Abstract
Objective
To assess the acceptability of sexually transmitted infection (STI) testing using self-collected vaginal swabs (SCVS) among college women.
Participants
First-year female students (N = 483).
Methods
Participants were offered free testing for three STIs using SCVS in April 2010 and later completed a survey regarding their testing decision and experiences.
Results
Sixty-four percent (n = 310) accepted testing; of these, 98% found it easy or very easy to understand the SCVS instructions, and 93% found it easy or very easy to collect the specimen. Among the 36% who did not participate in testing, most had scheduling conflicts or did not perceive a risk for STIs; only 26% felt uncomfortable about the SCVS procedure. Among all women, SCVS was preferred over other STI testing methods.
Conclusions
STI testing using SCVS was acceptable to the majority of college women and could increase the uptake of testing among sexually active college women.
Keywords: college students, sexually transmitted infections (STIs), STI testing, women women’s health
Many college students engage in sexual risk behavior, including unprotected oral, vaginal, and anal sex; inconsistent condom use; sex while intoxicated; and sex with multiple partners.1-3 Sexual risk behavior increases risk for sexually transmitted infections (STIs), which have negative health consequences. Nine million new cases of STIs occur each year among Americans aged 15-24.4 Women are more vulnerable to STIs than men; they are more biologically susceptible to STIs and more likely to have asymptomatic infections.5
Testing can identify STIs, facilitate timely treatment, reduce medical sequelae, and help prevent transmission to others. However, rates of STI/HIV testing among college students are low, ranging from 23% to 32% in previous studies.2,3,6,7 STI testing uptake may be low due to barriers such as shame, stigma, fear, denial, perceived negative social consequences, inaccurate beliefs about STIs, privacy concerns, cost, and inconveniences (e.g., travel, wait time).8-11
University health centers are opportune sites at which to offer STI testing services.9 A survey on the availability of STI testing services at US colleges found that 60% of colleges have a student health center, and, of these, 66% provide STI testing.12 Traditional STI testing methods for women include clinician-obtained endocervical swabs and urine samples. Clinician-obtained specimens may be perceived as invasive.10 Urine samples require quick processing, constant refrigeration, and mailing restrictions and may leak during storage or transportation.13,14
The self-collected vaginal swab (SCVS) is a newer method of testing that offers several advantages compared to traditional methods. SCVS are as sensitive as cervical swabs, and more sensitive than urine, in the detection of chlamydia and gonorrhea.15 SCVS are also easier to process and transport compared to urine samples.13 Using SCVS offers the ability to test for trichomoniasis, which may be more prevalent than either chlamydia or gonorrhea in some sub-populations,16 using the preferred specimen type (vaginal rather than cervical).15 In addition, SCVS are less expensive and more cost-effective for chlamydia screening relative to urine samples and cervical swabs.17 SCVS have been approved for use in health care settings by the United States Food and Drug Administration (FDA).18
SCVS have been found to be acceptable among adolescent and older women.13,19-22 It is also established that women prefer SCVS over clinician-obtained endocervical swabs.14,19-24 However, reports on women’s preferences with respect to SCVS compared to urine testing have been mixed.19,20,22,24-26 Furthermore, the use of SCVS for STI testing among college women in the university health setting has received little research attention. Therefore, the goals of this study were to assess (a) the acceptability of STI testing using SCVS among college women and (b) preferences for STI testing using SCVS versus urine samples.
Methods
Study Design
Data for this study were taken from the Women’s Health Project, a 13-month longitudinal study of health behaviors and relationships among first-year college women. Participants completed monthly surveys throughout their first year of college (August 2009-2010) and were invited to receive STI testing free of charge at the end of the academic year. The current study uses data from the baseline (August/September 2009) and wave nine surveys (April 2010) as well as the biological STI testing conducted through the Women’s Health Project.
Participants
Participants were 483 first-year female undergraduates attending a private university in upstate New York. Most (94%) were 18 years old at baseline (M = 18.1, SD = 0.3, range: 18-21). The racial distribution was 66% White, 11% Asian, 10% Black, and 13% other/multiple; 9% self-identified as Hispanic/Latina.
Procedures
The university’s institutional review board approved all study procedures.
Recruitment
Recruitment began with a mass mailing to 1,400 incoming female students one month before the Fall 2009 semester began. The mailing introduced the study and invited women to sign up on a website to receive further information. Campus flyers, word of mouth, and the psychology department research pool were also used to bolster recruitment. Most participants (61%) heard about the study through the mass mailing; 28% signed up through the research pool, and 11% came from word of mouth referrals and flyer response.
Survey data collection
Within their first three weeks on campus, interested women attended an orientation session, at which time research staff explained the purpose and requirements of the study, and participants provided written informed consent. The baseline survey was then administered on individual computers, and participants received $20 for completing it. All survey data were collected using online survey software, and identifying information was stored separately from survey responses to protect privacy. On the last day of April 2010, participants received emails with embedded links to the wave nine survey. They had one week to complete it remotely, for which they received $10. To encourage timely responding, participants were entered into a raffle for two $50 prizes based on response time.
STI testing
Participants were invited to provide a biological specimen for STI testing at the end of the academic year (i.e., during April 2010). In late March 2010, participants were emailed about the opportunity to sign up for free, confidential STI testing on one of five testing days; Saturdays were chosen to minimize conflicts with students’ class schedules. Participants received up to five emails, sent once per week, until they scheduled an appointment for testing.
Participants used SCVS to obtain biological specimens, which were tested for chlamydia, gonorrhea, and trichomoniasis. All three STIs can be detected accurately using a single SCVS.27 STI testing was conducted by a Clinical Laboratory Improvement Amendments (CLIA) and Georgia State certified laboratory. Specimens were tested for chlamydia and gonorrhea using the Becton Dickinson ProbeTec ET amplified DNA assay28 and for trichomoniasis using a laboratory-developed Taq-Man polymerase chain reaction, which tested for the presence of Trichomonas vaginalis DNA.27 At the time of the study, the chlamydia and gonorrhea tests were FDA-approved for use with endocervical, urethral, and urine specimens, but not SCVS; however, all three tests were validated by the laboratory.27 Sensitivity and specificity (with 95% confidence intervals in parentheses when available) were 92.0% (88.6%-94.6%) and 96.6% (95.9%-97.2%) for chlamydia,29 95.2% (92.5%-97.1%) and 98.8% (98.3%-99.1%) for gonorrhea,29 and 100% and 99.6% for trichomoniasis, respectively.27
Specimen collection appointments took 20-30 minutes and occurred at the university’s on-campus health center, a well-known location close in proximity to participants, who all lived on campus. First, the informed consent process was conducted, in which research staff explained the specimen collection process and other pertinent information. Participants were reminded that they could opt not to participate in testing and still continue with the larger study. After providing written informed consent, participants were given a vaginal swab kit in an opaque bag.
To protect privacy, biological specimens were labeled with unique identification codes rather than participants’ name. Participants were shown the swab (see Appendix A), given a list of detailed written instructions (including a diagram) for the SCVS procedure (see Appendix B), and escorted to an individual bathroom or exam room, where they self-collected their specimen. Participants were instructed to insert the swab about two inches into the vagina, rotate it for 15-30 seconds, carefully withdraw it, and secure it firmly in the plastic sleeve. Participants returned their swab kit (in an opaque bag for privacy) to research staff and were paid $20.
Given the high volume of testing, participants were advised they would not be notified of negative test results. However, women with positive tests for any of the three STIs were called with the results, advised to make an appointment at the health center, and reminded of the procedure for receiving treatment free of charge. After ensuring no drug allergies, a health center physician provided a prescription for the appropriate antibiotic. Positive test results for chlamydia and gonorrhea were reported to the county health department per state law.
Measures
At baseline (late August/early September 2009), women provided their age, race, and ethnicity. At wave nine (April 2010), women were asked if they participated in STI testing offered through the study. Women who indicated they participated (testers) were asked why; response options were (select all that apply): I wanted to know my STI status, I wanted the $20, I wanted to contribute to the Women’s Health Project, and other. Measures of SCVS acceptability were adapted from prior research.19,20,22 Testers were asked to rate the ease of (a) understanding the instructions for self-collecting your vaginal swab and (b) self-collecting your vaginal swab, on a Likert scale from 1 (very easy) to 4 (very difficult).20 Testers were also asked to rate their agreement with 10 statements22 (see Table 1 for items) about their SCVS experience on a Likert scale from 1 (strongly disagree) to 4 (strongly agree). The five positive statements (α = .90) and five negative statements (α = .84) were averaged to create summary variables. Lastly, testers were asked if they would test themselves more often for STIs if self-collected vaginal swabs were available.19
Table 1.
Participants’ Subjective Experience with STD Testing
| Item | N | M (SD) | Median | Range |
|---|---|---|---|---|
| Positive items (averaged) | 290 | 3.4 (0.6) | 3.4 | 1-4 |
| I felt comfortable | 290 | 3.3 (0.8) | 3 | 1-4 |
| I felt in control | 288 | 3.5 (0.6) | 4 | 1-4 |
| I felt I was taking care of my health | 289 | 3.5 (0.6) | 4 | 1-4 |
| I felt relaxed | 290 | 3.2 (0.8) | 3 | 1-4 |
| I trusted the test | 289 | 3.5 (0.6) | 4 | 1-4 |
| Negative items (averaged) | 290 | 1.8 (0.6) | 2 | 1-4 |
| I was anxious | 290 | 2.0 (0.9) | 2 | 1-4 |
| I was embarrassed | 290 | 1.7 (0.8) | 2 | 1-4 |
| It was inconvenient | 289 | 1.7 (0.8) | 2 | 1-4 |
| It was painful | 289 | 1.6 (0.8) | 1 | 1-4 |
| I worried I might do the test wrong | 290 | 2.1 (0.9) | 2 | 1-4 |
Note. Ns for each item differ due to missing data. Response options ranged from 1 (strongly disagree) to 4 (strongly agree).
Women who indicated they had not participated (non-testers) were asked why not; response options were (select all that apply): scheduling conflict, uncomfortable with vaginal swab testing procedure, concerned about confidentiality, I do not want to know if I have an STI, I do not think I am at risk for an STI, and other. Women who indicated a perceived lack of risk were ask why they perceived this; response options were (select all that apply) because I have: never had any sexual contact, not even oral sex; never had vaginal sex; never had anal sex; only been with one partner whom I believe does not have any STIs and is faithful to me; and other.
All women, regardless of testing participation, were asked which STI testing method they would prefer;20 response options were: self-collected vaginal swab, self-collected urine sample, pelvic examination by medical provider, no preference, and not sure.
Results
Of the 483 participants enrolled in the study, 310 (64%) agreed to STI testing. Of the 310 women who participated in STI testing, 3 (1%) tested positive for an STI: two for chlamydia, one for gonorrhea, and none for trichomoniasis. A total of 413 women (86%) completed the wave nine survey. Of these, 291 reported (correctly) that they participated in STI testing, and 119 reported (correctly) that they did not participate. One woman who incorrectly indicated she did not participate in STI testing did not receive the measures about testing experience; two others had missing data for all STI testing items. Overall, 94% of testers and 69% of non-testers completed the wave nine survey.
Among women who participated in testing, the most commonly endorsed motives were: (a) to learn their STI status (64%), (b) to contribute to the Women’s Health Project (82%), and (c) to receive the financial compensation (97%). As depicted in Figure 1, 98% of women reported it was easy or very easy to understand the instructions for obtaining the SCVS. Similarly, 93% reported it was easy or very easy to collect the specimen. Most testers (73%) reported they would test themselves for STIs more often if the SCVS method were available.
Figure 1.
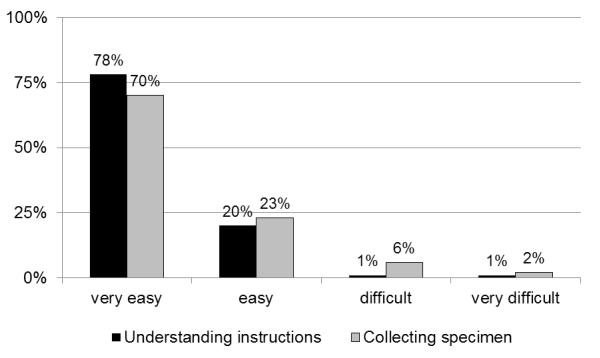
Ease of Understanding Instructions and Collecting Vaginal Swab Specimen
On average, testers agreed they felt comfortable and relaxed while obtaining the SCVS (median response for both items = 3, see Table 1). Testers strongly agreed they felt in control, felt like they were taking care of their health, and trusted the test (medians = 4). Testers disagreed that they felt anxious or embarrassed, found the test inconvenient, or worried they would do the test wrong (medians = 2). Testers strongly disagreed that obtaining the SCVS was painful (median = 1). On average, participants more strongly endorsed positive feedback about the SCVS procedure than negative feedback, t(289) = 24.61, p < .0001, d = 2.04.
Among women who did not participate in the testing, the most common reasons provided for this decision were (a) a scheduling conflict (62%), (b) the perception that they were not at risk for an STI (43%), (c) being uncomfortable with the vaginal swab testing procedure (26%), (d) concerns about confidentiality (5%), and (e) not wanting to know if they had an STI (1%). Among women not perceiving a risk for STIs, 42% reported they had never had oral, vaginal, or anal sex; 31% had had only one partner whom they believed was faithful and did not have an STI, 12% had never had vaginal sex, 8% were not currently sexually active, 6% were recently tested by their doctor, and 2% had other reasons.
Across all participants, SCVS was the most preferred method of STI testing (38%); 28% preferred urine samples, 3% preferred a pelvic exam by a medical provider, 17% had no preference, and 14% were not sure. Testers and non-testers differed in terms of their preferred method of STI testing (see Table 2), χ2 (4, N = 410), = 72.15, p < .001. Testers were more likely to prefer SCVS, whereas non-testers were more likely to prefer urine samples or be unsure.
Table 2.
Preferred Method of STD Testing by Testing Participation
| Preferred Method of STD Testing | Testers (N = 290) | Non-Testers (N = 120) | ||
|---|---|---|---|---|
|
| ||||
| N | % | N | % | |
|
|
||||
| Self-collected vaginal swab | 146 | 50 | 11 | 9 |
| Urine sample | 59 | 20 | 55 | 46 |
| Pelvic exam by medical provider | 12 | 4 | 1 | 1 |
| No preference | 44 | 15 | 26 | 22 |
| Not sure | 29 | 10 | 27 | 23 |
Comment
The objectives of this study were to examine the acceptability of STI testing using SCVS among college women as well as assess women’s preferences for STI testing methods. We offered women free, confidential STI testing for chlamydia, gonorrhea, and trichomoniasis. Sixty-four percent opted to participate in the STI testing, which is close to the 71% acceptance rate found in a similar study using SCVS.13 Most women who declined to test had a scheduling conflict or did not perceive they were at risk for STIs; only 26% of non-testers indicated they were uncomfortable with the SCVS procedure. Among testers, 73% reported they would pursue more frequent STI testing were the SCVS method available. Overall, the high rate of participation, the low rate of discomfort with SCVS, and the high rate of willingness to repeat the test in the future indicate that this testing method was acceptable to the majority of college women in our sample. Thus, the availability of SCVS may help health care providers increase rates of chlamydia screening among young women, consistent with Healthy Campus 202030 and Healthy People 202031 initiatives.
Consistent with a prior study,22 women reported positive feedback about STI testing using SCVS. On average, women felt comfortable, relaxed, and in control while collecting their specimens. Although using SCVS is a novel method of STI testing that none had used before, women trusted the testing process and results. Reported anxiety and embarrassment were low, despite undergoing STI testing, which has the potential for unsettling results, and using a new testing method for the first time. We attempted to allay concerns by normalizing SCVS and likening inserting the swab to inserting a tampon, which 81% of college women use routinely.32 Women strongly disagreed that collecting the specimen was painful. Women did express mild concern that they did not collect the specimen correctly, consistent with previous reports.22,25
This study also documents the feasibility of STI testing using SCVS. As in prior studies,33,34 all women self-collected their specimen successfully; none reported problems with specimen collection, nor did the laboratory identify any problems. Only 2% found the instructions difficult to understand, and only 7% found self-collecting the specimen to be difficult, corroborating previous research.14,19,20,23 Given that women likely had little to no prior experience with vaginal swabs, we found it helpful to explain the procedure in detail, anticipate questions ahead of time, and provide verbal and written instructions (including a diagram).
Across all participants, SCVS was preferred over urine samples for STI testing, consistent with some,19,20,25 but not all, prior research22,24,26 A minority of women prefer urine samples; reasons for this preference include finding urine samples easier and more natural than SCVS, not liking to insert things into their vagina, or fearing they will incorrectly collect the swab.25 In the current study, testers were more likely to prefer SCVS over urine samples, but non-testers were more likely to prefer urine samples over SCVS. Almost half (45%) of the non-testers indicated they had no preference or were unsure of their preference; this higher level of uncertainty may have been due to the novelty of the SCVS method. Whereas only 9% of non-testers preferred SCVS, 50% of testers did. A positive testing experience may have translated into a higher likelihood of preferring SCVS to other methods. Indeed, in a prior study that assessed acceptability of SCVS before and after testing, comfort, self-efficacy for collecting the specimen, and overall acceptability of SCVS increased from pre- to post-testing.33
Limitations
Participants in this study were first-year women from a single university in the Northeast, which may limit the generalizability of the results. Future research might employ multi-site sampling to include women from a variety of geographic regions and expand beyond first-year students. We found a low prevalence of STDs in our sample; it is possible that college women with a higher risk for STDs may have a different level of acceptability of SCVS. We did not offer participants the option to be tested through urine sample or clinician-obtained endocervical swab. The wave nine survey was not completed by all participants; however, we reached 94% of testers and 69% of non-testers.
Conclusions
Consistent with national data,1 we found a low prevalence (1%) of STIs in our sample. Overall, STI testing using SCVS was acceptable to the majority of college women. SCVS provide a quick, easy method for testing for common STIs without requiring a pelvic exam, which may result in anxiety and discomfort,35 thus causing some women to avoid needed STI testing. Due in part to revised guidelines for cervical cancer screening, some physicians have argued against yearly pelvic exams for asymptomatic women.36 Given this, SCVS affords an STI testing method that does not involve clinician-obtained endocervical swabs. Moreover, compared to urine tests, SCVS are more sensitive, less messy, less susceptible to laboratory errors, less expensive, and more cost-effective.13,15,17,18 Thus, use of SCVS provides college and university health providers with an additional and highly acceptable method of STI testing for women.
Supplementary Material
Acknowledgments
This research was supported by grant R21-AA018257 from the National Institute on Alcohol Abuse and Alcoholism to Michael P. Carey. The authors thank Annelise Sullivan for her assistance with data collection and the Center for AIDS Research (P30-AI050409) at Emory University for conducting the STI testing.
Footnotes
APPENDIX A. Vaginal Swab and Plastic Sleeve
APPENDIX B. Instructions for Self-collected Vaginal Swab
1. Wash your hands with soap and water before and after collecting your swab.
2. Decide which position (standing, squatting, or sitting) is most comfortable for you. Pull down your pants/underwear.
3. Remove the swab from the plastic sleeve by grasping the pink cap. Do not set the swab or sleeve down on anything while performing the collection.
4. Insert the swab about 2 inches into your vaginal canal. This is similar to the way you would insert a tampon. There should be no discomfort.
5. Rotate the swab for 15-30 seconds.
6. Carefully withdraw the swab from your vagina.
7. Immediately place the swab back in the plastic sleeve. Push the pink cap in to make sure the swab is securely inside the plastic sleeve.
8. Place the swab kit (swab and sleeve) in the brown privacy bag.
9. Return the bag to WHP staff.
References
- 1.American College Health Association . American College Health Association—National College Health Assessment II: Undergraduate reference group data report Fall 2011. American College Health Association; Hanover, MD: 2012. [Google Scholar]
- 2.Buhi ER, Marhefka SL, Hoban MT. The state of the union: Sexual health disparities in a national sample of US college students. J Am Coll Health. 2010;58:337–346. doi: 10.1080/07448480903501780. [DOI] [PubMed] [Google Scholar]
- 3.Trieu SL, Bratton S, Marshak HH. Sexual and reproductive health behaviors of California community college students. J Am Coll Health. 2011;59:744–750. doi: 10.1080/07448481.2010.540764. [DOI] [PubMed] [Google Scholar]
- 4.Weinstock H, Berman S, Cates W. Sexually transmitted diseases among American youth: Incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36:6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- 5.McCree DH, Rompalo AM. Behavioral Interventions for Prevention and Control of Sexually Transmitted Diseases. Springer; New York: 2007. Biological and behavioral risk factors associated with STDs/HIV in women: Implications for behavioral interventions. [Google Scholar]
- 6.Crosby RA, Miller KH, Staten RR, et al. Prevalence and correlates of HIV testing among college students: An exploratory study. Sex Health. 2005;2:19–22. doi: 10.1071/sh04047. [DOI] [PubMed] [Google Scholar]
- 7.Gullette DL, Lyons MA. Sensation seeking, self-esteem, and unprotected sex in college students. J Assoc Nurses AIDS Care. 2006;17:23–31. doi: 10.1016/j.jana.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Barth KR, Cook RL, Downs JS, et al. Social stigma and negative consequences: Factors that influence college students’ decisions to seek testing for sexually transmitted infections. J Am Coll Health. 2002;50:153–159. doi: 10.1080/07448480209596021. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert LK, Levandowski BA, Roberts CM. Characteristics associated with genital herpes testing among young adults: Assessing factors from two national data sets. J Am Coll Health. 2011;59:143–150. doi: 10.1080/07448481.2010.497522. [DOI] [PubMed] [Google Scholar]
- 10.Pavlin NL, Gunn JM, Parker R, et al. Implementing chlamydia screening: What do women think? A systematic review of the literature. BMC Public Health. 2006;6 doi: 10.1186/1471-2458-6-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tilson EC, Sanchez V, Ford CL, et al. Barriers to asymptomatic screening and other STI services for adolescents and young adults: Focus group discussions. BMC Public Health. 2004;4:21. doi: 10.1186/1471-2458-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koumans EH, Sternberg MR, Motamed C, et al. Sexually transmitted disease services at US colleges and universities. J Am Coll Health. 2005;53:211–217. [PubMed] [Google Scholar]
- 13.Doshi JS, Power J, Allen E. Acceptability of chlamydia screening using self-taken vaginal swabs. Int J STI AIDS. 2008;19:507–509. doi: 10.1258/ijsa.2008.008056. [DOI] [PubMed] [Google Scholar]
- 14.Wiesenfeld HC, Lowry DLB, Heine RP, et al. Self-collection of vaginal swabs for the detection of Chlamydia, gonorrhea, and trichomoniasis: Opportunity to encourage sexually transmitted disease testing among adolescents. Sex Transm Dis. 2001;28:321–325. doi: 10.1097/00007435-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Hobbs MM, van der Pol B, Totten P, et al. From the NIH: Proceedings of a workshop on the importance of self-obtained vaginal specimens for detection of sexually transmitted infections. Sex Transm Dis. 2008;35:8–13. doi: 10.1097/OLQ.0b013e31815d968d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller WC, Swygard H, Hobbs MM, et al. The prevalence of trichomoniasis in young adults in the United States. Sex Transm Dis. 2005;32:593–598. doi: 10.1097/01.olq.0000179874.76360.ad. [DOI] [PubMed] [Google Scholar]
- 17.Blake DR, Maldeis N, Barnes MR, et al. Cost-effectiveness of screening strategies for Chlamydia trachomatis using cervical swabs, urine, and self-obtained vaginal swabs in a sexually transmitted disease clinic setting. Sex Transm Dis. 2008;35:649–655. doi: 10.1097/OLQ.0b013e31816ddb9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang J, Husman C, DeSilva L, et al. Evaluation of self-collected vaginal swab, first void urine, and endocervical swab specimens for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae in adolescent females. J Pediatr Adolesc Gynecol. 2008;21:355–360. doi: 10.1016/j.jpag.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chernesky MA, Hook EW, Martin DH, et al. Women find it easy and prefer to collect their own vaginal swabs to diagnose Chlamydia trachomatis or Neisseria gonorrhoeae infections. Sex Transm Dis. 2005;32:729–733. doi: 10.1097/01.olq.0000190057.61633.8d. [DOI] [PubMed] [Google Scholar]
- 20.Gaydos CA, Dwyer K, Barnes M, et al. Internet-based screening for Chlamydia trachomatis to reach non-clinic populations with mailed self-administered vaginal swabs. Sex Transm Dis. 2006;33:451–457. doi: 10.1097/01.olq.0000200497.14326.fb. [DOI] [PubMed] [Google Scholar]
- 21.Richardson E, Sellors JW, Mackinnon S, et al. Prevalence of Chlamydia trachomatis infections and specimen collection preference among women, using self-collected vaginal swabs in community settings. Sex Transm Dis. 2003;30:880–885. doi: 10.1097/01.OLQ.0000091142.68884.2A. [DOI] [PubMed] [Google Scholar]
- 22.Serlin M, Shafer M, Tebb K, et al. What sexually transmitted disease screening method does the adolescent prefer? Adolescents’ attitudes toward first-void urine, self-collected vaginal swab, and pelvic examination. Arch Pediatr Adolesc Med. 2002;156:588–591. doi: 10.1001/archpedi.156.6.588. [DOI] [PubMed] [Google Scholar]
- 23.Holland-Hall CM, Wiesenfeld HC, Murray PJ. Self-collected vaginal swabs for the detection of multiple sexually transmitted infections in adolescent girls. J Pediatr Adolesc Gynecol. 2002;15:307–313. doi: 10.1016/s1083-3188(02)00197-3. [DOI] [PubMed] [Google Scholar]
- 24.Hoebe CJPA, Rademaker CW, Brouwers EEHG, et al. Acceptability of self-taken vaginal swabs and first-catch urine samples for the diagnosis of urogenital Chlamydia trachomatis and Neisseria gonorrhoeae with an amplified DNA assay in young women attending a public health sexually transmitted disease clinic. Sex Transm Dis. 2006;33:491–495. doi: 10.1097/01.olq.0000204619.87066.28. [DOI] [PubMed] [Google Scholar]
- 25.Newman SB, Nelson MB, Gaydos CA, et al. Female prisoners’ preferences of collection methods for testing for Chlamydia trachomatis and Neisseria gonorrhoeae infection. Sex Transm Dis. 2003;30:306–309. doi: 10.1097/00007435-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh Y, Howell MR, Gaydos JC, et al. Preference among female Army recruits for use of self-administrated vaginal swabs or urine to screen for Chlamydia trachomatis genital infections. Sex Transm Dis. 2003;30:769–773. doi: 10.1097/01.OLQ.0000079048.11771.46. [DOI] [PubMed] [Google Scholar]
- 27.Caliendo AM, Jordan JA, Green AM, et al. Real-time PCR improves detection of Trichomonas vaginalis infection compared with culture using self-collected vaginal swabs. Infect Dis Obstet Gynecol. 2005;13:145–150. doi: 10.1080/10647440500068248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Der Pol B, Ferrero DV, Buck-Barrington L, et al. Multicenter evaluation of the BDProbeTec ET system for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine specimens, female endocervical swabs, and male urethral swabs. J Clin Microbiol. 2001;39:1008–1016. doi: 10.1128/JCM.39.3.1008-1016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becton, Dickinson and Company [Accessed November 22, 2012];Package insert, ProbeTec™ ET Chlamydia trachomatis and Neisseria gonorrhoeae Amplified DNA Assays. Available at: http://www.bd.com/ds/technicalCenter/inserts/3300754JAA%28201007%29.pdf.
- 30.Healthy Campus 2020 [Accessed September 18, 2012];Student objectives. Available at: http://www.acha.org/HealthyCampus/student-obj.cfm.
- 31.Healthy People 2020 [Accessed September 18, 2012];Sexually transmitted diseases. Available at: http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=37.
- 32.Omar HA, Aggarwal S, Perkins KC. Tampon use in young women. J Pediatr Adolesc Gynecol. 1998;11:143–146. doi: 10.1016/s1083-3188(98)70134-2. [DOI] [PubMed] [Google Scholar]
- 33.Huppert JS, Hesse EA, Bernard MA, et al. Acceptability of self-testing for trichomoniasis increases with experience. Sex Transm Infect. 2011;87:494–500. doi: 10.1136/sextrans-2011-050037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berwald N, Cheng S, Augenbraun M, et al. Self-administered vaginal swabs are a feasible alternative to physician-assisted cervical swabs for sexually transmitted infection screening in the emergency department. Acad Emerg Med. 2009;16:360–363. doi: 10.1111/j.1553-2712.2009.00359.x. [DOI] [PubMed] [Google Scholar]
- 35.Bodden-Heidrich R, Walter S, Teutenberger S, et al. What does a young girl experience in her first gynecological examination? Study on the relationship between anxiety and pain. J Pediatr Adolesc Gynecol. 2000;13:139–142. doi: 10.1016/s1083-3188(00)00056-5. [DOI] [PubMed] [Google Scholar]
- 36.Westhoff CL, Jones HE, Guiahi M. Do new guidelines and technology make the routine pelvic examination obsolete? J Womens Health. 2011;20:5–10. doi: 10.1089/jwh.2010.2349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


