Abstract
Gout affects 8.3 million Americans according to NHANES 2007–2008, roughly 3.9% of the U.S. population. Gout has significant impact on physical function, productivity, health-related quality of life (HRQOL) and health care costs. Uncontrolled gout is also associated with significant utilization of emergent care services. Women are less likely to have gout than men, but in the postmenopausal years the gender difference in disease incidence decreases. Compared to Whites, racial/ethnic minorities, especially blacks, have higher prevalence of gout. On the other hand, blacks are less likely to receive quality gout care, leading to a disproportionate morbidity. Women are less likely than men to receive allopurinol, less likely to get joint aspirations for crystal analyses for establishing diagnosis, but those on urate-lowering therapy are as/more likely as men to get serum urate check within 6-months of initiation. While a few studies provide the knowledge related to gender and race/ethnicity disparities in gout, several knowledge gaps exist in gout epidemiology and outcomes differences by gender and race/ethnicity. These should be explored in future studies.
Keywords: Gout, Hyperuricemia, Race, Ethnicity, Gender, Disparity, Epidemiology, Prevalence, Genetic risk factors, Adverse effects
Introduction: Why Care About Disparities?
Racial and ethnic disparities in medical care are widespread, as demonstrated by multiple studies in multiple conditions and across a time horizon.1–10 Factors postulated to contribute to these disparities include lower socioeconomic status,11,12 greater barriers to health care access,13–15 lower health literacy and numeracy,16 poorer physician-patient communication,17–20 higher medication non-adherence,21 and greater risk averseness to treatments.22–27 To overcome these health care disparities requires multifaceted approaches that simultaneously target several risk factors. For example, to reduce health literacy and to overcome barriers to effective communication, one may need to provide health education to patients and devise ways to improve patient-physician communication. Whereas physician practice changes may help alleviate some disparities, policy changes are needed to address structural disparities such as issues related to poverty and barriers to health care access. Reduction of racial disparities will likely lead to more optimal care and improvement in health outcomes in racial and ethnic minorities.
The 2002 Institute of Medicine (IOM) report drew attention to racial and ethnic disparities in health care and called for “confronting Racial and Ethnic Disparities in Health Care”.28 Among other things recommended in the IOM report, the committee made three recommendations with respect to data collection and monitoring: (1) collection and reporting of data on health care access and utilization by patient’s race, ethnicity, socio-economic status, and where possible, language; (2) inclusion of measures of racial and ethnic disparities in assessing provider performance; and (3) monitoring of progress toward the elimination of health care disparities.29,30
Subsequently, the 2009 IOM’s comparative effectiveness research (CER) agenda listed “Comparison of effectiveness of literacy-sensitive disease management programs and usual care in reducing disparities in children and adults with low literacy and chronic disease” in the first quartile of the 100 top priority areas.31 As in the 2002 report, the 2009 agenda not only highlighted the importance of racial/ethnic disparities in health care, but also emphasized the challenge of knowledge gaps in implementation research aimed at reducing disparities. By identifying barriers to quality health care, high-quality research can lead to the identification of targets for intervention and thus improve the overall quality of care for racial/ethnic minorities. The Affordable Care Act (ACA) recognized minority health as a priority by establishing minority health offices.32 The Agency for Healthcare Research and Quality (AHRQ) similarly recognized the importance of minority health disparities through its issuance of Special Emphasis Notice.33
Gender disparities in health care have also been widely reported.34–38 The 2003 IOM report on Women’s Health Research noted that the underrepresentation of women in many past studies limits the generalizability of their findings.39 Women’s health is a priority area for AHRQ, and two key publications from the agency, the National Healthcare Quality Report (NHQR) and National Healthcare Disparities Report (NHDR), highlighted its importance. NHDR focuses on “prevailing disparities in health care delivery as it relates to racial factors and socioeconomic factors in priority populations”; priority populations include racial and ethnic minorities, and women, among others.40 The Affordable Care Act (ACA) also recognized women’s health as a priority by establishing women’s health offices.32
In this review, we summarize the evidence regarding racial/ethnic and gender disparities in gout, identify areas for further research including the types of studies needed to identify and close gaps in care of minorities and women with gout.
The Burden of Gout as a Musculoskeletal Disorder
Gout is a prototypical inflammatory arthritis, similar in many ways to rheumatoid arthritis (RA). However, in contrast to RA that is primarily driven by autoimmunity, gout is a metabolic disease characterized by the effects of hyperuricemia on joints, kidneys and other systems. According to recent studies, gout prevalence is estimated at 4% in the U.S.,41 which is at least 4-times that of RA, its closest rival.42 Gout has significant impact on patient’s lives and on their function,43 productivity44,45 and health-related quality of life (HRQOL).43,46,47
Recent studies have also highlighted gout as an independent risk factor for cardiovascular morbidity and mortality, making treatment of gout relevant not only for the rheumatologist and the nephrologist, but also for the internist and the cardiologist. Gout is an independent risk factor for myocardial infarction (MI) in men48 and women49. After adjustment for traditional risk factors, the use of diuretics and aspirin and serum creatinine level, patients with gout had a 1.35-times higher hazard for coronary heart disease mortality (95% confidence interval [CI], 1.06–1.72) versus those without gout.50 Gout was also associated with an adjusted hazard of 1.74 (95% CI, 1.03–2.93) for incident heart failure and higher relative risk of 3.70 (95% CI, 1.68–8.16) for abnormally low left ventricular ejection fraction.51 Gout was independently associated with 1.5-fold increase in risk of mortality in dialysis patients, after adjusting for black race, older age, female gender, hypertension, ischemic heart disease, congestive heart failure, and alcohol use.52 These studies highlight that gout is a systemic disease, and provide support that its impact goes far beyond the joint and the musculoskeletal system.
Disparity in Epidemiology of Gout
Racial/Ethnic Differences in Prevalence
Genetic studies in African-Americans have found the same loci to be associated with hyperuricemia as those noted in the European ancestry populations. Importantly, the most strongly-associated locus for serum uric acid levels among European ancestry populations was also the most strongly-associated locus in African-Americans.53,54 Despite this sharing of hyperuricemia-related loci across racial/ethnic groups, gout seems to be more prevalent in minorities. Several studies have provided evidence for disparities in the prevalence and incidence of gout by race/ethnicity.
In a recent study based on U.S. National Health and Nutrition Examination Survey (NHANES) conducted in 2007 and 2008, 3.9% of the U.S. population reported physician-diagnosed prevalent gout.41 Compared to whites whose gout prevalence was 4.0% (95% CI, 3.3%–4.8%), the prevalence of gout was higher in blacks at 5.0% (95% CI, 3.3%–6.6%). This observation mirrored the higher prevalence of hyperuricemia in blacks compared to whites, 25.7% versus 22.1% in NHANES 2007–2008.41 This study also showed that the prevalence of gout increased from 2.7% in NHANES-III (1988–1994) to 3.9% in NHANES 2007–2008 sample, signifying the rapidly increasing gout prevalence over time.
In a different cohort study, the incidence of gout was 3.11 versus 1.82 per 1,000 person-years among black and white males, respectively.55 This incidences resulted in a relative risk of 1.69 (95% CI, 1.02–2.80) in blacks versus whites. Incident hypertension was independently associated with the development of gout in univariate analysis, and when this variable was included as a time-dependent covariate in a Cox model, the excess risk for gout among black males was reduced and no longer statistically significant (adjusted RR = 1.30 [95% CI 0.77–2.19]).55 These data suggest that at least some of the difference in gout risk among black males derives from related co-morbidities.
In contrast, in a study from the Atherosclerosis Risk in Communities (ARIC) study, a population-based bi-racial cohort comprised of individuals aged 45–65 years at baseline (1987–1989), the graded association between BMI with incident gout (each P for trend <0.001) did not differ significantly between black and white females.56 Similarly, the association of weight gain and incident gout also did not differ significantly between black and white women.56
In a recent study of gout of Hmong Chinese from the state of Minnesota, the prevalence of self-reported gout among Hmong was reported to be 2-fold higher than in the general US population as identified in NHANES III (6.5% versus 2.9%; P < 0.001).57 Although women in both groups (Hmong Chinese and U.S. general population) reported similar gout prevalence of 1.9%, Hmong men were significantly more likely than their non-Hmong counterparts to report gout (11.5% versus 4.1%; P < 0.001).57 In another study comparing Hmong in Minnesota to the general white population, the authors found that in a multivariable analysis (adjusted for age, sex, hypertension, diuretic use and kidney disease), Hmong ethnicity was significantly associated with risk of tophaceous gout, with an odds ratio of 4.3 (95% CI: 1.5, 12.2).58 This was despite the fact that Hmong Chinese immigrants had a lower prevalence of diabetes mellitus, coronary vascular disease, and hyperlipidemia compared to whites at initial presentation.
A higher prevalence of hyperuricemia and gout was also noted in the New Zealand Maori population, with half the Polynesian population of New Zealand having hyperuricemia based on European and North American standards.59 Ten percent of the New Zealand Maori males older than 20 years had gout. The Maoris also had higher prevalence of obesity, diabetes mellitus, hypertension, and associated degenerative vascular disorders, these being not only diseases associated with higher mortality, but also being risk factors for gout.59 In a study comparing Maori and European students aged 13 to 16 years, Maori boys had a mean serum urate that was 1 mg/dl higher than that of European boys, and serum urate level among Maori girls were a mean of 0.5 mg/dl higher than their European female counterparts.60
In summary, these studies indicate that certain racial/ethnic groups had a higher prevalence of gout compared to whites of European Ancestry. This was especially true for blacks and the Hmong Chinese in the US and New Zealand Maoris. Blacks in US and Maoris in New Zealand had higher prevalence of certain risk factors for gout (hypertension, obesity etc.), which partially, but not totally, explain the difference in prevalence. On the other hand Hmong Chinese immigrants to US had a lower prevalence of most gout risk factors, denoting that genetic factors and/or diet may underlie these differences.
Racial/Ethnic Differences in Clinical and Genetic Risk Factors for Gout
In another analysis of the U.S. NHANES cohorts of 1988–1994 and 2007–2010, a linear trend of increasing prevalence of gout was seen for increasing body mass index (BMI), prevalence increasing from 1–2% in subjects with normal BMI 18.5–24.9 to 7% in those with BMI≥ 35 kg/m2.61 Obesity, defined as a BMI category over 30 kg/m2, was significantly associated with approximately twice the prevalence of gout, even after adjusting for serum uric acid and other comorbidities. In further analyses of the association of obesity with gout prevalence by race/ethnicity, obesity among non-Hispanic whites was associated with 1.6-fold increased gout prevalence compared to non-obese; the respective prevalence ratio was 2.2-times in non-Hispanic blacks. This denoted a slightly greater risk of gout with obesity in the racial/ethnic minorities compared with whites.
A New Zealand study reported that association of ABCG2 gene (rs2231142 allele) with susceptibility to gout varied according to race and ethnicity.62 Association of the minor allele of rs2231142 with gout was observed in the Pacific Islanders (OR = 2.80, P< 0.001), but not in the Maoris (OR = 1.08, P = 0.70), with heterogeneity in association evident between the Maori and Pacific Island residents (P = 0.001).62 Similarly, rs2231142 was associated with risk of gout in people of western (Tonga, Samoa, Niue, Tokelau; OR = 2.59, P < 0.001) but not eastern (Maori, Cook Island; OR = 1.12, P= 0.48) Polynesian origin. An association of rs2231142 with gout was observed in the Caucasian samples with an OR = 2.20 (P <0.00001). Thus, unlike SLC2A9, which is a strong risk factor for gout in both Maori and Pacific Island people, ABCG2 rs2231142 had a strong effect only in people of western Polynesian ancestry.62
Gender Differences in Prevalence of Gout
Women are protected against gout in the premenopausal period due to uricosurics effect of female sex hormones, and therefore men outnumber women in all age groups for prevalent gout, more in the younger than older age groups by a ratio of 5:1 to 10:1. Previous studies have reported gender differences in the prevalence of gout. In an analysis of the Framingham study, 2,283 men and 2,844 women aged 30–59, free of CHD at initial examination were analyzed.63 Hall et al. reported that the prevalence of one or more attacks of gouty arthritis over a 12-year period was reported by 1.5% overall, 2.8% men and 0.4% women.63
In a population-based study comparing the incidence of gout in Olmsted County, Minnesota the annual incidence rate of gout increased from 45.0/100,000 (95% CI: 30.7, 59.3) in 1977–78 to 62.3/100,000 (95% CI: 48.4, 76.2) in 1995–96, and a doubling of gout incidence in 2 decades.64 Gender differences were noted. The overall male to female ratio remained the same at 3.3 to 1 for both incidence cohorts. The incidence of gout rose with age in women and men, peaking in those 80 years and over.
In a study of 374,832 patients in 45 practices in England, Scotland and Wales during 1971–75 that included 374,832 people, gout incidence was reported at 0.3 per 1,000 per year. Age and gender data were available for 258,091. The prevalence of gout was higher in men at 6.1/1,000 men compared to 1.0/1,000 women.65 Among the different age strata, the prevalence (per 1,000) differed between men and women as well, but the gap narrowed with increasing age: Age 15–44, 1.7 vs. 0.1; 45–64, 10.6 vs. 1.3; and age ≥65, 12.2 vs. 3.0, respectively (Figure 1A).65
Figure 1. Epidemiology of gout by gender.
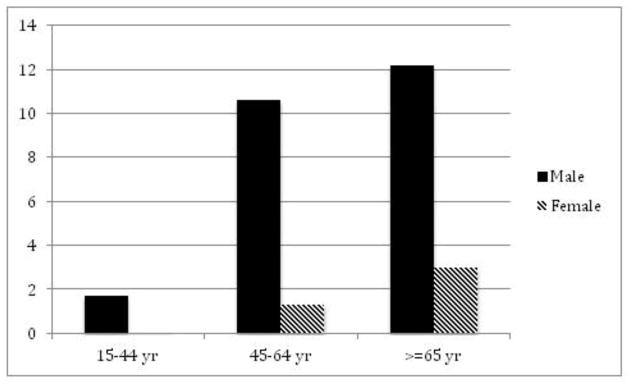
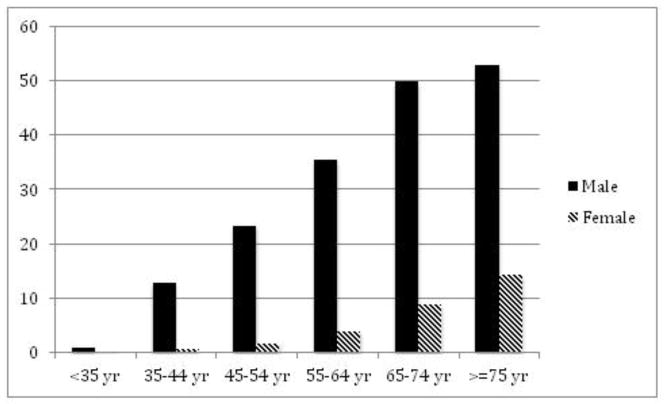
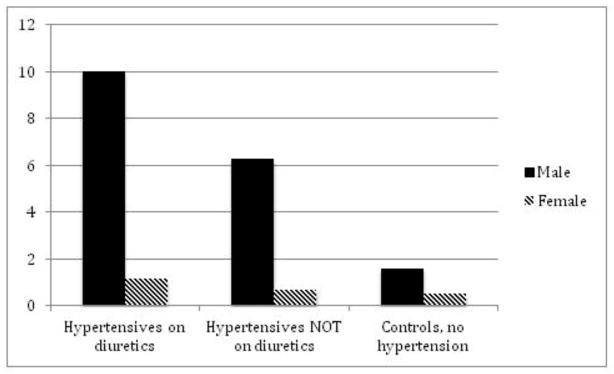
A. Prevalence of gout in Great Britain, 1971–75 by gender per 1,000 persons. (Data from Currie [65].) B. Prevalence of gout in England by gender in 1991 per 1,000 persons. (Data from Harris et al. [66].) C. Incidence of gout in Europe in hypertensives and controls by gender in a cohort study during a 12-year follow-up. (Data from Grodzicki et al. [67].)
In a separate study from 40 practices in UK including 300,000 patients, 2,865 were diagnosed with gout, leading to an overall prevalence of 9.5 per 1,000 patients.66 In that study, gout prevalence in men was 16.4/1,000 compared with 2.93/1,000 in women. Analyzed according to age strata, respective prevalence/1,000 in men and women were as follows: <35 years, 0.96 vs. 0.09; 35–44 years, 12.86 vs. 0.54; 45–54 years, 23.42 vs. 1.70; 55–64 years, 35.48 vs. 3.84; 65–74 years, 49.91 vs. 8.80; and ≥75 years, 53.08 vs. 14.31 (Figure 1B).66
In an analysis of the participants of the European Working Party on High Blood Pressure in the Elderly (EWHE), data from 2,295 hypertensives and 2,280 controls were analyzed with regards to diuretic use and the incidence of physician-diagnosed gout during follow-up.67 The incidence of gout was higher in hypertensives on diuretics versus hypertensives not on diuretics versus controls for men (10 vs. 6.29 vs. 1.6/1,000 patient years) and women (1.16 vs. 0.69 vs. 0.53/1,000 patient years), (Figure 1C). As clearly evident, the incidence of gout was higher in men compared to women.67 In a small study, the onset of gout in women was a mean of 7 years later than in men.68
In the recent study that reported NHANES data from 2007–2008, prevalence of gout in men was 5.9% (95% CI: 4.7, 7.1) and in women was 2.0% (95% CI: 1.5, 2.5).62 Mean serum urate was 6.14 (6.06, 6.23) mg/dl in men and 4.87 (4.79, 4.94) in women. Hyperuricemia, defined as serum urate > 7mg/dl was noted in 21.2% men (95% CI: 18.9, 23.5) and 5.7% women (95% CI: 4.4, 6.9).62
In summary, these data indicate that women have far lower prevalence of gout compared to men. The gender difference gets smaller with increasing age, but men still far outnumber women with gout, even among the elderly.
Gender Differences in Clinical and Genetic Risk Factors for gout
In a population-based study, 34,036 people in the Nagano province in Japan, were studied for hyperuricemia,69 the underlying biochemical abnormality in gout. 15,712 were males and 18,324 were females. The study showed that gender was related to serum urate levels across all age strata. Men had a mean serum urate of 5.82 mg/dl (standard deviation [SD], 1.14 mg/dl), and the women 4.33 mg/dl (SD, 0.91mg/dl).69 The gender difference in serum urate decreased with increasing age, such that in the 15–19 year olds men and women had serum urate levels of 6.1 and 4.6 mg/dl and in the 70+ age group, serum urate levels were 5.8 and 5.0, respectively.
In a small study of onset of gout in women versus men, there were no differences between women and men regarding multiple known (and potential) risk factors for gout including systemic hypertension, diabetes mellitus, hyperlipidemia, chronic renal failure, ischemic heart disease, or heavy alcohol consumption.68
In another study of 132,556 individuals ≥18 years enrolled in Taiwan’s National Health Insurance, 1,606 subjects (1,341 men and 265 women) developed incident gout. Hyperuricemia (sUA ≥7.7 mg/dL for men or ≥6.6 mg/dL for women) was associated with hazard ratio of 9.65 (95% CI, 8.53–10.9) for gout in men and 9.28 (95% CI, 7.00–12.3) in women.70 The association of risk factors with incident gout was similar in women versus men: hypertension, obesity, and hyperlipidemia were significantly associated with respective hazard ratios of 1.32 (95% CI, 1.17–1.48), 1.30 (95% CI, 1.15–1.47), and 1.12 (95% CI, 0.99–1.26) for men and 1.34 (95% CI, 1.02–1.77), 2.15 (95% CI, 1.67–2.76), and 1.70 (95% CI, 1.32–2.19) for women.
In the study comparing NHANES cohorts of 1988–1994 and 2007–2010, analyses of association of obesity with the risk of prevalent gout was also performed by gender.61 The association of obesity, defined as a BMI category over 30 kg/m2, with the risk of prevalent gout, seemed similar in men and women. Obesity was associated with 2.3-times higher risk of prevalent gout in men and 2.5-times in women, in a model adjusted for race, age- and race-adjusted analyses. In a model that additionally adjusted for hypertension, glomerular filtration rate, total cholesterol, low high density lipoprotein, and diabetes, the risk was 1.7-times in men and 1.9 times in women, respectively. Thus, no major differences in risk by obesity were seen for prevalent gout in men and women in the U.S.
In a genomic analysis of the German MI (myocardial infarction) family study, 1,563 unrelated individuals were studied.71 This included 683 individuals with gout (n=480 males, n=203 females) and unrelated subjects who neither had any indication of gout nor were medicated with urate-lowering therapies including uricosuric agents at any time during follow-up (n=871 males, n=692 females; controls). The study confirmed the association of 3 SNPs in two established genes with gout for the overall sample, namely, SLC2A9 (rs734553 and rs6855911) and ABCG2 (rs2231142). In separate analyses, the authors found the association of both SLC2A9 SNPs (s734553 and rs6855911) with gout in both females and males, whereas ABCG2 SNP rs2231142 showed significant association with gout only in males, but not in females. Thus, this study provides indication that certain genetic risk factors for gout may vary by gender.71
These studies indicate that several clinical and genetic risk factors for gout have similar impact on patients, regardless of gender. The risk imparted by common risk factors for gout including hypertension, obesity and renal failure, did not differ much by gender. Similar genetic polymorphisms were associated with gout in both males and females.
Disparity in Risk of Adverse Effects
Racial/Ethnic Differences
Very few studies have explored whether adverse effects of medications used for the treatment of gout differ by race/ethnicity.
One study examined the genetic markers for severe cutaneous adverse reactions (SCARs) in Koreans.72 Human leukocyte antigen (HLA) class I alleles of 25 cases of allopurinol-induced SCARs (20 cases of drug-induced hypersensitivity syndrome and five cases of Stevens-Johnson syndrome/toxic epidermal necrolysis) and 57 patients tolerant to allopurinol was genotyped.72 They found that B*5801 [92.0 vs. 10.5%, p<0.0001, odds ratio (OR)=97.8], Cw*0302 (92.0 vs. 12.3%, p<0.0001, OR=82.1), and A*3303 (88.0 vs. 26.3%, p<0.0001, OR=20.5) were significantly higher in SCARs compared with tolerant controls. In contrast, A*0201 was not found in SCARs patients despite relatively high frequency in tolerant controls (0% vs. 29.8%). This finding of strong positive association of HLA-B*5801 and negative association of HLA-A*0201 with the risk of allopurinol-induced SCARs in the Korean population is a landmark finding.72 In a recent double-blind randomized controlled trial (RCT) that examined the comparative efficacy and safety of febuxostat 40 mg, febuxostat 80 mg, or allopurinol 300 mg (200 mg in patients with moderate renal impairment) in 2,269 subjects, authors reported that a lower proportion of African-Americans had liver function test abnormality (ALT elevation 2-times or higher, 2% vs. 13%, respectively) compared to Caucasians.73 Rates of most other common adverse events, including upper respiratory symptoms, diarrhea, musculoskeletal symptoms etc. were not different by race/ethnicity.73
We could not find any studies that have documented gender differences in adverse effects of gout treatments.
Disparity in Treatment and Receipt of Quality Gout care
There is no evidence available to suggest differences in pharmacokinetics or pharmacodynamics of urate-lowering therapies by age, gender or race/ethnicity. A recent study that examined the pharmacokinetics, pharmacodynamics, and safety of once-daily oral febuxostat 80 mg in healthy male and female subjects after 7 days, found minimal differences in peak concentrations of febuxostat and percentage decrease in serum uric acid 24-hour mean concentration, between men and women.74 Similarly, a six-month randomized trial of febuxostat compared to allopurinol in patients with gout showed that the efficacy of febuxostat in lowering serum urate was similar in blacks and whites in all treatment groups, regardless of renal function.73 These studies support the notion that aggressive therapy of gout should be pursued in all patients, regardless of age, gender and race/ethnicity.
Racial/Ethnic differences
In a study of 663 U.S. veterans with gout who qualified for testing of at least one quality indicator for gout care, racial/ethnic disparities were seen.75 The cohort had a mean age of 68 years, and a Charlson comorbidity score of 2.5; 99% were men, and of those with known race/ethnicity, 96% were white. Physician adherence to three quality indicators (QIs) in gout patients was examined: (1) lowering of initial daily allopurinol dose to <300 mg/day in presence of renal insufficiency; (2) monitoring of serum urate level at least once during the first 6 months of continued use of a xanthine oxidase inhibitor, such as allopurinol; and (3) monitoring of complete blood cell count and creatine kinase at least every 6 months in patients with renal impairment receiving long-term prophylactic oral colchicine (≥0.5 mg/day for ≥6 months). In the multivariable-adjusted logistic analysis of predictors of overall physician adherence to all QIs applicable as the outcome, patient race was significantly associated with non-adherence to QIs (p=0.035).75 Compared to whites with gout, gout patients of non-white race were more likely to be non-adherent to QIs with an odds ratio of 1.41 (95% confidence interval, 0.52–3.84).75 This association was independent of age, comorbidity index, utilization characteristics (number of inpatient and outpatient visits, type of physician, most frequently seen physician) and health care access (percent service connection).
In an analyses of 3.9 million outpatient visits with a gout diagnosis in 2002 from the National Ambulatory Medical Care Survey in the US, African Americans were less likely than Caucasians to receive ULT with allopurinol (odds ratio [OR] 0.18; 95% CI, 0.04–0.78; p = 0.02) and only 10% were made by African Americans vs. 82% by Caucasians. 76 In another study, African Americans with gout are twice more likely to be non-adherent with ULT than Caucasian patients (OR 1.86; 95% CI, 1.52–2.27).77
Gender differences
Previous studies indicated that inappropriate prescribing is more common in women compared to men among veterans aged ≥65 years, as judged by the Beers criteria for diagnosis-adjusted prevalence of 33 potentially inappropriate medications.38 We examined whether published studies provide evidence of differences in quality of gout care by gender.
In a UK study of 40 practices with 300,000 people, compared to men with gout the prescription of allopurinol was lower in women with gout across all age strata, except the highest age group: <35 years, 48% vs. 0%; 35–44 years, 36% vs. 9%; 45–54 years, 46% vs. 24%; 55–64 years, 48% vs. 42%; 65–74, 50% vs. 45%; and ≥75 years, 46% vs. 50%.66
In an analysis of data from 1.4 million people in managed care plans in the U.S., Harrold et al. identified 6,133 patients with a diagnosis of gout (4,975 men and 1,158 women).78 They found that the use of diuretics was almost twofold greater in women than in men, 77% vs. 40% (p<0.001). Analyses were adjusted for age, total number of encounters with a gout diagnosis, comorbidities, use of diuretics and health plan. In adjusted analyses, compared to men, women were less likely to receive synovial fluid crystal analysis for diagnosis, less likely to receive allopurinol and more likely to receive glucocorticoids with odds ratios of 0.49 (0.34 to 0.72), 0.78 (0.67 to 0.90) and 1.30 (1.12 to 1.50), respectively. Women with gout were more likely to receive serum urate monitoring within 6 months after starting urate-lowering therapy, with an odds ratio of 1.36, 95% CI 1.11 to 1.67).
Knowledge Gaps and Future Research
A recent randomized trial reported similar efficacy and safety of febuxostat for blacks and whites with gout.73 In fact, fewer blacks compared to whites had liver function test abnormalities while taking febuxostat. Thus, lack of race/ethnicity differences in success rates of gout treatments implies that the quality of gout care should not differ by race/ethnicity, as noted in a recent editorial.79 Despite certain risk factors being more common in women (e.g. diuretics) than men, there is no evidence that treatment responses differ by gender either. As discussed above, blacks have higher prevalence of gout than Whites. This implies that one needs to aggressively treat gout among the minorities. Since gout is recognized as a disease mostly affecting men, gout in women may be under-recognized, even in the postmenopausal years.
There are several knowledge gaps related to disparities in gout. For example, it is not known whether the higher risk of gout in blacks compared to whites can be attributed to the differences in risk factors (renal disease, heart failure, serum urate level, obesity), or to genetic differences related to polymorphisms in genes that regulate urate production and excretion. Even less is known about the interplay of genetics and environment as it relates to differences in epidemiology of gout by race/ethnicity and gender. This implies that more studies of gout in women and minorities are needed. At the very least, results should be presented by gender and race/ethnicity for studies with large samples. In particular, RCTs should provide exploratory subgroup analyses by gender and race/ethnicity to highlight any differences in outcomes by these factors. Very few studies have examined various aspects of quality of gout care. As health care in the U.S. increasingly transitions to electronic health record-based systems, more sources of data will be available to examine aspects of quality of care, such as those related to safety and efficacy, among patients with gout.
Conclusions
In conclusion, recent interest in developing new gout therapeutics has also re-energized clinical research in gout. The association of gout with cardiac outcomes is yet another reason to justify the extra attention to the optimal control of gout. Racial/ethnic minorities may share a higher burden of gout, due to higher prevalence of comorbidities (hypertension, obesity, renal failure etc.), the use of predisposing medications such as diuretics, and delay in diagnosis and/or treatment. Women may have more delay in diagnosis with gout, since the disease is male-dominant. More research is needed to provide data in women and racial/ethnic minorities with gout, so that we can learn about similarities and differences (if any) by these demographic factors. While knowing these differences is important, appropriate treatment of gout in all patients regardless of gender and race/ethnicity is perhaps the most relevant and effective approach.
Acknowledgments
Dr. Singh is supported by research grants from the National Institute of Aging, National Cancer Institute, National Institute of Arthritis and Musculoskeletal Diseases, Agency for Health Quality and Research Center for Education and Research on Therapeutics, and the resources and the use of facilities at the Birmingham VA Medical Center, Alabama, USA. The views expressed in this article are those of the author and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Footnotes
Disclosure Dr. Singh has received research grants from Takeda and Savient and consultant fees from Savient, Takeda, Ardea, Regeneron, Allergan, URL Pharmaceuticals, and Novartis. Dr. Singh is a member of the executive of OMERACT (an organization that develops outcome measures in rheumatology and receives arms-length funding from 36 companies), a member of the American College of Rheumatology’s Guidelines Subcommittee of the Quality of Care Committee, and a member of the Veterans Affairs Rheumatology Field Advisory Committee.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Kimball MM, Neal D, Waters MF, Hoh BL. Race and Income Disparity in Ischemic Stroke Care: Nationwide Inpatient Sample Database, 2002 to 2008. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association. 2012 Jul 17; doi: 10.1016/j.jstrokecerebrovasdis.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Wellons MF, Fujimoto VY, Baker VL, et al. Race matters: a systematic review of racial/ethnic disparity in Society for Assisted Reproductive Technology reported outcomes. Fertil Steril. 2012 Aug;98(2):406–409. doi: 10.1016/j.fertnstert.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sclar DA, Robison LM, Schmidt JM, Bowen KA, Castillo LV, Oganov AM. Diagnosis of depression and use of antidepressant pharmacotherapy among adults in the United States: does a disparity persist by ethnicity/race? Clinical drug investigation. 2012 Feb 1;32(2):139–144. doi: 10.2165/11598950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Kreatsoulas C, Anand SS. Disparity in outcomes of surgical revascularization for limb salvage. Race and gender are synergistic determinants of vein graft failure and limb loss. doi: 10.1161/CIRCULATIONAHA.108.810341. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nguyen LL, Hevelone N, Rogers SO, Bandyk DF, Clowes AW, Moneta GL, Lipsitz S, Conte MS. Circulation. 2009;119:123–130. doi: 10.1177/1358863X09107006. [DOI] [PubMed] [Google Scholar]; Vasc Med. 2009 Nov;14(4):397–399. doi: 10.1177/1358863X09107006. [DOI] [PubMed] [Google Scholar]
- 5.Lam BL, Lee DJ, Zheng DD, Davila EP, Christ SL, Arheart KL. Disparity in prevalence of self-reported visual impairment in older adults among U.S. race-ethnic subgroups. Ophthalmic epidemiology. 2009 May-Jun;16(3):144–150. doi: 10.1080/09286580902863007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomson CR, Foley RN, Li Q, Gilbertson DT, Xue JL, Collins AJ. Race and end-stage renal disease in the United States Medicare population: the disparity persists. Nephrology (Carlton) 2008 Oct;13(7):651–656. doi: 10.1111/j.1440-1797.2008.01010.x. [DOI] [PubMed] [Google Scholar]
- 7.Hlaing WM. Race/ethnic disparity in hypertension-related hospitalization in Florida. Ethn Dis. 2007 Summer;17(3):453–460. [PubMed] [Google Scholar]
- 8.Hayanga AJ. Risk of pancreatic adenocarcinoma: disparity between African Americans and other race/ethnic groups. Cancer. 2005 Dec 1;104(11):2530–2531. doi: 10.1002/cncr.21494. author reply 2531. [DOI] [PubMed] [Google Scholar]
- 9.Hawker GA. The quest for explanations for race/ethnic disparity in rates of use of total joint arthroplasty. J Rheumatol. 2004 Sep;31(9):1683–1685. [PubMed] [Google Scholar]
- 10.Wenger NS, Pearson ML, Desmond KA, et al. Epidemiology of do-not-resuscitate orders. Disparity by age, diagnosis, gender, race, and functional impairment. Arch Intern Med. 1995 Oct 23;155(19):2056–2062. [PubMed] [Google Scholar]
- 11.Cox ED, Nackers KA, Young HN, Moreno MA, Levy JF, Mangione-Smith RM. Influence of race and socioeconomic status on engagement in pediatric primary care. Patient education and counseling. 2012 Jun;87(3):319–326. doi: 10.1016/j.pec.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao L, Robert SA. Examining the Racial Crossover in Mortality between African American and White Older Adults: A Multilevel Survival Analysis of Race, Individual Socioeconomic Status, and Neighborhood Socioeconomic Context. Journal of aging research. 2011;2011:132073. doi: 10.4061/2011/132073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu DI, Moreira DM, Gerber L, et al. Effect of race and socioeconomic status on surgical margins and biochemical outcomes in an equal-access health care setting: Results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Cancer. 2012 Mar 13; doi: 10.1002/cncr.27456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trinh QD, Sun M, Sammon J, et al. Disparities in access to care at high-volume institutions for uro-oncologic procedures. Cancer. 2012 Feb 1; doi: 10.1002/cncr.27440. [DOI] [PubMed] [Google Scholar]
- 15.FitzGerald JD, Soohoo NF, Losina E, Katz JN. Potential impact on patient residence to hospital travel distance and access to care under a policy of preferential referral to high-volume knee replacement hospitals. Arthritis care & research. 2012 Jun;64(6):890–897. doi: 10.1002/acr.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langford AT, Resnicow K, Roberts JS, Zikmund-Fisher BJ. Racial and ethnic differences in direct-to-consumer genetic tests awareness in HINTS 2007: sociodemographic and numeracy correlates. Journal of genetic counseling. 2012 Jun;21(3):440–447. doi: 10.1007/s10897-011-9478-2. [DOI] [PubMed] [Google Scholar]
- 17.Cene CW, Roter D, Carson KA, Miller ER, 3rd, Cooper LA. The effect of patient race and blood pressure control on patient-physician communication. Journal of general internal medicine. 2009 Sep;24(9):1057–1064. doi: 10.1007/s11606-009-1051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon HS, Street RL, Jr, Kelly PA, Souchek J, Wray NP. Physician-patient communication following invasive procedures: an analysis of post-angiogram consultations. Soc Sci Med. 2005 Sep;61(5):1015–1025. doi: 10.1016/j.socscimed.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 19.Johnson RL, Roter D, Powe NR, Cooper LA. Patient race/ethnicity and quality of patient-physician communication during medical visits. American journal of public health. 2004 Dec;94(12):2084–2090. doi: 10.2105/ajph.94.12.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manfredi C, Kaiser K, Matthews AK, Johnson TP. Are racial differences in patient-physician cancer communication and information explained by background, predisposing, and enabling factors? Journal of health communication. 2010 Apr;15(3):272–292. doi: 10.1080/10810731003686598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeon-Slaughter H. Economic factors in of patients’ nonadherence to antidepressant treatment. Social psychiatry and psychiatric epidemiology. 2012 Mar 14; doi: 10.1007/s00127-012-0497-6. [DOI] [PubMed] [Google Scholar]
- 22.Byrne MM, Souchek J, Richardson M, Suarez-Almazor M. Racial/ethnic differences in preferences for total knee replacement surgery. J Clin Epidemiol. 2006 Oct;59(10):1078–1086. doi: 10.1016/j.jclinepi.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Rosen AB, Tsai JS, Downs SM. Variations in risk attitude across race, gender, and education. Medical decision making: an international journal of the Society for Medical Decision Making. 2003 Nov-Dec;23(6):511–517. doi: 10.1177/0272989X03258431. [DOI] [PubMed] [Google Scholar]
- 24.Ibrahim SA, Siminoff LA, Burant CJ, Kwoh CK. Differences in expectations of outcome mediate African American/white patient differences in “willingness” to consider joint replacement. Arthritis and rheumatism. 2002 Sep;46(9):2429–2435. doi: 10.1002/art.10494. [DOI] [PubMed] [Google Scholar]
- 25.Hofmann JC, Wenger NS, Davis RB, et al. Patient preferences for communication with physicians about end-of-life decisions. SUPPORT Investigators. Study to Understand Prognoses and Preference for Outcomes and Risks of Treatment. Annals of internal medicine. 1997 Jul 1;127(1):1–12. doi: 10.7326/0003-4819-127-1-199707010-00001. [DOI] [PubMed] [Google Scholar]
- 26.Whittle J, Conigliaro J, Good CB, Lofgren RP. Racial differences in the use of invasive cardiovascular procedures in the Department of Veterans Affairs medical system. N Engl J Med. 1993 Aug 26;329(9):621–627. doi: 10.1056/NEJM199308263290907. [DOI] [PubMed] [Google Scholar]
- 27.Constantinescu F, Goucher S, Weinstein A, Fraenkel L. Racial disparities in treatment preferences for rheumatoid arthritis. Med Care. 2009 Mar;47(3):350–355. doi: 10.1097/MLR.0b013e31818af829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care (full printed version) Vol. 782. 2003. pp. 6–9. http://www.nap.edu/catalog/10260.html. [Google Scholar]
- 29.Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc. 2002 Aug;94(8):666–668. [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson AR. Unequal treatment: report of the Institute of Medicine on racial and ethnic disparities in healthcare. Ann Thorac Surg. 2003 Oct;76(4):S1377–1381. doi: 10.1016/s0003-4975(03)01205-0. [DOI] [PubMed] [Google Scholar]
- 31.Institute of Medicine. 100 Initial Priority Topics for Comparative Effectiveness Research. 2009 http://www.iom.edu/~/media/Files/ReportFiles/2009/ComparativeEffectivenessResearchPriorities/StandAloneListof100CERPriorities-forweb.pdf.
- 32.Cartwright-Smith L, Rosenbaum S, Mehta D. Disparities Reduction and Minority Health Improvement under the ACA. Vol. 3. Washington, D.C: The George Washington University School of Public Health and Health Services; 2011. pp. 1–6. [Google Scholar]
- 33.Notice Number: NOT-HS-11-013. Special Emphasis Notice (SEN): AHRQ Announces Interest in Grants focused on Health Issues of Minority Women. Release Date: April 13, 2011. http://grants.nih.gov/grants/guide/notice-files/NOT-HS-11-013.html. 2011.
- 34.Birnie D, Tung S, Simpson C, et al. Complications associated with defibrillation threshold testing: the Canadian experience. Heart rhythm: the official journal of the Heart Rhythm Society. 2008 Mar;5(3):387–390. doi: 10.1016/j.hrthm.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 35.Curtis LH, Al-Khatib SM, Shea AM, Hammill BG, Hernandez AF, Schulman KA. Sex differences in the use of implantable cardioverter-defibrillators for primary and secondary prevention of sudden cardiac death. JAMA. 2007 Oct 3;298(13):1517–1524. doi: 10.1001/jama.298.13.1517. [DOI] [PubMed] [Google Scholar]
- 36.Hernandez AF, Fonarow GC, Liang L, et al. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA. 2007 Oct 3;298(13):1525–1532. doi: 10.1001/jama.298.13.1525. [DOI] [PubMed] [Google Scholar]
- 37.Kaul P, Chang WC, Westerhout CM, Graham MM, Armstrong PW. Differences in admission rates and outcomes between men and women presenting to emergency departments with coronary syndromes. CMAJ. 2007 Nov 6;177(10):1193–1199. doi: 10.1503/cmaj.060711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bierman AS, Pugh MJ, Dhalla I, et al. Sex differences in inappropriate prescribing among elderly veterans. Am J Geriatr Pharmacother. 2007 Jun;5(2):147–161. doi: 10.1016/j.amjopharm.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Institute of Medicine. [Accessed 07/18/2012];Women’s Health Research: Progress, Pitfalls, and Promise. 9/23/2010. 2010 http://www.iom.edu/Reports/2010/Womens-Health-Research-Progress-Pitfalls-and-Promise/Report-Brief.aspx.
- 40.Healthcare Quality and Disparities in Women: Selected Findings From the 2010 National Healthcare Quality and Disparities Reports. [Accessed 08/05/2012];Fact Sheet. 2011 AHRQ Publication No. 11-0005-1-EF, March 2011. http://www.ahrq.gov/qual/nhqrwomen/nhqrwomen.htm.
- 41••.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011 Oct;63(10):3136–3141. doi: 10.1002/art.30520. This is the first comprehensive popoulation-based epidemiological study of prevalence of gout in the U.S. general population. [DOI] [PubMed] [Google Scholar]
- 42.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008 Jan;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 43.Singh JA, Strand V. Gout is associated with more comorbidities, poorer health-related quality of life and higher healthcare utilisation in US veterans. Ann Rheum Dis. 2008 Sep;67(9):1310–1316. doi: 10.1136/ard.2007.081604. [DOI] [PubMed] [Google Scholar]
- 44.Edwards NL, Sundy JS, Forsythe A, Blume S, Pan F, Becker MA. Work productivity loss due to flares in patients with chronic gout refractory to conventional therapy. J Med Econ. 2011;14(1):10–15. doi: 10.3111/13696998.2010.540874. [DOI] [PubMed] [Google Scholar]
- 45.Kleinman NL, Brook RA, Patel PA, et al. The impact of gout on work absence and productivity. Value Health. 2007 Jul-Aug;10(4):231–237. doi: 10.1111/j.1524-4733.2007.00173.x. [DOI] [PubMed] [Google Scholar]
- 46.Roddy E, Zhang W, Doherty M. Is gout associated with reduced quality of life? A case-control study. Rheumatology (Oxford) 2007 Sep;46(9):1441–1444. doi: 10.1093/rheumatology/kem150. [DOI] [PubMed] [Google Scholar]
- 47.Khanna D, Ahmed M, Yontz D, et al. The disutility of chronic gout. Qual Life Res. 2008 Jun;17(5):815–822. doi: 10.1007/s11136-008-9355-0. [DOI] [PubMed] [Google Scholar]
- 48.Krishnan E, Baker JF, Furst DE, Schumacher HR. Gout and the risk of acute myocardial infarction. Arthritis Rheum. 2006 Aug;54(8):2688–2696. doi: 10.1002/art.22014. [DOI] [PubMed] [Google Scholar]
- 49••.De Vera MA, Rahman MM, Bhole V, Kopec JA, Choi HK. Independent impact of gout on the risk of acute myocardial infarction among elderly women: a population-based study. Ann Rheum Dis. 2010 Jun;69(6):1162–1164. doi: 10.1136/ard.2009.122770. This is a high-quality cohort study assessing gout as a risk factor for cardiac outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krishnan E, Svendsen K, Neaton JD, Grandits G, Kuller LH, Group MR. Long-term cardiovascular mortality among middle-aged men with gout. Archives of Internal Medicine. 2008 May 26;168(10):1104–1110. doi: 10.1001/archinte.168.10.1104. [DOI] [PubMed] [Google Scholar]
- 51••.Krishnan E. Gout and the risk for incident heart failure and systolic dysfunction. BMJ Open. 2012;2(1):e000282. doi: 10.1136/bmjopen-2011-000282. This is a high-quality cohort study that established a link between gout and heart failure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen SD, Kimmel PL, Neff R, Agodoa L, Abbott KC. Association of incident gout and mortality in dialysis patients. J Am Soc Nephrol. 2008 Nov;19(11):2204–2210. doi: 10.1681/ASN.2007111256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Charles BA, Shriner D, Doumatey A, et al. A genome-wide association study of serum uric acid in African Americans. BMC Med Genomics. 2011;4:17. doi: 10.1186/1755-8794-4-17. This study highlights the genetic correlates of serum uric acid in minorities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rule AD, de Andrade M, Matsumoto M, Mosley TH, Kardia S, Turner ST. Association between SLC2A9 transporter gene variants and uric acid phenotypes in African American and white families. Rheumatology (Oxford) 2011 May;50(5):871–878. doi: 10.1093/rheumatology/keq425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hochberg MC, Thomas J, Thomas DJ, Mead L, Levine DM, Klag MJ. Racial differences in the incidence of gout. The role of hypertension. Arthritis Rheum. 1995 May;38(5):628–632. doi: 10.1002/art.1780380508. [DOI] [PubMed] [Google Scholar]
- 56.Maynard JW, McAdams Demarco MA, Baer AN, et al. Incident Gout in Women and Association with Obesity in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Med. 2012 Jul;125(7):717 e719–717 e717. doi: 10.1016/j.amjmed.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Portis AJ, Laliberte M, Tatman P, et al. High prevalence of gouty arthritis among the Hmong population in Minnesota. Arthritis Care Res (Hoboken) 2010 Oct;62(10):1386–1391. doi: 10.1002/acr.20232. [DOI] [PubMed] [Google Scholar]
- 58.Wahedduddin S, Singh JA, Culhane-Pera KA, Gertner E. Gout in the Hmong in the United States. J Clin Rheumatol. 2010 Sep;16(6):262–266. doi: 10.1097/RHU.0b013e3181eeb487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rose BS. Gout in Maoris. Semin Arthritis Rheum. 1975 Nov;5(2):121–145. doi: 10.1016/0049-0172(75)90002-5. [DOI] [PubMed] [Google Scholar]
- 60.Stanhope JM, Prior IA. Uric acid, joint morbidity, and streptococcal antibodies in Maori and European teenagers. Rotorua Lakes study 3. Ann Rheum Dis. 1975 Aug;34(4):359–363. doi: 10.1136/ard.34.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61••.Juraschek SP, Miller ER, 3rd, Gelber AC. Body mass index, obesity, and prevalent gout in the United States in 1988–1994 and 2007–2010: Body Mass Index, Obesity, and Gout. Arthritis Care Res (Hoboken) 2012 Jul 6; doi: 10.1002/acr.21791. This study used NHANES data and described the association between obesity and prevalent gout over time. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Phipps-Green AJ, Hollis-Moffatt JE, Dalbeth N, et al. A strong role for the ABCG2 gene in susceptibility to gout in New Zealand Pacific Island and Caucasian, but not Maori, case and control sample sets. Hum Mol Genet. 2010 Dec 15;19(24):4813–4819. doi: 10.1093/hmg/ddq412. [DOI] [PubMed] [Google Scholar]
- 63.Hall AP, Barry PE, Dawber TR, McNamara PM. Epidemiology of gout and hyperuricemia. A long-term population study. Am J Med. 1967 Jan;42(1):27–37. doi: 10.1016/0002-9343(67)90004-6. [DOI] [PubMed] [Google Scholar]
- 64.Arromdee E, Michet CJ, Crowson CS, O’Fallon WM, Gabriel SE. Epidemiology of gout: is the incidence rising? J Rheumatol. 2002 Nov;29(11):2403–2406. [PubMed] [Google Scholar]
- 65.Currie WJ. Prevalence and incidence of the diagnosis of gout in Great Britain. Ann Rheum Dis. 1979 Apr;38(2):101–106. doi: 10.1136/ard.38.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harris CM, Lloyd DC, Lewis J. The prevalence and prophylaxis of gout in England. J Clin Epidemiol. 1995 Sep;48(9):1153–1158. doi: 10.1016/0895-4356(94)00244-k. [DOI] [PubMed] [Google Scholar]
- 67.Grodzicki T, Palmer A, Bulpitt CJ. Incidence of diabetes and gout in hypertensive patients during 8 years of follow-up. The General Practice Hypertension Study Group. J Hum Hypertens. 1997 Sep;11(9):583–585. doi: 10.1038/sj.jhh.1000496. [DOI] [PubMed] [Google Scholar]
- 68.De Souza AW, Fernandes V, Ferrari AJ. Female gout: clinical and laboratory features. J Rheumatol. 2005 Nov;32(11):2186–2188. [PubMed] [Google Scholar]
- 69.Akizuki S. A population study of hyperuricaemia and gout in Japan--analysis of sex, age and occupational differences in thirty-four thousand people living in Nagano Prefecture. Ryumachi [Rheumatism] 1982 Jun;22(3):201–208. [PubMed] [Google Scholar]
- 70.Chen JH, Yeh WT, Chuang SY, Wu YY, Pan WH. Gender-specific risk factors for incident gout: a prospective cohort study. Clin Rheumatol. 2012 Feb;31(2):239–245. doi: 10.1007/s10067-011-1802-6. [DOI] [PubMed] [Google Scholar]
- 71.Kolz M, Johnson T, Sanna S, et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009 Jun;5(6):e1000504. doi: 10.1371/journal.pgen.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kang HR, Jee YK, Kim YS, et al. Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet Genomics. 2011 May;21(5):303–307. doi: 10.1097/FPC.0b013e32834282b8. [DOI] [PubMed] [Google Scholar]
- 73.Wells AF, MacDonald PA, Chefo S, Jackson RL. African American patients with gout: efficacy and safety of febuxostat vs allopurinol. BMC Musculoskelet Disord. 2012;13:15. doi: 10.1186/1471-2474-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khosravan R, Kukulka MJ, Wu JT, Joseph-Ridge N, Vernillet L. The effect of age and gender on pharmacokinetics, pharmacodynamics, and safety of febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase. J Clin Pharmacol. 2008 Sep;48(9):1014–1024. doi: 10.1177/0091270008322035. [DOI] [PubMed] [Google Scholar]
- 75.Singh JA, Hodges JS, Toscano JP, Asch SM. Quality of care for gout in the US needs improvement. Arthritis Rheum. 2007 Jun 15;57(5):822–829. doi: 10.1002/art.22767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krishnan E, Lienesch D, Kwoh CK. Gout in ambulatory care settings in the United States. J Rheumatol. 2008 Mar;35(3):498–501. [PubMed] [Google Scholar]
- 77.Solomon DH, Avorn J, Levin R, Brookhart MA. Uric acid lowering therapy: prescribing patterns in a large cohort of older adults. Ann Rheum Dis. 2008 May;67(5):609–613. doi: 10.1136/ard.2007.076182. [DOI] [PubMed] [Google Scholar]
- 78.Harrold LR, Yood RA, Mikuls TR, et al. Sex differences in gout epidemiology: evaluation and treatment. Ann Rheum Dis. 2006 Oct;65(10):1368–1372. doi: 10.1136/ard.2006.051649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh JA. Can racial disparities in optimal gout treatment be reduced? Evidence from a randomized trial. BMC Med. 2012;10:15. doi: 10.1186/1741-7015-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]


