Abstract
Objective
The oxidative stress in 20 sickle cell anemia patients taking hydroxyurea and 13 sickle cell anemia patients who did not take hydroxyurea was compared with a control group of 96 individuals without any hemoglobinopathy.
Methods
Oxidative stress was assessed by thiobarbituric acid reactive species production, the Trolox-equivalent antioxidant capacity and plasma glutathione levels.
Results
Thiobarbituric acid reactive species values were higher in patients without specific medication, followed by patients taking hydroxyurea and the Control Group (p < 0.0001). The antioxidant capacity was higher in patients taking hydroxyurea and lower in the Control Group (p = 0.0002 for Trolox-equivalent antioxidant capacity and p < 0.0292 for plasma glutathione). Thiobarbituric acid reactive species levels were correlated with higher hemoglobin S levels (r = 0.55; p = 0.0040) and lower hemoglobin F concentrations(r = -0.52; p = 0.0067). On the other hand, plasma glutathione levels were negatively correlated with hemoglobin S levels (r = -0.49; p = 0.0111) and positively associated with hemoglobin F values (r = 0.56; p = 0.0031).
Conclusion
Sickle cell anemia patients have high oxidative stress and, conversely, increased antioxidant activity. The increase in hemoglobin F levels provided by hydroxyurea and its antioxidant action may explain the reduction in lipid peroxidation and increased antioxidant defenses in these individuals.
Keywords: Hemoglobinopathies; Oxidative stress; Anemia, sickle cell; Hydroxyurea
Introduction
Sickle cell anemia (SCA) is one of the most common genetic disorders in the world. It is characterized by homozygous hemoglobin (Hb) S and represents the most severe form of sickle cell disease (SCD)(1). Hb S is caused by a mutation in the β-globin gene in which the sixth amino acid is changed from glutamic acid to valine due to a substitution of adenine for thymine(2). SCA presents a series of clinical manifestations which are influenced by genetic and environmental factors. These factors result in many phenotypes, mainly mediated by the polymerization of Hb S, hemolysis and cell adhesion to endothelium which leads to vascular occlusion(3,4). Blood transfusions, folic acid supplementation and hydroxyurea (HU) are the most common treatments in SCA. HU is an oral drug whose main effect in SCA is the increased synthesis of fetal Hb (Hb F) which reduces the frequency of vaso-occlusive episodes, pain crises, hospitalizations and blood transfusions(5).
Oxidative stress is one of the factors that modulates the phenotypic expression of SCA. This stress influences the vaso-occlusive process by increasing the adhesive properties of erythrocytes, leukocytes and platelets to the endothelium(6). Normal erythrocytes suffer oxidative stress resulting from the production of reactive oxygen species (ROS) due to the oxygen metabolism. This metabolic ROS production is increased in patients with hemoglobinopathies, causing oxidative damage such as lipid peroxidation. The release of Hb in plasma and ischemia-reperfusion cycles are characteristic of SCD, increasing oxidative stress and requiring a more effective antioxidant system(7,8).
The literature suggests that excess ROS has implications in the pathophysiology of SCA. Thus an evaluation of the oxidative stress in these patients may provide important information regarding the current use of medications, such as HU, and may lead to new therapeutic strategies. Therefore, considering the intense generation of ROS with the presence and hemolysis of Hb S in SCA, the influence of HU on oxidative stress was evaluated using cell damage markers and antioxidant capacity by comparing patients taking HU with those who were not taking the medication and a control group.
Methods
Subjects
Peripheral blood samples of 33 SCA patients (21 females and 12 males; mean age: 28 ± 15 years) from blood banks in São Paulo and Rio de Janeiro, southeastern Brazil were evaluated. All patients were treated with folic acid supplementation however only 20 of the patients were taking HU. A Control Group was formed of 96 individuals without hemoglobinopathies and not taking HU (57 females and 39 males; mean age: 23 ± 6 years) from southeastern Brazil.
All SCA patients were screened using a questionnaire. Pregnant women, smokers and individuals who drank significant quantities of alcohol were excluded from the study as were patients who had had strokes, pain or hemolytic crisis or had received blood transfusions within the two months prior to the start of the study. Patients who had taken medications known to affect the analyzed parameters (such as acetylsalicylic acid, antibiotics or vitamins) within the 24 hours prior to sample collection were also excluded from the study. All subjects gave their informed consent and the study was approved by the Data Safety Monitoring Board (DSMB) according to Brazilian regulations.
Genotype investigation
All samples were submitted to classical hemoglobin diagnosis techniques including electrophoresis at alkaline and acid pH, to evaluate the Hb migration profile and high performance liquid chromatography (HPLC) to measure Hb fractions(9-11). Genomic DNA was extracted employing the phenol-chloroform method for molecular analysis(12). The amplification of the segment that encodes Hb S was performed using specific primers (sense: 5'-GGCAGAGCCATCTATTGCTTA-3'; antisense: 5'-ACCTTAGGGTTGCCCATAAC-3') and cleavage was achieved by the action of restriction endonuclease FastDigest Ddel (Fermentas, USA).(13)
Biochemical analysis
Oxidative stress analysis was evaluated based on the detection of lipid peroxidation and antioxidant capacity markers in plasma. The lipid peroxidation levels were calculated by the thiobarbituric acid reactive species (TBARS) technique. This method is based on the reaction of malondialdehyde and other aldehydes, which are by-products of membrane damage caused by ROS, with thiobarbituric acid (TBA) at low pH and high temperature forming a complex with maximum light absorption at 535 nm(14).
The antioxidant capacity was evaluated using Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), a potent antioxidant similar to vitamin E. The Trolox-equivalent antioxidant capacity (TEAC) method is based on the reaction between 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid)(ABTS) (SIGMA, A1888), 2,2'-azino-bis (3-ethylbenzothiazoline6-sulfonate) and potassium persulfate (K2S2O8). This reaction produces the radical cation ABTS•+, a green/blue chromophore. The addiction of one antioxidant to this radical cation reduces ABTS, resulting in a solution discoloration that is evaluated at 734nm to determine the total antioxidant capacity(15).
Plasma glutathione (GSH) concentrations were determined in EDTA-treated plasma samples by the quantification in HPLC coupled to a coulometric electrochemical detector (Coulochem IIIESA, Bedford, MA) set with a potential of 650 mV(16). Under these conditions, GSH is clearly eluted in ~ 6 min. GSH was extracted from the plasma samples by adding perchloric acid to the plasma sample (10% final concentration). After vigorous stirring and 10min on ice, the mixture was centrifuged at 825 g for 10 min at 4°C. The extract was then filtered through Millex syringe filter units (0.22 µm) and directly injected into the HPLC. Calculations were based on a calibration curve previously constructed by injecting known GSH standards into the HPLC system.
Statistical analysis
Data were tested for normality and homoscedasticity using the Shapiro-Wilk and Levene tests, respectively. Analysis of variance (ANOVA) was employed complemented by Tukey's test for data with normal distribution. The Kruskal-Wallis test was employed followed by Dunn's test for non-parametric data. The correlations between Hb concentrations and lipid peroxidation and plasma GSH levels were achieved by the Pearson linear correlation test. The level of significance was assumed as 0.05 and analysis was made using the Statistica 8.0 software.
Results
Hemoglobin fraction concentration in SCA patients
The Hb profiles of SCA individuals were compared depending on whether patients took HU or not. Results showed higher Hb F levels (p = 0.018) and lower Hb S concentrations(p = 0.015) among individuals taking HU compared to those who were not taking this medication (Table 1).
Table 1.
Differences between hemoglobin profiles of the SCA patients taking HU or not
| Sickle cell anemia patients | p-value | ||
| (-HU) | (+HU) | ||
| Hb S | 86.63 ± 4.42 | 80.44 ± 7.47 | 0.015 |
| Hb A2 | 3.63 ± 1.28 | 4.11 ± 0.50 | 0.250 |
| Hb F | 6.47 ± 4.23 | 11.89 ± 6.92 | 0.018 |
-HU: patients not taking hydroxyurea; +HU: patients taking hydroxyurea.
Lipid peroxidation - thiobarbituric acid reactive species dosage
The generation of ROS was indirectly measured though the analysis of lipid peroxidation. The values were higher for SCA patients (1450 ± 549 ng/mL) compared to the Control Group (239 ± 159 ng/mL)(p < 0.0001). Patients receiving HU had lower lipid peroxidation than those without specific medication however they still had higher levels than the Control Group (p < 0.0001).
Correlation analysis was carried out comparing Hb S and Hb F levels and the TBARS dosage. Results showed that TBARS values in SCA patients were positively correlated with Hb S concentrations (r = 0.55; p = 0.0040) reflecting increased lipid peroxidation in the presence of Hb S (Figure 1A). Negative correlation was observed between TBARS and Hb F levels (r = -0.52; p = 0.0067), showing a protective effect of Hb F (Figure 1B).
Figure 1.
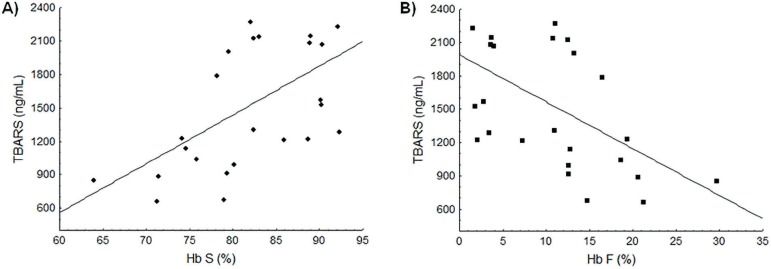
Association between the Hb S and Hb F concentrations and lipid peroxidation levels. (A) Positive linear correlation between Hb S and TBARS levels (r = 0.55; p-value = 0.0040). (B) Negative linear correlation between Hb F and TBARS levels (r = -0.52; p-value = 0.0067).
Antioxidant Capacity - Trolox-equivalent antioxidant capacity assay and plasma glutathione levels
The overall antioxidant capacity analyzed by TEAC showed higher values for SCA patients (2.04 ± 0.15 mM) than for the Control Group (1.93 ± 0.15 mM; p = 0.0028). Patients taking HU had higher TEAC values when compared to the group of patients that did not take HU (p = 0.0002). No correlation was observed between Hb concentrations and TEAC levels.
The GSH concentration in the Control Group was 0.41 ± 0.38 µM with this value being about two times higher in SCA patients (0.88 ± 0.69 µM; p = 0.0292). Patients taking HU had higher GSH levels that were significantly different to the Control Group (p < 0.0001). Higher Hb S concentrations were correlated with decreased GSH levels (r = -0.49; p = 0.0111: Figure 2A) while Hb F concentrations were positively correlated with GSH values (r = 0.56; p = 0.0031: Figure 2B).
Figure 2.
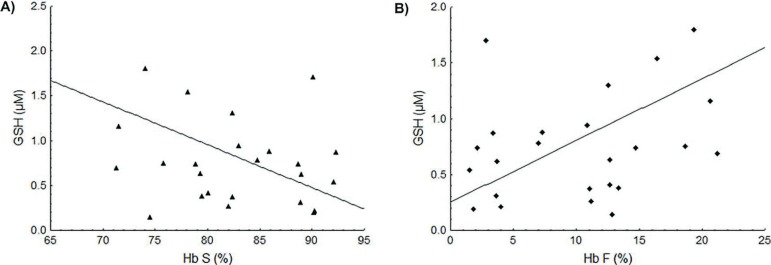
Association between the Hb S and Hb F concentrations and the GSH levels. (A) Negative linear correlation between Hb S and GSH levels (r = -0.49; p-value = 0.0111). (B) Positive linear correlation between Hb F and GSH levels (r = 0.56; p-value = 0.0031).
Discussion
The oxidative stress and antioxidant markers were evaluated in SCA patients who were either taking HU or not and the results were compared to the results of a control group. The correlation between Hb S levels and TBARS values, as well as lipid peroxidation in SCA patients suggest that oxidative stress may result from high levels of meta Hb Swhich is less stable than meta Hb A, leading to the formation of hemichromes and hemolysis with the release of heme iron. Oxidative stress may be even higher during vaso-occlusive crises and painful episodes(17).
Hb F levels were higher in SCA patients. It is known that in SCA patients, the concentration of Hb F ranges from 1% to 30% and it is inherited as a quantitative trait. A trend of increased Hb F levels in SCA patients taking HU was observed in this study which reflects the ability of HU to regulate Hb F expression(18).
SCA pathophysiology can be viewed as a cycle driven by hemolysis, oxidative stress, inflammation, cell adhesion to endothelium and vaso-occlusion. During hemolysis, iron is transformed from ferric to ferrous iron, thus producing meta Hb, with heme iron release. The hydrophobic character of free heme iron allows it to merge with cell membranes, increasing the susceptibility to oxidant-mediated destruction as well as the generation of ROS, characterizing lipid peroxidation. Thus, the level of TBARS is a good indicator of pro-oxidant stimuli, since it measures malondialdehyde and other aldehydes which are subproducts of cell membrane destruction(19).
Patients who are not taking HU but are taking folic acid, showed increased lipid peroxidation levels compared to patients taking HU. Folate acid is important in the formation of red blood cells because it participates in the purine and pyrimidine metabolism for DNA and RNA synthesis(20). In turn, HU is a deoxyribonucleotide reductase which is able to increase Hb F synthesis due to its myelotoxicity. Studies have shown that this drug leads to increased Hb F levels in approximately 50% of patients with SCD. High Hb F concentrations may decrease the severity of illness because of inhibition in the polymerization of Hb S. Hb F alter contact sites between Hb molecules, impairing polymer formation with consequent reduction in the sickling process(21-23). This is a potential reduction factor of hemolysis and the consequent cell damage caused by substances released during this process, for example, iron. Kaul et al. demonstrated that increased Hb F expression reduces oxidative stress in SCD transgenic mice(24). In the current work, this antioxidant property of HU is observed by lower TBARS values obtained in patients in the group taking this drug compared to patients who were not.
The generation of free radicals is counteracted by enzymaticand non-enzymatic antioxidants, including antioxidants from the diet such as vitamins C and E and GSH(25). Some studies have found decreased GSH concentrations in erythrocytes from SCD patients(26,27). However, Reid et al. and Kiessling et al. showed that GSH synthesis is not impaired in SCA patients, thus suggesting that lower GSH levels may be explained by the high demand(28,29). Reid et al. showed that GSH synthesis was increased in 57% of SCA patients compared to control subjects, indicating that the consumption of GSH may exceed its synthesis(28). On the other hand, GSH levels may also be influenced by diet, for example, due to deficiencies in the amino acid precursors of GSH synthesis, especially cysteine(30).
Maintaining adequate levels of GSH is important for many critical cell functions with disruptions in these processes being observed in several human diseases. GSH deficiency is manifested primarily as an increased susceptibility to oxidative stress and cell damage, the results of which may be an important factor in the progression of the disease. On the other hand, high GSH levels may increase antioxidant capacity and resistance to oxidative stress(31).
Regarding treatment, HU was associated with increased antioxidant capacity. Liu et al. tested the activity of HU against free radicals such as DPPH (2,2-diphenyl-1-picril-hydrazyl) and hydroxyl radicals; they found that HU has antioxidant activity(32). The higher values of GSH and TEAC in patients treated with HU corroborate this finding, since the antioxidant properties of HU may have curbed the consumption of the antioxidant systems evaluated.
Conclusion
The results of this study show that the influence of Hb S on the oxidative status is reflected by increased lipid peroxidation and antioxidant status in SCA patients. High Hb F concentrations are associated with less oxidative stress. Treatment using HU decreased lipid peroxidation and contributed to the body's antioxidant defenses.
Acknowledgments
The authors would like to thank the following Brazilian foundations: Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Ministry of Health, for the financial support.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interest
References
- 1.Frenette PS, Atweh GF. Sickle cell disease: old discoveries, new concepts, and future promise. J Clin Invest. 2007;117(4):850–858. doi: 10.1172/JCI30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2 Higgs DR, Wood WG. Genetic complexity in sickle cell disease. Proc Natl Acad Sci USA. 2008;105(33):11595–11596. doi: 10.1073/pnas.0806633105. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comment on: Proc Natl Acad Sci USA 20081053311869–11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Figueiredo MS. Fatores moduladores da gravidade da evolução clínica da anemia falciforme. Rev Bras Hematol Hemoter. 2007;29(3):215–217. [Google Scholar]
- 4.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 5.Kovacic P. Hydroxyurea (therapeutics and mechanism): metabolism, carbamoyl nitroso, nitroxyl, radicals, cell signaling and clinical applications. Med Hypotheses. 2011;76(1):24–31. doi: 10.1016/j.mehy.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Chaves MA, Leonart MS, do Nascimento AJ. Oxidative process in erythrocytes of individuals with hemoglobin S. Hematology. 2008;13(3):187–192. doi: 10.1179/102453308X343356. [DOI] [PubMed] [Google Scholar]
- 7.Kuypers FA. Red cell membrane lipids in hemoglobinopathies. Curr Mol Med. 2008;8(7):633–638. doi: 10.2174/156652408786241429. [DOI] [PubMed] [Google Scholar]
- 8.Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT. Vasculopathy in sickle cell disease: biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol. 2009;84(9):618–625. doi: 10.1002/ajh.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marengo-Rowe AJ. Rapid electrophoresis and quantitation of haemoglobin on cellulose acetate. J Clin Pathol. 1965;18(6):790–792. doi: 10.1136/jcp.18.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vella F. Acid agar gel electrophoresis oh human hemoglobins. Am J Clin Pathol. 1968;49(3):440–442. doi: 10.1093/ajcp/49.3_ts.440. [DOI] [PubMed] [Google Scholar]
- 11.Bio-Rad Laboratories . Instruction Manual: Variant β-thalassemia short program. Hercules, California: Bio-Rad Laboratories; 1999. [Google Scholar]
- 12.Sambrook J, Fritcsh EF, Manatis T. Molecular cloning: a laboratory manual. 2nd ed. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 13.Saiki RK, Scharf S, Faloona F, Mullis KB, Horn GT, Erlich HA, et al. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 14.Percário S. Dosagem do dialdeído malônico. Newslab. 2004;6:46–50. [Google Scholar]
- 15.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9-10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 16.Rodríguez-Ariza A, Toribio F, López-Barea J. Rapid determination of glutathione status in fish liver using high-performance liquid chromatography and electrochemical detection. J Chromatogr B Biomed Appl. 1994;656(2):311–318. doi: 10.1016/0378-4347(94)00111-1. [DOI] [PubMed] [Google Scholar]
- 17.Fibach E, Rachmilewitz E. The role of oxidative stress in hemolytic anemia. Curr Mol Med. 2008;8(7):609–619. doi: 10.2174/156652408786241384. [DOI] [PubMed] [Google Scholar]
- 18.Green NS, Barral S. Genetic modifiers of HbF and response to hydroxyurea in sickle cell disease. Pediatric Blood Cancer. 2011;56(2):177–181. doi: 10.1002/pbc.22754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belcher JD, Beckman JD, Balla G, Balla J, Vercellotti G. Heme degradationand vascular injury. Antioxid Redox Signal. 2010;12(2):233–248. doi: 10.1089/ars.2009.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brasil. Ministério da Saúde. Agencia de Vigilância Sanitária - ANVISA . Manual de diagnóstico e tratamento de doenças falciformes [Internet] Brasília: Ministério da Saúde; 2001. [cited 2011 Jan 12]. Available from: http://www.ebah.com.br/content/ABAAAA2R4AB/manual-diagnosticotratamento-doencas-falciformes. [Google Scholar]
- 21.Steinberg MH. Management of sickle cell disease. N Engl J Med. 1999;340(13):1021–1030. doi: 10.1056/NEJM199904013401307. [DOI] [PubMed] [Google Scholar]
- 22 Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med. 1995;332(20):1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]; Comment in: N Engl J Med 19963345333–334. [Google Scholar]; N Engl J Med. 1995;332(20):1372–1374. doi: 10.1056/NEJM199505183322010. [DOI] [PubMed] [Google Scholar]; N Engl J Med. 1995;333(15):1008–1009. author reply 1009. [Google Scholar]
- 23.Silva LB, Gonçalves RP, Martins MF. Estudo da correlação entre os níveis de hemoglobina fetal e o prognóstico dos pacientes com anemia falciforme. Rev Bras Hematol Hemoter. 2009;31(6):417–420. [Google Scholar]
- 24.Kaul DK, Liu XD, Choong S, Belcher JD, Vercellotti GM, Hebbel RP. Anti-inflammatory therapy ameliorates leukocyte adhesion and microvascular flow abnormalities in transgenic sickle mice. Am J Physiol Heart Circ Physiol. 2004;287(1):H293–301. doi: 10.1152/ajpheart.01150.2003. [DOI] [PubMed] [Google Scholar]
- 25.Hernanz A, Fernández-Vivancos E, Montiel C, Vazquez JJ, Arnalich F. Changes in the intracellular homocysteine and glutathione content associated with aging. Life Sci. 2000;67(11):1317–1324. doi: 10.1016/s0024-3205(00)00722-0. [DOI] [PubMed] [Google Scholar]
- 26.Chan AC, Chow CK, Chiu D. Interaction of antioxidants andtheir implication in genetic anemia. Proc Soc Exp Biol Med. 1999;222(3):274–282. doi: 10.1177/153537029922200310. [DOI] [PubMed] [Google Scholar]
- 27.Reid M, Jahoor F. Glutathione in disease. Curr Opin Clin Nutr Metab Care. 2001;4(1):65–71. doi: 10.1097/00075197-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Reid M, Badaloo A, Forrester T, Jahoor F. In vivo rates of erythrocyte glutathione synthesis in adults with sickle cell disease. Am J Physiol Endocrinol Metab. 2006;291(1) doi: 10.1152/ajpendo.00287.2005. [DOI] [PubMed] [Google Scholar]
- 29.Kiessling K, Roberts N, Gibson JS, Ellory JC. A comparison in normal individuals and sickle cell patients of reduced glutathione precursors and their transport between plasma and red cells. Hematol J. 2000;1(4):243–249. doi: 10.1038/sj.thj.6200033. [DOI] [PubMed] [Google Scholar]
- 30.Cresenzi CL, Lee JI, Stipanuk MH. Cysteine is the metabolic signal responsible for dietary regulation of hepatic cysteine dioxygenase and glutamate cysteine ligase in intact rats. J Nutr. 2003;133(9):2697–2702. doi: 10.1093/jn/133.9.2697. [DOI] [PubMed] [Google Scholar]
- 31.Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009;390(3):191–214. doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu YH, Wu WC, Lu YL, Lai YJ, Hou WC. Antioxidant and amine oxidase inhibitory activities of hydroxyrea. Biosci Biotechnol Biochem. 2010;74(6):1256–1260. doi: 10.1271/bbb.100096. [DOI] [PubMed] [Google Scholar]


