Abstract
Aim
(1) To investigate the recurrence of periocular basal cell carcinoma (BCC) reported as completely excised on histology. (2) To identify risks associated with recurrence. (3) To recommend a rational follow-up protocol.
Methods
This is a cohort study by case note review of consecutive patients undergoing excision of periocular BCC between 2000 and 2006 at University Hospitals of Leicester. All lesions were excised with 3 mm clinical margin and the defect reconstructed only after the excision margin was declared clear.
Results
A total of 413 episodes of surgical excision were recorded for 270 patients over the 7-year period of 2000–2006. All of them have 5 years follow-up. Mean age 73.7 (±12.5). In all, 67% were nodular BCC and 45.4% located in the lower eyelid. The main outcome measure was the recurrence rate. None of the patients with primary nodular BCC suffered recurrence. The recurrence rate for primary morphoeaform BCC following complete excision is 3.8%. In total, 8.1% of patients had several lesions simultaneously whereas 7.8% patients had BCC in multiple locations subsequently (metachronous). Three patients who had previously recurrent BCC (rBCC) treated elsewhere or not using this method had orbital/lacrimal drainage system involvement requiring exenteration.
Conclusion
We recommend that patients with a single, completely excised primary solid or nodular BCC can be discharged after one 6-monthly review, although they should be instructed to monitor for the development of further lesions. The incidence of recurrence for primary morphoeaform BCC is 3.8% and for rBCC is 3.6% over 5 years and these patients should stay under review for this period.
Keywords: basal cell carcinoma, recurrence, follow-up
Introduction
Basal cell carcinoma (BCC) is the most common periocular skin cancer affecting mainly Caucasians. In the UK, 53 000 new cases of BCC are estimated every year and figures are continuing to rise on a yearly basis. More worrying, the largest increase in incidence is seen in the 30–39 year age groups.1
Traditionally, 5 years of clinical follow-up for periocular (high-risk site) BCC is recommended to ensure early detection of recurrence2 or new primary lesions.3 Primary BCCs (pBCCs) recur <10% of the time, with two-thirds recurring in the first 3 years.4, 5 However, the studies are relatively dated, use a wide variety of techniques with different methods of excision margin control,5, 6 and none describe the histological processing of the specimens.
In the light of increasing incidence of BCC and limited resources in the UK health-care system, it is not always possible to follow every patient for 5 years post excision. A national survey with self-completion questionnaire showed variability in follow-up. In all, 27% of respondents reported that they would not review further after excision of a ‘well-defined' BCC from inside a central ‘T' area on the face; 37% reported that they would review on one occasion; and 36% reported that they review more than once.7
Follow-up of excised BCC constitutes a large workload in oculoplastic follow-up clinics. Although we acknowledge that there is always a risk of recurrence with all types BCC, it is in our experience that the majority do not recur and therefore do not require follow-up over a long period of time. To examine this hypothesis, we conducted a study to look at 5 years' complete follow-up of periocular BCC in Leicester.
We aimed to investigate the recurrence of histologically proven completely excised BCC and to identify risks associated with recurrence in order to recommend a follow-up protocol.
Materials and methods
A detailed retrospective analysis was undertaken of the medical records of all patients who underwent excision of periocular BCC between 2000 and 2006 in Leicester Royal Infirmary. Incision biopsies were excluded. All of them have complete 5 years follow-up. Data collected included demographics, location and nature of lesion, histology, evidence of recurrence, time since last excision, and duration of follow-up.
At our centre, a two-stage procedure is used. The first stage is excision with a 3 mm margin from the perceived edge of lesion subjected to rapid turnaround paraffin section. The second stage involves reconstruction a few days later when the histology results are available. If the margins are clear, then reconstruction is performed. If involved, then further excision is undertaken with either a frozen section or paraffin examination until a clear margin is obtained, followed then by reconstruction.
Two patients who declined further excision are excluded from the study.
Histological technique
Routinely a rapid paraffin technique is used in which the sample is processed with a turnover of 24–48 h. The specimen is received in formalin within 1–2 h of surgery and is therefore not necessarily fixed. The whole of the excised tissue is blocked out in a bread slice manner with the end slices being levelled × 3 towards the resection margins. The blocks are then processed overnight (Leica ASP 300 processor, Nussloch, Germany) in the conventional manner and are ready for examination the following morning. This differs from the traditional method in that modern processors will take unfixed material so there is no need to wait for tissue to be fixed before cutting the blocks. Standard H&E slides are prepared and examined by conventional microscopy. If the end blocks are involved by tumour, they are reversed 180 degrees so that the resection margin can be seen en face.
In regards with frozen section, we adopt the en face or bread-loafing technique depending on the width of the re-excision of the involved margin: if narrow the whole tissue is sectioned enface, if wider than about 3 mm then the whole sample is cut in bread loaf manner. The frozen section findings are confirmed with subsequent routine paraffin preparation of the specimen. Since 2009 we have provision for Mohs', which is sparingly used given the long waiting list for it and restrict its use to post radiotherapy or recurrent morpheaform type.
Results
During the 7-year period 2000 to 2006 at the University Hospitals of Leicester (tertiary referral centre), there were 413 BCC excised from 270 patients; 140 female and 130 male. Mean age 73.7±12.5 (range 28–99) and the median age 77. Histologically, the majority were nodular BCC (277 or 67.1%), 20 (4.8%) were multifocal/superficial, 39 (9.4%) of the infiltrative/morpheaform type, and 2 (0.5%) described as micronodular. Focal squamous differentiation was seen in 1 patient (0.2%) and 74 (17.9%) were of mixed pattern histology. As expected, the majority (45.4%) were located on the lower eyelid, 18.6% on the medial canthus, 11.4% on the upper eyelid, 5.8% on the temple, 4% on the cheek, and 2.4% on the brow. All patients had 5 years follow-up. A total of 385 are primary cases whereas 28 were recurrent cases referred from elsewhere or before such techniques was adopted in our unit.
None of the patients included in the study had Gorlin syndrome nor were they immunosuppressed.
In order to achieve complete clearance of the BCC, some patients required several attempts at excision. In 36 out of 413 lesions (8.7%), more than one procedure was required before complete excision was confirmed histologically. Table 1 shows the percentage of each histological subtype requiring more than one excision to achieve clearance.
Table 1. Percentage of patients with each histological subtype requiring more than one excision to achieve clearance.
| Histological type of BCC | Percentage requiring extra excision |
|---|---|
| Nodular | 5.8 (16/277) |
| Mixed | 10.8 (8/74) |
| Infiltrative/morpheaform | 20.5 (8/39) |
| Multifocal | 15 (3/20) |
| Micronodular | 50 (1/2) |
As we do not calculate recurrent tumours sent from other units where primary excision methods are different, there is only one case of recurrence in our group leading to incidence of 0.26% (1/385). Table 2 showed characteristics of recurrent BCC (rBCC). None of the patients with primary nodular BCC suffered recurrence, but the recurrence rate for primary morpheaform/infiltrative BCC is 1/26 (3.8%).
Table 2. Characteristics of recurrent basal cell carcinoma.
| Patient | Type of BCC | Location | Number of excisions at same site previously |
|---|---|---|---|
| A | Infiltrating, morpheaform | Upper eyelid | 4 |
| B | Morpheaform | Medial canthus | 0 |
Eighteen patients had two lesions, three patients had three lesions, and one patient had four lesions and above; an incidence for multiple lesions in a simultaneous setting of 22/270 (8.1%). Simultaneous BCC can be of different histological types. BCC also occurred in multiple locations subsequently (metachronous lesions) in 21/270 (7.8%) of patients.
Three patients had extensive lesions involving orbit that required exenteration. All of these were recurrent lesions referred from other units and were of the aggressive morpheaform or infiltrative subtype. All three patients are still alive at the time of writing this paper.
One patient had 10 lesions from the age of 52 onwards attributed to excessive sunbed use.
Discussion
We present here the largest series of BCC excised with non-Mohs' rapid paraffin technique. Our series is unique in that clinical follow-up is used as opposed to questionnaire, which may be subject to bias.8 The reported recurrence rate in current literature is 1–3% in pBCC and 5–7% for rBCC.8, 9, 10 Our incidence of 0.26% with pBCC improves on this. Table 3 showed recurrence rates of previous studies that have 5 years follow-up results.
Table 3. Recurrence rate of previous studies.
| Year of study and author | Study sample (patients, lesions) | Excision method | 5 Years follow-up recurrence rate (primary BCC) | 5 Years recurrence rate (recurrent BCC) |
|---|---|---|---|---|
| Mohs11 | 1414 Lesions (1124 primary, 290 recurrent) | Mohs (fixed tissue and fresh tissue techniques) | 0.6% | 7.6% |
| 1992–2001 Wong et al12 | 97 Lesions (primary lesions) | En face frozen section control | 2.1% (2/97) | 4.4% (2/21 lesions) |
| Glatt et al46 | 81 Cases | Frozen section (FS) | 2.5% (5 year)a | |
| 1993–1996 Malhotra et al8 | 346 Patients | Mohs' surgery | 0% (cases are not consecutive, 5 years follow-up based on questionnaire, there are significant proportion of loss to follow-up, which involved older and infiltrative lesions) | 7.8% (7/90) |
| 1994–1997 Hamada et al16 | 55 Patients | 2 mm excision, further 2 mm re-excision if necessary | 0% (primary nodular) | |
| 2000–2006 Our results | 428 Lesions, 270 patients | 3 mm excision and check for histology clearance, further excision with paraffin/FFS control | 0.26% (overall), 0% (primary nodular), 3.8% (primary morpheaform) | 3.8% |
5 years recurrence rate without differentiating primary BCC and recurrent BCC.
Although recently Mohs' micrographic surgery has been hailed as the best method of removing BCC with minimal recurrence,8, 11, 12, 13 it may be too costly14 and time consuming to be used for all periocular BCC.15 In addition, it may not be available in every hospital. It is reassuring that our technique of excising BCC and examining with rapid turnover paraffin techniques, occasionally combined with fresh-frozen section control, achieves results comparable to another recent study.16
Close communication between the surgeon and the pathologist enables accurate orientation of the specimen in relation to a map of the lesion12, 17 facilitating clearance. In general, we require a clear histological margin of >0.1 mm for nodular BCC and >1 mm for morpheaform/infiltrative subtypes. Cases with a narrow histological clearance margin are discussed at the skin cancer multi-disciplinary meeting.
In all, 8.7% (36/413) of the tumours required more than one excision to achieve complete excision, which is not surprisingly lower than with the 2 mm margin used by Hamada et al16 (22.7%). Periocular tumours frequently extend well beyond the obvious clinical margin,18, 19, 20 and the subtle extension of infiltrative and morpheaform BCC is probably one of the main reasons for their recurrence.21 An average of 7.2 mm of subclinical tumour extension was found in 51 morpheaform BCC in one study as compared with 2.1 mm of extension in 138 well- circumscribed periocular lesion.22 In our study, 20.5% of the morpheaform BCC required more than one surgical excision to achieve complete clearance.
Guardiano et al23 attempted to use dermoscopy to improve yield and did not find the evidences supportive. Fluorescein confocal mosaicing microscopy24 and multispectral polarized light imaging25 show promising results but are still largely experimental.
We choose a two-stage approach to ensure all lesions are completely excised, obviating the uncertainty of possible incomplete excision when customary 3 mm or 4 mm margins are used and simultaneous closure of wound is undertaken.26 We opt for ensuring complete excision for all our BCC as reports show recurrence rates varying between 30 and 50% for incompletely excised BCC depending on the length of follow-up.27 Recurrence can be difficult to diagnose in the periocular area as the recurrent tumour may arise in the deeper margins and can spread subdermally or into the orbit. The treatment of a recurrent tumour can induce greater morbidity and cost than immediate re-excision of incompletely excised tumours.28 Therefore, most guidelines on the management of BCC recommend re-excision of incompletely excised tumours whereas acknowledging that this is not always achieved in practice.27, 29, 30 Most of our patients are happy to attend the surgery twice knowing that their tumour will be completely excised. The effort to ensure complete initial excision is ‘rewarded' in the low recurrence rates in this study. In addition, the two-stage procedure facilitates planning of the appropriate theatre time for the reconstruction.
For those lesions in which complete clearance is not achieved with the first excision, fresh-frozen section may be used for margin control if it is just one margin and not of morpheaform, as it has been used widely with proven efficacy.20, 31 It is rapid (<2 h) and allows the defect to be reconstructed in the same theatre session once the margin is confirmed clear. With frozen section, the pathologist dissects a 0.5 to 1.0 mm margin of tissue for cryosectioning.12 All fresh-frozen section are further analysed in formalin subsequently as the literature suggests frozen section may be more prone to inaccurate interpretation than formal paraffin section. Difference between the findings on fresh-frozen section and formal histology section was noted in two of our patients. One was because the section, which contained BCC had been trimmed through leading to a negative paraffin results. In another case, the lesion was multifocal and the fresh-frozen section was negative whereas the paraffin section was positive. A further excision was undertaken in this case.
Three cases (3/413 or 7.3%) had extensive recurrent disease involving orbit at presentation to our unit and required exenteration. All were aggressive infiltrative, morpheaform disease. Two patients presented with ocular dysmotility and one with a mass fixed to bone. None of these patients have died of disease or show intracranial spread. Our series is consistent with the reported literature incidence of 0.8 to 3.6%.32, 33, 34, 35, 36, 37
In this study, recurrence for pBCC is very uncommon once the excision is histologically complete.16, 38 Our single case of recurrence of pBCC in 6 years of study was in a case of morpheaform BCC in the medial canthus, which required more than one excision. We therefore propose that those with pBCC with non-aggressive (nodular) histology could be discharged after one 6-monthly follow-up, once complete excision is confirmed, but advised to monitor for new lesions elsewhere. However, patients with infiltrative, morpheaform, micronodular, mixed, or rBCC should be followed-up for at least 5 years. Recurrent BCC confirmed by biopsy often has a mixture of scar tissue and basaloid cells, making pathologic identification of margins difficult.32 In addition, recurrent eyelid BCC often exhibit more aggressive biological behaviour associated with a less favourable prognosis and a worse outcome.39, 40
Although we agree as with others that follow-up is of value for some patients, it is not possible in the modern NHS with limited capacity and resources. Equally, a large proportion of patients do not wish to be followed-up41 and in some clinics there is a high rate of non-attendance.42 Prolonged follow-up of all patients with periocular BCC12, 43, 44 is both costly and inconvenient. Our views concur with the current evidence-based recommendations from the BAD (British Association of Dermatologists) guidelines advising that long-term hospital-based follow-up after treatment of BCC for patients other than those with Gorlin's syndrome is neither necessary nor recommended.45
The retrospective nature means that tumour size, which was not constantly documented has not been assessed as a risk factor for recurrence.
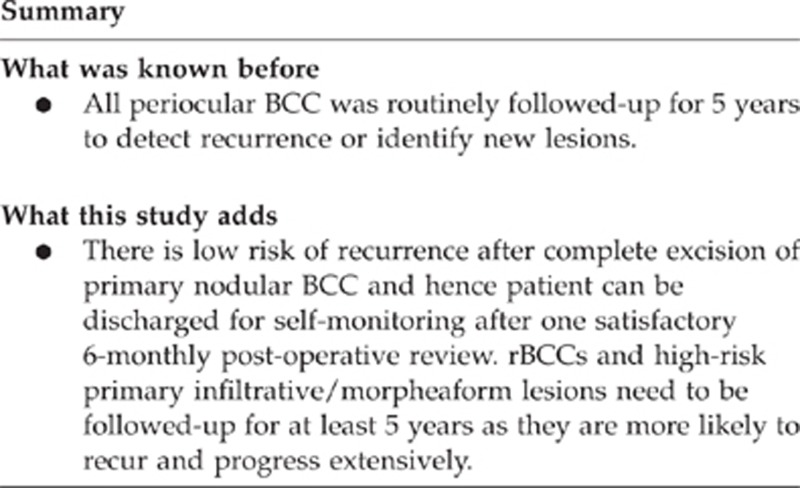
In conclusion, we found no episodes of recurrence after proven complete excision of primary nodular BCC during 5 years follow-up period. rBCCs and high-risk primary infiltrative/morpheaform lesions need to be followed-up for at least 5 years as they are more likely to recur and progress extensively. We therefore recommend that patients with primary nodular BCC be discharged after one 6-monthly follow-up once complete excision is confirmed and wound healing satisfactory. However, all patients should be advised to monitor for new lesions elsewhere and seek medical attention if any suspicious lumps are identified.
The authors declare no conflict of interest.
Footnotes
The paper has been presented as rapid fire session in British Oculoplastic Society meeting in June 2011 and at the European Oculoplastic Surgery Society (ESOPRS) meeting in September 2011.
References
- Bath-Hextall F, Leonardi-Bee J, Smith C, Meal A, Hubbard R. Trends in incidence of skin basal cell carcinoma. Additional evidence froma UK primary care database study. Int J Cancer. 2007;121:2105–2108. doi: 10.1002/ijc.22952. [DOI] [PubMed] [Google Scholar]
- Epstein E. Value of follow-up after treatment of basal cell carcinoma. Arch Dermatol. 1973;108:798–800. [PubMed] [Google Scholar]
- Robinson JK. Risk of developing another basal cell carcinoma. A 5-year prospective study. Cancer. 1987;60:118–120. doi: 10.1002/1097-0142(19870701)60:1<118::aid-cncr2820600122>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Czarnecki D, Staples N, Nar A, Giles G, Meehan C. Recurrent nonmelanoma skin cancer in Southern Australia. Int J Dermatol. 1996;35:410–412. doi: 10.1111/j.1365-4362.1996.tb03022.x. [DOI] [PubMed] [Google Scholar]
- Silverman MK, Kopf AW, Grin CM, Bart RS, Levenstein MJ. Recurrence rates of treated basal cell carcinomas. Part I: overview. J Dermatol Surg Oncol. 1991;17:713–718. doi: 10.1111/j.1524-4725.1991.tb03424.x. [DOI] [PubMed] [Google Scholar]
- Rowe DE, Carroll RJ, Day CL. Long-term recurrence rates in previously untreated (primary) basal cell carcinomas: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315–328. doi: 10.1111/j.1524-4725.1989.tb03166.x. [DOI] [PubMed] [Google Scholar]
- Bower CPR, Lear JT, de Berker DA. Basal cell carcinoma follow- up practices by dermatologists: a national survey. Br J Dermatol. 2001;145:949–956. doi: 10.1046/j.1365-2133.2001.04488.x. [DOI] [PubMed] [Google Scholar]
- Malhotra R, Huilgol SC, Huynh NT, Selva D. The Australian Mohs database, part II. Periocular basal cell carcinoma outcome at 5-year follow-up. Ophthalmology. 2004;111:631–636. doi: 10.1016/j.ophtha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer. A critical review of the literature and meta-analysis. Arch Dermatol. 2000;136:1524–1530. doi: 10.1001/archderm.136.12.1524. [DOI] [PubMed] [Google Scholar]
- Rowe DE, Carroll RJ, Day CL. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424–431. doi: 10.1111/j.1524-4725.1989.tb03249.x. [DOI] [PubMed] [Google Scholar]
- Mohs FE. Micrographic surgery for the microscopically controlled excision of eyelid cancers. Arch Ophthalmol. 1986;104:901–909. doi: 10.1001/archopht.1986.01050180135046. [DOI] [PubMed] [Google Scholar]
- Wong V, Marshall JA, Whitehead KJ, Williamson RM, Sullivan TJ. Management of periocular basall cell carcinoma with modified en face frozen section controlled excision. Ophthal Plast Reconstr Surg. 2002;18 (6:430–435. doi: 10.1097/00002341-200211000-00008. [DOI] [PubMed] [Google Scholar]
- Malhotra R, Huilgol S, Huynh N, Selva D. The Australian Mohs database part I. Periocular basal cell carcinoma experience over 7 years. Ophthalmology. 2004;111:624–630. doi: 10.1016/j.ophtha.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Essers BA, Dirksen CD, Nieman FH, Smeets NW, Krekels GA, Prins MH, et al. Cost-effectiveness of Mohs micrographic surgery vs surgical excision for basal cell carcinoma of the face. Arch Dermatol. 2006;142:187–194. doi: 10.1001/archderm.142.2.187. [DOI] [PubMed] [Google Scholar]
- Hsuan JD, Harrad RA, Potts MJ, Collins C. Small margin excision of periocular basal cell carcinoma: 5 year results. Br J Ophthalmol. 2004;888:358–360. doi: 10.1136/bjo.2003.018531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S, Kersey T, Thaller VT. Eyelid basal cell carcinoma: non-Mohs excision, repair, and outcome. Br J Ophthalmol. 2005;89:992–994. doi: 10.1136/bjo.2004.058834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway RM, Themel S, Holbach LM. Surgery for primary basal cell carcinoma including the eyelid margins with intraoperative frozen section control: comparative interventional study with a minimum clinical follow up of 5 years. Br J Ophthalmol. 2004;88:236–238. doi: 10.1136/bjo.2003.025056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceilley RI, Anderson RL. Microscopically controlled excision of malignant neoplasms on and around eyelids followed by immediate surgical reconstruction. J Dermatol Surg Oncol. 1978;4:44. doi: 10.1111/j.1524-4725.1978.tb00380.x. [DOI] [PubMed] [Google Scholar]
- Nerad JA, Whitaker DC. Periocular basal cell carcinoma in adults 35 years of age and younger. Am J Ophthalmol. 1988;106:723. doi: 10.1016/0002-9394(88)90708-8. [DOI] [PubMed] [Google Scholar]
- Chalfin J, Putterman AM. Frozen section control in the surgery of basal cell carcinoma of the eyelid. Am J Ophthalmol. 1979;87:802. doi: 10.1016/0002-9394(79)90358-1. [DOI] [PubMed] [Google Scholar]
- Miller SJ. Biology of basal cell carcinoma. J Am Academy of Dermatology. 1991;24:1–13. doi: 10.1016/0190-9622(91)70001-i. [DOI] [PubMed] [Google Scholar]
- Salasche SA, AMonette RA. Morpheaform basal-cell epitheliomas. A study of subclinical extensions in a series of 51 cases. J Dermatol Surg Oncol. 1981;7:387–394. doi: 10.1111/j.1524-4725.1981.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Guardiano RA, Gramde DJ. A direct comparison of visual inspection, curettage, and epiluminescence microscopy in determing tumor extent before the initial margins are determined for Mohs' micrographic surgery. Dermatol Surg. 2010;36:1240–1244. doi: 10.1111/j.1524-4725.2010.01616.x. [DOI] [PubMed] [Google Scholar]
- Karen JK, Gareau DS, Dusza SW, Tudisco M, Rajadhyaksha M, Nehal KS. Detection of basal cell carcinomas in Mohs excisions with fluorescence confocal mosaicing microscopy. Br J Dermatol. 2009;160 (6:1242–1250. doi: 10.1111/j.1365-2133.2009.09141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky AN, Neel V, Anderson RR. Demarcation of nonmelanoma skin cancer margins in thick excisions using multispectral polarized light imaging. J Invest Dermatol. 2003;121:259–266. doi: 10.1046/j.1523-1747.2003.12372.x. [DOI] [PubMed] [Google Scholar]
- Doxanas MT, Green WR, Iliff CE. Factors in the successful surgical management of basal cell carcinoma of the eyelid. Am J Ophthalmol. 1981;91 (6:720–736. doi: 10.1016/0002-9394(81)90005-2. [DOI] [PubMed] [Google Scholar]
- Telfer NR, Colver GB, Morton A. British Association of Dermatologists. Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35–48. doi: 10.1111/j.1365-2133.2008.08666.x. [DOI] [PubMed] [Google Scholar]
- Cannon PS, Huilgol SC, Selva D, Malhotra R. Basal cell carcinoma. Ophthalmology. 2009;116 (11:2266–2267. doi: 10.1016/j.ophtha.2009.06.034. [DOI] [PubMed] [Google Scholar]
- National Health & Medical Research Council Non-melanoma skin cancer: guidelines for the treatment and management in Australia Commonwealth of Australia 2003. Available at http://www.nhmrc.gov.au .
- Drake LA, Ceilley RI, Cornelison RL, Dobes WL, Dorner W, Goltz RW, et al. Guidelines of care for basal cell carcinoma. The American Academy of Dermatology Committee on Guidelines of Care. J Am Acad Dermatol. 1992;26:117–120. doi: 10.1016/s0190-9622(08)80524-5. [DOI] [PubMed] [Google Scholar]
- Frank HT. Frozen section control of excision of eyelid basal cell carcinomas: 8 ½ years' experience. Br J Ophthalmol. 1989;73:328–332. doi: 10.1136/bjo.73.5.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard GR, Nerad JA, Carter KD, Whitaker DC. Clinical characteristics associated with orbital invasion of cutaneous basal cell and squamous cell tumors of the eyelid. American Journal of Ophthalmology. 1992;113:123–133. doi: 10.1016/s0002-9394(14)71523-5. [DOI] [PubMed] [Google Scholar]
- Payne JW, Duke JR, Butner R, Eifrig DE. Basal cell carcinoma of the eyelids. A long-term follow-up study. Arch Ophthalmol. 1969;81:553. doi: 10.1001/archopht.1969.00990010555016. [DOI] [PubMed] [Google Scholar]
- Perlman GS, Hornblass A. Basal cell carcinoma of the eyelids. A review of patients treated by surgical excision. Ophthalmic Surg. 1976;7:23. [PubMed] [Google Scholar]
- Henderson J, Farrow GM.The tumor surveyIn: Henderson JW (ed).Orbital Tumors2nd ednThieme Stratton: New York; 198067–74. [Google Scholar]
- Reese AB. Expanding lesions of the orbit. Trans Ophthalmol Soc UK. 1971;91:85. [PubMed] [Google Scholar]
- Shields JA, Bakewell B, Augsburger JJ, Flanagan JC. Classification and incidence of space-ocupying lesions of theorbit. A survey of 465 biopsies. Arch Ophthalmol. 1984;102:1606. doi: 10.1001/archopht.1984.01040031296011. [DOI] [PubMed] [Google Scholar]
- Stefanous S. Five-year cycle of basal cell carcinoma management re-audit. Orbit. 2009;28 (4:258–263. [PubMed] [Google Scholar]
- Cook BE, Bartley GB. Treatment options and future prospects for the management of eyelid malignancies: an evidence-based update. Ophthalmology. 2001;109:2088–2098. doi: 10.1016/s0161-6420(01)00796-5. [DOI] [PubMed] [Google Scholar]
- Margo CE, Waltz K. Basal cell carcinoma of the eyelid and periocular skin. Surv Ophthalmol. 1993;38:169–192. doi: 10.1016/0039-6257(93)90100-l. [DOI] [PubMed] [Google Scholar]
- Charmann CR, NacFarlane CS, Perkins W. Follow-up of patients following basal cell carcinoma excision: what do patients want. Br J Dermatol. 2003;149 (Suppl. 64:104–105. [Google Scholar]
- Mc Loone NM, Tolland J, Walsh M, Dolan OM. Follow-up of basal cell carcinomas: an audit of current practice. JEADV. 2006;20:698–701. doi: 10.1111/j.1468-3083.2006.01576.x. [DOI] [PubMed] [Google Scholar]
- Levin F, Khalil M, McCormick SA, Della Rocca D, Maher E, Della Rocca RC. Excision of periocular basal cell carcinoma with stereoscopic microdissection of surgical margins for frozen- section control. Arch Ophthalmol. 2009;127:1011–1015. doi: 10.1001/archophthalmol.2009.222. [DOI] [PubMed] [Google Scholar]
- Steinkogler FJ, Scholda CD. The necessity of long -term follow up after surgeryfor basal cell carcinomas of the eyelid. Ophthalmic Surg. 1993;24:755–758. [PubMed] [Google Scholar]
- Park AJ, Strick N, Watson JD. Basal cell carcinomas: do they need to be followed up. J R Coll Surg Edin. 1994;39:109–111. [PubMed] [Google Scholar]
- Glatt HJ, Olson JJ, Putterman AM. Conventional frozen sections in periocular basal- cell carcinoma: a review of 236 cases. Ophthalmic Surg. 1992;23 (1:6–8. [PubMed] [Google Scholar]


