Abstract
Purpose
Vitreous biopsy for the cytological assessment of suspected intraocular lymphoma and vitritis of uncertain aetiology is a standard investigation. The types of specimens generated and the diagnostic rate are variable within and between centres. There are many reasons for this but one observation that has not been considered previously is the differential distribution of cells in the vitreous gel. To test this possibility, five consecutive patients with suspected vitreous involvement by lymphoma or vitritis of uncertain aetiology underwent a core vitreous biopsy immediately before a planned full pars plana vitrectomy (PPV) and the cellularity of the two sampling techniques compared.
Methods
A prospective study of five consecutive patients requiring vitreous sampling to secure a firm diagnosis. For each of five patients, the core vitreous biopsy specimen was received in a universal tube and the PPV specimen was received in a vitreous cassette. Fluid (0.25 ml) was removed from both specimens, centrifuged and haematoxylin and eosin (H&E) stained slides prepared per sampling method. The slides were examined with a light microscope, the most cellular field selected and the number of cells per mm2 counted and compared between sampling techniques.
Results
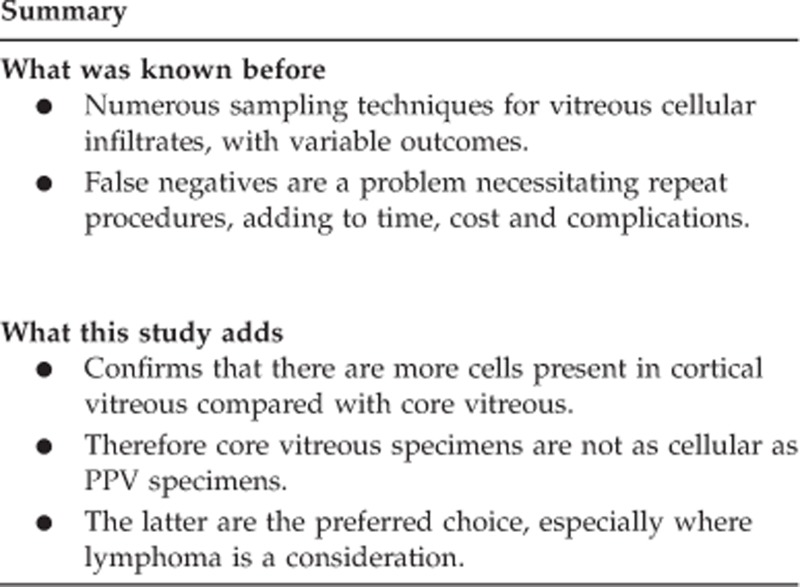
PPV specimen's, revealed a cellularity range that was 7.4 to 78 × (average 31 ×) greater than a core vitreous biopsy. In the two cases of a final diagnosis of intraocular lymphoma, the vitreous core biopsy was non-diagnostic. Furthermore, the PPV specimen generated additional cellular material for numerous ancillary investigations to permit a secure diagnosis.
Conclusions
The results of this differential vitreous sampling study has strengthened our anecdotal slit lamp clinical observations that inflammatory cells and lymphoma cells are concentrated more in the cortical vitreous. Therefore, vitreous cells have less chance to be sampled if a single core vitreous biopsy is performed. Indeed, the two cases of confirmed lymphoma generated a non-diagnostic core vitreous biopsy. In our centre, this study has lead to PPV being performed as a gold standard on all patients with suspected intraocular lymphoma or vitritis of uncertain aetiology.
Keywords: vitreous, lymphoma, sampling
Introduction
Vitreous biopsy for diagnostic cytological analysis has been used since the mid-1970s in a clinical setting of vitreous inflammation and suspected intraocular lymphoma.1, 2 There are essentially two vitreous sampling methods in vogue. One is a vitreous biopsy that targets the core vitreous (1–2 ml obtained) and the other is a formal diagnostic pars plana vitrectomy (PPV) that samples the core and the cortical vitreous, resulting in a larger volume of fluid (50–100 ml), collected in a vitrectomy cassette/bag comprising the dispersed vitreous gel and balanced salt solution (BSS).3
Although investigators have focused on stopping steroid treatment temporarily to enhance cell viability, prompt processing of the sample, use of tissue culture medium and the use of cytokine assay and PCR for monoclonal testing to improve the diagnostic yield in cases of suspected intraocular lymphoma,3, 4 one simple observation has been overlooked in our experience. In our institution, careful clinical observations have noted a differential distribution of vitreous cells in patients presenting with vitreous cellular infiltrates. There appear to be more cells in the cortical vitreous rather than the core vitreous in cases of vitreous inflammation and vitreous lymphoma. Some evidence highlights false negative rates with core vitreous biopsy, with patients exposed to repeated procedures and risk of complications.3, 4, 5 We wanted to test whether the cellularity of vitreous biopsy obtained from a core vitreous biopsy would differ to that obtained from the cortical vitreous (sampled with a PPV). There are important reasons why this knowledge would be useful: to influence sampling technique; to reduce repeat procedures because of false negatives hence preventing repeat patient hospital visits with cost saving; to allow sufficient material to be obtained for ancillary investigations to secure a firm diagnosis and to reduce treatment delay in cases of lymphoma.
Materials and methods
This was a prospective study of five consecutive patients who required a vitreous biopsy to establish the vitreous pathology. All patients were consented routinely for a PPV and core vitreous biopsy. This paper has no patient images and the data in the tables have undergone anonimisation. All investigations were done according to the Declaration of Helsinki. No specific ethics approval was required for this study because vitreous core biopsies and PPV are well established, standard sampling techniques for the investigation of vitreous cellular techniques. The clinical findings and indications for the vitreous biopsies are summarised in Table 1.
Table 1. Clinical summary of patients presentations and investigative work.
| Patient number | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age at presentation | 71 years | 71 years | 70 years | 18 years | 76 years |
| Ophthalmic presentation | Right granulomatous uveitis, progressing to bilateral vitritis | Left vitritis/vitreous infiltrate | Right granulomatous vitritis | Left hypopyon uveitis, haemorrhagic focal chorioretinitis at macula | Bilateral vitritis/vitreous infiltrate |
| Systemic presentation | Nil | Nil | Nil | Nil | Nil |
| Relevant past ocular and medical history | Pernicious anaemia | Type 2 diabetes mellitus | Hodgkin's lymphoma—in remission for 2 years | Nil | Testicular B-cell lymphoma |
| Investigations before diagnostic vitrectomy | Bloods and CXR—nil significant | Bloods and CXR—nil significant | Bloods & CXR—nil significant | Bloods, blood cultures, sputum cultures, CXR, abdominal US,-all nil significant | None |
| Treatment(s) before vitrectomy | Topical steroids & ocular antihypertensives Bilateral orbital floor steroid injections | None | None | None | None |
| Clinical diagnosis/suspicion | Idiopathic chronic vitritis | Intraocular lymphoma | Intraocular lymphoma | Endogenous fungal endophthalmitis | Intraocular lymphoma |
| Indication for vitrectomy | Exclude lymphoma | Exclude lymphoma | Exclude lymphoma | Investigate possible infective aetiologies | Exclude lymphoma |
| Interval from presentation to vitrectomy | 8 months | 2 months | 1 month | 2 days (initially refused all treatment) | 1 month |
| Intra-operative complications | None | None | Retinal tear | Giant retinal tear | None |
| Postoperative complications | None | None | None | None | None |
| Investigations on vitrectomy specimen | Histopathology, PCR TEM | Histopathology microbiology PCR | Histopathology PCR | Histopathology, microbiology, PCR TEM | Histopathology PCR |
Abbreviations: CXR, chest X-ray; PCR, polymerase chain reaction; TEM, transmission electron microscopy; US, ultrasound.
Surgical technique for obtaining the specimens
All patients underwent the same procedure, using 20 gauge PPV instrumentation. After conjunctival peritomy an infusion port, connected to BSS,was placed in the inferotemporal quadrant. The three-way tap on the infusion line was kept closed to prevent dilution of the core vitreous biopsy. Two further sclerostomies were placed in the superonasal and superotemporal quadrants, taking care to minimise vitreous fluid egress and one of these was temporarily closed with a 20-gauge scleral plug. The aspiration line of the unprimed vitrectomy cutter was connected to a 3-ml luer-lock syringe and the cutter passed through the open sclerostomy into the mid-vitreous cavity. Under direct visualisation through the pupil the vitrectomy cutter was activated, and the assistant applied gentle suction using the syringe to aspirate neat core vitreous. The surgeon maintained intraocular pressure by pressing on the eye with a squint hook to counter the loss of intraocular volume. The largest volume of vitreous that could be safely removed without risking injury to other intraocular structures was aspirated, typically between 1 and 2 ml. The infusion flow was then turned on to re-inflate the eye while gradually reducing the pressure from the squint hook. The vitrectomy cutter was then removed from the eye, the sclerostomy closed with a scleral plug and the infusion tap closed once more. Any vitreous remaining in the cutter aspiration line was aspirated into the syringe, and the contents of the syringe transferred into 5 ml of an alcohol-based fixative for transport to the laboratory. This vitreous biopsy therefore consisted of core vitreous.
Full bimanual PPV was then completed, including induction of posterior vitreous detachment by aspiration if necessary, and trimming of the posterior hyaloid membrane close to the vitreous base. This ensures as complete removal of cortical vitreous as possible. Before suturing of the sclerostomies a full inspection of the retina, with scleral indentation, was performed. Any retinal tears were treated with cryopexy and gas tamponade if appropriate. At the end of the procedure an equal volume of alcohol-based fixative was added to the vitrectomy cassette, containing typically 50 to 100 ml of vitreous washings (ie vitreous and BSS), and the cassette sealed for transport to the laboratory. The PPV specimen therefore comprised diluted core and cortical vitreous. In some cases an aliquot of unfixed vitreous was sent for microbiology cultures and viral PCR, before vitreous fixation.
Histopathological handling of specimens
All specimens had been fixed immediately in theatre ensuring maximum cellular viability. The fixative used in our ophthalmic pathology laboratory is ‘Shandon' cytofix6 (which is a combination of ethanol, methyl alcohol and isopropyl alcohol). Our experience has shown that use of this alcohol-based fixative actually liquefies viscous vitreous, enhancing cell retrieval when cell blocking (unpublished).
The core vitreous biopsy and the PPV sample had 0.25 ml removed from each specimen and this was used to prepare a cytospin. Preparation of the cytospins served two purposes: to allow the ophthalmic pathologist to quickly assess the type of disease process being dealt with and to permit a cell count comparison. Cytospins were prepared by centrifuging the 0.25 ml samples at 1500 rpm for 5 min, followed by conventional haematoxylin and eosin (H&E) staining, cover-slipped and viewed with a light microscope. For cell counts, the most cellular area was selected on the cytospin preparation and the number of cells per mm2 counted, with the aid of an eyepiece graticule.
After the cell counts, the rest of the specimens were placed into universal tubes, centrifuged at 4000 rpm for 30 min and a cell block using the Shandon cytoblock system prepared,6 followed by standard processing to paraffin wax blocks, serial sections cut, stained with H&E and viewed with a light microscope.
Results
Cellularity and diagnostic outcome comparison between core vitreous biopsy and PPV
PPV specimens revealed a cellularity range that was 7.4 to 78 × (average 31 × ) greater than the paired core vitreous biopsy (per 0.25 ml per mm2). The cell blocks prepared from the core vitreous biopsy were non-diagnostic in three cases. Importantly, two of these non-diagnostic cases showed unequivocal lymphoma on the cell blocks prepared from the corresponding PPV specimen. Only in one case did the core vitreous biopsy reveal a diagnosis that was identical to the diagnosis secured on the PPV specimen (granulomatous vitritis).
Additional investigation comparison between core vitreous biopsy and PPV
In all cases, the greater cellularity of the PPV specimen permitted additional investigations to secure a firm diagnosis. These investigations included immunohistochemistry, DNA extraction for IgH monoclonality PCR assessment (in those cases of lymphoma), stains for infectious agents, DNA extraction for PCR for infectious agents (mycobacteria) and transmission electron microscopy in all those cases of granulomatous vitritis, performed on the PPV cell block material. These investigations were not possible on the core vitreous biopsy because of a lack of diagnostic material. The results of all these additional investigations are summarised in Table 2.
Table 2. Results of the cellularity of PPV specimens vs those from a core vitreous tap and the diagnostic outcomes.
| Patient number | Core vitreous biopsy cytopsin cell count/mm2 | Pars plana vitrectomy cytospin cell count/mm2 | Core vitreous biopsy cell block histological diagnosis | Pars plana vitrectomy cell block histological diagnosis (PPV cell block) | Ancillary investigations results | Final clinico-pathological diagnosis |
|---|---|---|---|---|---|---|
| 1 | 12 | 89 | Granulomatous inflammation | Granulomatous vitritis—no infectious agent detected | Mycobacterial PCR-negative (on PPV cell block) Nothing significant found on transmission electron microscopy (on PPV cell block) | Granulomatous Vitritis? sarcoid |
| 2 | 1 | 18 | Non-diagnostic-insufficient cells | Primary CNS-type intraocular diffuse large B-cell lymphoma | Microbiology negative (unfixed vitreous sample) Monoclonal B-cell population on PCR (on PPV cell block) | Primary CNS-type intraocular diffuse large B-cell lymphoma |
| 3 | 15 | 280 | Chronic inflammation | Paraneoplastic granulomatous vitritis and retinitis | Mycobacteria PCR negative (on PPV cell block) | Paraneoplastic granulomatous vitritis and retinitis, secondary to recurrent classical Hodgkin's lymphoma (see Mudhar et al.13) |
| 4 | 3 | 235 | Non-diagnostic-insufficient cells | Granulomatous vitritis—no infectious agent detected | Microbiology culture and viral PCR negative (unfixed vitreous) Mycobacterial PCR negative (on PPV cell block) Nothing significant found on transmission electron microscopy (on PPV cell block) | Granulomatous vitritis-no infectious agent detected |
| 5 | 1 | 33 | Non-diagnostic-insufficient cells | Metastatic diffuse large B-cell lymphoma of testis | Monoclonal B-cell population on PCR (on PPV cell block) | Metastatic diffuse large B-cell lymphoma of testis |
Abbreviations: PCR, polymerase chain reaction; PPV, pars plana vitrectomy; TEM, transmission electron microscopy.
Repeat biopsy rate
None of the patients in this series have had repeat biopsies as the original PPV.
Complication rate
Two of the five patients developed an intra-operative retinal tear requiring treatment with cryopexy and intraocular tamponade. No other intra- or postoperative complications were encountered.
Discussion
This small study has highlighted a few important points. First, it appears to support our anecdotal clinical observations that cells appear to concentrate more in the cortical vitreous compared with the core vitreous in our five consecutive patients presenting with vitreous cellular infiltrates. This may be attributable to two factors. It is well established that the core vitreous tends to be more liquid compared with the gel-like cortical/pre-retinal vitreous with its higher concentration of Type 2 collagen fibres.7 Cells gaining access to the vitreous will first encounter the gel-like cortical vitreous before the core vitreous and therefore, could become entrapped within it, retarding their migration to the core vitreous. A second reason why more cells may be seen in the cortical vitreous is their proximity to an aerobic source, namely the retina. The cells in the core are furthest away from the retina and therefore liable to become relatively ischaemic. It is well known that lymphoma cells removed from the vitreous are liable to rapid degeneration unless fixed or exposed to tissue culture medium in a timely manner.3, 4, 8
In this study, the core vitreous biopsy and PPV were performed and fixed by the same surgeon and the specimens handled by the same biomedical scientist in the histopathology lab according to standard operating protocols. No tissue was lost during the transport and handling of the fluids.
The vitreo-retinal literature contains papers indicating ‘false negative core vitreous biopies' in cases of suspected intraocular lymphoma.3, 4, 8 In such cases, it is first important to mention that a false negative result for lymphoma can be due to the lymphoma being confined to the subretinal space, before it gains access to the vitreous. Once lymphoma gains access to the vitreous gel, this study supports the interpretation that a core vitreous biopsy has a higher chance of a false negative result due to a combination of vitreous physico-chemical and aerobic influences, as speculated above.
In cases that required PCR testing for B-cell monoclonality,9 unequivocal monoclonal bands were obtained from DNA extracted from the PPV paraffin processed blocks. Some authorities recommend keeping an aliquot of unprocessed vitreous aside for lymphoid population monoclonal testing.8 However, the methodology used in this study illustrates that paraffin processed cell block DNA is of a sufficient quality to allow an unequivocal confirmation of a diagnosis of lymphoma and supports other work that has also shown this.10
Our findings have implications for the manner in which a patient with a suspicion of intraocular lymphoma is biopsied. In our institution, core vitreous biopsy was the investigation of choice during the period 2000 to 2004. Three positive diagnoses of lymphoma were made during this time span, in cases that were clinically thought to be highly suspect of lymphoma. This compares with eight cases of lymphoma diagnosed from 2005 to 2009 that underwent PPV. However, this needs to be balanced against a higher vitrectomy rate from 2005 to 2009.
Core vitreous biopsy is quick and easy to perform, but the volume of the sample obtained is limited by the potential risk of inadvertent trauma to intraocular structures. Data on complications of vitreous biopsy are sparse, but one series of 53 cases reported one occurrence of retinal detachment.11 PPV requires more specialised equipment and training, and takes longer to perform. The potential complications of PPV are well understood and include cataract, raised intraocular pressure and retinal tears or detachment. One recent large retrospective series of vitrectomy for all indications reported intra-operative retinal tears in 15.2% of eyes, and postoperative retinal detachment in 1.7%.12 These authors reported that surgical induction of PVD was a significant risk factor for intra-operative retinal tear formation. Two of our five patients developed an intra-operative retinal tear as a result of this surgical step; however, induction of PVD is essential to enable complete removal of cortical vitreous and to maximise the cellular yield.
It is likely that the rate of intra-operative complications is higher for full PPV than core vitreous biopsy, particularly in relation to retinal tear formation. With appropriate intra-operative detection and management, however, the consequences of iatrogenic retinal tears are minimised and the risk of postoperative retinal detachment is very low for both sampling techniques. We believe that the higher diagnostic yield from PPV justifies the additional surgical complexity and risk of intra-operative complications and in our institution we have adopted PPV as the gold standard for cases where there is a strong clinical suspicion of intraocular lymphoma or vitritis of uncertain aetiology.
The authors declare no conflict of interest.
References
- Akpek EK, Ahmed I, Hochberg FH, Soheilian M, Dryja TP, Jakobiec FA, et al. Intraocular-central nervous system lymphoma: clinical features, diagnosis, and outcomes. Ophthalmology. 1999;106 (9:1805–1810. doi: 10.1016/S0161-6420(99)90341-X. [DOI] [PubMed] [Google Scholar]
- Michels RG, Knox DL, Erozan YS, Green WR. Intraocular reticulum cell sarcoma: diagnosis by pars plana vitrectomy. Arch Ophthalmol. 1975;93 (12:1331–1335. doi: 10.1001/archopht.1975.01010020961005. [DOI] [PubMed] [Google Scholar]
- Gonzales JA, Chan C-C. Biopsy techniques and yields in diagnosing primary intraocular lymphoma. Int Ophthalmol. 2007;27:241–250. doi: 10.1007/s10792-007-9065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcup SM, Chan C-C, Buggage RR, Nuessenblatt MD, Byrnes GA, Rubin BI. Improving the diagnostic yield of vitrectomy for intraocular lymphomas. Arch Ophthalmol. 2000;118 (3:446. [PubMed] [Google Scholar]
- Blumenkranz MS, Ward T, Murphy S, Mieler W, Williams GA, Long J. Applications and limitations of vitreoretinal biopsy techniques in intraocular large cell lymphoma. Retina. 1992;12 (suppl 3:S64–S70. doi: 10.1097/00006982-199212031-00014. [DOI] [PubMed] [Google Scholar]
- Shandon Cytoblock Cell Block Preparation System booklet. Anatomical Pathology International, Clinical Diagnostics, Astmoor, Runcorn, Cheshire UK, , www.thermo.com/shandon .
- Forrester JV, Dick AD, McMenamin PG, Lee WR.The Eye Basic Sciences in Practice Saunders: London; 2002. Chapters 1, 2, 3, 9. [Google Scholar]
- Coupland SE. The pathologist's perspective on vitreous opacities. Eye. 2008;22 (10:1318–1329. doi: 10.1038/eye.2008.31. [DOI] [PubMed] [Google Scholar]
- White VA, Gascoyne RD, Paton KE. Use of polymerase chain reaction to detect B- and T- cell gene rearrangements in vitreous specimens from patients with intraocular lymphoma. Arch Ophthalmol. 1999;117 (6:761–765. doi: 10.1001/archopht.117.6.761. [DOI] [PubMed] [Google Scholar]
- Raparia K, Chung C-C, Chevez-Barrios P. Intraocular lymphoma-diagnostic approach and immunophenotypic findings in vitrectomy specimens. Arch Pathol Lab Med. 2009;133 (8:1233–1237. doi: 10.5858/133.8.1233. [DOI] [PubMed] [Google Scholar]
- Lobo A, Lightman S. Vitreous aspiration needle tap in the diagnosis of intraocular inflammation. Ophthalmology. 2003;110 (3:595–599. doi: 10.1016/S0161-6420(02)01895-X. [DOI] [PubMed] [Google Scholar]
- Ramkissoon YD, Aslam SA, Shah SP, Wong SC, Sullivan PM. Risk of iatrogenic peripheral retinal breaks in 20-G pars plana vitrectomy. Ophthalmology. 2010;117 (9:1825–1830. doi: 10.1016/j.ophtha.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Mudhar HS, Fernando M, Sheard R, Rennie I. Paraneoplastic granulomatous vitritis and retinitis as a presentation of recurrent classical Hodgkin's lymphoma. Int Ophthalmol. 2010;30 (4:341–343. doi: 10.1007/s10792-009-9340-9. [DOI] [PubMed] [Google Scholar]


