Abstract
Osteoarthritis (OA) is the most common form of arthritis in the US, and a leading cause of disability. It is typically defined in epidemiologic studies on the basis of radiographic findings and consideration of symptoms. Its incidence and prevalence are rising, likely related to the aging of the population and increasing obesity. Risk factors for OA include a number of person-level factors, such as age, sex, obesity, and genetics, as well as joint-specific factors that are likely reflective of abnormal loading of the joints. A number of methodologic challenges exist in studying OA that can hamper our ability to identify pertinent relationships.
Keywords: Osteoarthritis, epidemiology, risk factors, pain
Introduction
Osteoarthritis (OA) is the most common form of arthritis,1 and one of the most common diagnoses in general practice.2 Given its predilection for lower extremity joints such as the knee and hip, OA is the leading cause of lower extremity disability among older adults.3
Defining Osteoarthritis
OA is frequently defined on the basis of radiography, with the most commonly used radiographic grading system being the Kellgren and Lawrence (KL) grade, which scores OA severity on a scale of 0-4; definite radiographic OA is KL grade ≥2.4 The KL grading system has been used for the hand, hip, and knee; however, at the knee, it is only used to define tibiofemoral OA. Patellofemoral radiographic OA can also be assessed if appropriate x-ray views are obtained. The Osteoarthritis Research Society International atlas provides a means to score individual radiographic features, such as osteophytes and joint-space narrowing in a semi-quantitative manner,5 while other methods are available to quantify joint-space width on x-rays.6 Numerous joint structures can be examined by MRI that are not otherwise visualized on radiographs. While an MRI definition of OA has been proposed, it requires validation.7 However, individual structural lesions on MRI are well-described, including cartilage lesions, osteophytes, bone marrow lesions, synovitis, effusion, and subchondral bone attrition.8,9 Due to the enhanced sensitivity of MRI, of knees without radiographic evidence of tibiofemoral OA (KL 0) in adults aged ≥50, 89% had at least one such abnormality in the tibiofemoral joint on MRI, with similar prevalences in painful and painless knees.10
Symptomatic OA indicates the presence of radiographic OA in combination with knee symptoms attributable to OA. Not all individuals with radiographic OA have concomitant symptoms. OA may be described in a joint-specific manner (e.g., knee OA, hip OA), or, when several joint areas are involved, it may be considered as being generalized (e.g., involvement with OA of at least one of each joint area: knee, hip, and hand), although a standard definition for generalized OA does not yet exist.
Incidence and Prevalence of OA
One estimate of the lifetime risk of developing symptomatic knee OA was ~40% in men and 47% in women, with higher risks among those who are obese.11 Age- and sex-standardized incident rates for symptomatic hand, hip, and knee OA have been estimated to be 100, 88, and 240 cases per 100,000 person-years, respectively, with incidence rates rising sharply after age 50, and leveling off after age 70 (Figure 1).12 However, the interpretation of the leveling off or decline in OA incidence at older ages should be made with caution given the potential biases related to competing risks and depletion of susceptibles (see below in Methodologic Challenges).13 Recent estimates of hand OA incidence derived from the Framingham Osteoarthritis Study were ~34-35% for OA incidence in any hand joint for both sexes, while symptomatic hand OA incidence was 4% for men and 9.7% for women over a 9-year period.14
Figure 1.
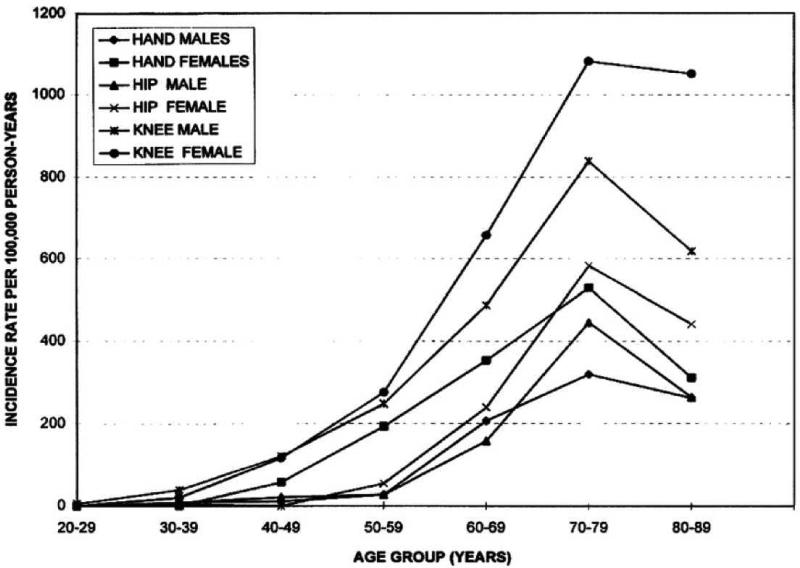
Incidence of hand, hip, and knee OA 1991-1992 by age and sex in a community health plan. Reference: Oliveria SA, et al. Arthritis Rheum. 1995;38:1134-1141.
There has been a rise in OA prevalence with an estimated 27 million US adults in 2005 having clinical OA of their hand, knee, or hip joint, an increase from 21 million in 1995.1 Such increases are likely due to aging of the population and rising prevalence of obesity. In Framingham, the age-standardized prevalence of radiographic hand OA was 44.2% in women and 37.7% in men,14 and 19% had knee OA among adults age ≥45.15 From the Johnston County Osteoarthritis Project, ~28% of African Americans and Caucasians age ≥45 had knee OA and 28% had hip OA.16,17 This latter estimate is higher than the 7% prevalence noted in the Study of Osteoporotic Fractures among Caucasian women age >65.18
Symptomatic OA prevalence estimates are lower since it requires the presence of radiographic OA with pain, aching or stiffness in the joint. The age-standardized prevalence of symptomatic hand OA was 14.4% and 6.9% in women and men, respectively, in younger Framingham cohorts,14 and increased to 26.2% and 13.4%, respectively, among those age ≥71 in an older Framingham cohort.19 The prevalence of symptomatic knee OA among adults age ≥45 was ~7% in Framingham,15 while in the Johnston County OA Project, it was ~17%.16 Symptomatic hip OA was present in ~10% of the Johnston County cohort.17 There has also been an increase in prevalence of symptomatic knee OA over the past 20 years by 4.1% and 6% among women and men in the Framingham cohort, respectively.20
Racial/ethnic differences in the prevalence of OA and specific patterns of joint involvement have been noted. In the Johnston County OA Project, African American men had a higher prevalence of radiographic hip OA than Caucasian men (32.2% vs. 23.8%), while there was no difference between African American and Caucasian women (40.3% vs. 39.4%).17 Individual radiographic features at the hip and knee were also noted to differ between the two groups.21,22 In the Beijing Osteoarthritis Study, hand and hip OA were less prevalent among Chinese than Caucasians (age-standardized prevalences 44.5-47% vs. 75.2-85% and 0.8% vs. 3.8-4.5%, respectively), but knee OA was more prevalent among Chinese women than Caucasian women ( 46.6% vs. 34.8%).23-25 A higher prevalence of lateral tibiofemoral knee OA was also noted in Beijing Chinese in comparison with Framingham Caucasian subjects.26
Risk Factors for Radiographic OA
OA can be thought of as the phenotypic manifestation of a series of different pathways leading to a common end-stage pathology (Figure 2). As such, the disease has a multifactorial etiology, with different sets of risk factors (at a person and or joint level) acting together to cause OA onset in any given individual. Person-level factors are generally those that are thought to act at a systemic level on all relevant joints or are a characteristic of the individual, while joint-level factors generally refer to those that are joint-specific, and may be unique to a particular joint.
Figure 2.
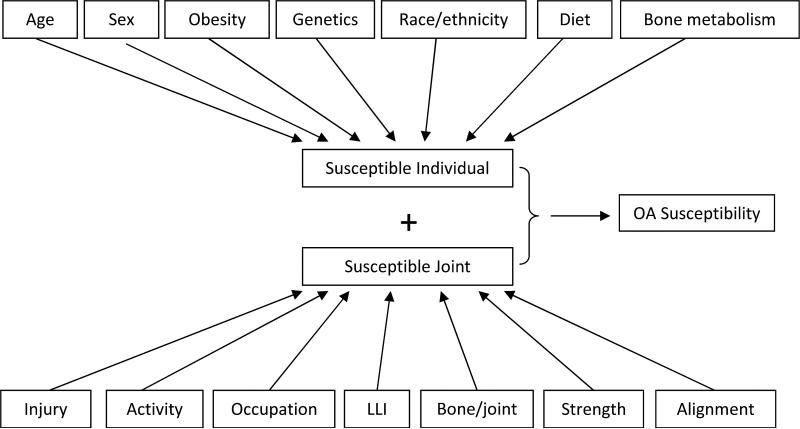
Potential risk factors for susceptibility to OA incidence and progression, each with varying degrees of evidence to support their association (see text for details). LLI=leg-length inequality
Person-level Risk Factors
Age and Sex
Age is one of the strongest risk factors for OA.1 The exact mechanism is not known, but is likely related to a combination of changes in the capacity for joint tissues to adapt to biomechanical insults, and age being a proxy for the accumulation of a sufficient set of risk factors over the years.
Female sex is associated with higher prevalence and greater severity of OA.27 The increase in OA prevalence and incidence at the time of menopause has led to hypotheses regarding the role of estrogen in OA, such as the loss of estrogen potentially unmasking the symptoms of OA by enhancing pain sensitivity. However, results from observational studies and clinical trials have been conflicting regarding estrogen effects on OA.28-30 In the Heart and Estrogen/Progestin Replacement Study, there was no difference in knee pain in those randomized to receive estrogen replacement therapy compared with those receiving placebo.29 On the other hand, in the Women's Health Initiative, unopposed estrogen therapy was associated with borderline significant lower rate of joint arthroplasty, but no such association was noted for estrogen-plus-progestin, compared with placebo.30 A review of sex differences in MRI features of OA and biomarkers of joint metabolism noted variable findings.31 Women may have thinner and more reduced volume of knee cartilage than men (even after taking into account differences in height, weight, and bone size), whether women have a more accelerated rate of cartilage volume loss than men is not clear.
Obesity
Obesity has long been identified as a risk factor for knee OA.32 In a meta-analysis, those who were obese or overweight had 2.96 times higher risk of incident knee OA compared with those who were normal weight (95% CI 2.56-3.43).33 Assuming the prevalence of obesity in a hypothetical population to be 25%, the population attributable risk percent due to obesity would therefore be 29% (95% CI 24-34%); this would be higher where obesity prevalence is higher.34 Further, those who were only overweight (not obese) had over 2 times the chance of developing knee OA compared with their normal weight counterparts.33 Risk of incident knee OA increases with increasing BMI, regardless of knee alignment.35 Decreasing BMI by 2 units or more over 10 years (~5 kg) was associated with 50% lower risk of developing symptomatic knee OA among women,36 findings supported by a recent meta-analysis.37 Duration of exposure to high BMI during adulthood confers risk of incident knee OA, suggesting the importance of weight control throughout life as a means of primary prevention of knee OA.38 Obesity also contributes to symptoms in knee OA, with the Arthritis, Diet, and Activity Promotion Trial (ADAPT) and Intensive Diet and Exercise for Arthritis (IDEA) trial both demonstrating improvements in pain accompanying weight loss related to dietary and exercise interventions.39,40
In contrast to data supporting the role of obesity in knee OA development, high BMI was not associated with progressive radiographic knee OA in one study.35 However, using the same data, Zhang et al demonstrated that high BMI increased the risk of both mild radiographic OA (KL=2) and moderate-severe radiographic OA (KL=3 or 4) among knees that were KL=0 at baseline, respectively.41 Since knees that develop KL=3 or 4 over time must have gone through the KL=2 stage, this provides indirect evidence that obesity increases risk of incident knee OA and also accelerates knee OA progression.
The effects of obesity on OA may be through both mechanical effects and systemic effects (e.g., metabolic or inflammatory). While there is no doubt about an effect of increased load related to overall body weight, there may be differential systemic effects dependent upon degree of fat versus lean mass; unfortunately BMI does not differentiate between the two. Recently, total body fat measured by DXA was associated with decreased cartilage thickness, while lean mass was associated with increased cartilage thickness.42 Adipose tissue is known to be metabolically active, secreting adipokines such as adiponectin, leptin, and resistin, but their role in OA is not yet clear.43,44
Obesity is also associated with both incident radiographic and symptomatic hand OA,45,46 further supporting potential metabolic or inflammatory effects of obesity. In contrast, the association between obesity and hip OA has been variable, and where noted, less strong than for the knee or hand.47-51
Genetics
The heritable component of OA has been estimated to be 40-65%, and stronger for hand and hip OA than for knee OA.52-54 To date, three loci, GDF5, which encodes the growth differentiation factor 5 (a bone morphogenetic protein expressed in skeletal and articular structures), chromosome 7q22, and MCF2L have been associated with OA at genome-wide significance levels.55-57 A recent large, well-powered study from the arcOGEN Consortium identified five new susceptibility loci for OA with genome-wide significance.58 Two SNPs were on chromosome 3 which are in linkage disequilibrium with each other within an exon of nucleostemin-encoding GNL3; one on chromosome 9 close to ASTN2; one on chromosome 6 between FILIP1 and SENP6; one on chromosome 12 close to KLHDC5 and PTHLH; and another on chromosome 12 close to CHST11.58 Of note, the previously identified loci did not achieve genome-wide significance in this arcOGEN sample.
Pain severity related to OA may also have genetic contributions. A functional polymorphism (Val158Met) in the COMT gene, which has been associated with pain sensitivity in other clinical conditions, was associated with hip OA-related pain in one cohort study, but has not yet been replicated in other cohorts.59 Other genes associated with pain sensitivity have also been studied in relation to OA pain. TRPV1 was associated with symptomatic knee OA in a meta-analytic approach,60 while an association with a SCN9 SNP could not be replicated.61
BMD
The material properties of bone may influence OA susceptibility. Nevitt et al recently confirmed the previous observation that higher systemic bone mineral density (BMD) was associated with an increased risk of incident OA.62 Whether this finding is related to factors contributing to bone remodeling or peak bone mass that may be genetically determined,63 or whether the higher systemic BMD represents higher BMI load over the years prior to OA onset, itself a strong risk factor for OA, is not clear. Paradoxically, BMD was not associated with progressive OA in the same study.62 Low BMD has been associated cross-sectionally with reduced joint space width at the hip, which could be a reflection of effects of existing OA.64 That is, once symptomatic OA has developed, an individual may decrease their physical activity and therefore loading of the joint, which in turn can contribute to low BMD. Further, there is evidence to suggest that although the apparent density of bone in OA may be increased, the bone itself is less mineralized, resulting in lower material density.65
Nutritional Factors
The effects of readily modifiable dietary factors in humans have been inconclusive. Studies of the relationship between vitamin D and OA have been conflicting.66-68 A recent randomized controlled trial of vitamin D's effects on knee OA did not demonstrate a beneficial effect on cartilage loss by MRI.69 One difficulty in the conduct of such a study is that it is unethical to conduct a fully placebo-controlled trial; whether the 400iu/d given to the control arm was sufficient to account for the negative results is not clear. Antioxidant vitamins such as vitamins C and E have also been studied in relation to OA, with conflicting results.70-75 Vitamin K, which has potential bone and cartilage effects, has been associated cross-sectionally with hand and knee OA, incident radiographic knee OA and MRI-based cartilage lesions, and with potentially less hand OA progression among those who were deficient at baseline in a randomized trial, though the overall trial results were null.76-79 Selenium and iodine deficiency have been associated with Kashin-Beck osteoarthropathy. In two observational cohort studies, both low and high levels of selenium have been associated with OA.80,81
Joint-level Risk factors
Occupation, Physical Activity, and Injury
Repetitive joint use may predispose to OA. For example, squatting among Beijing Chinese,82 and jobs requiring kneeling or squatting were associated with increased risk of knee OA, particularly among those who were overweight or whose jobs required carrying or lifting, as well as worse cartilage morphology scores on MRI at the patellofemoral joint.83-85 A recent meta-analysis noted a 1.6 times increased risk of knee OA related to occupational activities, with most activities conferring increased risk other than standing.86 Occupational lifting and prolonged standing have been associated with hip OA.87-89 Occupations involving manual dexterity, particularly repeated pincer grip, have been associated with features of hand OA.90,91 This is also supported by an increase in OA found in the interphalangeal joint of the thumb and in the second and third PIP and MCP joints of the hand used to eat with chopsticks compared with other joints of that same hand or any joint in the opposite hand among Beijing Chinese.92
Physical activity may have benefits for the joint by strengthening periarticular muscles to help stabilize the joint, but may potentially be detrimental if it places undue load on the joint, particularly one that is already vulnerable due to other risks. General population studies have shown that habitual levels of activity are not associated with incident radiographic/symptomatic OA or new knee replacement, whereas more vigorous levels of activity appeared to increase the risk of OA.93-95 A recent study reported that daily walking of greater than 10,000 steps per day may be associated with worsening of certain MRI features; however certain biases could not be ruled out.96
Although studies focused on former athletes have had conflicting results,97-100 the mechanism by which vigorous or elite-level (or equivalent) physical activity/sports may be associated with increased risk of OA may be related to factors other than simple load-bearing. In one study of athletes, the increased risk of OA appeared to be related to knee injury among soccer players and increased BMI as well as squatting among weight-lifters.101 A number of studies have demonstrated the importance of knee injury such as related to meniscal tears requiring meniscectomy or anterior cruciate ligament injury as a risk factor for OA onset.102,103 Two recent meta-analyses report knee injury to confer a 4-fold increased risk of developing knee OA.33,104
Beyond certain sports, some occupational activities may increase risk of meniscal tears as well, which are known to confer high risk of knee OA.105 For example, floor layers, who spend much time kneeling were more likely to have degenerative meniscal tears than graphic designers without any knee demands.106 Although the prevalence of meniscal abnormalities increases as the radiographic severity of knee OA increases,107 surgical intervention has not been shown to reduce these risks.108 These studies support the importance of maintaining an intact meniscus to protect against development of OA.
Muscle Strength
The effect of knee injury on OA risk may be partially related to muscle strength. Muscle weakness and atrophy can occur as a consequence of OA related to disuse due to pain avoidance, but whether it is a risk factor for development of OA is not clear. In some studies, quadriceps muscle weakness was associated with increased risk of structural knee OA.109,110 In another study, discrepant findings were noted for low knee extensor strength being associated with incident symptomatic knee OA, but not with incident radiographic OA.111 In this study, the patellofemoral joint was included in the evaluation of incident symptomatic whole knee OA, but not included in the definition of incident radiographic tibiofemoral OA. On the other hand, greater quadriceps strength in the setting of malalignment and laxity was associated with increased risk of tibiofemoral OA progression in one study, 112 but no association with tibiofemoral progression was noted in another study, in which it was also associated with less cartilage loss in the lateral patellofemoral joint,113 suggesting a more complex interrelationship.
Muscle strength could potentially play a role in hand OA as well. For example, greater grip strength was associated with increased risk of developing radiographic hand OA.114 However, potentially as a consequence of existing hand OA, a cross-sectional study found an inverse association between grip strength and prevalent first carpometacarpal joint OA, and between pinch strength and prevalent metacarpophalangeal joint OA.115
Alignment
Dynamic alignment (i.e., the alterations in the knee that occur during gait) may be pertinent for understanding the specific load effects the joint is experiencing. In epidemiologic studies, however, static alignment from full-limb x-rays (mechanical axis) or from posteroanterior knee x-rays (i.e., anatomic axis) is typically assessed due to feasibility. Prior studies have had conflicting findings regarding the effects of alignment on incident OA,116,117 although more recent studies have reported that varus malalignment assessed by full-limb radiographs increased the incidence of both radiographic knee OA and cartilage damage.118,119 Nevertheless, a best evidence synthesis concluded there was lack of sufficient evidence to draw a conclusion.120
Knee malalignment is one of the strongest predictors for progressive knee OA.119 These findings may imply that the association between alignment and OA development is a vicious cycle: joint space narrowing (e.g., due to cartilage and meniscal abnormalities) and bony contour alterations occurring in OA may themselves lead to joint malalignment, while malalignment itself can further alter joint loading and accelerate disease progression. However, no study to date has documented slowing of disease progression if alignment is corrected. Interestingly, in post-hoc analysis of data from a randomized placebo-controlled trial of doxycycline in obese middle-aged women with unilateral knee OA, varus malalignment was found to negate the potential chondroprotective effects of doxycycline.121 Using a computational modeling approach with finite or discrete element analysis, knees that developed incident symptomatic OA demonstrated higher maximal contact stress and larger area of engagement with higher contact stresses at baseline than control knees that did not develop symptomatic OA, suggesting a local biomechanical role in the development of symptomatic knee OA.122
Leg -Length Inequality
Leg-length inequality (LLI) is an easily modifiable abnormality. Persons with LLI of ≥2cm in the Johnston County OA Project were almost twice as likely to have prevalent radiographic knee OA, but no association was noted for incident knee OA.123,124 Similar findings were noted using data from The MOST Study, in which persons with LLI of ≥1cm were almost twice as likely to have prevalent radiographic knee OA in the shorter limb.125 An association with incident radiographic knee OA was not found in that study, although LLI was associated with incident symptomatic knee OA. This discordance may be related, as discussed above, to the inclusion of the patellofemoral joint in the definition of symptomatic whole knee OA, but excluded from incident radiographic tibiofemoral OA.
Bone/Joint Morphology
The anatomy or the shape of a joint may contribute to OA risk given that biomechanical load distribution through the joint is partially dependent upon the geometric shape over which that load is distributed in addition to the material properties of the joint tissues receiving that load. This has perhaps been best studied and described in the hip in relation to OA, where, using active shape modeling, the two-dimensional shape of the hip has been associated with OA.126,127 Even mild acetabular dysplasia has been associated with risk of incident hip OA.128 Pistol grip deformity, or cam-type femoral acetabular impingement (FAI) syndrome, as well as the pincer-type FAI, have been associated with hip OA and hip pain.129,130 More recently, using MRI data, three-dimensional bone shape has been shown to predict onset of knee OA.131,132
Recent insights into risk factors for knee pain
Clinically, symptoms related to knee OA are known to be activity-related in early stages, progressing to more persistent symptoms in late stages of disease that are punctuated with intermittent increased pain.133 In The MOST Study, ~40% with persons with or at high risk of knee OA had fluctuating knee pain; these individuals had less severe KL grades on radiograph, less depressive symptoms, and less widespread pain.134 In the Longitudinal Examination of Arthritis Pain, an observational cohort study of 287 adults with hip or knee OA in which pain assessments were conducted weekly over 12 weeks, psychological factors fluctuated with pain severity,135 supporting an important link between the pain experience and psychological state. Indeed, because numerous factors, many of which may not be assessed in a particular study, can contribute to the pain experience, such as genetics, sociocultural environment, medications, among others in addition to psychological factors, a so-called “structure-symptom” discordance is often described in OA.
However, when such between-person variability and confounding factors are accounted for by using a within-person knee-matched study design (in which one knee has pain while the other does not), a strong association between radiographic severity and knee pain can be discerned, even at the earliest stages of radiographic knee OA (Figure 3).136 Such findings indicate that certain structural lesions within the knee may be a cause of knee pain. Furthermore, specific MRI features of OA that can change over time, including bone marrow lesions, synovitis, and effusions, have been associated with knee pain fluctuation.137 As structural lesions worsened, the likelihood that the knee would be painful increased. Similarly, a decrease in the structural abnormalities of a knee was associated with that knee's pain having subsided. A recent systematic review supports an association of MRI-detected bone marrow lesions and synovitis with the pain experience of OA.138
Figure 3.
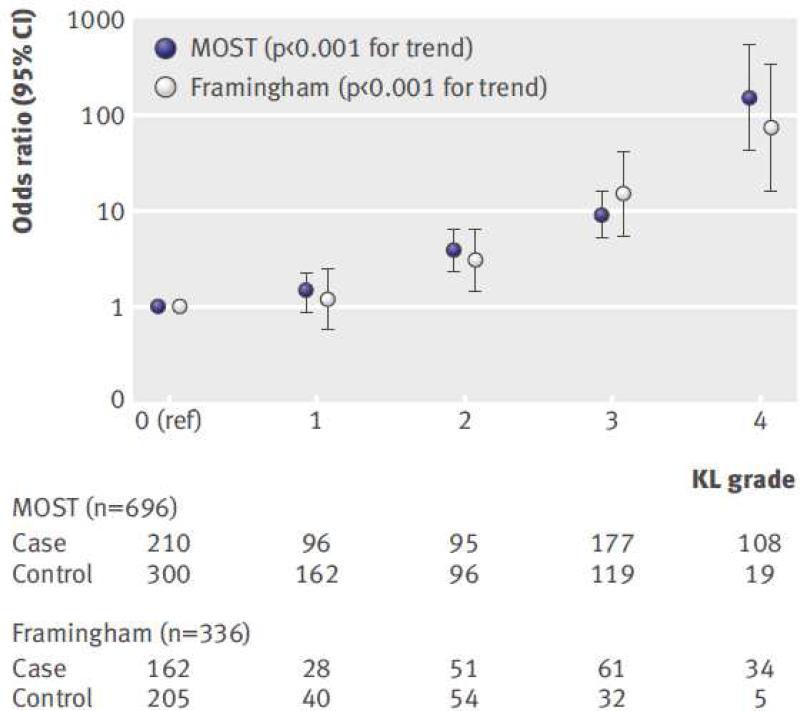
Associations of frequent knee pain with Kellgren and Lawrence (KL) grade among people with two knees discordant for frequent knee pain status. Number of case knees (i.e., with knee pain) and control knees (i.e., without knee pain) are shown beneath the graph for each KL grade. Note the y-axis is logarithmically scaled.
Reference: Neogi T, et al. BMJ 2009;339:b2844 doi:10.1136/bmj.b2844
Methodologic Challenges in the Study of Incident and Progressive Radiographic Knee OA
There are a number of methodologic challenges to the conduct, analyses and interpretation of results from studies of OA, as discussed elsewhere13,41 and reviewed briefly here.
“Depletion of susceptibles”
Most risk factors for OA, such as obesity or BMD, are chronic in nature. These chronic factors are likely to be present long before subjects are enrolled into a study. If those chronic risk factors have already caused a substantial proportion of subjects to develop knee OA, then it is quite possible that participants who are still exposed to such a risk factor without yet having developed OA are less susceptible to knee OA than are individuals who have never been exposed to such a risk factor. For example, long-standing exposures such as obesity may have caused OA at an earlier age than those being studied, but that true effect cannot be discerned since those individuals who already have knee OA are excluded from studies of incident disease. Individuals who have been obese for a long time and who are free of OA at the study onset may in fact be less susceptible to developing OA. Thus observational studies evaluating the association between a chronic exposure and incident knee OA may not be able to detect the true magnitude of effect. Such a phenomenon has been observed in other fields. For example, studies that have assessed BMI in midlife (in one's 40s, 50s, and 60s) find that higher BMI are associated with an increased risk of death over the subsequent decades (in one's 60s, and 70s, and 80s). However, many investigations of BMI at age ≥ 70 find less clear associations with mortality.139 One potential explanation for such findings is depletion of susceptibles among the elderly. Since the risk of knee OA increases rapidly around middle 50s to 60's, one would ideally study subjects younger than this age to identify risk factors for incident knee OA. If OA studies consist of a large proportion of subjects who are older than the typical age of onset, the overall effect of a specific chronic risk factor is likely to be underestimated due to depletion of those that were susceptible to OA.
Loss to follow-up and competing risks
The risk of developing new onset OA is difficult to determine due to a number of challenges. OA is a chronic disease whose onset is typically unknown. In most OA cohort studies, repeated study visits with imaging may occur after a substantial amount of time passes between each study visit. As a result, there is a potential for loss to follow-up. For example, in two large cohort studies in which knee x-rays were repeated after 4 and 9 years, respectively, both studies reported that ~40% did not have x-rays at the follow-up visit.140,141 Given that OA is a disease with onset in middle- or older ages, death due to other causes rather than OA (competing risks) makes risk estimation difficult and prone to bias. In most cases, estimates of the risk of OA can only be obtained among subjects who provide both baseline and follow-up data. If loss to follow-up is associated with the occurrence of OA (as might be expected when older adults or obese participants are lost to follow-up, for example), the estimate of OA risk based on those who are followed with complete data could be an underestimate.
Potential discordant findings for risk factors for incident and progressive knee OA
Some risk factors associated with incident disease are not associated with or are even paradoxically protective against progressive OA. In observational studies of OA progression, eligible knees consist of those that already have knee OA, i.e., KL=2 or KL=3, representing a “mixture” of differing degrees of severity that may vary among exposed and non-exposed groups. The outcome is also heterogeneous: knees that progress from KL=3 to KL=4 are considered the same as those that progress from KL=2 to KL=3 or to KL=4 over the same period of time. Finally, studies of OA progression are, in essence, conducted to assess an association between a risk factor that causes OA initiation to progress to more severe OA. This results in conditioning on an “intermediate” stage of OA when assembling the study sample, i.e., by limiting the study sample to those who already had mild to moderate knee OA at baseline. This “blocks” the potential effect of a risk factor on the risk of OA progression if the risk factor of interest was present before any OA pathology occurred.41 Conditioning on an intermediate stage of OA can also result in collider bias. For example, in a hypothetical study of obesity as a risk factor for progressive radiographic OA, the assembled knees with KL=2 or KL=3 would be divided into those knees that belong to obese persons and those that belong to non-obese persons. Those knees with OA among the non-obese participants must have developed OA due to some other risk factors. Without accounting for those risk factors that led to the development of OA in those knees, the results of the study will be confounded, and will tend to be negatively biased (towards the null).
Discerning Independent Effects
Over the past several years, MRI has enabled identification of various pathologic changes in the joint. However, little is known about the true natural history of the occurrence of these structural lesions detected on MRI, particularly in relation to one another. There is often an attempt to include all structural lesions into a statistical regression model to compare the effect of each structural lesion on the outcome of interest. Without knowing the causal pathway and chronology of occurrence of these lesions, standard approaches of automatically mutually adjusting for all factors can not only lead to biased effect estimates, but the effect estimates for each structural lesions are not directly comparable with one another, resulting in incorrect interpretations of study findings.142
Conclusions
OA poses a substantial public health burden given its prevalence that continues to rise. A number of risk factors have been recognized, including some modifiable ones such as obesity and avoiding joint injury. There are numerous methodologic challenges to studying risk factors for OA, and therefore prevention of OA and its progression also remain challenging. There is a need for ongoing epidemiologic and intervention studies for the prevention of incident and progressive OA, as well as pain related to OA, with adoption of novel approaches to avoid some of the methodologic challenges identified.
Key Points.
OA is the most common form of arthritis, with OA of the knee, hand, or hip having a similar prevalence of ~20-30% of adults in various populations
Person-level factors associated with OA include increasing age, female sex, overweight/obesity, and race/ethnicity which may represent genetic or sociocultural influences
Joint-level factors associated with OA are reflective of mechanisms related to abnormal loading of the joints
A number of methodologic challenges to the study of OA exist which have affected our ability to identify important OA relationships
There is a need for ongoing epidemiologic and intervention studies for the prevention of incident and progressive OA and related pain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsiao CJ, Cherry DK, Beatty PC, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2007 summary. Natl Health Stat Report. 2010:1–32. [PubMed] [Google Scholar]
- 3.Guccione AA, Felson DT, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84:351–8. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kellgren JH, Lawrence JS. Atlas of standard radiographs. Oxford University Press; Oxford (UK): 1963. [Google Scholar]
- 5.Altman RD, Hochberg M, Murphy WA, Jr., Wolfe F, Lequesne M. Atlas of individual radiographic features in osteoarthritis. Osteoarthritis Cartilage. 1995;3(Suppl A):3–70. [PubMed] [Google Scholar]
- 6.Buckland-Wright JC, Macfarlane DG, Lynch JA, Jasani MK, Bradshaw CR. Joint space width measures cartilage thickness in osteoarthritis of the knee: high resolution plain film and double contrast macroradiographic investigation. Ann Rheum Dis. 1995;54:263–8. doi: 10.1136/ard.54.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter DJ, Arden N, Conaghan PG, et al. Definition of osteoarthritis on MRI: results of a Delphi exercise. Osteoarthritis Cartilage. 2011;19:963–9. doi: 10.1016/j.joca.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter DJ, Guermazi A, Lo GH, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage. 2011;19:990–1002. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterfy CG, Guermazi A, Zaim S, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Guermazi A, Niu J, Hayashi D, et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study). BMJ. 2012;345:e5339. doi: 10.1136/bmj.e5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–13. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995;38:1134–41. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 13.Neogi T, Zhang Y. Osteoarthritis prevention. Curr Opin Rheumatol. 2011;23:185–91. doi: 10.1097/BOR.0b013e32834307eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haugen IK, Englund M, Aliabadi P, et al. Prevalence, incidence and progression of hand osteoarthritis in the general population: the Framingham Osteoarthritis Study. Ann Rheum Dis. 2011;70:1581–6. doi: 10.1136/ard.2011.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987;30:914–8. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 16.Jordan JM, Helmick CG, Renner JB, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34:172–80. [PubMed] [Google Scholar]
- 17.Jordan JM, Helmick CG, Renner JB, et al. Prevalence of hip symptoms and radiographic and symptomatic hip osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2009;36:809–15. doi: 10.3899/jrheum.080677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nevitt MC, Lane NE, Scott JC, et al. Radiographic osteoarthritis of the hip and bone mineral density. The Study of Osteoporotic Fractures Research Group. Arthritis Rheum. 1995;38:907–16. doi: 10.1002/art.1780380706. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Niu J, Kelly-Hayes M, Chaisson CE, Aliabadi P, Felson DT. Prevalence of symptomatic hand osteoarthritis and its impact on functional status among the elderly: The Framingham Study. Am J Epidemiol. 2002;156:1021–7. doi: 10.1093/aje/kwf141. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen US, Zhang Y, Zhu Y, Niu J, Zhang B, Felson DT. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med. 2011;155:725–32. doi: 10.1059/0003-4819-155-11-201112060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braga L, Renner JB, Schwartz TA, et al. Differences in radiographic features of knee osteoarthritis in African-Americans and Caucasians: the Johnston county osteoarthritis project. Osteoarthritis Cartilage. 2009;17:1554–61. doi: 10.1016/j.joca.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson AE, Braga L, Renner JB, et al. Characterization of individual radiographic features of hip osteoarthritis in African American and White women and men: the Johnston County Osteoarthritis Project. Arthritis Care Res (Hoboken) 2010;62:190–7. doi: 10.1002/acr.20067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nevitt MC, Xu L, Zhang Y, et al. Very low prevalence of hip osteoarthritis among Chinese elderly in Beijing, China, compared with whites in the United States: the Beijing osteoarthritis study. Arthritis Rheum. 2002;46:1773–9. doi: 10.1002/art.10332. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Xu L, Nevitt MC, et al. Comparison of the prevalence of knee osteoarthritis between the elderly Chinese population in Beijing and whites in the United States: The Beijing Osteoarthritis Study. Arthritis Rheum. 2001;44:2065–71. doi: 10.1002/1529-0131(200109)44:9<2065::AID-ART356>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Xu L, Nevitt MC, et al. Lower prevalence of hand osteoarthritis among Chinese subjects in Beijing compared with white subjects in the United States: the Beijing Osteoarthritis Study. Arthritis Rheum. 2003;48:1034–40. doi: 10.1002/art.10928. [DOI] [PubMed] [Google Scholar]
- 26.Felson DT, Nevitt MC, Zhang Y, et al. High prevalence of lateral knee osteoarthritis in Beijing Chinese compared with Framingham Caucasian subjects. Arthritis Rheum. 2002;46:1217–22. doi: 10.1002/art.10293. [DOI] [PubMed] [Google Scholar]
- 27.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769–81. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Hanna FS, Wluka AE, Bell RJ, Davis SR, Cicuttini FM. Osteoarthritis and the postmenopausal woman: Epidemiological, magnetic resonance imaging, and radiological findings. Semin Arthritis Rheum. 2004;34:631–6. doi: 10.1016/j.semarthrit.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Nevitt MC, Felson DT, Williams EN, Grady D. The effect of estrogen plus progestin on knee symptoms and related disability in postmenopausal women: The Heart and Estrogen/Progestin Replacement Study, a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2001;44:811–8. doi: 10.1002/1529-0131(200104)44:4<811::AID-ANR137>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 30.Cirillo DJ, Wallace RB, Wu L, Yood RA. Effect of hormone therapy on risk of hip and knee joint replacement in the Women's Health Initiative. Arthritis Rheum. 2006;54:3194–204. doi: 10.1002/art.22138. [DOI] [PubMed] [Google Scholar]
- 31.Maleki-Fischbach M, Jordan JM. New developments in osteoarthritis. Sex differences in magnetic resonance imaging-based biomarkers and in those of joint metabolism. Arthritis Res Ther. 2010;12:212. doi: 10.1186/ar3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 33.Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010;18:24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W. Risk factors of knee osteoarthritis--excellent evidence but little has been done. Osteoarthritis Cartilage. 2010;18:1–2. doi: 10.1016/j.joca.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Niu J, Zhang YQ, Torner J, et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61:329–35. doi: 10.1002/art.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Felson DT, Zhang Y, Anthony JM, Naimark A, Anderson JJ. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham Study. Ann Intern Med. 1992;116:535–9. doi: 10.7326/0003-4819-116-7-535. [DOI] [PubMed] [Google Scholar]
- 37.Christensen R, Bartels EM, Astrup A, Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2007;66:433–9. doi: 10.1136/ard.2006.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wills AK, Black S, Cooper R, et al. Life course body mass index and risk of knee osteoarthritis at the age of 53 years: evidence from the 1946 British birth cohort study. Ann Rheum Dis. 2012;71:655–60. doi: 10.1136/ard.2011.154021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50:1501–10. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 40.Messier SP, Nicklas BJ, Legault C, et al. The Intenstive Diet and Exercise for Arthritis Trial: 18-month clinical outcomes. Arthritis Rheum. 2011;63:S281. [Google Scholar]
- 41.Zhang Y, Niu J, Felson DT, Choi HK, Nevitt M, Neogi T. Methodologic challenges in studying risk factors for progression of knee osteoarthritis. Arthritis Care Res (Hoboken) 2010;62:1527–32. doi: 10.1002/acr.20287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding C, Stannus O, Cicuttini F, Antony B, Jones G. Body fat is associated with increased and lean mass with decreased knee cartilage loss in older adults: a prospective cohort study. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2012.136. [DOI] [PubMed] [Google Scholar]
- 43.Sandell LJ. Obesity and osteoarthritis: is leptin the link? Arthritis Rheum. 2009;60:2858–60. doi: 10.1002/art.24862. [DOI] [PubMed] [Google Scholar]
- 44.Sowers MR, Karvonen-Gutierrez CA. The evolving role of obesity in knee osteoarthritis. Curr Opin Rheumatol. 2010;22:533–7. doi: 10.1097/BOR.0b013e32833b4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carman WJ, Sowers M, Hawthorne VM, Weissfeld LA. Obesity as a risk factor for osteoarthritis of the hand and wrist: a prospective study. Am J Epidemiol. 1994;139:119–29. doi: 10.1093/oxfordjournals.aje.a116974. [DOI] [PubMed] [Google Scholar]
- 46.Oliveria SA, Felson DT, Cirillo PA, Reed JI, Walker AM. Body weight, body mass index, and incident symptomatic osteoarthritis of the hand, hip, and knee. Epidemiology. 1999;10:161–6. [PubMed] [Google Scholar]
- 47.Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9:132. doi: 10.1186/1471-2474-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heliovaara M, Makela M, Impivaara O, Knekt P, Aromaa A, Sievers K. Association of overweight, trauma and workload with coxarthrosis. A health survey of 7,217 persons. Acta Orthop Scand. 1993;64:513–8. doi: 10.3109/17453679308993681. [DOI] [PubMed] [Google Scholar]
- 49.Karlson EW, Mandl LA, Aweh GN, Sangha O, Liang MH, Grodstein F. Total hip replacement due to osteoarthritis: the importance of age, obesity, and other modifiable risk factors. Am J Med. 2003;114:93–8. doi: 10.1016/s0002-9343(02)01447-x. [DOI] [PubMed] [Google Scholar]
- 50.Tepper S, Hochberg MC. Factors associated with hip osteoarthritis: data from the First National Health and Nutrition Examination Survey (NHANES-I). Am J Epidemiol. 1993;137:1081–8. doi: 10.1093/oxfordjournals.aje.a116611. [DOI] [PubMed] [Google Scholar]
- 51.van Saase JL, Vandenbroucke JP, van Romunde LK, Valkenburg HA. Osteoarthritis and obesity in the general population. A relationship calling for an explanation. J Rheumatol. 1988;15:1152–8. [PubMed] [Google Scholar]
- 52.Felson DT, Couropmitree NN, Chaisson CE, et al. Evidence for a Mendelian gene in a segregation analysis of generalized radiographic osteoarthritis: the Framingham Study. Arthritis Rheum. 1998;41:1064–71. doi: 10.1002/1529-0131(199806)41:6<1064::AID-ART13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 53.Palotie A, Vaisanen P, Ott J, et al. Predisposition to familial osteoarthrosis linked to type II collagen gene. Lancet. 1989;1:924–7. doi: 10.1016/s0140-6736(89)92507-5. [DOI] [PubMed] [Google Scholar]
- 54.Spector TD, Cicuttini F, Baker J, Loughlin J, Hart D. Genetic influences on osteoarthritis in women: a twin study. BMJ. 1996;312:940–3. doi: 10.1136/bmj.312.7036.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Evangelou E, Valdes AM, Kerkhof HJ, et al. Meta-analysis of genome-wide association studies confirms a susceptibility locus for knee osteoarthritis on chromosome 7q22. Ann Rheum Dis. 2011;70:349–55. doi: 10.1136/ard.2010.132787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valdes AM, Evangelou E, Kerkhof HJ, et al. The GDF5 rs143383 polymorphism is associated with osteoarthritis of the knee with genome-wide statistical significance. Ann Rheum Dis. 2011;70:873–5. doi: 10.1136/ard.2010.134155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Day-Williams AG, Southam L, Panoutsopoulou K, et al. A variant in MCF2L is associated with osteoarthritis. Am J Hum Genet. 2011;89:446–50. doi: 10.1016/j.ajhg.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Consortium a. Collaborators a Identification of new susceptibility loci for osteoarthritis (arcOGEN): a genome-wide association study. Lancet. 2012;380:815–23. doi: 10.1016/S0140-6736(12)60681-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Meurs JB, Uitterlinden AG, Stolk L, et al. A functional polymorphism in the catechol-O-methyltransferase gene is associated with osteoarthritis-related pain. Arthritis Rheum. 2009;60:628–9. doi: 10.1002/art.24175. [DOI] [PubMed] [Google Scholar]
- 60.Valdes AM, De Wilde G, Doherty SA, et al. The Ile585Val TRPV1 variant is involved in risk of painful knee osteoarthritis. Ann Rheum Dis. 2011;70:1556–61. doi: 10.1136/ard.2010.148122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valdes AM, Arden NK, Vaughn FL, et al. Role of the Nav1.7 R1150W amino acid change in susceptibility to symptomatic knee osteoarthritis and multiple regional pain. Arthritis Care Res (Hoboken) 2011;63:440–4. doi: 10.1002/acr.20375. [DOI] [PubMed] [Google Scholar]
- 62.Nevitt MC, Zhang Y, Javaid MK, et al. High systemic bone mineral density increases the risk of incident knee OA and joint space narrowing, but not radiographic progression of existing knee OA: the MOST study. Ann Rheum Dis. 2010;69:163–8. doi: 10.1136/ard.2008.099531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Naganathan V, Zochling J, March L, Sambrook PN. Peak bone mass is increased in the hip in daughters of women with osteoarthritis. Bone. 2002;30:287–92. doi: 10.1016/s8756-3282(01)00635-4. [DOI] [PubMed] [Google Scholar]
- 64.Jacobsen S, Jensen TW, Bach-Mortensen P, Hyldstrup L, Sonne-Holm S. Low bone mineral density is associated with reduced hip joint space width in women: results from the Copenhagen Osteoarthritis Study. Menopause. 2007;14:1025–30. doi: 10.1097/gme.0b013e318038d34a. [DOI] [PubMed] [Google Scholar]
- 65.Li B, Aspden RM. Composition and mechanical properties of cancellous bone from the femoral head of patients with osteoporosis or osteoarthritis. J Bone Miner Res. 1997;12:641–51. doi: 10.1359/jbmr.1997.12.4.641. [DOI] [PubMed] [Google Scholar]
- 66.Felson DT, Niu J, Clancy M, et al. Low levels of vitamin D and worsening of knee osteoarthritis: results of two longitudinal studies. Arthritis Rheum. 2007;56:129–36. doi: 10.1002/art.22292. [DOI] [PubMed] [Google Scholar]
- 67.Chaganti RK, Parimi N, Cawthon P, Dam TL, Nevitt MC, Lane NE. Association of 25-hydroxyvitamin D with prevalent osteoarthritis of the hip in elderly men: the osteoporotic fractures in men study. Arthritis Rheum. 2010;62:511–4. doi: 10.1002/art.27241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McAlindon T, Felson DT. Nutrition: risk factors for osteoarthritis. Ann Rheum Dis. 1997;56:397–400. doi: 10.1136/ard.56.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McAlindon TE, Dawson-Huges B, Driban J, et al. Clinical trial of vitamin D to reduce pain and structural progression of knee osteoarthritis (OA). Arthritis Rheum. 2010;62:S294. [Google Scholar]
- 70.McAlindon TE, Jacques P, Zhang Y, et al. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 1996;39:648–56. doi: 10.1002/art.1780390417. [DOI] [PubMed] [Google Scholar]
- 71.Chaganti R, Tolstykh I, Javaid K, et al. Association of baseline vitamin C with incident and progressive radiographic knee OA: The MOST Study. Arthritis Rheum. 2008;58:S897. [Google Scholar]
- 72.Peregoy J, Wilder FV. The effects of vitamin C supplementation on incident and progressive knee osteoarthritis: a longitudinal study. Public Health Nutr. 2011;14:709–15. doi: 10.1017/S1368980010001783. [DOI] [PubMed] [Google Scholar]
- 73.De Roos AJ, Arab L, Renner JB, et al. Serum carotenoids and radiographic knee osteoarthritis: the Johnston County Osteoarthritis Project. Public Health Nutr. 2001;4:935–42. doi: 10.1079/phn2001132. [DOI] [PubMed] [Google Scholar]
- 74.Jordan JM, De Roos AJ, Renner JB, et al. A case-control study of serum tocopherol levels and the alpha- to gamma-tocopherol ratio in radiographic knee osteoarthritis: the Johnston County Osteoarthritis Project. Am J Epidemiol. 2004;159:968–77. doi: 10.1093/aje/kwh133. [DOI] [PubMed] [Google Scholar]
- 75.Wluka AE, Stuckey S, Brand C, Cicuttini FM. Supplementary vitamin E does not affect the loss of cartilage volume in knee osteoarthritis: a 2 year double blind randomized placebo controlled study. J Rheumatol. 2002;29:2585–91. [PubMed] [Google Scholar]
- 76.Neogi T, Booth SL, Zhang YQ, et al. Low vitamin K status is associated with osteoarthritis in the hand and knee. Arthritis Rheum. 2006;54:1255–61. doi: 10.1002/art.21735. [DOI] [PubMed] [Google Scholar]
- 77.Neogi T, Felson DT, Sarno R, Booth SL. Vitamin K in hand osteoarthritis: results from a randomised clinical trial. Ann Rheum Dis. 2008;67:1570–3. doi: 10.1136/ard.2008.094771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Misra D, Booth SL, Tolstykh I, et al. Vitamin K deficiency is associated with incident knee osteoarthritis. Am J Med. 2012 doi: 10.1016/j.amjmed.2012.10.011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oka H, Akune T, Muraki S, et al. Association of low dietary vitamin K intake with radiographic knee osteoarthritis in the Japanese elderly population: dietary survey in a population-based cohort of the ROAD study. J Orthop Sci. 2009;14:687–92. doi: 10.1007/s00776-009-1395-y. [DOI] [PubMed] [Google Scholar]
- 80.Engstrom G, Gerhardsson de Verdier M, Nilsson PM, et al. Incidence of severe knee and hip osteoarthritis in relation to dietary intake of antioxidants beta-carotene, vitamin C, vitamin E and Selenium: A population-based prospective cohort study. Arthritis Rheum. 2009;60:S235–6. doi: 10.1136/ard.2008.089748. [DOI] [PubMed] [Google Scholar]
- 81.Jordan JM, Fang F, Arab L, et al. Low selenium levels are associated with increased risk for osteoarthritis of the knee. Arthritis Rheum. 2005;52:S455. [Google Scholar]
- 82.Zhang Y, Hunter DJ, Nevitt MC, et al. Association of squatting with increased prevalence of radiographic tibiofemoral knee osteoarthritis: the Beijing Osteoarthritis Study. Arthritis Rheum. 2004;50:1187–92. doi: 10.1002/art.20127. [DOI] [PubMed] [Google Scholar]
- 83.Amin S, Goggins J, Niu J, et al. Occupation-related squatting, kneeling, and heavy lifting and the knee joint: a magnetic resonance imaging-based study in men. J Rheumatol. 2008;35:1645–9. [PMC free article] [PubMed] [Google Scholar]
- 84.Coggon D, Croft P, Kellingray S, Barrett D, McLaren M, Cooper C. Occupational physical activities and osteoarthritis of the knee. Arthritis Rheum. 2000;43:1443–9. doi: 10.1002/1529-0131(200007)43:7<1443::AID-ANR5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 85.Felson DT, Hannan MT, Naimark A, et al. Occupational physical demands, knee bending, and knee osteoarthritis: results from the Framingham Study. J Rheumatol. 1991;18:1587–92. [PubMed] [Google Scholar]
- 86.McWilliams DF, Leeb BF, Muthuri SG, Doherty M, Zhang W. Occupational risk factors for osteoarthritis of the knee: a meta-analysis. Osteoarthritis Cartilage. 2011;19:829–39. doi: 10.1016/j.joca.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 87.Croft P, Coggon D, Cruddas M, Cooper C. Osteoarthritis of the hip: an occupational disease in farmers. BMJ. 1992;304:1269–72. doi: 10.1136/bmj.304.6837.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Croft P, Cooper C, Wickham C, Coggon D. Osteoarthritis of the hip and occupational activity. Scand J Work Environ Health. 1992;18:59–63. doi: 10.5271/sjweh.1608. [DOI] [PubMed] [Google Scholar]
- 89.Yoshimura N, Sasaki S, Iwasaki K, et al. Occupational lifting is associated with hip osteoarthritis: a Japanese case-control study. J Rheumatol. 2000;27:434–40. [PubMed] [Google Scholar]
- 90.Hadler NM, Gillings DB, Imbus HR, et al. Hand structure and function in an industrial setting. Arthritis Rheum. 1978;21:210–20. doi: 10.1002/art.1780210206. [DOI] [PubMed] [Google Scholar]
- 91.Lawrence JS. Rheumatism in cotton operatives. Br J Ind Med. 1961;18:270–6. doi: 10.1136/oem.18.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hunter DJ, Zhang Y, Nevitt MC, et al. Chopstick arthropathy: the Beijing Osteoarthritis Study. Arthritis Rheum. 2004;50:1495–500. doi: 10.1002/art.20145. [DOI] [PubMed] [Google Scholar]
- 93.Hannan MT, Felson DT, Anderson JJ, Naimark A. Habitual physical activity is not associated with knee osteoarthritis: the Framingham Study. J Rheumatol. 1993;20:704–9. [PubMed] [Google Scholar]
- 94.McAlindon TE, Wilson PW, Aliabadi P, Weissman B, Felson DT. Level of physical activity and the risk of radiographic and symptomatic knee osteoarthritis in the elderly: the Framingham study. Am J Med. 1999;106:151–7. doi: 10.1016/s0002-9343(98)00413-6. [DOI] [PubMed] [Google Scholar]
- 95.Wang Y, Simpson JA, Wluka AE, et al. Is physical activity a risk factor for primary knee or hip replacement due to osteoarthritis? A prospective cohort study. J Rheumatol. 2011;38:350–7. doi: 10.3899/jrheum.091138. [DOI] [PubMed] [Google Scholar]
- 96.Dore DA, Winzenberg TM, Ding C, et al. The association between objectively measured physical activity and knee structural change using MRI. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2012-201691. [DOI] [PubMed] [Google Scholar]
- 97.Lane NE, Oehlert JW, Bloch DA, Fries JF. The relationship of running to osteoarthritis of the knee and hip and bone mineral density of the lumbar spine: a 9 year longitudinal study. J Rheumatol. 1998;25:334–41. [PubMed] [Google Scholar]
- 98.Panush RS, Schmidt C, Caldwell JR, et al. Is running associated with degenerative joint disease? JAMA. 1986;255:1152–4. [PubMed] [Google Scholar]
- 99.Marti B, Knobloch M, Tschopp A, Jucker A, Howald H. Is excessive running predictive of degenerative hip disease? Controlled study of former elite athletes. BMJ. 1989;299:91–3. doi: 10.1136/bmj.299.6691.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spector TD, Harris PA, Hart DJ, et al. Risk of osteoarthritis associated with long-term weight-bearing sports: a radiologic survey of the hips and knees in female ex-athletes and population controls. Arthritis Rheum. 1996;39:988–95. doi: 10.1002/art.1780390616. [DOI] [PubMed] [Google Scholar]
- 101.Kujala UM, Kettunen J, Paananen H, et al. Knee osteoarthritis in former runners, soccer players, weight lifters, and shooters. Arthritis Rheum. 1995;38:539–46. doi: 10.1002/art.1780380413. [DOI] [PubMed] [Google Scholar]
- 102.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145–52. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 103.Roos EM, Ostenberg A, Roos H, Ekdahl C, Lohmander LS. Long-term outcome of meniscectomy: symptoms, function, and performance tests in patients with or without radiographic osteoarthritis compared to matched controls. Osteoarthritis Cartilage. 2001;9:316–24. doi: 10.1053/joca.2000.0391. [DOI] [PubMed] [Google Scholar]
- 104.Muthuri SG, McWilliams DF, Doherty M, Zhang W. History of knee injuries and knee osteoarthritis: a meta-analysis of observational studies. Osteoarthritis Cartilage. 2011;19:1286–93. doi: 10.1016/j.joca.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 105.Englund M, Guermazi A, Roemer FW, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60:831–9. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rytter S, Egund N, Jensen LK, Bonde JP. Occupational kneeling and radiographic tibiofemoral and patellofemoral osteoarthritis. J Occup Med Toxicol. 2009;4:19. doi: 10.1186/1745-6673-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Englund M, Guermazi A, Gale D, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359:1108–15. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–69. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 109.Brandt KD, Heilman DK, Slemenda C, et al. Quadriceps strength in women with radiographically progressive osteoarthritis of the knee and those with stable radiographic changes. J Rheumatol. 1999;26:2431–7. [PubMed] [Google Scholar]
- 110.Slemenda C, Heilman DK, Brandt KD, et al. Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998;41:1951–9. doi: 10.1002/1529-0131(199811)41:11<1951::AID-ART9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 111.Segal NA, Torner JC, Felson D, et al. Effect of thigh strength on incident radiographic and symptomatic knee osteoarthritis in a longitudinal cohort. Arthritis Rheum. 2009;61:1210–7. doi: 10.1002/art.24541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sharma L, Dunlop DD, Cahue S, Song J, Hayes KW. Quadriceps strength and osteoarthritis progression in malaligned and lax knees. Ann Intern Med. 2003;138:613–9. doi: 10.7326/0003-4819-138-8-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 113.Amin S, Baker K, Niu J, et al. Quadriceps strength and the risk of cartilage loss and symptom progression in knee osteoarthritis. Arthritis Rheum. 2009;60:189–98. doi: 10.1002/art.24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chaisson CE, Zhang Y, Sharma L, Felson DT. Higher grip strength increases the risk of incident radiographic osteoarthritis in proximal hand joints. Osteoarthritis Cartilage. 2000;8(Suppl A):S29–32. doi: 10.1053/joca.2000.0333. [DOI] [PubMed] [Google Scholar]
- 115.Dominick KL, Jordan JM, Renner JB, Kraus VB. Relationship of radiographic and clinical variables to pinch and grip strength among individuals with osteoarthritis. Arthritis Rheum. 2005;52:1424–30. doi: 10.1002/art.21035. [DOI] [PubMed] [Google Scholar]
- 116.Brouwer GM, van Tol AW, Bergink AP, et al. Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis Rheum. 2007;56:1204–11. doi: 10.1002/art.22515. [DOI] [PubMed] [Google Scholar]
- 117.Hunter DJ, Niu J, Felson DT, et al. Knee alignment does not predict incident osteoarthritis: the Framingham osteoarthritis study. Arthritis Rheum. 2007;56:1212–8. doi: 10.1002/art.22508. [DOI] [PubMed] [Google Scholar]
- 118.Sharma L, Chmiel JS, Almagor O, et al. The role of varus and valgus alignment in the initial development of knee cartilage damage by MRI: the MOST study. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2011-201070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sharma L, Song J, Dunlop D, et al. Varus and valgus alignment and incident and progressive knee osteoarthritis. Ann Rheum Dis. 2010;69:1940–5. doi: 10.1136/ard.2010.129742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tanamas S, Hanna FS, Cicuttini FM, Wluka AE, Berry P, Urquhart DM. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61:459–67. doi: 10.1002/art.24336. [DOI] [PubMed] [Google Scholar]
- 121.Mazzuca SA, Brandt KD, Chakr R, Lane KA. Varus malalignment negates the structure-modifying benefits of doxycycline in obese women with knee osteoarthritis. Osteoarthritis Cartilage. 2010;18:1008–11. doi: 10.1016/j.joca.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Segal NA, Anderson DD, Iyer KS, et al. Baseline articular contact stress levels predict incident symptomatic knee osteoarthritis development in the MOST cohort. J Orthop Res. 2009;27:1562–8. doi: 10.1002/jor.20936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Golightly YM, Allen KD, Helmick CG, Schwartz TA, Renner JB, Jordan JM. Hazard of Incident and Progressive Knee and Hip Radiographic Osteoarthritis and Chronic Joint Symptoms in Individuals with and without Limb Length Inequality. J Rheumatol. 2010;37:2133–40. doi: 10.3899/jrheum.091410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Golightly YM, Allen KD, Renner JB, Helmick CG, Salazar A, Jordan JM. Relationship of limb length inequality with radiographic knee and hip osteoarthritis. Osteoarthritis Cartilage. 2007;15:824–9. doi: 10.1016/j.joca.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Harvey WF, Yang M, Cooke TD, et al. Association of leg-length inequality with knee osteoarthritis: a cohort study. Ann Intern Med. 2010;152:287–95. doi: 10.1059/0003-4819-152-5-201003020-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gregory JS, Waarsing JH, Day J, et al. Early identification of radiographic osteoarthritis of the hip using an active shape model to quantify changes in bone morphometric features: can hip shape tell us anything about the progression of osteoarthritis? Arthritis Rheum. 2007;56:3634–43. doi: 10.1002/art.22982. [DOI] [PubMed] [Google Scholar]
- 127.Lynch JA, Parimi N, Chaganti RK, Nevitt MC, Lane NE. The association of proximal femoral shape and incident radiographic hip OA in elderly women. Osteoarthritis Cartilage. 2009;17:1313–8. doi: 10.1016/j.joca.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lane NE, Lin P, Christiansen L, et al. Association of mild acetabular dysplasia with an increased risk of incident hip osteoarthritis in elderly white women: the study of osteoporotic fractures. Arthritis Rheum. 2000;43:400–4. doi: 10.1002/1529-0131(200002)43:2<400::AID-ANR21>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 129.Doherty M, Courtney P, Doherty S, et al. Nonspherical femoral head shape (pistol grip deformity), neck shaft angle, and risk of hip osteoarthritis: a case-control study. Arthritis Rheum. 2008;58:3172–82. doi: 10.1002/art.23939. [DOI] [PubMed] [Google Scholar]
- 130.Reid GD, Reid CG, Widmer N, Munk PL. Femoroacetabular impingement syndrome: an underrecognized cause of hip pain and premature osteoarthritis? J Rheumatol. 2010;37:1395–404. doi: 10.3899/jrheum.091186. [DOI] [PubMed] [Google Scholar]
- 131.Bredbenner TL, Eliason TD, Potter RS, Mason RL, Havill LM, Nicolella DP. Statistical shape modeling describes variation in tibia and femur surface geometry between Control and Incidence groups from the osteoarthritis initiative database. J Biomech. 2010;43:1780–6. doi: 10.1016/j.jbiomech.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Neogi T, Bowes M, Niu J, et al. MRI-based 3D bone shape predicts incident knee OA 12 months prior to its onset. Osteoarthritis Cartilage. 2011;19(Suppl 1):S51–S2. [Google Scholar]
- 133.Hawker GA, Stewart L, French MR, et al. Understanding the pain experience in hip and knee osteoarthritis--an OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16:415–22. doi: 10.1016/j.joca.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 134.Neogi T, Nevitt MC, Yang M, Curtis JR, Torner J, Felson DT. Consistency of knee pain: correlates and association with function. Osteoarthritis Cartilage. 2010;18:1250–5. doi: 10.1016/j.joca.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wise BL, Niu J, Zhang Y, et al. Psychological factors and their relation to osteoarthritis pain. Osteoarthritis Cartilage. 2010;18:883–7. doi: 10.1016/j.joca.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Neogi T, Felson D, Niu J, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. doi: 10.1136/bmj.b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang Y, Nevitt M, Niu J, et al. Fluctuation of knee pain and changes in bone marrow lesions, effusions and synovitis on MRI: The Most Study. Arthritis Rheum. 2011;63:691–9. doi: 10.1002/art.30148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yusuf E, Kortekaas MC, Watt I, Huizinga TW, Kloppenburg M. Do knee abnormalities visualised on MRI explain knee pain in knee osteoarthritis? A systematic review. Ann Rheum Dis. 2010 doi: 10.1136/ard.2010.131904. First online: 10.1136/ard.2010.131904. [DOI] [PubMed] [Google Scholar]
- 139.Manson JE, Bassuk SS, Hu FB, Stampfer MJ, Colditz GA, Willett WC. Estimating the number of deaths due to obesity: can the divergent findings be reconciled? J Womens Health (Larchmt) 2007;16:168–76. doi: 10.1089/jwh.2006.0080. [DOI] [PubMed] [Google Scholar]
- 140.Cooper C, Snow S, McAlindon TE, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000;43:995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 141.Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40:728–33. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 142.Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiology. 1992;3:143–55. doi: 10.1097/00001648-199203000-00013. [DOI] [PubMed] [Google Scholar]


