Abstract
The study investigated squeezing reaction time (RT) in response to a visual cue during rhythmic voluntary breathing at 0.6 Hz paced by a metronome, breath holding, or at rest in 13 healthy subjects. Rhythmic voluntary breathing slowed down RT, only in the expiratory phase with accompanied changes in the length of respiratory phases, while breath-holding reduced RT. The prolonged RT during voluntary expiratory phases and the absence of changes in RT during voluntary inspiratory phases are most likely related to disproportionally increased cognitive demands during the expiratory phase of voluntary breathing. The absence of changes in RT during voluntary inspiration is likely to be compensated by respiratory-motor facilitation mechanisms in this phase. Shortened RT during breath holding is possibly associated with increased attention.
Keywords: Reaction time, voluntary breathing, respiratory, finger, human
Introduction
Previous research has demonstrated adaptive and dynamic interactions between the respiratory and motor systems in motor tasks involving muscles that are not mechanically linked to respiratory activities (e.g., finger muscles). These interactions occur during both automatic breathing, i.e., resting (Rassler et al. 1999; Mateika and Gordon 2000; Rassler et al. 2000; Lamberg et al. 2003) and voluntary breathing (Li and Laskin 2006; Li and Yasuda 2007). Mutual influences were observed between resting breathing and regulation of finger flexion posture. The reaction to perturbation by a discrete extension torque impulse was faster at the beginning of inspiration but more precise when the torque impulse was applied in mid-expiration. The end of the applied continuous torque steps often coincided with the respiratory phase-transition (Rassler et al. 2000). Similarly, phase-dependent interactions were reported during discrete object manipulation with a predictable weight (Mateika and Gordon 2000). Regardless of where in the respiratory cycle the auditory cue was provided to start the task, the inspiratory duration was shorter and the rate of prehensile force development was faster in lifting an object of 1000g than of 150 g. The observed phase-dependent interactions during resting breathing could partially be explained by motor and respiratory inputs to spinal motor neurons being mutually facilitatory during the inspiratory phase of resting breathing (Gandevia 1976).
Volitional breathing has been shown to influence motor functions of non-respiratory muscles (Li and Laskin 2006; Li and Yasuda 2007; Ikeda et al. 2009) but with a different pattern than respiratory muscles. Initiation of self-paced forced expiration or inspiration while maintaining a static force output (i.e., with visual feedback) increased force variability of isometric finger forces, as compared to resting breathing (Li and Yasuda 2007). Both discrete volitional inspiration and expiration coincided with an abrupt increase in force (i.e., not phase-dependent), subsequently resulting in increased force variability. Voluntary breathing is synchronized with enhanced respiratory-related neuronal activity at multiple cortical areas, including the primary motor cortex, premotor cortex and supplementary motor areas, as manifested by brain imaging studies (Colebatch et al. 1991; Maskill et al. 1991; Ramsay et al. 1993; Fink et al. 1995; Evans et al. 1999; Smejkal et al. 1999; Smejkal et al. 2000; Macey et al. 2003; Macey et al. 2004). It is likely that such enhanced, respiratory-related, neuronal activities in the motor cortex act in concert with the motor drive to non-respiratory muscles (cf. (Guz 1997)). This is demonstrated by voluntary breathing related change in corticospinal excitability of finger muscles (Li and Rymer 2011), thus resulting in observed motor behaviors of these muscles.
As compared to phase-dependent interactions between respiratory and motor systems during resting breathing, the absence of phase-dependent interactions during discrete forced breathing (Li and Yasuda 2007) may be attributed to different tasks. In the Li and Yasuda study, subjects maintained constant finger forces when a discrete forced inspiration/expiration was initiated. The purpose of this experiment was to examine potential phase-dependent interactions between rhythmic voluntary breathing and a discrete motor task, as observed in respiratory and motor interactions at resting breathing, by adopting an established squeezing reaction time paradigm (Li et al. 2005; Li et al. 2009). Reaction time (RT) measures the time interval between the onset of an imperative stimulus (e.g., visual cue) and the onset of movement. Subtle changes in corticospinal excitability, for example by motor imagery (Li et al. 2005; Li et al. 2009), could be reflected in changes in reaction time. Voluntary breathing was paced by a metronome at an accelerated rate of 0.6 Hz (normal, 0.2–0.3 Hz, i.e., 12–18 breaths per min; 36 breaths per min at 0.6 Hz), which increased cognitive demands and effort to voluntarily speed up and deepen breathing. A visual cue was randomly turned on during either expiratory or inspiratory phases of rhythmic voluntary breathing. Subjects were instructed to react to the light by squeezing a handle (i.e., finger flexion). Therefore, interactions between a discrete motor activity (i.e., squeezing) and rhythmic respiratory activities could be compared between resting breathing and rhythmic voluntary breathing. Specifically, we hypothesized that rhythmic voluntary breathing, given increased cognitive demands and effort in both phases, would lead to prolonged reaction time (RT) in both phases. We also hypothesized that RT would be shorter in the inspiratory than expiratory phase, according to the observed facilitation of reaction of finger flexion in the inspiratory phase at rest (Rassler et al. 2000). Another aim of this study was to evaluate the effect of a visual signal delivered during either the inspiratory or expiratory phase on the breathing pattern.
Methods
Subjects
Thirteen healthy subjects (26.0 ± 4.5 years old; 8 men and 5 women) participated in the experiment. All subjects were right-handed based on their preferential use of the right hand during writing and eating. All subjects gave informed consent and all procedures were approved by our Institutional Review Board and conformed to the Declaration of Helsinki.
Apparatus
Subjects sat comfortably with their arms resting symmetrically on a table. Chair height was adjusted to optimize arm positioning. The right wrist was held at approximately 0° using an aluminum wrist splint support. The right hand grasped a customized handle with the thumb and fingers attached to two vertical bars. In this configuration, the carpal bones were stabilized at about 0° with the metacarpophalangeal (MCP) joints at about 90° of flexion, the proximal interphalangeal (IP) joints at about 45° of flexion and the distal IP at about 20° of flexion. The handle consisted of two 4.5 × 14.5 cm2 vertical plates in parallel connected to a 16 × 7 cm2 aluminum base. The distance between the two vertical plates was adjustable to accommodate the hand size of individual subjects. The top ends of the vertical plates were secured with a rod to ensure rigidity of the handle.
Subjects wore a facemask connected to a pneumotach (Series 1110A, Hans Rudolph, Inc., Kansas City, MO). The pneumotach provided real-time measurement of airflow rate during breathing.
A computer monitor was positioned on the testing table 1.2 m in front of the subject. An 8 × 8 cm2 grey square was displayed in the center of the computer screen. Subjects were instructed to squeeze the handle when the square changed from grey to blue. Afterwards, the square would remain blue until the end of the trial.
Electromyographic (EMG) activity of right hand muscles was collected to calculate reaction time (Fig 1). Disposable surface electrodes (DelSys Inc., Boston, MA) were placed over the muscle bellies of the flexor digitorum superficialis (FDS) and extensor digitorum communis (EDC) muscles. The EMG and breathing signals were amplified and digitized with a 1000 Hz sampling rate by a 16 bit A/D board (PCI 6229, National Instruments, Austin, TX). All data were saved to a desktop computer and analyzed using a customized Matlab program.
Fig 1.
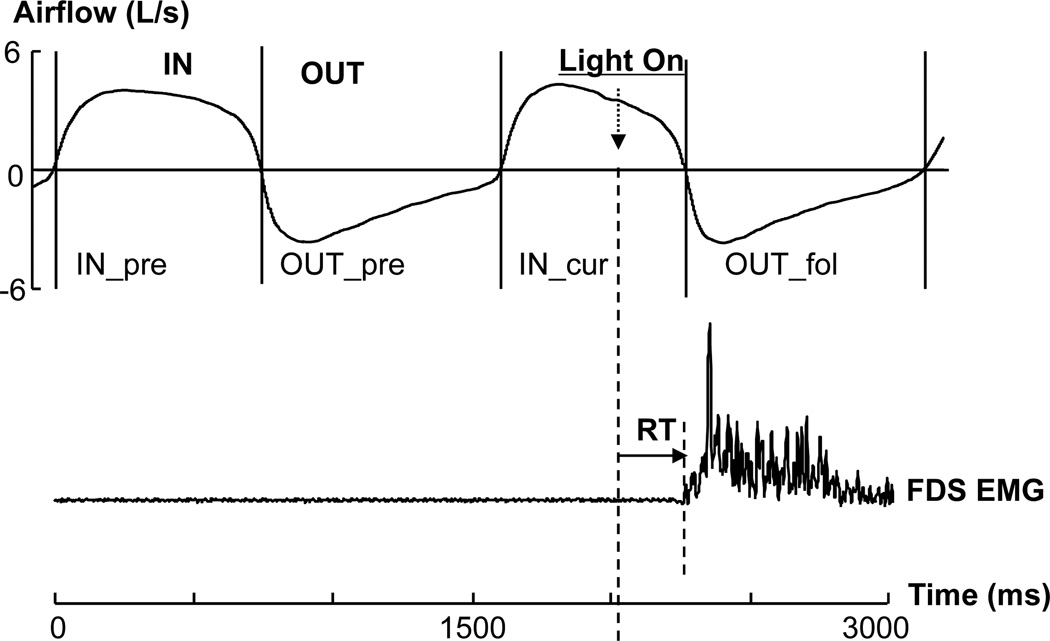
A typical trial. The visual signal was turned on during an inspiratory phase (IN_cur). The time lengths of the preceding inspiration phase (IN_pre), expiration phase (OUT_pre) and the following expiration phase (OUT_fol) were measured for comparisons. The RT was defined as the interval between the light-on moment and the flexor digitorum superficialis (FDS) EMG onset. IN: inspiration; OUT: expiration. Note: the light-on moment may occur at any point of a respiratory phase.
Procedure
Subjects were asked to perform the following three breathing strategies in a randomized order: 1) REST: normal resting breathing without specific instructions; 2) VLUT: voluntary rhythmic breathing following a metronome at 0.6 Hz; peak airflow rates were required to exceed 40% of corresponding maximum airflows, i.e., to speed up and deepen breathing; 3) HOLD: subjects were instructed to breathe normally, and then voluntarily hold their breath after a trial began; it was not necessary to start breath-holding in a certain phase of respiration; subjects were allowed to breathe normally after squeezing the handle, i.e., it was not necessary to hold their breath until the end of a trial. Prior to the breathing conditions, subjects were instructed to perform inspiration or expiration as fast and as deep as possible. This was repeated three times for each respiratory phase, and the highest value was recorded as the maximum airflow rate.
For the three breathing conditions, subjects were instructed to squeeze the handle with their right hand as quickly as possible when they noticed the visual signal (i.e., the square changing from grey to blue) and then release voluntarily. Each subject had 10–15 practice trials before the main experiment. Each trial lasted 10 seconds. In the REST and HOLD conditions, trials began with an audible beep. In the VLUT condition, subjects were instructed to follow metronome beeps at 0.6 Hz (i.e., one beep per breath). The experimenter started to run the computer program when subjects synchronized voluntary rhythmic breathing with the metronome. This synchronization usually took about 5 seconds prior to the start of a trial. Sufficient time to recover from breath-holding and voluntary breathing was allowed after the end of each trial. The visual signal to squeeze was randomly triggered between 3.5 and 7.5 seconds of a 10-seconds trial in the REST and HOLD conditions. As such, subjects did not hold their breath for longer than 7.5 seconds during trials in the HOLD condition. In the VLUT condition, the visual signal was triggered at the moment when the airflow rate reached 40% of the maximal airflow rate of the corresponding respiratory phase. The visual signal to squeeze occurred with equal frequency during the expiratory and inspiratory phases of respiration (see results for details). There were 25 trials in HOLD and 50 trials in REST and VLUT.
Rhythmic voluntary breathing at 0.6 Hz usually leads to hyperventilation for most subjects. Wearing a facemask helped prevent hyperventilation in this situation, though the mask was uncomfortable for some subjects. There were no complaints of dizziness, lightheadedness, or hand numbness during breath-holding or rhythmic voluntary breathing.
Data Analysis
Two dependent variables were measured from recorded EMG and respiratory signals: reaction time (RT) and the time course of respiratory phases, as illustrated in Figure 1. RT was measured from the finger flexor EMG signal. The digitized EMG signals were rectified and filtered by the 10th order low-pass digital Butterworth filter with 150 Hz cut-off frequency. The background EMG (EMGBG) was defined as the mean rectified EMG calculated from −100 ms to the moment of the visual signal (t0). The onset of EMG signal (tEMG) was defined as the time that it took the baseline EMG to increase by 2 standard deviations (SD). RT was calculated from the interval between tEMG and t0. RT was then averaged across tested trials for each condition. Any trial with detectable EMG activity prior to the visual signal was discarded and repeated.
A breathing cycle consists of two phases: inspiratory (IN) and expiratory (OUT) phases (Figure 1). To compare the effect of the reaction time task on the respiratory cycle, we measured the time length of two phases in two consecutive cycles: the light-on cycle and its preceding cycle. When the visual signal was turned on during the IN phase, the time length was measured in a sequence as follows: the inspiratory (IN_pre) and expiratory (OUT_pre) phases of the preceding cycle and the inspiratory (IN_cur) and expiratory (OUT_fol) phases of the current cycle. When the visual signal was turned on during the expiration phase, the sequence was OUT_pre, IN_pre, OUT_cur, and IN_fol. During voluntary rhythmic breathing, breathing phases were further fractionated into early and late segments, which were separated by the peak airflow during a breathing phase. Segmental lengths were calculated separately when the light was turned on during the early or late phase.
Statistics
Repeated-Measures ANOVAs were used to evaluate task performances. A factor of BREATH (3 levels, REST, VLUT and HOLD) was used to compare the effect of breathing condition on RT. The phase-dependent effect of breathing on RT was further examined using factors of BREATH (REST and VLUT) and PHASE (IN and OUT). Factors of PHASE and CYCLE (preceding and current cycles) were used to compare the effect of reaction time tasks on respiratory phases. Whenever necessary, Tukey HSD post-hoc test was performed. The level of significance was set at p≤0.05.
Results
During resting breathing, the airflow rates were 1.1 ± 0.3 (L/s) in the inspiration phase and −0.9 ± 0.3 (L/s) in the expiration phase, respectively. During voluntary rhythmic breathing paced by a metronome at 0.6 Hz, subjects breathed faster and deeper. The airflow rates were 3.5 ± 0.8 L/s in the inspiration phase and −4.2 ± 0.8 L/s in the expiration phase. The length of both inspiratory and expiratory phases was shortened during voluntary breathing relative to resting breathing, but not proportionally. During normal breathing the length of inspiration was 1498 ± 95 seconds and expiration was 2045 ± 155 seconds, whereas during voluntary rhythmic breathing, the length of inspiration was 772 ± 25 seconds and expiration was 874 ± 23 seconds. The time of expiration decreased significantly from 57.4% of a breathing cycle during resting breathing to 53.1% during voluntary rhythmic breathing (p=0.024). Changes in breathing conditions clearly modulated RT (Fig 2A). According to a one-way ANOVA (F(2,12)=19.17, p<0.001), RT was faster in the HOLD condition (268 ms) and slowest in the VLUT condition (308 ms). Both were significantly different from RT in the REST condition (291 ms) (p <0.001).
Fig 2.
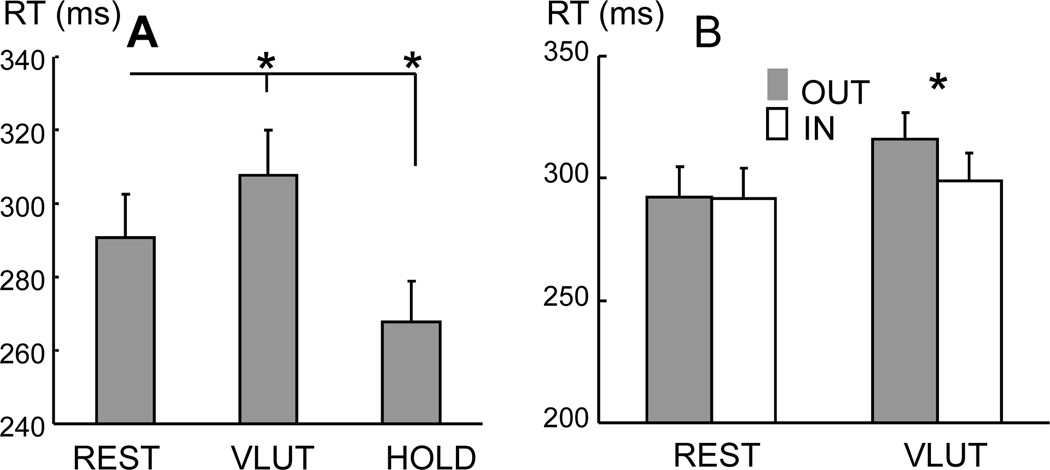
(A) The RT during different breathing conditions. The breathing conditions were REST, VLUT, and HOLD. (B) RT was further measured based on the light-on phase: inspiration (IN) and expiration (OUT). Mean and standard errors are plotted. Asterisk indicates significance (p<0.05). Asterisk indicates significance (p<0.05).
To further investigate the phase-dependent effect of breathing on RT, RT was averaged for each subject based on the phase when the light (i.e., the visual cue) was turned on: IN or OUT phases for both REST and VLUT conditions (Fig 2B). A 2-way ANOVA, with factors of BREATH and PHASE, showed an interaction of BREATH × PHASE (F(1,12)=5.85, p=0.032), but no main effect of the two. The post-hoc test showed that RT was slower in the OUT phase of the VLUT condition (316 ms) than in any of the other phases (p=0.002). No difference in RT among other phases was found.
During voluntary rhythmic breathing, the visual signal (i.e., the light) was turned on randomly and equally distributed to the IN (49.8%) and OUT (50.2%) phases. To investigate the effect of reaction time tasks on breathing cycles, we calculated the time length of four breathing phases of two consecutive cycles in both REST and VLUT conditions. When the light was turned on during the expiratory phase (Fig 3A Left panel), a two-way ANOVA, with factors of CYCLE and PHASE, showed the main effects of CYCLE (F(1,12)=7.00, p=0.021) and PHASE (F(1,12)=5.38, p=0.039), but no significant interactions. The average time length of the current cycle (i.e., the average of OUT_cur and IN_fol) was shorter than that of the preceding cycle (i.e., the average of OUT_pre and IN_pre). However, no change in time length was found in the comparison of individual breathing phases between the preceding and current cycles.
Fig 3.
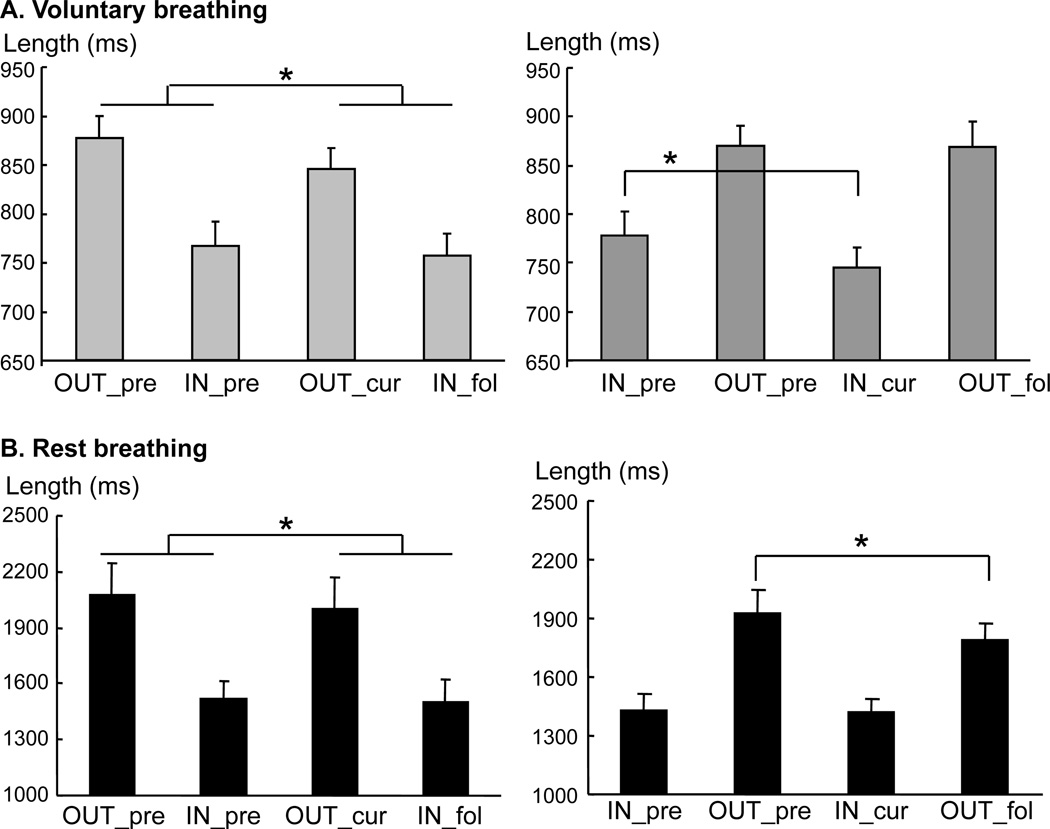
Changes in length of breathing phases during rhythmic voluntary breathing (A) and resting breathing (B) when the light was turned on during the expiratory (OUT) phase (Left panels) and the inspiratory (IN) phase (Right panels). Note that the light-on breathing cycle was shortened during the OUT phase in both conditions, while the inspiratory phase was shortened during the IN phase during rhythmic voluntary breathing. No such change was observed during resting breathing (B). Asterisk indicates significance (p<0.05).
A different pattern of effect on breathing phases was observed when the light was turned on during the inspiratory phase (Fig 3A Right panel). A 2-way ANOVA, with factors of CYCLE and PHASE, showed a main effect of PHASE (F(1,12)=5.52, p=0.037) and an interaction of CYCLE × PHASE (F(1,12)=4.77, p=0.05) but no main effect of CYCLE. The time length in VLUT was shortened in the current inspiratory phase (IN_cur :745 ms) compared to the preceding inspiration phase (IN_pre: 777 ms); while it was similar across the expiratory phase of the two cycles (OUT_pre: 870 ms vs. OUT_fol: 868 ms). Detailed analysis (Fig 4) showed that the length of the inspiratory phase was shortened regardless of whether the light was turned on during the early or late inspiratory phase (p<0.05). However, when the light was turned on during the late expiratory phase, the length of the late expiratory phase and the following early inspiratory phase was shortened (p<0.05, i.e., a quick phase shift). This resulted in a shortened cycle length.
Fig 4.
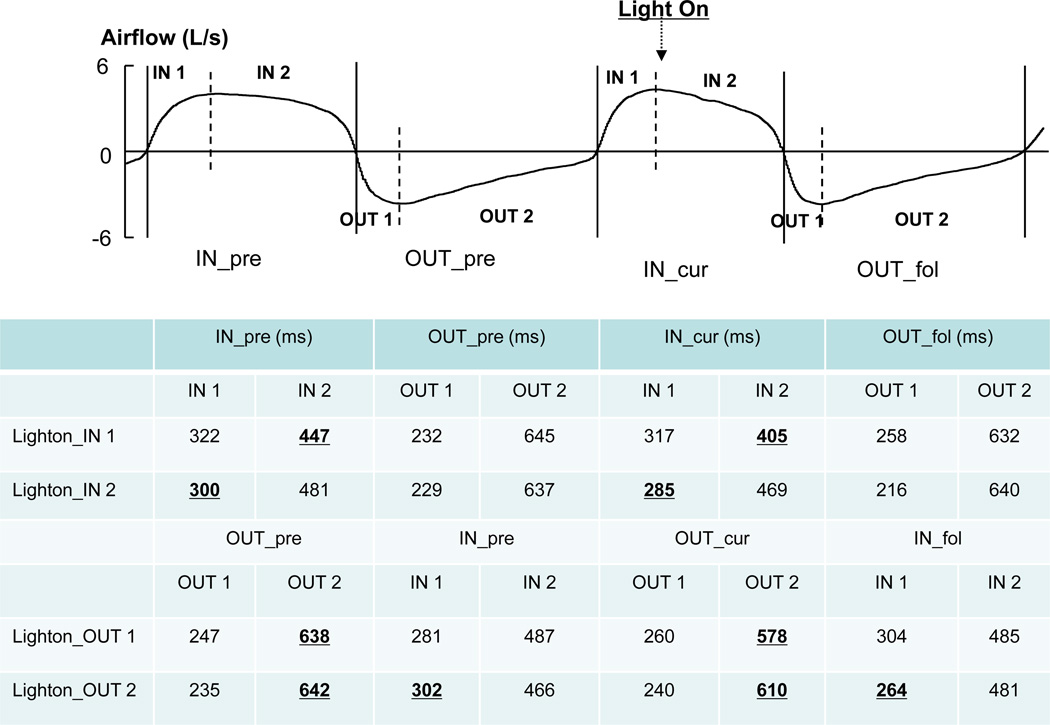
The segmental lengths of breathing phases during voluntary rhythmic breathing. As illustrated in the figure, a phase (e.g., IN_cur) is further fractioned into two (early, IN1; and late, IN2) segments separated by the peak airflow during the phase (vertical dotted lines). The segmental lengths are also measured when the light is turned on in the early segment (e.g., Lighton_IN1) or in the late segment (e.g., Lighton_IN2). Note: same abbreviations are used as in Figure 1. Note: underlined bold numbers in corresponding column indicate significant difference (p<0.05). For example, the second row shows that when the light was turned on during the late inspiratory phase (lighton_IN2), the length of the early inspiratory phase was shortened (300ms vs 285ms).
In contrast, resting breathing had different effects on respiratory cycles. When the visual signal was turned on during the inspiratory phase (Fig 3B, Right panel), the following expiratory phase was shortened more than the preceding expiratory phase (OUT_fol: 1786ms vs. OUT_pre: 1926ms), while there was no change in the inspiratory phase between the current and preceding cycles (IN_cur: 1417ms vs IN_pre: 1431ms). This was confirmed by a 2-way ANOVA, with factors of CYCLE and PHASE, showing a main effect of PHASE (F(1,12)=77.60, p<0.001) and a CYCLE × PHASE interaction (F(1,12)=6.37, p=0.027), but no main effect of CYCLE. When the light was turned on during the expiratory phase (Fig 3B, Left panel), the length of current breathing cycle was shortened (3435 ms vs 3322 ms). A 2-way ANOVA, performed with factors of CYCLE and PHASE, showed a main effect of PHASE (F(1,12)=51.31, p<0.001) and CYCLE (F(1,12)=6.67, p=0.024), but no interaction.
Discussion
The present study demonstrated clearly that rhythmic voluntary breathing following a metronome increased reaction time (RT), while breath-holding decreased RT. More specifically, comparing rhythmic voluntary breathing to resting breathing, reactions were slowed when the visual signal was turned on during the expiratory phase but not changed when the signal occurred during the inspiratory phase. The increased RT was accompanied by a shortened breathing cycle (i.e., the expiratory phase + its following inspiratory phase), relative to its preceding cycle. The absence of changes in RT during the inspiratory phase of voluntary breathing was associated with a shortened inspiratory phase, but no change in the current breathing cycle was found. During resting breathing, no difference in RT was found between breathing phases. Reaction time tasks did not change the length of the breathing cycle. When the light was turned on during the inspiratory phase of resting breathing the following expiratory phase was shortened.
Phase-dependent interactions between rhythmic voluntary breathing and discrete squeezing reaction time tasks by non-respiratory finger muscles are consistent with previous reports of phase-dependent interactions between respiratory and motor systems during resting breathing (Ebert et al. 2000; Mateika and Gordon 2000; Rassler 2000; Rassler et al. 2000). The results of phase-dependent interactions may reflect phase-dependent difficulty during rhythmic voluntary breathing. During resting breathing, inspiration is active while expiration is primarily passive, relying mainly on recoil forces of the chest wall. Rhythmic voluntary breathing at 0.6 Hz requires both active expiration and inspiration. The expiratory phase was significantly shortened from 57.4% during resting breathing to 53.1% of a breathing cycle during voluntary breathing at 0.6 Hz. This result suggests that active expiration has disproportionally shorter duration than active inspiration in this respiratory challenge. An earlier study with positron emission tomographic (PET) imaging found that the cortical areas activated during volitional expiration are more extensive, but overlap with those involved in volitional inspiration (Ramsay et al. 1993). Recent TMS studies also showed that changes in corticospinal excitability for finger muscles were associated with volitional breathing (i.e., deeper and faster; Li and Rymer 2011) rather than normal resting breathing (Filippi et al. 2000). It is likely that, as compared to normal breathing, active expiration requires disproportionally more cortical excitation than active inspiration. Arguably, this makes squeezing RT tasks more difficult, resulting in an elongated RT during this phase.
Voluntary inspiration, like voluntary expiration, is expected to result in elongation of RT. Unchanged RT in this phase is likely attributed to facilitation from voluntary breathing. During resting breathing, reaction to an external perturbation torque in maintaining a finger flexion posture is faster in the inspiratory phase (Rassler et al. 2000). This facilitation effect is likely to be strengthened during active inspiration and thus balances its potential elongating effect on RT, resulting in an unchanged RT during the inspiratory phase. It has been reported that inspiratory motor neurons are active in both respiratory phases to maintain airway patency, with phasic increases in their excitability during the inspiratory phase (Rafferty and Gardner 1996; Butler and Gandevia 2008; Murray et al. 2010), while timing of respiratory phases/cycles is loosely controlled (Rafferty and Gardner 1996) to maintain tidal volume. It is conceivable that excitability of inspiratory motor neurons increases during voluntary expiration and phasically higher during voluntary inspiration. Therefore, RT remains unchanged during the inspiratory phase and elongates during the expiratory phase of rhythmic voluntary breathing. Our results of change in timing of respiratory phases/cycles were also consistent with previous findings (Rafferty and Gardner 1996).
Other factors could also potentially modulate RT in this experiment. Subjects were required to use extra attention to follow the metronome during voluntary breathing. This possible dual tasking effect, however, is expected to impose an overall negative effect on reaction time. Our results of equally distributed triggering in the expiratory and inspiratory phase and of phase-dependent effect of voluntary breathing on RT argue against this possibility. Rhythmic auditory stimuli from the metronome during voluntary breathing may not impose a facilitatory effect on RT via the inter-sensory facilitation (Terao et al. 1997) that occurs when there is a discrete auditory cue (e.g., from TMS click sound). Arguably, background rhythmic auditory stimuli may be distracting, thus requiring more attention, and impose an overall negative effect on RT. It is also likely that proximal muscles (e.g., shoulder adductors and flexors) are activated via mechanical linkage to chest wall movement during voluntary breathing, especially in the expiratory phase. Expiration-associated increases in activation of these muscles may be transferred to the distal muscles reflexively via a preprogrammed, proximal-distal synergy (Latash 2000; Chabran et al. 2001; Ginanneschi et al. 2006). Conceivably, this reflexive increase in distal flexor muscles (e.g., finger flexors) could assist in squeezing RT tasks, leading to shorter RT during the expiratory phase than during the inspiratory phase of voluntary breathing. However, our results of shorter RT in the inspiratory phase than in the expiratory phase of voluntary breathing suggest that reflexive increase in activation of distal muscles is not likely to influence RT during voluntary breathing.
Reaction time was shorter during breath holding than the other breathing conditions. Breath holding was very brief, ranging from 3.5 to 7.5 seconds of a trial in this experiment. Subjects were asked to hold their breath until the visual signal was turned on. This brief period of breath holding was not likely to cause significant hemodynamic changes. Subjects had no complaints, such as dizziness. A previous study demonstrated no systemic hemodynamic changes (i.e., blood gas and blood pressure) after 10 minutes of resistive loaded (50 cmH2O l−1 s−1) breathing challenges (Fontanari et al. 1996). On the other hand, breath holding as short as 3 seconds can lead to significant focal changes on fMRI (Abbott et al. 2005). Breath holding can activate areas associated with selective attention, such as the anterior and posterior cingulate. Therefore, a brief period of breath holding could improve attention and concentration to a given task, thus allowing subjects to react faster.
Prolongation of RT with voluntary expiration in this study seems to contradict a previous finding that voluntary expiration increases finger flexor strength (Li and Laskin 2006). However, these phenomena may both be due to the increased effort required for voluntary expiration resulting in greater expiration-related cortical activation (Ramsay et al. 1993). This more extensive cortical activation could have greater influence on finger flexor muscles in force production via a respiratory-motor enhancement mechanism (Guz 1997), resulting in greater finger flexor strength (Li and Laskin 2006). In contrast, cognitive effort during voluntary expiration conceivably produced prolonged RT in this experiment.
Taken together, our study demonstrates mutual influences between squeezing reaction time tasks by non-respiratory muscles and rhythmic voluntary breathing. Elongation of reaction time during voluntary expiratory phases and no change during voluntary inspiratory phases is most likely related to disproportionally increased cognitive demands during the expiratory phase of voluntary breathing. The possible negative effect of voluntary inspiration on reaction time is likely to be compensated by respiratory-motor facilitation mechanisms in this phase. Shortened reaction time during breath holding is possibly associated with increased attention.
Acknowledgement
The study was supported in part by the National Institutes of Health [R01NS060774 and R24 HD050821-08 under subcontract with Rehabilitation Institute of Chicago to S.L.]. We thank Stephen Hampton, MD for editing the manuscript.
References
- Abbott DF, Opdam HI, Briellmann RS, Jackson GD. Brief breath holding may confound functional magnetic resonance imaging studies. Hum Brain Map. 2005;24:284–290. doi: 10.1002/hbm.20086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, Gandevia SC. The output from human inspiratory motoneurone pools. J Physiol. 2008;586:1257–1264. doi: 10.1113/jphysiol.2007.145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabran E, Maton B, Ribreau C, Fourment A. Electromyographic and biomechanical characteristics of segmental postural adjustments associated with voluntary wrist movements. Influence of an elbow support. Exp Brain Res. 2001;141:133–145. doi: 10.1007/s002210100823. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Adams L, Murphy K, Martin AJ, Lammertsma AA, Tochon-Danguy HJ, et al. Regional cerebral blood flow during volitional breathing in man. J Physiol. 1991;443:91–103. doi: 10.1113/jphysiol.1991.sp018824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert D, Rassler B, Hefter H. Coordination between breathing and forearm movements during sinusoidal tracking. Eur J Appl Physiol. 2000;81:288–296. doi: 10.1007/s004210050045. [DOI] [PubMed] [Google Scholar]
- Evans KC, Shea SA, Saykin AJ. Functional MRI localisation of central nervous system regions associated with volitional inspiration in humans. J Physiol. 1999;520(Pt 2):383–392. doi: 10.1111/j.1469-7793.1999.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi MM, Oliveri M, Vernieri F, Pasqualetti P, Rossini PM. Are autonomic signals influencing cortico-spinal motor excitability? A study with transcranial magnetic stimulation. Brain Res. 2000;881:159–164. doi: 10.1016/s0006-8993(00)02837-7. [DOI] [PubMed] [Google Scholar]
- Fink GR, Adams L, Watson JD, Innes JA, Wuyam B, Kobayashi I, et al. Hyperpnoea during and immediately after exercise in man: evidence of motor cortical involvement. J Physiol. 1995;489(Pt 3):663–675. doi: 10.1113/jphysiol.1995.sp021081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanari P, Vuillon-Cacciuttolo G, Balzamo E, Zattara-Hartmann MC, Lagier-Tessonnier F, Jammes Y. Resistive loaded breathing changes the motor drive to arm and leg muscles in man. Neurosci Lett. 1996;210:130–134. doi: 10.1016/0304-3940(96)12564-7. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Changes in teh pattern of breathing caused by chest vibration. Resp Physiol. 1976;26:163–171. doi: 10.1016/0034-5687(76)90094-3. [DOI] [PubMed] [Google Scholar]
- Ginanneschi F, Dominici F, Biasella A, Gelli F, Rossi A. Changes in corticomotor excitability of forearm muscles in relation to static shoulder positions. Brain Res. 2006;1073–1074:332–338. doi: 10.1016/j.brainres.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Guz A. Brain, breathing and breathlessness. Respir Physiol. 1997;109:197–204. doi: 10.1016/s0034-5687(97)00050-9. [DOI] [PubMed] [Google Scholar]
- Ikeda ER, Borg A, Brown D, Malouf J, Showers KM, Li S. The valsalva maneuver revisited: the influence of voluntary breathing on isometric muscle strength. J Strength Cond Res. 2009;23:127–132. doi: 10.1519/JSC.0b013e31818eb256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberg EM, Mateika JH, Cherry L, Gordon AM. Internal representations underlying respiration during object manipulation. Brain Res. 2003;982:270–279. doi: 10.1016/s0006-8993(03)03120-2. [DOI] [PubMed] [Google Scholar]
- Latash ML. The organization of quick corrections within a two-joint synergy in conditions of unexpected blocking and release of a fast movement. Clin Neurophysiol. 2000;111:975–987. doi: 10.1016/s1388-2457(00)00263-7. [DOI] [PubMed] [Google Scholar]
- Li S, Laskin JJ. Influences of ventilation on maximal isometric force of the finger flexors. Muscle Nerve. 2006;34:651–655. doi: 10.1002/mus.20592. [DOI] [PubMed] [Google Scholar]
- Li S, Rymer WZ. Voluntary breathing influences corticospinal excitability of nonrespiratory finger muscles. J Neurophysiol. 2011;105:512–521. doi: 10.1152/jn.00946.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Stevens JA, Kamper DG, Rymer WZ. The movement-specific effect of motor imagery on the premotor time. Motor Control. 2005;9:119–128. doi: 10.1123/mcj.9.2.119. [DOI] [PubMed] [Google Scholar]
- Li S, Stevens JA, Rymer WZ. Interactions between imagined movement and the initiation of voluntary movement: a TMS study. Clin Neurophysiol. 2009;120:1154–1160. doi: 10.1016/j.clinph.2008.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Yasuda N. Forced ventilation increases variability of isometric finger forces. Neurosci Lett. 2007;412:243–247. doi: 10.1016/j.neulet.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey KE, Macey PM, Woo MA, Harper RK, Alger JR, Keens TG, et al. fMRI signal changes in response to forced expiratory loading in congenital central hypoventilation syndrome. J Appl Physiol. 2004;97:1897–1907. doi: 10.1152/japplphysiol.00359.2004. [DOI] [PubMed] [Google Scholar]
- Macey PM, Macey KE, Henderson LA, Alger JR, Frysinger RC, Woo MA, et al. Functional magnetic resonance imaging responses to expiratory loading in obstructive sleep apnea. Respir Physiol Neurobiol. 2003;138:275–290. doi: 10.1016/j.resp.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Maskill D, Murphy K, Mier A, Owen M, Guz A. Motor cortical representation of the diaphragm in man. J Physiol. 1991;443:105–121. doi: 10.1113/jphysiol.1991.sp018825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateika JH, Gordon AM. Adaptive and dynamic control of respiratory and motor systems during object manipulation. Brain Res. 2000;864:327–337. doi: 10.1016/s0006-8993(00)02221-6. [DOI] [PubMed] [Google Scholar]
- Murray NPS, McKenzie DK, Gandevia SC, Butler JE. Voluntary and involuntary ventilation do not alter the human inspiratory muscle loading reflex. J Appl Physiol. 2010;109:87–94. doi: 10.1152/japplphysiol.01128.2009. [DOI] [PubMed] [Google Scholar]
- Rafferty GF, Gardner WN. Control of the respiratory cycle in conscious humans. J Appl Physiol. 1996;81:1744–1753. doi: 10.1152/jappl.1996.81.4.1744. [DOI] [PubMed] [Google Scholar]
- Ramsay SC, Adams L, Murphy K, Corfield DR, Grootoonk S, Bailey DL, et al. Regional cerebral blood flow during volitional expiration in man: a comparison with volitional inspiration. J Physiol. 1993;461:85–101. doi: 10.1113/jphysiol.1993.sp019503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassler B. Mutual nervous influences between breathing and precision finger movements. Eur J Appl Physiol. 2000;81:479–485. doi: 10.1007/s004210050071. [DOI] [PubMed] [Google Scholar]
- Rassler B, Bradl U, Scholle H. Interactions of breathing with the postural regulation of the fingers. Clin Neurophysiol. 2000;111:2180–2187. doi: 10.1016/s1388-2457(00)00483-1. [DOI] [PubMed] [Google Scholar]
- Rassler B, Nietzold I, Waurick S. Phase-dependence of breathing and finger tracking movements during normocapnia and hypercapnia. Eur J Appl Physiol Occup Physiol. 1999;80:324–332. doi: 10.1007/s004210050599. [DOI] [PubMed] [Google Scholar]
- Smejkal V, Druga R, Tintera J. Control of breathing and brain activation in human subjects seen by functional magnetic resonance imaging. Physiol Res. 1999;48:21–25. [PubMed] [Google Scholar]
- Smejkal V, Druga R, Tintera J. Brain activation during volitional control of breathing. Physiol Res. 2000;49:659–663. [PubMed] [Google Scholar]
- Terao Y, Ugawa Y, Suzuki M, Sakai K, Hanajima R, Gemba-Shimizu K, et al. Shortening of simple reaction time by peripheral electrical and submotor-threshold magnetic cortical stimulation. Exp Brain Res. 1997;115:541–545. doi: 10.1007/pl00005724. [DOI] [PubMed] [Google Scholar]


