Abstract
Background
Urinary tract infections (UTI) are often misdiagnosed in nursing home (NH) residents leading to unnecessary antimicrobial exposure. The diagnosis is particularly challenging among residents with advanced dementia who have minimal verbal ability to communicate symptoms.
Design
twelve-month prospective study
Setting
25 NHs
Participants
Two-hundred and sixty-six residents with advanced dementia.
Measurements
Charts were abstracted monthly for documentation of suspected UTI episodes to determine whether episodes met minimum criteria to initiate antimicrobials according to consensus guidelines.
Results
Seventy-two residents experienced 131 suspected UTI episodes. Presenting signs and symptoms for these episodes were: mental status change, 44.3%; fever, 20.6%; hematuria, 6.9%; dysuria, 3.8%; costovertebral tenderness, 2.3%; frequency, 1.5%; rigors, 1.5; urgency; 0% and suprapubic pain, 0%. Only 21 (16.0%) episodes met minimal criteria to initiate antimicrobials based on signs and symptoms. Among the 110 episodes that lacked minimum criteria to justify antimicrobial initiation, 82 (74.5%) were treated with antimicrobials. Urinalyses and urine culture results were available for 101 episodes, of which 80 (79.2%) had positive results on both tests. The proportion of episodes with a positive urinalysis and culture was similar for those that met or did not meet minimum criteria (83.3% versus 78.3% p = 0.06).
Conclusion
The symptoms and signs necessary to meet minimum criteria to support antimicrobial initiation for UTIs are frequently absent among NH residents with advanced dementia. Antimicrobials are prescribed for the majority of suspected UTIs that do not meet these minimum criteria. Urine specimens are frequently positive regardless of symptoms. These observations underscore the need to reconsider the diagnosis and the initiation of treatment for suspected UTIs in advanced dementia.
Keywords: advanced dementia, urinary tract infection, antimicrobials, criteria
INTRODUCTION
Urinary tract infections (UTIs) are the most common infection diagnosed in nursing homes (NH) residents, and account for the majority of antimicrobial prescriptions in this setting.1-4 However, approximately one-third of UTIs in NH residents are misdiagnosed leading to unnecessary antimicrobial exposure.5 The diagnosis of a UTI requires the presence of symptoms (e.g., fever, dysuria, frequency), as well as positive findings on urinalyses and urine cultures.6 Prior work clearly shows that antimicrobial therapy is not warranted for asymptomatic bacteriuria, yet this problem continues to account for the majority of antimicrobial misuse in the NH setting.6
Over 5 million Americans suffer with Alzheimer’s disease or a related dementia.7 An increasing proportion of these patients are surviving to the advanced stage of their disease, the majority of whom are cared for in NHs. Antimicrobials in the NH setting are often started empirically based the residents’ clinical signs and symptoms. The clinical assessment of NH residents with advanced dementia to determine whether or not they have symptoms of UTI, is particularly challenging. These residents, by definition, have minimal to no verbal communication (i.e., speech limited to less than five words). 8 Thus, their ability to meaningfully express the typical genitourinary symptoms of UTI (e.g., dysuria, costovertebral pain) is very limited. Moreover, these residents have profound cognitive deficits and urinary incontinence. Thus, mental status changes are hard to discern and urinary frequency is extremely difficult to detect. Despite these challenges, NH residents with advanced dementia are commonly treated with antimicrobials for suspected UTIs.9 Antimicrobial misuse is particularly concerning in these residents because they are three times more likely to acquire antimicrobial-resistant bacteria compared to other residents.10
In 2001, the Society for Healthcare Epidemiology of America (SHEA) endorsed minimum clinical criteria to initiate antimicrobials in the general NH population for suspected infections, including UTIs.11 In 2009, we initiated an NIH-funded prospective study of infections management in NH home residents with advanced dementia entitled: the Study of Pathogen Resistance and Antimicrobial Exposure in Advanced Dementia (SPREAD). As part of this study, we used the SHEA criteria to determine if minimum criteria were met to initiate antimicrobials for suspected UTIs. The objective of this report was to describe the presentation of suspected UTIs in NH residents with advanced dementia and how they align to minimum criteria. A better understanding of the clinical challenges facing NH practitioners in diagnosing UTIs in these residents is needed to inform strategies aimed at preventing inappropriate antimicrobial use in this vulnerable population.
METHODS
Setting and subjects
Data were obtained from the ongoing Study of Pathogen Resistance and Exposure to Antimicrobials in Dementia (SPREAD). SPREAD’s overriding goals are to examine antimicrobial exposure in NH residents with advanced dementia to further our understanding as to how such exposure contributes to antimicrobial resistance. Results from the first 266 residents recruited into this study are presented in this report. The Institutional Review Board of the Hebrew SeniorLife approved the conduct of this study.
From September, 2009 through November, 2011, residents with advanced dementia were recruited from 25 NHs. Participating NHs had to be within 60 miles of the Boston, Massachusetts and have a minimum of 45 beds. Resident eligibility criteria included (1) age over 65 years, (2) dementia (any type, determined from the medical record), (3) an available, English-speaking proxy to provide informed consent and (4) a Global Deterioration Scale score of 7 (ascertained by interview with a nurse caring for the resident).8 A Global Deterioration Scale score of 7 is characterized by profound memory deficits (unable to recognize family), limited verbal communication (< 5 words), incontinence, and inability to ambulate.
Data collection and variables
Data analyzed in this report were collected by research nurses from the residents’ medical record at baseline and monthly thereafter for up to 12 months. Among residents who died during the study period, a medical record review was conducted within 14 days of death. A brief baseline interview with the residents’ nurse was conducted to quantify the resident’s functional status as measured using the Bedford Alzheimer’s Nursing Severity-Subscale (BANS-S) (range 7-28); higher scores indicate more functional disability.12 Other baseline resident characteristics included age, gender and race.
At baseline, monthly, and on the death assessment, all suspected UTIs documented in the resident’s medical record by a nurse, nurse practitioner, physician assistant, or physician were identified. Documentation required that the provider specifically state that the resident had an infection for which the suspected source was the urinary tract. For each suspected UTI episode, documentation of the following was ascertained: 1. whether the resident had a foley catheter, 2. temperature data, and 3. the presence of the following symptoms: new dysuria, frequency, urgency, hematuria, costovertebral tenderness, suprapubic pain, change in mental status (i.e., “mental status change”, “ lethargy” or “alteration from cognitive status from baseline”), or rigors. Temperature data included; highest recorded temperature, source (oral, rectal, axillary), whether the resident had an oral temperature of >99°F (>37.2°C) more than once, and whether the resident had a temperature of >2°F (>1.1°C) over baseline temperature.
Whether or not urinalyses and/or urine cultures were obtained was determined, and if so, the results of these tests were ascertained. Urinalyses were considered positive if microscopic analysis showed > 10,000 white blood cells per liter or a urine dipstick was positive for either white blood cells, leukocyte esterase or nitrites.13 Urine cultures were considered positive if it grew > 105 colony forming units of at least one bacterial organism in residents without a foley catheter and > 103 colony forming units of at least one bacterial organism in residents with a foley catheter. 6,14
Criteria for antimicrobial initiation
Whether or not the minimum criteria for antimicrobial initiation were present was based on the published SHEA guidelines (Table 1). For residents without foley catheters, the minimum signs or symptoms included: i. the presence of dysuria, or ii. fever with at least one of the following: frequency, urgency, hematuria, costoverterbral tenderness, suprapubic pain, a mental status change, or rigors.11 While mental status and rigors were not included in the original SHEA guidelines for non-catheterized NH residents, we chose to include them given the inability of residents with advanced dementia to express other symptoms. For residents with an indwelling foley catheter, minimum signs or symptoms included the presence of at least one of the following: fever, rigors, or change in mental status. For all residents fever was defined as either: i. a single oral temperature of >100°F (>37.8°C), ii. repeated (≥2 times) oral temperatures of >99°F (>37.2°C), or iii. an increase in temperature of >2°F (>1.1°C) over baseline temperature.15 For residents without any localizing findings, a suspected UTI was only considered if an alternate infectious source was not identified.
Table 1.
Minimum criteria for initiation of antimicrobials use for a suspected urinary tract infection in nursing home residents with advanced dementia.11
| Criteria for initiation of antimicrobials for suspected urinary tract infections |
|---|
| a. No indwelling foley catheter |
| Acute dysuria OR Fever (a single oral temperature of >100°F (>37.8°C), ii. oral temperatures of >99°F (>37.2°C), repeated ≥2 times, or iii. an increase in temperature of >2°F (>1.1°C) over baseline temperature.). |
| AND ≥1 of the following: |
| b. Indwelling foley catheter |
≥1 of the following:
|
| 3. Change in mental status1 |
At the start of this study, the presence of a mental status change and rigors were added to Society for Healthcare Epidemiology of America endorsed minimum clinical criteria to initiate antimicrobials for suspected urinary tract infections in the general NH population in order to slightly liberalize these criteria for residents with advanced dementia.
Statistical Analysis
Descriptive statistics were used to describe all resident characteristics and features of suspected UTIs using frequencies for categorical variables and means with standard deviations for continuous variables. An odds ratio (OR) and 95% and confidence interval (CI) were generated to examine the association between laboratory evidence consistent with a diagnosis of a UTI (i.e. whether or not both the urinalysis and culture urine were positive), and presence of minimum criteria to initiate antimicrobials (e.g., clinical evidence suggestive of a UTI). All analyses were conducted using STATA 10.0 (College Station, TX).
RESULTS
Subjects
Subjects included in this report were derived from the first 700 NH residents who met eligibility criteria for the SPREAD study, of whom 266 (38%) residents were recruited. Proxy refusal was the sole reason for non-participation except for one resident whose physician refused to allow enrollment. Eligible residents who did not participate did not differ from participants with respect to age and gender (data not shown).
Demographic characteristics of the 266 subjects included in this report were as follows: mean age, 86.6 ± (standard deviation (SD) 7.4 years), 85.7% female, and 92.5% white. The mean BANS-S score was 21.8 + (SD) 2.4 indicating very severe functional disability.10 A total of 11 (4.1%) of residents had urinary catheters. At the time of this report, 87 (32.7%) residents had died and the mean follow-up time for the entire cohort was 221.2 + (SD) 131.8 days.
Characteristics of suspected urinary tract infections
At total of 72 (27.1%) of 266 residents experienced 131 suspected UTIs during the follow-up period. Ten (13.9%) residents had a urinary catheter. Residents had a mean of 2 suspected UTI episodes (range 1-9 episodes). A total of 15 (11.5%) suspected UTIs episodes occurred in residents with a foley catheter and 116 (88.5%) occurred among residents without a foley catheter.
Characteristics of the 131 episodes are shown in Table 2. Mental status changes were the sole symptom or sign documented for 47 (35.9%) episodes. Only 21 (16.0%) of all episodes met the minimum criteria to initiate antimicrobials based on documented signs or symptoms according to our expanded version of the original SHEA guidelines (i.e. mental status changes and rigors included as adjunct symptoms for non-catheterized residents).
Table 2.
Characteristics of suspected urinary tract infection episodes in nursing home residents with advanced dementia
| No. (%) suspected urinary tract infection episodes | |||
|---|---|---|---|
| All episodes N=131 | Episodes in residents with a foley catheter N=15 | Episodes in residents without a foley catheter N=116 | |
| Signs or symptoms | |||
| Fever | 27(20.6) | 5 (33.3) | 22 (19.0) |
| Dysuria | 5 (3.8) | 1 (6.7) | 4 (3.4) |
| Frequency | 2 (1.5) | 0 (0) | 2 (1.7) |
| Urgency | 0 (0) | 0 (0) | 0 (0) |
| Hematuria | 9 (6.9) | 3 (13.3) | 6 (5.2) |
| Costoverterbral tenderness | 3 (2.3) | 1 (6.7) | 2 (1.7) |
| Suprapubic pain | 0 (0) | 0 (0) | 0 (0) |
| Mental status change | 58 (44.3) | 3 (13.3) | 56 (48.3) |
| Rigors | 2 (1.5) | 1 (6.7) | 1 (0.9) |
| Minimum signs/symptoms to support antimicrobial initiation | 21 (16.0) | 6 (40.0) | 15 (12.9) |
Table 2 shows data stratified by whether the suspected UTI occurred among residents with and without a foley catheter. A mental status change was more commonly documented (48.3% vs. 13.3%) and fever was less commonly documented (19.0% vs. 33.3%) for episodes in non-catheterized vs. catheterized residents. The proportion of episodes for which the minimum criteria to initiate antimicrobial were met based on signs and symptoms was also lower among non-catheterized residents (12.9% vs. 40.0%).
Urinalyses and urine cultures
Overall, among 131 suspected UTI episodes, urinalyses and cultures were available for 101 episodes among 52 residents (7 residents had a urinary catheter). Of these episodes, 80 (79.2%) were positive for both tests. This percentage was not statistically different between episodes that met (N=15/18; 83.3%) as compared to those that did not meet (N=65/83, 78.3%) minimum signs or symptoms to initiate antimicrobials (OR 1.3, 95% CI 0.3-8.2; P=0.6).
Taken together, only 15 (11.4%) of all 131 suspected UTIs episodes in NH residents with advanced dementia had both the minimum signs or symptoms and positive laboratory findings to meet the complete diagnostic criteria for a UTI.
Antimicrobial Exposure
A total of 102 (77.9%) of all suspected UTIs were treated with antimicrobials. Twelve episodes occurred among 9 residents with urinary catheters. Among all 102 suspected UTIs, 82 (80.4%) lacked minimum criteria to justify antimicrobial initiation. Among the 110 episodes that did not meet minimum criteria, 82 (74.5%) were treated with antimicrobials (Figure).
Figure.
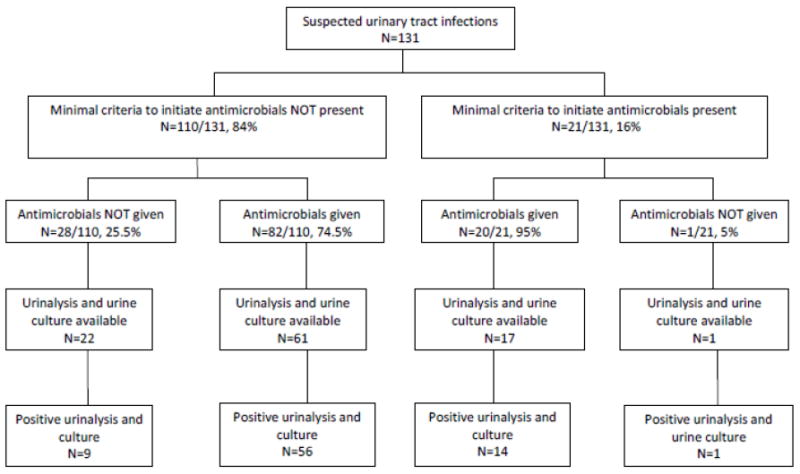
Flowchart for episodes of suspected urinary tract infections (N=131) among nursing home residents with advanced dementia.(N=266) showing: whether or not minimum criteria to initiate antimicrobials were met based on signs and symptoms, whether or not the episode was treated with antimicrobials, and results of urine tests among episodes for which urine specimens were available.
DISCUSSION
This report demonstrates the difficulties associated with diagnosing UTIs among NH residents with advanced dementia and the extent to which antimicrobials are initiated unnecessarily. Even with our liberalized adaption of the SHEA guidelines, 84% of suspected UTIs lacked the minimum clinical criteria to support antimicrobial initiation, yet 74.5% of these episodes were treated with antimicrobials. The usefulness of urinary specimens in diagnosing UTIs in these residents was also questionable, as urinalyses and urine cultures were positive in the vast majority of episodes regardless of whether or not minimum signs or symptoms were present. These observations underscore the need to reconsider the diagnosis and treatment of UTIs in advanced dementia.
Based on the SHEA criteria, at a minimum, either fever or dysuria must be present to support antimicrobial initiation for a suspected UTI.11 Fever was present in approximately one in five suspected UTI episodes, and thus a helpful objective clinical sign when present. However, older patients with known infections sometimes do not manifest a fever.15 Given that they cannot reliably express dysuria, strict application of the SHEA criteria would require the presence of fever plus another symptom or sign. However, the usefulness of adjunctive symptoms such as urgency, costovertebral tenderness and suprapubic pain, is also limited because they are rarely reported and the validity of the few instances that they are documented is questionable given that the resident were effectively mute.8,16 Mental status changes were the most common documented symptom associated with a suspected UTI. However, the reliance on mental status changes as supportive evidence for a UTI17, may be valid but is problematic. Advanced dementia residents have such profound cognitive deficits at baseline that a mental change is challenging to detect and often transient. Moreover, the reason for a mental status change is impossible to discern in the absence of more specific signs and symptoms.
The original SHEA criteria were developed for the general NH population and did not take into consideration the unique characteristics of residents with profound cognitive impairment who are non-verbal.8,11 Since dysuria and adjunctive symptoms/signs are also hard to discern, fever alone may be adequate evidence to justify antimicrobial initiation for a suspected UTI, so long as there are no additional symptoms (e.g., new cough) to suggest an alternative source of infection. However, if this approach is adopted, then diligence to discontinue antimicrobials for a suspected UTI should be enforced as soon as the urinalysis and urine culture results are confirmed negative.6 If the test results are positive, the healthcare provider must still apply clinical judgment to assess whether the combination of signs or symptoms with positive urine tests reflect a true UTI versus another etiology for the fever in the presence of asymptomatic bacteriuria.
Finally, treatment decisions for infections in advanced dementia, including UTIs, should also incorporate the residents’ preferences regarding the goal of their medical care (e.g., comfort only vs. life prolongation) as ascertained from their health care proxies. The work-up and treatment of UTIs is potentially burdensome in this frail population who are near the end of life. Urine specimens often need to be obtained by bladder catheterization, which can be an uncomfortable procedure. Antimicrobials have the potential for interactions with other drugs and adverse effects (e.g., Clostridium difficile) and can be challenging to administer in these residents who often have swallowing problems. Thus, for the majority of NH with advanced dementia for whom the goal of care is comfort18, the potential disadvantages of the work-up and treatment of suspected UTI may outweigh the advantages, particularly when the likelihood of a true UTI is low.
There are several limitations of this report that warrant discussion. First, we relied on documentation of signs and symptoms in the residents’ charts which may or may not have captured the actual clinical situation. However, appropriate medical documentation is generally considered a standard of care. Second, our study was limited to NHs in the Boston area and may not be generalizable to other regions.
This study demonstrates that UTIs are commonly suspected in NH residents with advanced dementia but that the great majority of these episodes likely do not reflect a true UTI, resulting in substantial inappropriate antimicrobial exposure. Rates of antimicrobial-resistant bacteria in the NH population are rapidly rising and residents with advanced dementia are among the subgroup of residents in NH at highest risk of harboring these resistant bacteria.10,19 From both an infectious disease and palliative care perspective, it is imperative to minimize inappropriate treatment of UTIs in advanced dementia. This report provides insight into future research initiatives for optimizing the criteria required to diagnose a UTI in the unique NH population with advanced dementia and ultimately in decreasing inappropriate antimicrobial use.
Acknowledgments
This research was supported by NIH-NIA R01 AG032982 (EMCD and SLM) and K24AG033640 (SLM)
Sponsor’s Role: None.
Footnotes
Author contribution: Drs. D’Agata and Mitchell conceived the study concept and design, acquired subjects and data, analyzed and interpreted the data and, prepared the manuscript. Dr. Loeb assisted in the study design, analyzed and interpreted the data and prepared the manuscript.
Conflict of Interest: none
References
- 1.Wang L, Lansing B, Symons K, et al. Infection rate and colonization with antibiotic-resistant organisms in skilled nursing facility residents with indwelling devices. Eur J Clin Microbiol Infect Dis. 2012 Jan 25; doi: 10.1007/s10096-011-1504-7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loeb M, Brazil K, Lohfeld L, et al. Optimizing antibiotics in residents of nursing homes: protocol of a randomized trial. BMC Health Serv Res. 2002;2:17. doi: 10.1186/1472-6963-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pettersson E, Vernby A, Mölstad S, et al. Infections and antibiotic prescribing in Swedish nursing homes: a cross-sectional study. Scand J Infect Dis. 2008;40:393–398. doi: 10.1080/00365540701745279. [DOI] [PubMed] [Google Scholar]
- 4.Daneman N, Gruneir A, Newman A, et al. Antibiotic use in long-term care facilities. J Antimicrob Chemother. 2011;66:2856–2863. doi: 10.1093/jac/dkr395. [DOI] [PubMed] [Google Scholar]
- 5.Loeb M, Simor AE, Landry L, et al. Antibiotic use in Ontario facilities that provide chronic care. J Gen Intern Med. 2001;16:376–383. doi: 10.1046/j.1525-1497.2001.016006376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicolle LE, Bradely S, Colgan R, et al. Infectious Disease Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643–654. doi: 10.1086/427507. [DOI] [PubMed] [Google Scholar]
- 7.Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: Prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 8.Reisberg B, Ferris SH, Georgotas A, et al. The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 9.D’Agata E, Mitchell S. antimicrobial utilization patterns among nursing home residents with advanced dementia. Arch Int Med. 2008;168:357–362. doi: 10.1001/archinternmed.2007.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pop-Vicas A, Mitchell SL, Kandel R, et al. Multidrug-resistant bacteria in a longterm care facility: Prevalence and risk factors. J Am Geriatr Soc. 2008;56:1276–1280. doi: 10.1111/j.1532-5415.2008.01787.x. [DOI] [PubMed] [Google Scholar]
- 11.Loeb M, Bentley DW, Bradley S, et al. Development of minimum criteria for the initiation of antibiotics in residents of long-term-care facilities: Results of a consensus conference. Infect Control Hosp Epidemiol. 2001;22:120–124. doi: 10.1086/501875. [DOI] [PubMed] [Google Scholar]
- 12.Volicer L, Hurley C, Lathi DC, et al. Measurement of severity in advanced Alzheimer’s disease. J Gerontol. 1994;49:M223–M226. doi: 10.1093/geronj/49.5.m223. [DOI] [PubMed] [Google Scholar]
- 13.Little P, Turner S, Rumsby K, et al. Dipsticks and diagnostic algorithms in urinary tract infections: Development and validation, randomized trial, economic analysis, observational cohort and qualitative study. Health Technol Assess. 2009;13:1–73. doi: 10.3310/hta13190. [DOI] [PubMed] [Google Scholar]
- 14.Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 15.High KP, Bradley SF, Gravenstein S, et al. Clinical practice guideline for the evaluation of fever and infection in older adult residents of long-term care facilities: 2008 update by the Infectious Disease Society of America. Clin Infect Dis. 2009;48:149–171. doi: 10.1086/595683. [DOI] [PubMed] [Google Scholar]
- 16.Drinka P. Treatment of bacteriuria without urinary signs, symptoms, or systemic infectious illness (S/S/S) J Am Med Dir Assoc. 2009;10:516–519. doi: 10.1016/j.jamda.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Juthani-Mehta M, Quagilarello V, Perrelli E, et al. Clincial features to identify urinary tract infection in nursing home residents: A cohort study. J Am Geriatr Soc. 2009;57:963–970. doi: 10.1111/j.1532-5415.2009.02227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361:1529–1538. doi: 10.1056/NEJMoa0902234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pop-Vicas A, D’Agata EMC. The rising influx of multi-drug resistant gram-negative bacteria into a tertiary care hospital. Clin Infect Dis. 2005;40:1792–1798. doi: 10.1086/430314. [DOI] [PubMed] [Google Scholar]


