Abstract
Inflammation and its mediators, including cytokines and reactive oxigen species, are believed to contribute to neurodegeneration. In the mouse brain, we found that the interleukin 13 receptor alpha 1 chain (IL-13Rα1) was expressed in the dopaminergic (DA) neurons of the substantia nigra pars compacta which are preferentially lost in human Parkinson’s disease (PD). Mice deficient for Il13ra1 exhibited resistance to loss of DA neurons in a model of chronic peripheral inflammation using bacterial lipopolysaccharide. Interleukin-13, as well as interleukin-4, potentiated the cytotoxic effects of t-butyl hydroperoxide and hydrogen peroxide on mouse dopaminergic MN9D cells. Collectively, our data indicate that expression of IL-13Rα1 on DA neurons can increase their susceptibility to oxidative stress-mediated damage thereby contributing to their preferential loss. In humans, Il13ra1 lies on the X chromosome within the PARK12 locus of susceptibility to PD suggesting that IL-13Rα1 may have a role in the pathogenesis of this neurodegenerative disease.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder and affects approximately 1% of the population over the age of 60 (1). Clinical symptoms are believed to arise primarily from the progressive reduction of dopamine signaling in the basal ganglia resulting from the loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNc). Current treatments for PD focus on symptom management usually through the use of levadopa to increase brain dopamine levels. There are currently no therapies to address the underlying neurodegeneration. Genetic studies have identified at least 9 rare, monogenic causes of PD and over 13 loci (2). The majority of PD cases remain however sporadic and idiopathic with environmental factors, including exposure to pesticides and toxins, thought to contribute to disease development in these cases (3).
Recently, inflammation and its mediators, including cytokines and reactive oxygen species (ROS), were also proposed to contribute to the neuronal loss occurring in idiopathic PD (4). Yet, how these factors might cause the preferential loss of DA neurons observed in PD is not entirely understood. By examining the location of genes encoding immunoregulatory factors with Mendelian linkage to neurodegenerative diseases, we observed that the interleukin 13 receptor alpha 1 gene (Il13ra1) lies at Xq24 within the PARK12 locus of PD susceptibility on the human X chromosome (5, 6).
The IL-13 receptor alpha 1 chain (IL-13Rα1) is recognized for its role in mediating allergic responses. It has been demonstrated by ablation of Il13ra1 in mice that IL-13Rα1 is essential for allergen-induced airway hyperreactivity, mucus hypersecretion, and eotaxin production (7, 8). IL-13Rα1 heterodimerizes with the IL-4R alpha chain (IL-4Rα) forming a complex capable of binding IL-13 or IL-4. To date, this complex is the only known signal transducer for IL-13 while IL-4 can also signal through an IL-4Rα/gamma chain complex. IL-13 and IL-4 receptor binding results in the initiation of a gene program by phosphorylation of the transcription factor STAT6 via Janus Kinase (9).
To test the possibility that IL-13Rα1 may have a role in PD, we compared the effects of chronic peripheral inflammation with bacterial lipopolysaccharides on dopaminergic neuronal loss in the SNpc of mice null for IL-13Rα1 and their wild-type littermates. We also tested the effects of IL-13 and IL-4 on dopaminergic neuronal loss in vitro.
Materials and Methods
Animals
Mouse husbandry and procedures were performed under the guidelines of the Institutional Animal Care and Use Committee. Il13ra1 null mice on the C57/B6 background were obtained from Regeneron Pharmaceuticals, Inc. (7) In these animals the disrupted I13Ra1 contains a LacZ reporter cassette that can be used for β-galactosidase staining. As in humans, mouse I13ra1 also localized to the X chromosome. All experiments were carried out on male null mice (Il13ra −/Y) and wild-type littermates (wt or Il13ra +/Y).
In situ hybridization
ISH was carried out as previously described by us (10) using the digoxigenin-UTP (DIG) probe kit (Roche). Il13ra1 RNA was isolated by PCR using the following primers (listed 5′-3′): forward: TTCTTGCCGACGCTGTCT and reverse: GGGTCC TCAAACAATAGGCA.
β-galactosidase reporter assay
Mouse brains containing a lacz-interrupted Il13ra1 gene were harvested, cryosectioned to 30 μm and placed onto slides. Sections were fixed with 0.125% glutaraldehyde, permeabilized with 0.01% Na-deoxycholate and 0.02% NP-40 and signal was detected by incubating with 1mg/mL x-gal at 30°C for 4 h while rocking. Slides were washed, dried and mounted.
Immunohistochemistry
Mouse brains were harvested, fixed and cryoprotected as described above. Free floating brain sections were permeabilized with 0.2% Triton X-100 washed and probed with primary antibodies against TH (1:1000; Millipore), Iba1 (1:300; Wako), GFAP (1:300; Dako) or LacZ (1:300; MBL) overnight at 4°C. TH DAB staining and neuronal counting was carried out as previously described by us (11, 12).
For immunofluorescence, sections were incubated with streptavidin-Alexa596 (1:500, Invitrogen) for LacZ detection or Alexa488 secondary antibody (1:500, Invitrogen) for TH, Iba1 and GFAP, washed incubated with 4′,6-diamidino-2-phenylindole (DAPI) at 1 ng/mL, washed, mounted and visualized with a Zeiss Radiance 2100 confocal system.
Chronic inflammatory model
Mice were injected (i.p.) twice weekly with saline or bacterial lipopolysaccharide (LPS; 100 μg/kg from Escherichia coli O111:B4; Sigma-Aldrich) for six months as previously described (13).
Cell viability assays
The mouse dopaminergic MN9D cells were obtained from Dr. Alfred Heller, The University of Chicago, and were maintained at 37°C with 5% CO2 and cultured in collagen-coated plates in DMEM with 10% FCS. For assessment of oxidative damage, the cells were treated with PBS or IL-13 or IL-4 at the indicated doses in the presence of PBS, H2O2 (80 μM) or tBOOH (10μM). Cell viability was assayed 24 h later by the LDH assay (Roche) according to the manufacturer’s instructions. Results are the average of at least 3 independent experiments performed in triplicate.
Real Time PCR
RNA was isolated, purified, quantified, and used as a template for cDNA as previously described (10) A dilutional analysis of the samples indicated a correlation coefficient for concentration and cycle threshold (Ct) of 0.99. Reactions were performed on a 7900HT Fast Real Time PCR machine (Applied Biosystems) using SYBR Green (Invitrogen Life Technologies). The delta Ct (dCt) method was performed to determine relative concentrations, using the Ct of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the normalizing value for calculation of relative values (2dCt). Primers used (listed 5′-3′): IL-13 forward, CTT GCC TTG GTG GTC TCG; IL-13 reverse CGT TGC ACA GGG GAG TCT; IL-4 forward CAT CGG CAT TTT GAA CGA G; IL-4 reverse, CGA GCT CAC TCT CTG TGG TG.
Statistics
One-factor analyses of variance (ANOVA) followed by Dunnett’s post hoc test was used to determine significance (p<0.05).
Results
Il13ra1 is expressed by DA neurons in the SNc
To map the expression of Il13ra1 in the brain, we performed in situ hybridization (ISH) of Il13ra1 using RNA antisense and sense control probes on wild-type and Il13ra1 knockout mouse brain sections (Fig. 1A and B). Il13rα1 mRNA was detected in the SNc and the ventral tegmental area (VTA) but not in the glomerular layer of the olfactory bulb containing dopaminergic neurons or in the locus coeruleus containing noradrenergic neurons (Suppl Fig. 1A and B). This distribution was similar to that for Il13ra1 reported in the Allen Brain Atlas (http://www.brain-map.org). An RNA probe for tyrosine hydroxylase (TH), the first and rate limiting enzyme in the synthesis of catecholamines, was used as a marker for DA neurons (Fig. 1C). We observed that Il13ra1 and TH labeling showed similar patterns at comparable levels of the ventral midbrain indicating that Il13ra1 mRNA is expressed in the DA neurons of the SNc and VTA. No detectable Il13ra1 signal was found in the SNc of Il13ra −/Y mice by ISH carried out with the anti-sense probe (Fig. 1D). Expression of Il13ra1 in the SNc was also confirmed by utilizing an Il13ra1 knockout mouse containing a Lacz reporter cassette expressed within the Il13ra1 gene locus. Brain sections from these mice assayed for β-galactosidase activity showed staining in the SNc and VTA (Fig. 1E). To confirm the expression of IL-13Rα1 in DA neurons in the SNc, double immunohistochemistry was performed on the Il13ra −/Y mice containing the LacZ reporter using antibodies against TH and LacZ (Fig. 1F and Suppl. Fig. 2). Over 80% of the TH neurons were found positive for LacZ. A 3-D rendering produced from Z-stacks of confocal images demonstrating colocalization in one cell is shown in Suppl Fig. 2A. Conversely, LacZ expression did not colocalize with the microglial marker, Iba1 or the astrocyte marker, GFAP (Suppl Fig. 2 B and C).
Figure 1.
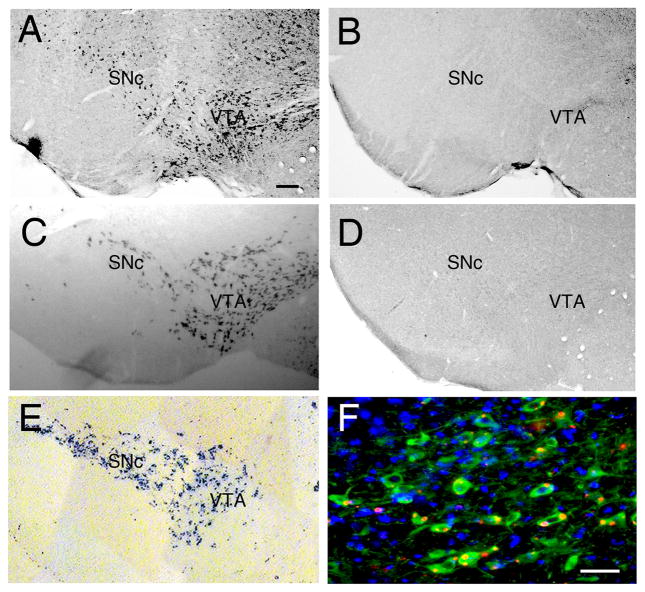
Mapping Il13ra1 expression in SNc and VTA. A, In situ hybridization (ISH) of Il13ra1 mRNA using anti sense RNA probes in representative wild type (Il13ra+/Y) mouse brains shows expression of Il13ra1 is restricted to the SNc and VTA. B, representative ISH of Il13ra1 mRNA sense probe on a brain section from a wild type mouse used as a control. C, Representative ISH with TH antisense probe used as a marker for DA neurons on a brain section from a wild type mouse. D, In situ hybridization (ISH) of Il13ra1 mRNA using anti-sense RNA probes in a representative Il13ra−/Y mouse brain shows no detectable Il13ra1 expression. E, β-galactosidase assay for the detection of LacZ reporter in a brain from a Il13ra −/Y mouse reveals SNc and VTA restricted expression of Il13ra1. F, The Il13ra1 reporter, LacZ, localizes to DA neurons in the SNc of Il13ra −/Y mice. Representative confocal microscopy images shown were obtained by immunohistochemistry: TH (green), LacZ (red), merge, nuclei stained with DAPI (blue). Scale bar = 200 μm in A–E, =50μm in F
Mice lacking Il13ra1 are protected in a chronic inflammatory model of DA neuron loss
We next examined if IL-13Rα1 affects DA neuron survival in vivo. We chose the chronic peripheral inflammatory model of DA neuron loss (13). We used eight week-old Il13ra −/Y and +/Y mice (n=11 per group) that were injected twice weekly for six months with saline or 100 μg/kg LPS (i.p.). Out of 23 animals treated with LPS, one Il13ra −/Y mouse died 2 weeks before termination of the study. DA neurons of the SNc were then labeled by immunohistochemistry using a TH antibody and counted (Fig. 2A and B). Vehicle treated Il13ra −/Y mice and their wild-type littermates used in the study showed a similar number of SNc TH+ neurons (10132 ± 192 wild type animals treated with vehicle (saline) versus 9626 ± 346 for Il13ra −/Y mice treated with vehicle; n=11 per group, NS). Similar to what was originally described (13), 6 months of LPS treatment induced a 44% reduction in SNc DA neurons in wt mice while the number of SNc DA neurons was reduced by only 10% in Il13ra −/Y mice (5711 ± 330 for wt mice receiving LPS and 8511 ± 476 for Il13ra −/Y mice treated with LPS; n=11 per group, p<0.05) (Fig. 2A and B).
Figure 2.
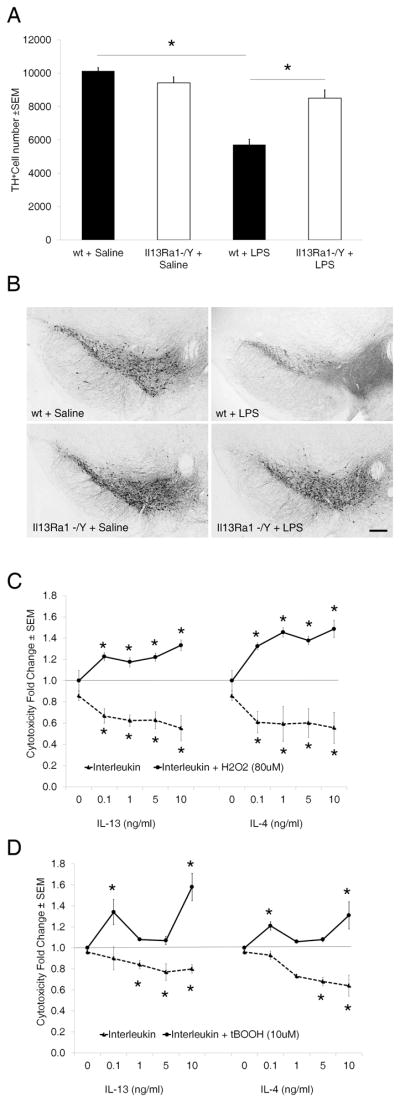
Il13ra1 enhances neuronal loss in vivo and in vitro. A, Il13ra −/Y mice show decreased DA neuron loss in a chronic peripheral inflammatory model of PD. Histogram of the total number of TH positive neurons in the SNc of Il13ra −/Y and +/Y mice chronically injected (i.p.) with LPS (100μg/kg) twice weekly for six months versus control mice injected with saline used as vehicle (n=11 mice/group). Values (mean +/− SEM) were normalized to the Il13ra +/Y/saline group and asterisk indicates p < 0.05 versus all other groups. B, Representative images showing immunohistochemical labeling of DA neurons using a TH antibody for Il13ra −/Y and +/Y mice injected with saline or LPS as indicated. Scale bar = 200 μm. C, IL-13 and IL-4 potentiate oxidative stress induced cell death in MN9D cells. Cells were treated with saline, IL-13 or IL-4 at the doses indicated followed by H2O2. Cell viability was assessed by the LDH assay 24 h later. Values (mean +/− SEM) are normalized to PBS only group and are from three independent experiments. Asterisks indicate p < 0.05 versus saline or 80 μM H2O2 group. D, Similar experiments were carried out in the presence of tBOOH.
Semiquantitative RT-PCR performed on RNA extracted from the SNc of mice treated with LPS showed that compared to the level of saline treated animals, arbitrarily fixed to 1 (± 0.19), levels were 0.62 (± 0.26), 1.23 (± 0.58), and 2.5 (± 0.99) at 0.5 hr, 1hr and 2 hr, respectively (p<0.05 at 2 hr). This is similar to other studies showing that peripheral LPS treatment elevated the transcription of IL-13 centrally (14, 15). No IL-4 transcript was detected in the same samples.
IL-13Rα1 signaling increases the sensitivity of dopaminergic cells to oxidative stress
The above results led us to employ a dopaminergic cell culture model to more closely examine IL-13Rα1-mediated death. We used the mouse dopaminergic line MN9D which expresses both IL-13Rα1 and IL-4Rα as determined by PCR (not shown) and measured cell viability by the LDH assay following treatment with IL-13 or IL-4 alone or in the presence of oxidative stress.
Neither IL-13 or IL-4 alone induced cellular loss. Instead they decreased cell mortality in a dose dependent manner over 24 hr (Fig. 2C and D). Since neuroinflammation results in the production of neurotoxic factors that include ROS we then tested the effects of IL-13 and IL-4 on cell survival in the presence of two different oxidative agents, H2O2 and tBOOH. Based on dose curve responses (not shown) we chose to use the maximal dose of agent that caused minimal damage (80 μM for H2O2 and 10 μM for tBOOH). Under these conditions, both cytokines potentiated oxidative stress-induced cytotoxicity. In the presence of H2O2, toxicity increased in a dose dependent manner and at the maximal dose of cytokine tested (10 ng/ml) reached 33% and 49% for IL-13 and IL-4, respectively (Fig. 2C). The dose curve response was U shaped for both cytokines in the presence of tBOOH and was highest at the smallest and at the highest doses tested with a maximum of 58% and 31% increase for IL-13 and IL-4, respectively (Fig. 2D). These difference were larger when compared to the effects of the cytokine alone. These data indicate that IL-13 and IL-4 can contribute to the loss of DA neurons by increasing susceptibility to oxidative damage and that this phenomenon may underlie the resistance of Il13ra −/Y DA neurons to chronic inflammation observed in vivo.
Discussion
We show here for the first time that IL-13Rα1 is expressed in DA neurons and that mice deficient for IL-13Rα1 have increased resistance to loss of SNc DA neurons in a chronic model of peripheral LPS inflammation.
Expression of IL-13Rα1 was detected in dopaminergic neurons of the VTA and the SNc and was not found in noradrenergic neurons of the locus coeruleus nor in the glomerular dopaminergic neurons of the olfactory bulb although we cannot exclude that expression may occur at a lower level also in these cells. Since preferential loss of SNc neurons, and to a minor extent of VTA neurons (16), is a hallmark of Parkinson’s Disease, we tested the hypothesis that IL-13Rα1 may contribute to the vulnerability of these cells to stress.
Neuronal degeneration in PD includes mitochondrial dysfunction, oxidative stress, and protein misfolding (17–21). Recently, inflammatory processes mediated by glial or infiltrating T cells have also been proposed to contribute to PD by generating toxic mediators (4, 22–25). These include cytokines as well as ROS that can sustain inflammation but lack specificity for DA neurons. Thus, we utilized a mouse model in which chronic inflammation induced by peripheral injection of LPS was previously shown to induce persistent neuroinflammation, oxidative stress and the eventual loss of DA neurons (13). We chose this model over the toxin-based PD models of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 6-hydroxydopamine because although inflammation occurs in these toxin-based models, the specificity for DA neuronal loss is conferred by the neurotoxins (26) and because the LPS model mimics a condition in which a peripheral and non-neuronal specific insult induce central nervous system damage. Thus, this type of model may be highly relevant towards understanding the correlation between environmental factors and idiopathic PD. Our finding that using this model, IL-13Rα1 deficient mice showed significantly decreased loss of DA neurons in the SNc suggests that IL-13Rα1 may be a factor contributing to the selective loss of these cells in PD.
In the brain, IL-13 was demonstrated previously in the microglia of LPS-treated mice and in the cerebrospinal fluid (27–30). A role for this cytokine in modulating neuroinflammation is being investigated by other groups with a focus on the ability of IL-13 to contribute to the death of activated microglia cells thus reducing the contribution of these cells to neuronal loss. Indeed, both IL-13 and IL-4 are considered anti-inflammatory and can lower the production of several pro-inflammatory cytokines including some previously shown to contribute to the inflammation-associated loss of DA neurons (31–37). Thus, our findings that mice devoid of IL-3Rα1 have increased rather than reduced resistance to loss of TH+ neurons in the SNc appears at first paradoxical. Yet, despite its ability to ultimately reduce inflammation, IL-13 and possibly IL-4, may act directly on neurons expressing IL-13Rα1 to cause damage. This action might occur only under pathological conditions and in an inflammatory environment as neither IL-13 nor IL-4 alone diminished MN9D cell survival but both significantly potentiated the toxic effects of H2O2 and tBOOH. These findings are in agreement with other studies showing that IL-13 and IL-4 can exert cytotoxic effects primarily by enhancing the action of cytotoxic factors such as LPS, IFN-γ and thrombin (27, 29, 38–40). However, the mechanisms mediating this phenomenon remain to be determined.
The chromosomal localization of Il13Ra1 supports the possibility that it may be involved in human PD. In fact, human Il13Ra1 lies on the X chromosome at Xq24 within the Xq21-q25 region found to contain the PARK12 locus of PD susceptibility (5, 6). Although PARK12 is sizable, this locus is of particular interest since an X-linked factor helps explain the observation that the incidence of PD in men is about twice that in women (41). Finally, further studies could determine whether IL-13Rα1 may also be an important factor in the hypothesized link between allergic rhinitis and increased susceptibility to PD (42).
In summary, our study shows that neuronal IL-13Rα1 can increase the susceptibility to oxidative damage and that its expression in DA neurons may contribute to their preferential loss during PD. Therefore, IL-13Rα1, IL-13, IL-4 and other regulators of their respective signaling pathways may represent novel therapeutic targets for PD.
Supplementary Material
Acknowledgments
This work was supported by the Ellison Medical Foundation, National Institutes of Health Grants AG028040, DA030908, and MH48866, and by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan. I.S. was supported by The Kristjan Jaak Scholarship program from the Archimedes Foundation, Estonia. This is The Scripps Research Institute manuscript number 21373.
References
- 1.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 2.Lesage S, Brice A. Parkinson’s disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet. 2009;18:R48–59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- 3.Kasten M, Chade A, Tanner CM. Epidemiology of Parkinson’s disease. In: Vinken PJ, Bruyn GW, editors. Handbook of clinical neurology. Vol. 83. 2007. pp. 129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott WK, Nance MA, Watts RL, Hubble JP, Koller WC, Lyons K, Pahwa R, Stern MB, Colcher A, Hiner BC, Jankovic J, Ondo WG, Allen FH, Jr, Goetz CG, Small GW, Masterman D, Mastaglia F, Laing NG, Stajich JM, Slotterbeck B, Booze MW, Ribble RC, Rampersaud E, West SG, Gibson RA, Middleton LT, Roses AD, Haines JL, Scott BL, Vance JM, Pericak-Vance MA. Complete genomic screen in Parkinson disease: evidence for multiple genes. JAMA. 2001;286:2239–2244. doi: 10.1001/jama.286.18.2239. [DOI] [PubMed] [Google Scholar]
- 6.Pankratz N, Nichols WC, Uniacke SK, Halter C, Rudolph A, Shults C, Conneally PM, Foroud T. Genome screen to identify susceptibility genes for Parkinson disease in a sample without parkin mutations. Am J Hum Genet. 2002;71:124–135. doi: 10.1086/341282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramalingam TR, Pesce JT, Sheikh F, Cheever AW, Mentink-Kane MM, Wilson MS, Stevens S, Valenzuela DM, Murphy AJ, Yancopoulos GD, Urban JF, Jr, Donnelly RP, Wynn TA. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor alpha1 chain. Nat Immunol. 2008;9:25–33. doi: 10.1038/ni1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME. Distinct roles for IL-13 and IL-4 via IL-13 receptor alpha1 and the type II IL-4 receptor in asthma pathogenesis. Proc Natl Acad Sci U S A. 2008;105:7240–7245. doi: 10.1073/pnas.0802465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murata T, Noguchi PD, Puri RK. IL-13 induces phosphorylation and activation of JAK2 Janus kinase in human colon carcinoma cell lines: similarities between IL-4 and IL-13 signaling. J Immunol. 1996;156:2972–2978. [PubMed] [Google Scholar]
- 10.Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S, Henriksen S, Zorrilla EP, de Lecea L, Bartfai T. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006;314:825–828. doi: 10.1126/science.1132191. [DOI] [PubMed] [Google Scholar]
- 11.Conti B, Sugama S, Lucero J, Winsky-Sommerer R, Wirz SA, Maher P, Andrews Z, Barr AM, Morale MC, Paneda C, Pemberton J, Gaidarova S, Behrens MM, Beal F, Sanna PP, Horvath T, Bartfai T. Uncoupling protein 2 protects dopaminergic neurons from acute 1,2,3,6-methyl-phenyl-tetrahydropyridine toxicity. J Neurochem. 2005;93:493–501. doi: 10.1111/j.1471-4159.2005.03052.x. [DOI] [PubMed] [Google Scholar]
- 12.Sugama S, Wirz SA, Barr AM, Conti B, Bartfai T, Shibasaki T. Interleukin-18 null mice show diminished microglial activation and reduced dopaminergic neuron loss following acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment. Neuroscience. 2004;128:451–458. doi: 10.1016/j.neuroscience.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Frank-Cannon TC, Tran T, Ruhn KA, Martinez TN, Hong J, Marvin M, Hartley M, Trevino I, O’Brien DE, Casey B, Goldberg MS, Tansey MG. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J Neurosci. 2008;28:10825–10834. doi: 10.1523/JNEUROSCI.3001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erickson MA, Banks WA. Cytokine and chemokine responses in serum and brain after single and repeated injections of lipopolysaccharide: multiplex quantification with path analysis. Brain Behav Immun. 2011;25:1637–1648. doi: 10.1016/j.bbi.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovac A, Erickson MA, Banks WA. Brain microvascular pericytes are immunoactive in culture: cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide. J Neuroinflammation. 2011;8:139. doi: 10.1186/1742-2094-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uhl GR, Hedreen JC, Price DL. Parkinson’s disease: loss of neurons from the ventral tegmental area contralateral to therapeutic surgical lesions. Neurology. 1985;35:1215–1218. doi: 10.1212/wnl.35.8.1215. [DOI] [PubMed] [Google Scholar]
- 17.Di Monte DA, Lavasani M, Manning-Bog AB. Environmental factors in Parkinson’s disease. Neurotoxicology. 2002;23:487–502. doi: 10.1016/s0161-813x(02)00099-2. [DOI] [PubMed] [Google Scholar]
- 18.Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008;4:600–609. doi: 10.1038/ncpneuro0924. [DOI] [PubMed] [Google Scholar]
- 19.Jenner P. Oxidative mechanisms in nigral cell death in Parkinson’s disease. Mov Disord. 1998;13(Suppl 1):24–34. [PubMed] [Google Scholar]
- 20.Parker WD, Jr, Swerdlow RH. Mitochondrial dysfunction in idiopathic Parkinson disease. Am J Hum Genet. 1998;62:758–762. doi: 10.1086/301812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan JM, Wong ES, Lim KL. Protein Misfolding and Aggregation in Parkinson Disease. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2490. [DOI] [PubMed] [Google Scholar]
- 22.Block ML, Hong JS. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem Soc Trans. 2007;35:1127–1132. doi: 10.1042/BST0351127. [DOI] [PubMed] [Google Scholar]
- 23.McGeer PL, McGeer EG. Glial reactions in Parkinson’s disease. Mov Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- 24.Nagatsu T, Sawada M. Inflammatory process in Parkinson’s disease: role for cytokines. Curr Pharm Des. 2005;11:999–1016. doi: 10.2174/1381612053381620. [DOI] [PubMed] [Google Scholar]
- 25.Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beal MF. Experimental models of Parkinson’s disease. Nat Rev Neurosci. 2001;2:325–334. doi: 10.1038/35072550. [DOI] [PubMed] [Google Scholar]
- 27.Bluthe RM, Bristow A, Lestage J, Imbs C, Dantzer R. Central injection of interleukin-13 potentiates LPS-induced sickness behavior in rats. Neuroreport. 2001;12:3979–3983. doi: 10.1097/00001756-200112210-00025. [DOI] [PubMed] [Google Scholar]
- 28.Shin WH, Lee DY, Park KW, Kim SU, Yang MS, Joe EH, Jin BK. Microglia expressing interleukin-13 undergo cell death and contribute to neuronal survival in vivo. Glia. 2004;46:142–152. doi: 10.1002/glia.10357. [DOI] [PubMed] [Google Scholar]
- 29.Yang MS, Ji KA, Jeon SB, Jin BK, Kim SU, Jou I, Joe E. Interleukin-13 enhances cyclooxygenase-2 expression in activated rat brain microglia: implications for death of activated microglia. J Immunol. 2006;177:1323–1329. doi: 10.4049/jimmunol.177.2.1323. [DOI] [PubMed] [Google Scholar]
- 30.Yang MS, Park EJ, Sohn S, Kwon HJ, Shin WH, Pyo HK, Jin B, Choi KS, Jou I, Joe EH. Interleukin-13 and -4 induce death of activated microglia. Glia. 2002;38:273–280. doi: 10.1002/glia.10057. [DOI] [PubMed] [Google Scholar]
- 31.Joshi BH, Hogaboam C, Dover P, Husain SR, Puri RK. Role of interleukin-13 in cancer, pulmonary fibrosis, and other T(H)2-type diseases. Vitam Horm. 2006;74:479–504. doi: 10.1016/S0083-6729(06)74019-5. [DOI] [PubMed] [Google Scholar]
- 32.Kuperman DA, Schleimer RP. Interleukin-4, interleukin-13, signal transducer and activator of transcription factor 6, and allergic asthma. Curr Mol Med. 2008;8:384–392. doi: 10.2174/156652408785161032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–190. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 34.Brown KD, Zurawski SM, Mosmann TR, Zurawski G. A family of small inducible proteins secreted by leukocytes are members of a new superfamily that includes leukocyte and fibroblast-derived inflammatory agents, growth factors, and indicators of various activation processes. J Immunol. 1989;142:679–687. [PubMed] [Google Scholar]
- 35.Minty A, Chalon P, Derocq JM, Dumont X, Guillemot JC, Kaghad M, Labit C, Leplatois P, Liauzun P, Miloux B, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362:248–250. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- 36.Morgan JG, Dolganov GM, Robbins SE, Hinton LM, Lovett M. The selective isolation of novel cDNAs encoded by the regions surrounding the human interleukin 4 and 5 genes. Nucleic Acids Res. 1992;20:5173–5179. doi: 10.1093/nar/20.19.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKenzie AN, Culpepper JA, de Waal Malefyt R, Briere F, Punnonen J, Aversa G, Sato A, Dang W, Cocks BG, Menon S, et al. Interleukin 13, a T-cell-derived cytokine that regulates human monocyte and B-cell function. Proc Natl Acad Sci U S A. 1993;90:3735–3739. doi: 10.1073/pnas.90.8.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park KW, Baik HH, Jin BK. Interleukin-4-induced oxidative stress via microglial NADPH oxidase contributes to the death of hippocampal neurons in vivo. Curr Aging Sci. 2008;1:192–201. doi: 10.2174/1874609810801030192. [DOI] [PubMed] [Google Scholar]
- 39.Park KW, Baik HH, Jin BK. IL-13-induced oxidative stress via microglial NADPH oxidase contributes to death of hippocampal neurons in vivo. J Immunol. 2009;183:4666–4674. doi: 10.4049/jimmunol.0803392. [DOI] [PubMed] [Google Scholar]
- 40.Yadav MC, Burudi EM, Alirezaei M, Flynn CC, Watry DD, Lanigan CM, Fox HS. IFN-gamma-induced IDO and WRS expression in microglia is differentially regulated by IL-4. Glia. 2007;55:1385–1396. doi: 10.1002/glia.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 42.Bower JH, Maraganore DM, Peterson BJ, Ahlskog JE, Rocca WA. Immunologic diseases, anti-inflammatory drugs, and Parkinson disease: a case-control study. Neurology. 2006;67:494–496. doi: 10.1212/01.wnl.0000227906.99570.cc. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


