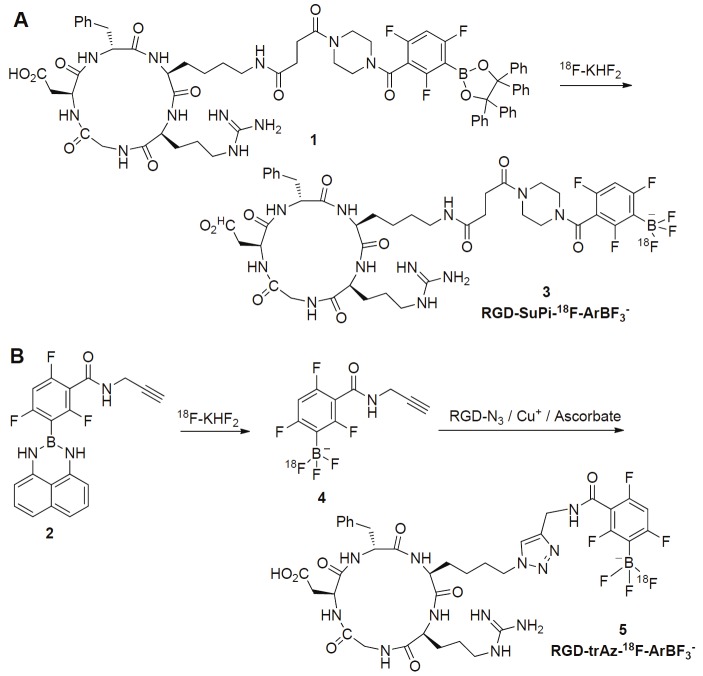
Synthetic scheme for the preparation of RGD-SuPi-18F-ArBF3 - and RGD-trAz-18F-ArBF3 -: A. a tetraphenylpinacolateboronate ester conjugate to RGD is directly converted to the RGD-SuPi-18F-ArBF3 -. B. The alkyne borimidine 2 is converted first to the corresponding 18F-ArBF3 - alkyne 4 in situ, which is then directly conjugated to the RGD-azide to provide RGD-trAz-18F-ArBF3 -.
In the synthesis of RGD-SuPi-18F-ArBF3 -, the protocol mixed the RGD borate precursor (compound 1, Figure 1) and the pre-concentrated 18F-fluoride together, followed by incubation at room temperature for 1 h. The crude mixture was subjected to HPLC purification to obtain the final product. The total synthetic time was 2 h with approximately 4% yield (non-decay corrected yield). In the synthesis of RGD-trAz-18F-ArBF3 -, the borate precursor equipped with alkyne functional group (compound 2, Figure 1) was mixed with concentrated 18F for fluorination, followed by addition of RGD-N3 peptide to the same reactor. After purification with HPLC and C18 cartridge, RGD-trAz-18F-ArBF3 - was obtained with the non-decay corrected yield of 6.8% (total synthetic time is 3.7 hours). These two tracers were studied with microPET using U87MG tumor models. The tumor uptakes of RGD-SuPi-18F-ArBF3 - and RGD-trAz-18F-ArBF3 - have no significant difference at time points examined and were comparable to other publications on 18F labeled RGD peptides [8,26]. Furthermore, the authors claimed the bone uptake for both probes were minimum based on the microPET analysis, which indicated negligible defluorination. Clearly, the preliminary data warrant further evaluation and improvement of these newly developed 18F labeling methods.
We would also like to point out that the labeling of RGD-SuPi-18F-ArBF3 - was performed in strong acidic conditions. Although it is acceptable for the labeling of RGD peptides, other ligands (such as proteins and antibodies) may be sensitive to acidic conditions. In addition, both reactions are carrier-added (KHF2), and relatively low specific activities for the products were obtained. This could be a limitation for the syntheses of PET probes with high toxicity or when the target has low density in vivo. Further modification of the labeling protocol may be necessary to obtain higher specific activity in those cases. Although the Log(Pow) of RGD-trAz-18F-ArBF3 - was -3.8 in terms of octanol-water partition, substantial uptake in the liver, spleen, and gallbladder was observed after injection. This indicates the pharmacokinetic properties of these peptides could be further improved to achieve increased tumor-to-background contrasts and reduced radiation exposure to normal organs. The Galacto- or PEG (polyethylene glycol)-linker could be employed to improve imaging quality as demonstrated previously [7,27].
In summary, much effort has been made to investigate 18F-labeling chemistry for biologically important peptides. Traditional labeling tactics such as 18F-SFB and 18F-NPFP have greatly advanced the PET imaging field. However, the preparation of these labeling moieties often requires multiple step procedures, sophisticated synthetic modules, and skilled radiochemists. The disadvantages severely hamper the use of these labeling approaches in routine clinical production of peptide-based PET probes. Therefore, the discovery of general and reliable protocols for the rapid incorporation of 18F-fluorine atoms into biomolecules would be highly beneficial, especially if these protocols can be carried out directly in the irradiated 18O-water. In the study published in the American Journal of Nuclear Medicine and Molecular Imaging, cyclo-RGD-18F-aryltrifluoroborate conjugates were prepared based on one-step and one-pot-two-step methods. This paper represents recent efforts on the design and development of novel PET tracers based on “Kit like” 18F labeling method. Certainly, continued efforts are needed to further optimize these methods in order to establish ideal “Kit-labeling” procedures of 18F-labeled peptides for clinical applications.
References
- 1.Wolbarst AB, Hendee WR. Evolving and experimental technologies in medical imaging. Radiology. 2006;238:16–39. doi: 10.1148/radiol.2381041602. [DOI] [PubMed] [Google Scholar]
- 2.Allen-Auerbach M, Weber WA. Measuring response with FDG-PET: methodological aspects. Oncologist. 2009;14:369–377. doi: 10.1634/theoncologist.2008-0119. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Conti PS. Radiopharmaceutical chemistry for positron emission tomography. Adv Drug Deliv Rev. 2010;62:1031–1051. doi: 10.1016/j.addr.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 5.Beer AJ, Haubner R, Goebel M, Luderschmidt S, Spilker ME, Wester HJ, Weber WA, Schwaiger M. Biodistribution and pharmacokinetics of the αvβ3-selective tracer 18F-galacto-RGD in cancer patients. J Nucl Med. 2005;46:1333–1341. [PubMed] [Google Scholar]
- 6.Haubner R, Weber WA, Beer AJ, Vabuliene E, Reim D, Sarbia M, Becker KF, Goebel M, Hein R, Wester HJ, Kessler H, Schwaiger M. Noninvasive visualization of the activated αvβ3 integrin in cancer patients by positron emission tomography and [18F] Galacto-RGD. PLoS Med. 2005;2:e70. doi: 10.1371/journal.pmed.0020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haubner R, Kuhnast B, Mang C, Weber WA, Kessler H, Wester HJ, Schwaiger M. [18F] Galacto-RGD: synthesis, radiolabeling, metabolic stability, and radiation dose estimates. Bioconjug Chem. 2004;15:61–69. doi: 10.1021/bc034170n. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Park R, Shahinian AH, Tohme M, Khankaldyyan V, Bozorgzadeh MH, Bading JR, Moats R, Laug WE, Conti PS. 18F-labeled RGD peptide: initial evaluation for imaging brain tumor angiogenesis. Nucl Med Biol. 2004;31:179–189. doi: 10.1016/j.nucmedbio.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Wu Z, Li ZB, Cai W, He L, Chin FT, Li F, Chen X. 18F-labeled mini-PEG spacered RGD dimer (18F-FPRGD2): synthesis and microPET imaging of αvβ3 integrin expression. Eur J Nucl Med Mol Imaging. 2007;34:1823–1831. doi: 10.1007/s00259-007-0427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Z, Li ZB, Chen K, Cai W, He L, Chin FT, Li F, Chen X. microPET of tumor integrin αvβ3 expression using 18F-labeled PEGylated tetrameric RGD peptide (18F-FPRGD4) J Nucl Med. 2007;48:1536–1544. doi: 10.2967/jnumed.107.040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Cai H, Hassink M, Blackman ML, Brown RC, Conti PS, Fox JM. Tetrazine-trans-cyclooctene ligation for the rapid construction of 18F labeled probes. Chem Commun (Camb) 2010;46:8043–8045. doi: 10.1039/c0cc03078c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selvaraj R, Liu S, Hassink M, Huang CW, Yap LP, Park R, Fox JM, Li Z, Conti PS. Tetrazine-trans-cyclooctene ligation for the rapid construction of integrin αvβ3 targeted PET tracer based on a cyclic RGD peptide. Bioorg Med Chem Lett. 2011;21:5011–5014. doi: 10.1016/j.bmcl.2011.04.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glaser M, Morrison M, Solbakken M, Arukwe J, Karlsen H, Wiggen U, Champion S, Kindberg GM, Cuthbertson A. Radiosynthesis and biodistribution of cyclic RGD peptides conjugated with novel [18F] fluorinated aldehyde-containing prosthetic groups. Bioconjug Chem. 2008;19:951–957. doi: 10.1021/bc700472w. [DOI] [PubMed] [Google Scholar]
- 14.McParland BJ, Miller MP, Spinks TJ, Kenny LM, Osman S, Khela MK, Aboagye E, Coombes RC, Hui AM, Cohen PS. The biodistribution and radiation dosimetry of the Arg-Gly-Asp peptide 18F-AH111585 in healthy volunteers. J Nucl Med. 2008;49:1664–1667. doi: 10.2967/jnumed.108.052126. [DOI] [PubMed] [Google Scholar]
- 15.Kenny LM, Coombes RC, Oulie I, Contractor KB, Miller M, Spinks TJ, McParland B, Cohen PS, Hui AM, Palmieri C, Osman S, Glaser M, Turton D, Al-Nahhas A, Aboagye EO. Phase I trial of the positron-emitting Arg-Gly-Asp (RGD) peptide radioligand 18F-AH111585 in breast cancer patients. J Nucl Med. 2008;49:879–886. doi: 10.2967/jnumed.107.049452. [DOI] [PubMed] [Google Scholar]
- 16.Cai W, Zhang X, Wu Y, Chen X. A thiol-reactive 18F-labeling agent, N-[2-(4-18F-fluorobenzamido)ethyl] maleimide, and synthesis of RGD peptide-based tracer for PET imaging of αvβ3 integrin expression. J Nucl Med. 2006;47:1172–1180. [PMC free article] [PubMed] [Google Scholar]
- 17.Ting R, Harwig C, auf dem Keller U, McCormick S, Austin P, Overall CM, Adam MJ, Ruth TJ, Perrin DM. Toward [18F] -labeled aryltrifluoroborate radiotracers: in vivo positron emission tomography imaging of stable aryltrifluoroborate clearance in mice. J Am Chem Soc. 2008;130:12045–12055. doi: 10.1021/ja802734t. [DOI] [PubMed] [Google Scholar]
- 18.Hohne A, Yu L, Mu L, Reiher M, Voigtmann U, Klar U, Graham K, Schubiger PA, Ametamey SM. Organofluorosilanes as model compounds for 18F-labeled silicon-based PET tracers and their hydrolytic stability: experimental data and theoretical calculations (PET = positron emission tomography) Chemistry. 2009;15:3736–3743. doi: 10.1002/chem.200802437. [DOI] [PubMed] [Google Scholar]
- 19.Studenov AR, Adam MJ, Wilson JS, Ruth TJ. New radiolabelling chemistry: synthesis of phosphorus–[18F] fluorine compounds. J Label Compd Radiopharm. 2005;48:497–500. [Google Scholar]
- 20.McBride WJ, Sharkey RM, Karacay H, D’Souza CA, Rossi EA, Laverman P, Chang CH, Boerman OC, Goldenberg DM. A novel method of 18F radiolabeling for PET. J Nucl Med. 2009;50:991–998. doi: 10.2967/jnumed.108.060418. [DOI] [PubMed] [Google Scholar]
- 21.McBride WJ, D’Souza CA, Sharkey RM, Karacay H, Rossi EA, Chang CH, Goldenberg DM. Improved 18F labeling of peptides with a fluoride-aluminum-chelate complex. Bioconjug Chem. 2010;21:1331–1340. doi: 10.1021/bc100137x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu S, Liu H, Jiang H, Xu Y, Zhang H, Cheng Z. One-step radiosynthesis of 18F-AlF-NOTA-RGD2 for tumor angiogenesis PET imaging. Eur J Nucl Med Mol Imaging. 2011;38:1732–1741. doi: 10.1007/s00259-011-1847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Lin TP, Liu S, Huang CW, Hudnall TW, Gabbai FP, Conti PS. Rapid aqueous [18F] -labeling of a bodipy dye for positron emission tomography/fluorescence dual modality imaging. Chem Commun (Camb) 2011;47:9324–9326. doi: 10.1039/c1cc13089g. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Chansaenpak K, Liu S, Wade CR, Conti PS, Gabbai FP. Harvesting 18F-fluoride ions in water via direct 18F-19F isotopic exchange: radiofluorination of zwitterionic aryltrifluoroborates and in vivo stability studies. Med Chem Comm. 2012;3:1305–1308. [Google Scholar]
- 25.Li Y, Guo J, Tang S, Lang L, Chen X, Perrin DM. One-step and One-Pot-Two-Step Radiosynthesis of Cyclo-RGD-18F-Aryltrifluoroborate Conjugates for Functional Imaging. Am J Nucl Med Mol Imaging. 2013;1:44–56. [PMC free article] [PubMed] [Google Scholar]
- 26.Li ZB, Wu Z, Chen K, Chin FT, Chen X. Click chemistry for 18F-labeling of RGD peptides and microPET imaging of tumor integrin αvβ3 expression. Bioconjug Chem. 2007;18:1987–1994. doi: 10.1021/bc700226v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu S, Liu Z, Chen K, Yan Y, Watzlowik P, Wester HJ, Chin FT, Chen X. 18F-labeled galacto and PEGylated RGD dimers for PET imaging of αvβ3 integrin expression. Mol Imaging Biol. 2010;12:530–538. doi: 10.1007/s11307-009-0284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


