Abstract
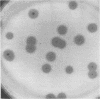
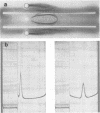
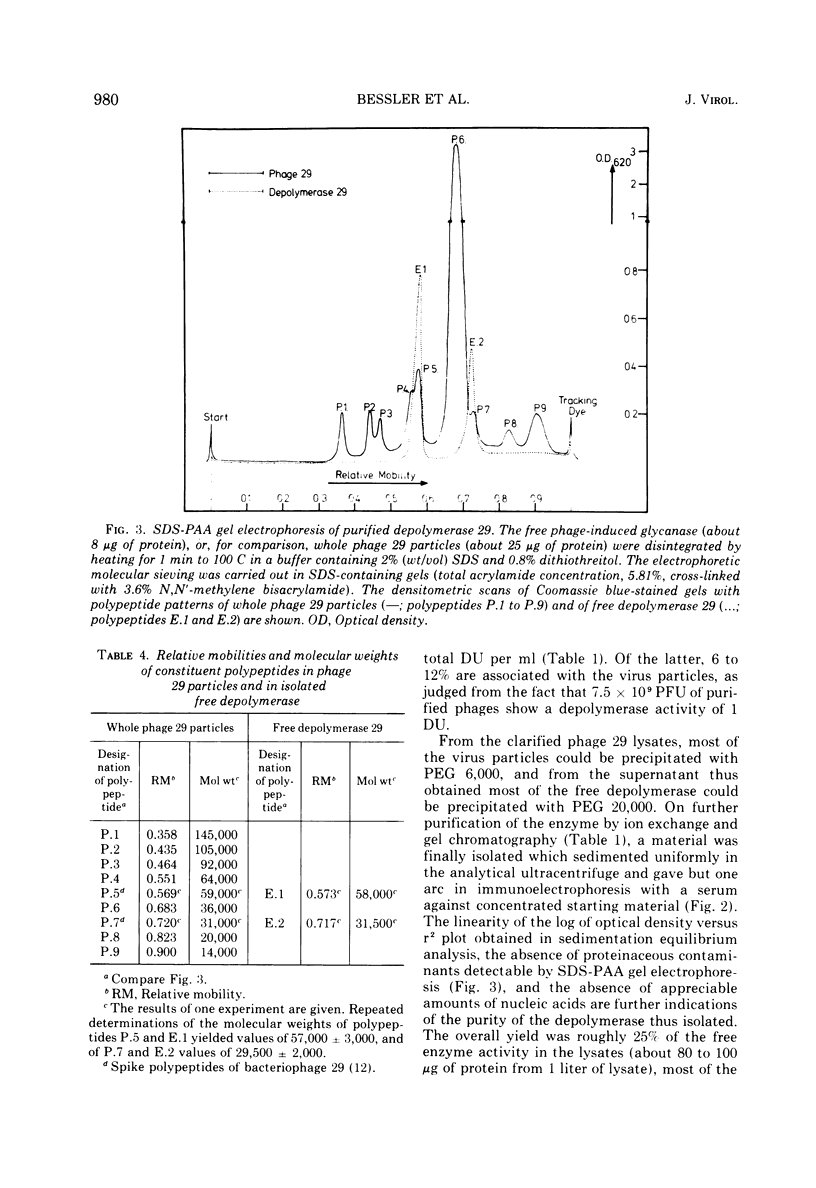
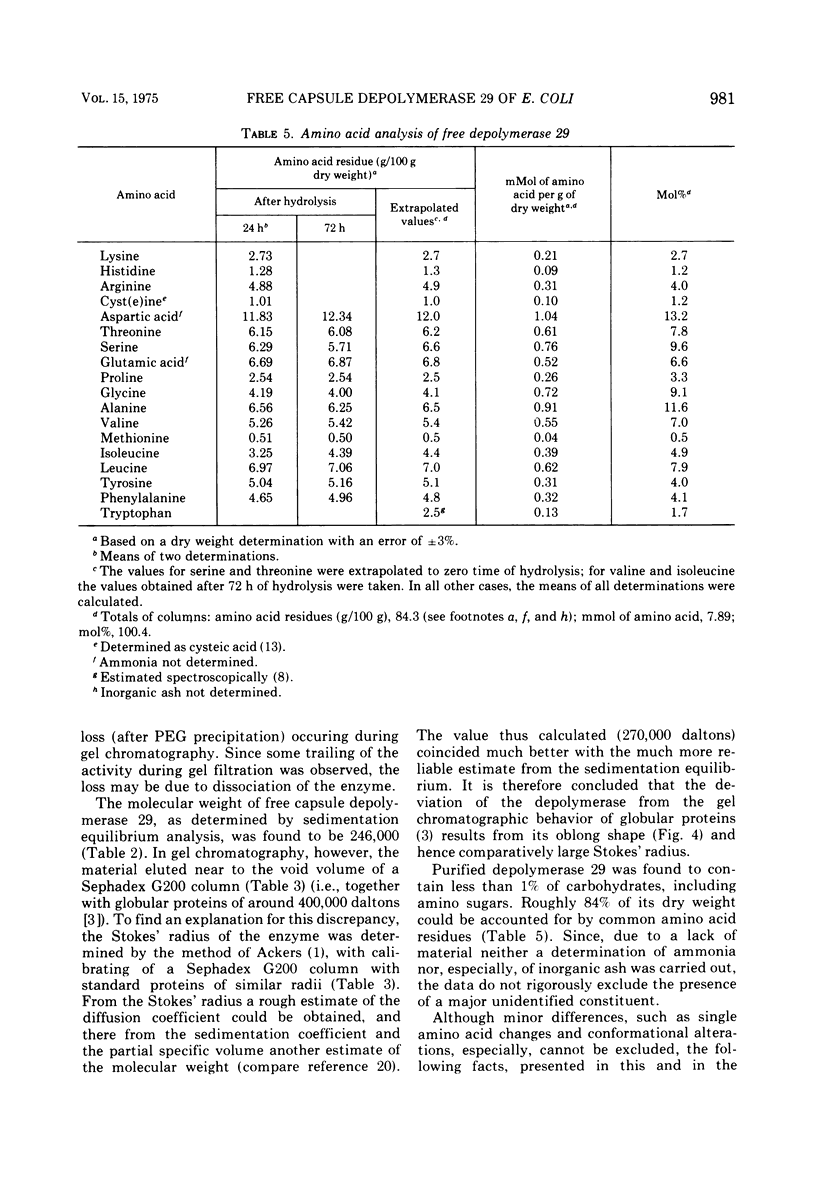
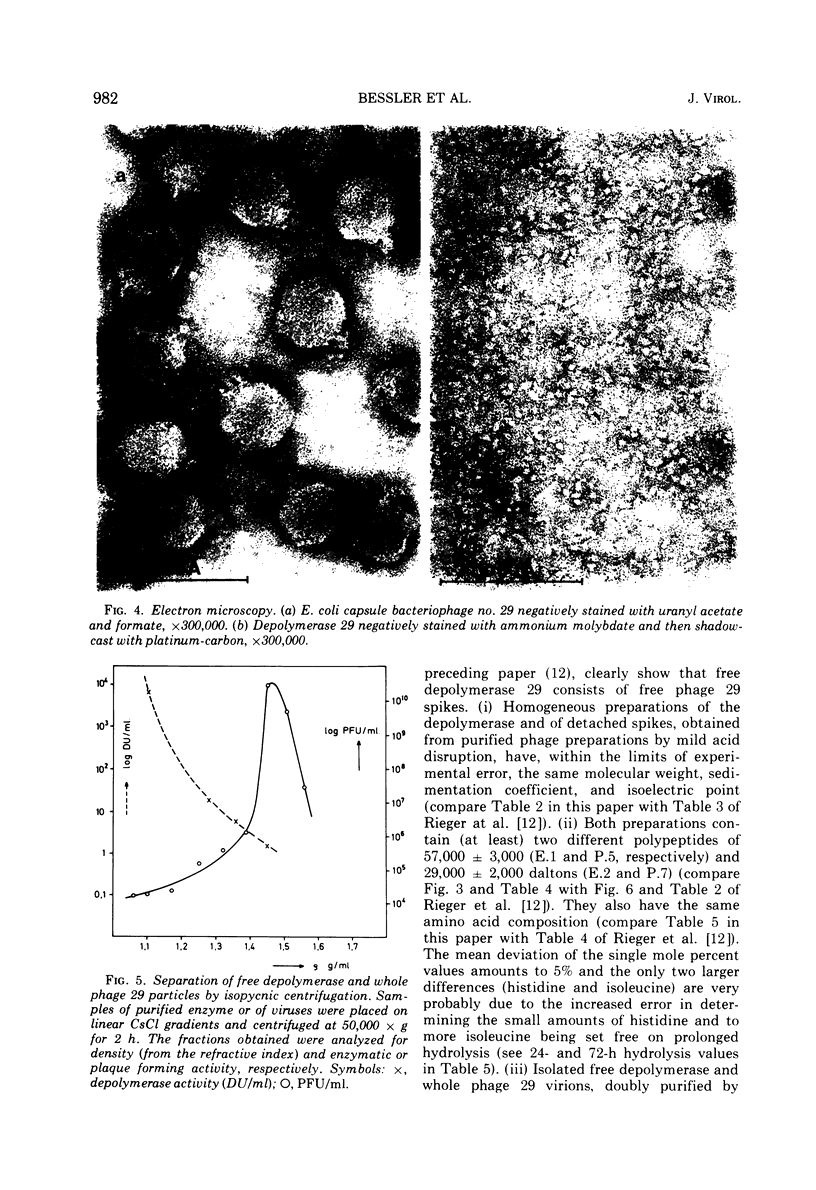
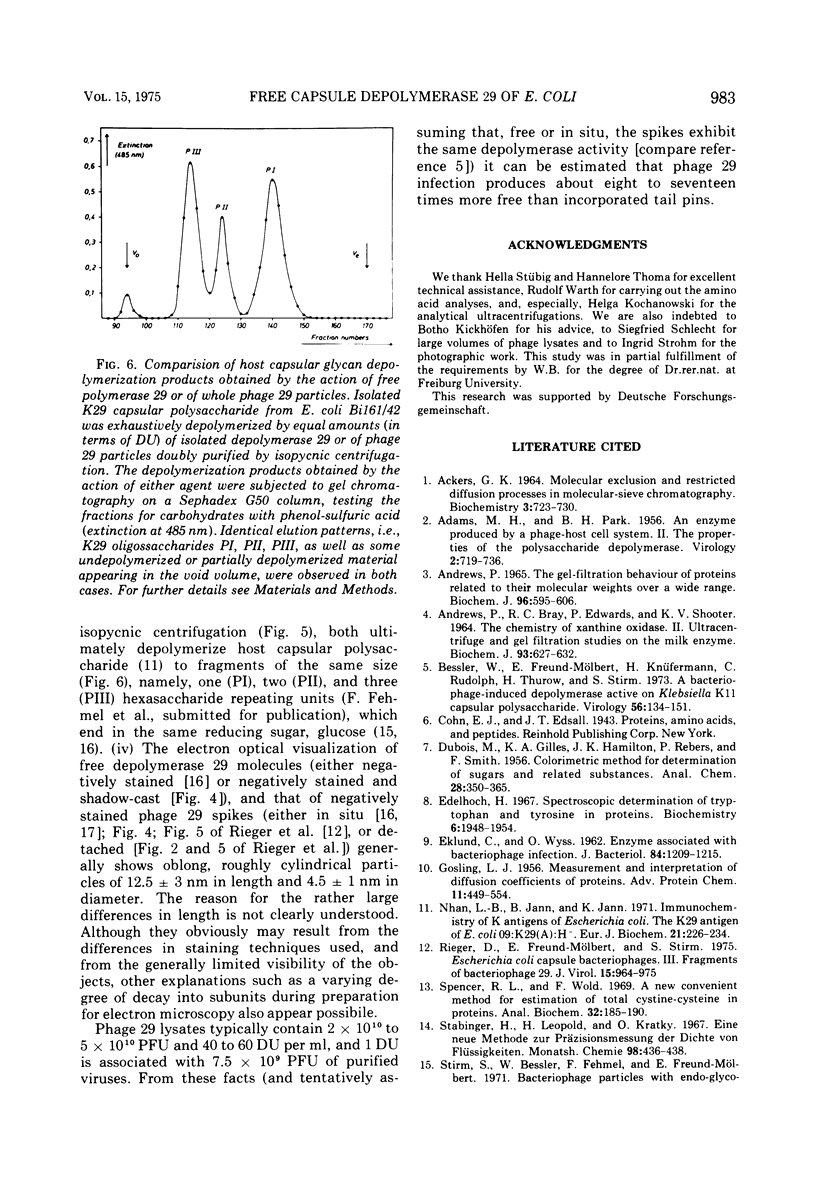
The free host capsule depolymerase, induced by Escherichia coli capsule bacteriophage no. 29, and causing the formation of haloes around its plaques, has been purified to homogeneity. As judged from the following facts, this "enzyme" consists of free phage 29 spikes. (i) Detached phage organelles and depolymerase 29 particles exhibit the same molecular weight (about 245,000, as determined from the sedimentation equilibrium), contain polypeptide chains of the same two sizes (57,000 plus or minus 3,000 and 29,500 plus or minus 2,000, as determined by SDS-PAA gel electrophoresis), and have (within experimental error) the same sedimentation coefficient, isoelectric point, and amino acid composition. (ii) Isolated depolymerase and phage spikes in situ both catalyze the hydrolysis of glucosidic bonds in host capsular polysaccharide, leading ultimately to the formation of oligosaccharide fragments of one, two, and three hexasaccharide repeating units. (iii) Depolymerase 29 and phage 29 spikes have roughly the same electron optical dimensions. As tentatively estimated from the total and the virus-associated capsule depolymerase activity in the lysates, phage 29 infection seems to produce eight to seventeen times more free than incorporated spikes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K. MOLECULAR EXCLUSION AND RESTRICTED DIFFUSION PROCESSES IN MOLECULAR-SIEVE CHROMATOGRAPHY. Biochemistry. 1964 May;3:723–730. doi: 10.1021/bi00893a021. [DOI] [PubMed] [Google Scholar]
- ADAMS M. H., PARK B. H. An enzyme produced by a phage-host cell system. II. The properties of the polysaccharide depolymerase. Virology. 1956 Dec;2(6):719–736. doi: 10.1016/0042-6822(56)90054-x. [DOI] [PubMed] [Google Scholar]
- Andrews P., Bray R. C., Edwards P., Shooter K. V. The chemistry of xanthine oxidase. 11. Ultracentrifuge and gel-filtration studies on the milk enzyme. Biochem J. 1964 Dec;93(3):627–632. doi: 10.1042/bj0930627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessler W., Freund-Mölbert E., Knüfermann H., Rduolph C., Thurow H., Stirm S. A bacteriophage-induced depolymerase active on Klebsiella K11 capsular polysaccharide. Virology. 1973 Nov;56(1):134–151. doi: 10.1016/0042-6822(73)90293-6. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Eklund C., Wyss O. ENZYME ASSOCIATED WITH BACTERIOPHAGE INFECTION. J Bacteriol. 1962 Dec;84(6):1209–1215. doi: 10.1128/jb.84.6.1209-1215.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le-Ba-Nhan, Jann B., Jann K. Immunochemistry of K antigens of Escherichia coli. The K29 antigen of E. coli 09:K29(A):H-. Eur J Biochem. 1971 Jul 29;21(2):226–234. doi: 10.1111/j.1432-1033.1971.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Rieger D., Freund-Mölbert E., Stirm S. Escherichia coli capsule bacteriophages. III. Fragments of bacteriophage 29. J Virol. 1975 Apr;15(4):964–975. doi: 10.1128/jvi.15.4.964-975.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer R. L., Wold F. A new convenient method for estimation of total cystine-cysteine in proteins. Anal Biochem. 1969 Oct 15;32(1):185–190. doi: 10.1016/0003-2697(69)90123-7. [DOI] [PubMed] [Google Scholar]
- Stirm S., Bessler W., Fehmel F., Freund-Mölbert E., Thurow H. Isolation of spike-formed particles from bacteriophage lysates. Virology. 1971 Jul;45(1):303–308. doi: 10.1016/0042-6822(71)90138-3. [DOI] [PubMed] [Google Scholar]
- Stirm S., Freund-Mölbert E. Escherichia coli capsule bacteriophages. II. Morphology. J Virol. 1971 Sep;8(3):330–342. doi: 10.1128/jvi.8.3.330-342.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W., Wilkinson J. F. Depolymerases for bacterial exopolysaccharides obtained from phage-infected bacteria. J Gen Microbiol. 1965 Jun;39(3):373–383. doi: 10.1099/00221287-39-3-373. [DOI] [PubMed] [Google Scholar]
- Yurewicz E. C., Ghalambor M. A., Duckworth D. H., Heath E. C. Catalytic and molecular properties of a phage-induced capsular polysaccharide depolymerase. J Biol Chem. 1971 Sep 25;246(18):5607–5616. [PubMed] [Google Scholar]