Abstract
Processing of social and emotional information has been shown to be disturbed in schizophrenia. The biological underpinnings of these abnormalities may be explained by an abnormally functioning mirror neuron system. Yet the relationship between mirror neuron system activity in schizophrenia, as measured using an electroencephalography (EEG) paradigm, and socio-emotional functioning has not been assessed. The present research measured empathy and mirror neuron activity using an established EEG paradigm assessing the integrity of the Mu rhythm (8–13 Hz) suppression over the sensorimotor cortex during observed and actual hand movement in 16 schizophrenia-spectrum disorder (SSD) participants (n=8 actively psychotic and n=8 in residual illness phase) and 16 age- and gender-matched healthy comparison participants. Actively psychotic SSD participants showed significantly greater mu suppression over the sensorimotor cortex of the left hemisphere than residual phase SSD and healthy comparison individuals. The latter two groups showed similar levels of mu suppression. Greater left-sided mu suppression was positively correlated with psychotic symptoms (i.e., greater mu suppression/mirror neuron activity was highest among subjects with the greater severity of psychotic symptoms). SSD subjects tended to have significantly higher levels of Personal Distress (as measured by the Interpersonal Reactivity Index) than healthy participants. The present study suggests that abnormal mirror neuron activity may exist among patients with schizophrenia during the active (psychotic) phase of the illness, and correlates with severity of psychosis.
Keywords: EEG, Empathy, Psychosis, Social cognition, Emotion
1. Introduction
Social-cognitive and emotion-processing dysfunctions are common features of schizophrenia (Bigelow et al., 2006; Burns, 2006; Shamay-Tsoory et al., 2007; Paradiso et al., 2003; Crespo Facorro et al., 2001) that often appear before the onset of florid psychotic symptoms (Edwards et al., 2001; Brüne, 2005; Bertrand et al., 2008) and that affect functional outcome (Couture et al., 2006). Studies of social cognition in schizophrenia have focused primarily on Theory of Mind (ToM), emotion processing, agency judgment, and empathy (Brunet-Gouet and Decety, 2006; Andreasen et al., 2008; Park et al., 2009). While ToM refers to cognitive aspects of mentalizing or the ability to draw accurate conclusions about others’ cognitions and emotions (Frith and Frith, 2003; Keysers and Gazzola, 2006), empathy has both cognitive and affective components and generally refers to the capacity to recognize and share the feelings experienced by another.
One proposed theory for the ability to understand mental states of others is through simulation theory, which is generally hypothesized to be a mechanism for experiencing others’ sensory, motor, perceptual, and emotional experiences as if they were one’s own (Preston and de Waal, 2002). One proposed mechanism for this simulation approach is through the mirror neuron system, a set of specialized neurons that become active both during motor action and during the observation of another individual’s motor action (Rizzolatti and Craighero, 2004; Keysers and Gazzola, 2006). This phenomenon was first described in a series of experiments that used deep brain electrodes in the inferior premotor cortex (F5) of monkeys (di Pellegrino et al., 1992), and was later also shown to include the inferior parietal lobule (IPL) (Keysers and Gazzola, 2006). Numerous functional magnetic resonance imaging (fMRI) studies in humans have since replicated these findings in homologous brain regions such as the posterior inferior frontal gyrus, the rostral inferior parietal lobule, and the precentral gyrus (Rizzolatti and Craighero, 2004; Iacoboni and Mazziotta, 2007). Since its original discovery, studies of mirror neuron system activity have extended to the sensory domains and most recently there have been studies examining the role of the mirror neuron system in the emotional domain. For example, two studies have shown that self-reported empathy is associated with activity in the mirror neuron system (Zaki et al., 2009; Hooker et al., 2010).
Patients with schizophrenia tend to show dysfunctional empathizing abilities (Brüne, 2005; Montag et al., 2007; Shamay-Tsoory et al., 2007; Benedetti et al., 2009; Derntl et al., 2009; Herold et al., 2009). These may be related to structural and functional deficits in the mirror neuron system and imitation network (Bertrand et al., 2008; Fujiwara et al., 2008; Mier et al., 2010; Park et al., 2011). The mirror neuron system may support appreciation of the self/other boundaries and understanding others’ intentions, and its breakdown may originate psychotic symptoms (Frith and Corcoran, 1996; Brüne, 2005; Langdon et al., 2010). For example, people with schizophrenia tend to make false interpretations of other people’s intentions, which may result in misperception of benign social cues as threats (paranoid delusions) or hallucinations (Abu-Akel, 2003; Arbib and Mundhenk, 2005; Bentall et al., 2009).
Prior to the discovery of mirror neurons, French epileptologists Gastaut and Bert reported a comparable phenomenon using electroencephalography (EEG) in humans) (Gastaut and Bert, 1954). The electrical activity observed was “mu rhythm” (i.e., 8–13 Hz) suppression over bilateral sensorimotor cortices when the person’s own hand moved and at about 50% of that by simply watching another person’s hand move (Pineda et al., 2000; Muthukumaraswamy et al., 2004; Pfurtscheller et al., 2006). Mu activity is typically highest over the somatosensory cortices during rest and is most strongly suppressed with actual or observed ipsilateral or contralateral hand movements. It is speculated that mu suppression is greatest over the left hemisphere during mimicry of hand and facial movements (Dawson et al., 1985; Cochin et al., 1999). Mu suppression has also been shown to be stronger for watching a live rather than video demonstration of hand movement (Järveläinen et al., 2001). Mu suppression is considered a good estimate of performing and observing hand movement activity in others (Cochin et al., 1998; Babiloni et al., 1999) and is thought to underlie mirror neuron activity (Pineda et al., 2000; Muthukumaraswamy et al., 2004).
This EEG mirror neuron paradigm has been used to examine the functioning of the mirror neuron system in persons with autism (Oberman et al., 2005; Martineau et al., 2008; Oberman et al., 2008). People with autism spectrum disorders exhibit mu suppression during the “self” hand movement condition, but not when watching another person performing this same action (Oberman et al., 2005). This lack of activity in the neural regions engaged during hand moving while viewing others’ actions suggests impairment in the functioning of the mirror neuron system. This finding was replicated in children with autism using functional magnetic resonance imaging (fMRI) (Martineau et al., 2010) and in high functioning adults with autism (Bernier et al., 2007).
While people with schizophrenia and autism spectrum disorders tend to exhibit below average performance on cognitive empathy tasks (Baron-Cohen, 2004; Bora et al., 2008), they report on average higher scores on affective empathy questionnaires (as evidenced by high levels of personal distress on the Interpersonal Reactivity Index, IRI) (Lombardo et al., 2007; Montag et al., 2007; Shamay-Tsoory et al., 2007; Dziobek et al., 2008; Lee et al., 2011). This finding is rather notable in light of the fact that in schizophrenia elevated personal distress may actually precede the onset of cognitive empathy deficits (Achim et al., 2011).
Previous studies of mirror neuron function in schizophrenia using various neuroimaging methods have suggested that people with schizophrenia have reduced mirror neuron activity (Enticott et al., 2008) that may relate to lower ability to distinguish between actions of self and others (Schurmann et al., 2007) or empathizing deficits (Varcin et al., 2010). It has also been suggested that the degree of altered empathy and social cognition in schizophrenia may be related to the state of the illness including active psychosis (Andreasen et al., 1986; Frith and Corcoran, 1996; Fahim et al., 2004; Salvatore et al., 2007). The combination of higher than normal self-agency and low self-awareness is then thought to lead to the development of delusions and psychosis (Frith, 2005). In contrast with this view, other investigators, based on higher than normal empathizing or mirroring abilities found to occur in schizophrenia (Abu-Akel and Bailey, 2000; Quintana et al., 2001), have suggested that intact ToM or ability to empathize is necessary for the development of psychosis (Walston et al., 2000). In a recent fMRI study by Quintana et al. (2001), patients with schizophrenia exhibited greater activation than healthy comparison participants in the face movement areas of the motor and pre-motor cortex when exposed to facial expressions in contrast to color circles.
While people with schizophrenia may show social cognition abnormalities overlapping with autism, it is not clear to what extent the underlying biology in these two conditions also overlaps. Several studies have now shown that people with autism have reduced mirror neuron activity, which may explain the empathy deficits thought to be at the core of social cognition impairment in this disorder (Perkins et al., 2010). To begin to examine the biological basis of empathy in patients with schizophrenia-spectrum disorders (SSD) relative to healthy comparison participants through the measurement of mirror neuron activity, the present research reports on the use of EEG to non-invasively measure mu suppression over the sensorimotor cortices during an observed hand movement paradigm as described by Oberman et al. (2005). Two related but separate hypotheses were tested: (1) whether, as with autism, mu suppression during observed hand movement would be reduced in the SSD group compared to healthy participants; and (2) whether mu suppression during observed hand movement would be atypical only among patients with active psychosis (i.e., a state phenomenon) compared to patients with residual illness and healthy participants. Correlations aimed at determining the extent to which mirror neuron activity covaried with measures of cognitive and affective empathy as well as clinical symptom dimension (i.e., psychotic, disorganized, and negative) scores were also computed.
2. Methods
2.1. Subjects
Initial enrollment included 25 SSD participants, recruited from Dr. Andreasen’s longitudinal study or from the inpatient unit at the University of Iowa Hospitals and Clinics; as well as 22 healthy comparison subjects, recruited via local advertisement in the Iowa City community. The diagnosis of SSD (i.e., schizophrenia, schizoaffective disorder, delusional disorder) was made by board certified psychiatrists using criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR; American Psychiatric Association, 2000). Exclusionary criteria for SSD subjects included recent use of a long-acting benzodiazepine and self-reported drug or alcohol abuse or dependence within the past three months. Exclusionary criteria for comparison subjects included family history of SSD, other psychotic disorder, or autism; treatment with psychotropic medications, including benzodiazepines, for a psychiatric disorder; substance use within the past three months; and history of seizure, head injury with loss of consciousness greater than five minutes, or other neurological disorder.
Despite specific instruction to remain as still as possible during data acquisition, five SSD datasets and six healthy comparison datasets were eventually excluded due to excessive motion artifact (e.g., eye blinking and jaw movement). Four additional SSD datasets were excluded due to complications related to drowsiness, paranoia, tardive dyskinesia and difficulty focusing/following instructions, respectively. To ensure proper age-matching, we did not enroll healthy comparison participants until we determined that EEG data from the matching SSD dataset was useable. In the end, a total of 16 SSD subjects (including 14 with schizophrenia, one with schizoaffective disorder, and one with delusional disorder) as well as 16 healthy comparison subjects had useable EEG data and were included in our analyses. This study was approved by the Institutional Review Board at the University of Iowa, and written informed consent was obtained from all subjects after the procedures had been fully explained.
2.2. Clinical assessments
All subjects completed clinical interviews using the Scale for the Assessment of Negative and Positive Symptoms (SANS/SAPS) (Andreasen, 1990). Scores from the SANS/SAPS were divided into three dimensions: (1) psychoticism (scale 0–10), based on global ratings of delusions and hallucinations; (2) disorganization (scale 0–15), based on global ratings of bizarre (disorganized) behavior, positive formal thought disorder, and inappropriate affect; and (3) negative symptoms (scale 0–25), based on global ratings of alogia, anhedonia, avolition, affective flattening, and attention.
2.3. Empathy measures
Empathy was assessed as a trait, or general tendency over the lifespan, using the Interpersonal Reactivity Index (IRI), a well-validated self-report questionnaire (Davis, 1980, 1983). The IRI is a multi-dimensional measure of empathy that assesses four components: cognitive empathy, or the ability to take the mental perspective of another person (Perspective Taking subscale); feelings of sympathy or compassion for others, often considered to be an aspect of affective empathy (Empathic Concern subscale); feelings of vicarious negative arousal in response to others’ emotional states (Personal Distress subscale); and the ability to take the mental perspective of characters in a book or movie (Fantasy subscale) (Davis, 1980). Personal Distress and Empathic Concern subscales can be combined into an Affective Empathy domain, while Perspective Taking and Fantasy subscales can be combined to make a Cognitive Empathy domain (Shamay-Tsoory et al., 2007).
2.4. Procedure
The EEG protocol included five conditions. Four of these conditions, which were used with the permission of Oberman et al., have been described elsewhere (Oberman et al., 2005). Briefly, subjects were instructed to: (1) complete a baseline eyes-open task of observing a video of visual white noise (i.e., snow); (2) observe a video of two bouncing balls; (3) observe a video of a person moving his/her right hand; and (4) observe the movement of their own right hand. An additional condition, which we added to the pre-established protocol just described, required subjects to: (5) observe a “live” person (the research assistant) moving his right hand in the same manner as the other two hand movement conditions (i.e., opening and closing from the palm, with the fingers and thumb held straight, at the rate of approximately 1 Hz).
Conditions were partially counterbalanced insofar as the relative order of the ball video and the three hand conditions was alternated. In all cases, the hand video was followed first by the own hand condition and then by the experimenter hand condition; in half of the cases, the ball video preceded the hand conditions whereas, in the other half of the cases, the ball video was presented after the hand conditions. All conditions were presented at least once for two minutes each, and were occasionally repeated at the discretion of the research assistant (e.g., in the case of subjects with appreciable eye blink artifact) in order increase the likelihood of collecting a sufficient amount of clean data.
In order to assess potential attention differences between groups that may have affected the findings, subjects were asked to complete a continuous performance task. Specifically, during three of the conditions (ball video, hand video observation, live hand observation), the stimuli (i.e., the balls or hand) would periodically stop moving for a duration of approximately 1 s, and subjects were instructed to count the number of these pauses that occurred. Four SSD subjects (25%) and three healthy comparison subjects (19%) made errors while counting. Subjects made two types of errors: (a) miscounting the number of pauses (e.g., counting five pauses when there were actually six); and (b) misunderstanding the instructions, and counting the total number of cycles (instead of the number of times that the stimuli paused upon completing a cycle). There were no significant differences between groups either in whether an error was made or in the type of error made.
2.5. EEG data collection and analysis
For all subjects, EEG data were obtained and digitalized on a Cadwell Easy II EEG machine by a trained research assistant in the Department of Psychiatry. EEG data were recorded using silver-silver/chloride electrodes from 19 scalp locations based on the international 10/20 system of electrode placement (FP1/2, F3/4, C3/4, P3/4, O1/2, F7/8, T3/4, T5/6, Fz, Cz, Pz) using linked ears as a reference. Electrode impedances were under 20kΩ. Data were collected digitally with a sampling rate of 200 Hz and a digital bandpass filter (1–70 Hz).
Each EEG recording was initially collected and labeled using Cadwell Easy EEG software, Version 2.1 (Cadwell Laboratories, Inc., Kennewick, WA) and then extracted, examined for artifact removal, and analyzed using NeuroGuide software (http://www.appliedneuroscience.com). Non-overlapping, artifact-free epochs of at least 50 s were selected from each recording. Split-half and test–retest reliability tests were conducted and all epochs contained ≥90% reliability on both tests (Thatcher et al., 2003).
A bipolar montage was used, at the suggestion of the EEG director at our institution, in an effort to reduce artifact and minimize the influence of non-sensorimotor electrodes, including possible occipital alpha effects on central rhythms. For all 18 bipolar derivations, a Fast Fourier Transform (FFT), computed on 2-s intervals and producing a 0.5 Hz frequency resolution, was used to calculate absolute power (uV Sq) for the mu rhythm (8–13 Hz) band. F3-C3 and F4-C4 were selected for data analysis because they correspond to the sensorimotor cortex and were analogous to the electrodes reported on by Oberman et al. (2005).
2.6. Statistical analyses
Independent t-tests were used initially to compare mean differences in demographic and clinical, and empathy measures between SSD and comparison subjects. Mu suppression was calculated by the log ratio of power during the four experimental conditions (i.e., ball video, observed video hand movement, observed live hand movement, and self-hand movement) relative to the power during the baseline visual white noise condition, as described in Oberman et al. (2005). The final log values of each condition were initially analyzed within each group using one-sample t-tests (to assess whether the degree of mu suppression was significantly less than zero). A linear mixed model analysis, with group (SSD vs. healthy comparison), condition, and side (F3-C3 vs. F4-C4) as fixed effects, was used to test for differences in the mean of the log ratio of mu suppression between groups. In addition to the main effects, the model also included all two-factor and three-factor interaction effects. To test for specific comparisons of interest, such as comparisons between SSD and healthy comparisons for a condition at each side of the brain, a test of mean contrast based on the fitted mixed model was performed with the p-value adjusted using Bonferroni’s method to account for the number of tests performed (Bonferroni adjusted p=unadjusted p×number of tests; with number of tests equaling 8, i.e., 4 conditions×2 sides).
A similar linear mixed model analysis was performed where there were 3 groups (healthy comparisons, and SSD subjects divided into an “active” versus “residual” subgroup), and included only the hand movement conditions (live, video, and self). The 16 SSD subjects were divided into active (n=8) versus residual (n=8) subgroups based on their endorsement of symptoms of psychosis (described in detail in Section 3.3). For this second mixed model, Bonferroni adjusted p-values were computed to account for 12 comparisons (2 pair-wise group comparisons×3 conditions×2 sides).
Spearman correlation analyses between the degree of mu suppression and SANS/SAPS scores in subjects were planned to examine the degree to which change in EEG activity co-varied with psychotic, disorganized, and negative symptom severity. Similarly, correlation analyses were computed to explore the degree of IRI subscales with mu suppression and SANS/SAPS scores. Analyses were corrected for multiple comparisons with a Bonferroni correction.
3. Results
3.1. Demographics and clinical assessments
Each of the 16 SSD subjects had an age- and gender-matched comparison subject (Table 1). All comparison subjects were right-handed whereas within the SSD group, 13 were right-handed, one was lefthanded, and two reported writing and throwing with opposite hands and were hence classified as ambidextrous. All SSD subjects were prescribed antipsychotic medications, while six (38%) were prescribed antidepressant medications. Medication compliance was not specifically assessed; however, one SSD subject (outpatient, diagnosed with schizophrenia, in the residual phase of his illness) volunteered that he was not currently taking his prescribed medications. Five (31%) of the SSD subjects were hospitalized at the time of assessment, and 11 (69%) were outpatients. There were no significant empathy differences between SSD subjects assessed as inpatients versus outpatients, however the inpatients had higher SAPS scores (6.8, S.D.=2.68 vs 2.0 S.D.=2.49, d.f.=14, p=0.011). As expected, the SSD group scored significantly higher than healthy comparison subjects on the SANS/SAPS dimensions of negative symptoms and psychosis, and showed a non-significant tendency toward higher degrees of disorganized symptoms (Table 1). SSD subjects also scored significantly higher than comparison subjects on the Affective Empathy domain of the IRI, which was predominately driven by the Personal Distress subscale (Table 1). Interestingly, none of the SSD subjects in our sample were first-onset and the average duration of illness was 15.8 years (S.D.=8.8, range=2-38).
Table 1.
Demographics, clinical symptoms and empathy assessments of schizophrenia spectrum disorder (SSD) and healthy comparison subjects.
| SSD (n=16) |
Comparisons (n=16) |
|
|---|---|---|
| Mean age in years (S.D.) | 37.0 (9.8) | 36.6 (9.7) |
| Years of education (S.D.) * | 13.9 (2.1) | 15.4 (1.4) |
| Age of onset (S.D.) | 20.7 (5.1) | – |
| Duration of illness in years (S.D.) | 15.8 (8.8) | – |
| Gender (male/female) | 14/2 | 14/2 |
| Diagnosis, n (%) | ||
| Schizophrenia | 14 (88) | – |
| Schizoaffective | 1 (6) | – |
| Delusional disorder | 1 (6) | – |
| Current use of medication, n (%) | ||
| Antipsychotics | 16 (100) | 0 |
| Antidepressants | 6 (38) | 0 |
| SANS/SAPS, mean (S.D.) | ||
| Negative symptoms ** | 9.2 (3.2) | 0.1 (0.5) |
| Disorganized symptoms | 1.1 (2.2) | 0 |
| Psychotic symptoms ** | 3.5 (3.4) | 0 |
| Global delusions ** | 1.7 (1.8) | 0 |
| Global hallucinations ** | 1.8 (2.0) | 0 |
| Interpersonal Reactivity Index, mean (S.D.) | ||
| Affective empathy *** | 36.3 (5.6) | 26.2 (9.4) |
| Empathic concern (EC) | 21.0 (3.3) | 18.8 (5.8) |
| Personal distress (PD) *** | 15.3 (4.7) | 7.4 (5.7) |
| Cognitive empathy | 31.9 (4.9) | 31,6 (7.1) |
| Fantasy (FS) | 14.8 (4.4) | 13.3 (5.1) |
| Perspective taking (PT) | 17.1 (4.6) | 18.4 (5.3) |
Abbreviations: SANS=Scale for the Assessment of Negative Symptoms; SAPS=Scale for the Assessment of Positive Symptoms.
Significant at p<0.05.
Significant at p<0.01.
Significant at p=0.001.
3.2. Mu suppression
SSD subjects exhibited significant bilateral sensorimotor mu suppression across all three hand movement conditions. In contrast, observing inanimate ball movement did not elicit mu suppression, which is consistent with Oberman et al. (2005). Among healthy comparison subjects, observing video hand movement and self-hand movement elicited significant bilateral sensorimotor mu suppression whereas, in response to observing live hand movement, significant right-sided (F4-C4) mu suppression was evident but left-sided (F3-C3) mu suppression only trended toward significance (t=−2.1, d.f.=15, p<0.06). As with the SSD group, healthy comparison subjects did not demonstrate mu suppression in response to the ball movement condition.
A mixed model analysis showed a significant group × condition × side interaction effect (p=0.029), indicating that size of the two group differences tended to differ with condition and side. The results of the pair-wise comparison between groups revealed greater left-sided (F3-C3) mu suppression in the SSD group relative to healthy comparison subjects during the live hand movement observation condition (t= −2.1, d.f.=54, p=0.038). However, when a Bonferroni correction was applied to account for the 8 tests performed (4 conditions at 2 sides); the adjusted p value was no longer significant.
Correlation analyses assessed the relationship between left-sided mu suppression during live hand movement observation versus SANS/SAPS and empathy scores. Greater left-sided mu suppression (i.e., a more negative log ratio value) was significantly correlated with higher scores on the SANS/SAPS psychotic (Spearman’s rho= −0.48, n=32, p=0.006) and negative (Spearman’s rho=−0.52, n=16, p=0.040) symptoms dimensions; however, only the psychosis finding remained significant after controlling for multiple comparisons. There were no correlations between mu suppression and IRI subscale scores or IRI subscale scores and SANS/SAPS scores. The correlation between mu suppression and psychosis was driven predominately by the global hallucinations subscale (Spearman’s rho= −0.59, p=0.016, see Fig. 1).
Fig. 1.
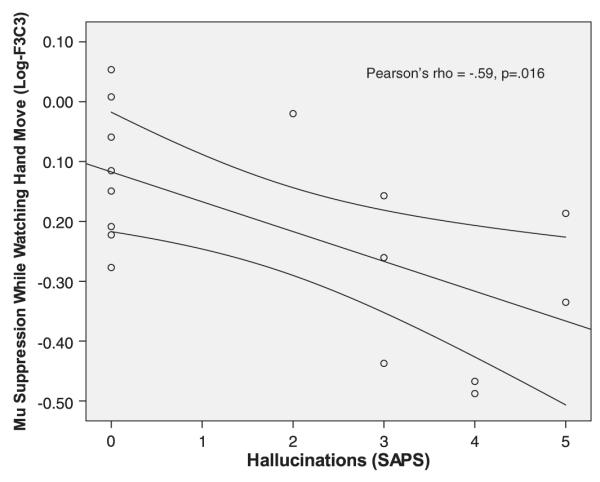
Correlation of left-sided mu suppression while schizophrenia spectrum subjects observed hand movement in another person. Scatter plot of the correlation between the SAPS hallucinations subscale and left-sided (F3-C3) mu suppression while schizophrenia-spectrum disorder subjects observed “live” movement of the researcher’s hand.
3.3. Mu suppression in active versus residual psychosis
Results of correlations guided these analyses for which independent variables (group membership) were based on a median split on SAPS psychotic symptoms scores in the SSD groups. There were eight subjects with “active” psychosis (i.e., SAPS psychotic symptoms dimension scores≥3) and eight subjects with “residual” psychosis (SAPS psychotic symptoms scores≤2). A mixed model analysis for the three group comparisons showed a significant group×condition×side interaction effect (p=0.028), indicating that the size of group differences may differ with condition and side. Results of the pair-wise group comparisons revealed significantly greater left-sided mu suppression during the live hand observation for the active psychosis group compared to the residual SSD and healthy comparisons subjects that remained significant even after controlling for multiple comparisons (t=3.0, d.f.=54, p=0.048; Fig. 2). There were no significant group differences in mu activity at baseline or with mu suppression during the video hand and own self-hand movements.
Fig. 2.
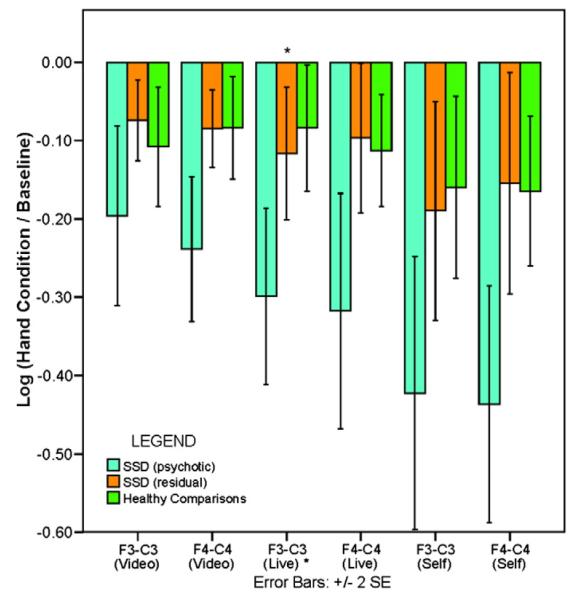
Mu suppression in schizophrenia spectrum disorder (SSD) subjects with active psychotic symptoms versus SSD subjects with residual psychotic symptoms and healthy comparisons during observed and self-hand movement. Mu suppression in schizophrenia-spectrum disorder (SSD) subjects with active psychosis (light blue) versus those with residual psychotic symptoms (orange) and healthy comparisons (green) during the observing video hand movement, observing live hand movement, and self-hand movement conditions. The bars represent the mean log ratio of power in the mu frequency range (8–13 Hz) during each of the three hand movement conditions relative to the power during the baseline (i.e., visual white noise) condition for scalp locations F3-C3 (left) and F4-C4 (right). The error bars represent±2 SE. For all values, a mean log ratio less than zero indicates mu suppression. Compared to SSD subjects with residual psychosis and healthy comparisons, SSD subjects with active psychosis demonstrated significantly greater left-sided (i.e., F3-C3) sensorimotor mu suppression during the observing live hand movement condition. * p<0.05.
Differences between the actively psychotic versus residual phase SSD subjects on empathy measures were found to be significant for IRI Fantasy subscale scores, which were higher among actively psychotic SSD participants (p=0.022) (Table 2). Five out of the eight subjects in the actively psychotic phase of their illness were hospitalized at the time of assessments, suggesting that although they were taking antipsychotic medication, their symptoms had not abated. Five of the eight SSD subjects in the residual phase of their illness were on an antidepressant medication compared with only one of eight in the actively psychotic SSD subgroup.
Table 2.
Demographics, clinical symptoms and empathy assessments of schizophrenia spectrum disorder subjects with actively psychotic symptoms (SSD-AP) versus those with residual psychotic symptoms (SSD-RP).
| SSD-AP (n=8) |
SSD-RP (n=8) |
|
|---|---|---|
| Mean age in years (S.D.) | 37.6 (11.8) | 36.4 (8.0) |
| Years of education (S.D.) | 14.0 (2.1) | 13.8 (2.2) |
| Age of onset (S.D.) | 21.3 (7.1) | 20.1 (2.4) |
| Duration of illness in years (S.D.) | 15.9 (11.2) | 15.8 (6.5) |
| Gender (male/female) | 7/1 | 7/1 |
| Diagnosis, n (%) | ||
| Schizophrenia | 6 | 8 |
| Schizoaffective | 1 | 0 |
| Delusional disorder | 1 | 0 |
| Current use of medication, n (%) | ||
| Antipsychotics | 8 | 8 |
| Antidepressants * | 1 | 5 |
| SANS/SAPS, mean (S.D.) | ||
| Negative symptoms | 9.9 (3.1) | 8.6 (3.4) |
| Disorganized symptoms | 1.9 (2.9) | 0.3 (0.7) |
| Psychotic symptoms *** | 6.4 (2.1) | 0.6 (0.9) |
| Global delusions ** | 3.0 (1.5) | 0.4 (0.7) |
| Global hallucinations ** | 3.4 (1.6) | 0.3 (0.7) |
| Interpersonal Reactivity Index, mean (S.D.) | ||
| Affective empathy | 36.1 (7.1) | 36.5 (4.1) |
| Empathic concern (EC) | 21.4 (4.0) | 20.6 (2.8) |
| Personal distress (PD) | 14.8 (4.2) | 15.9 (5.4) |
| Cognitive empathy | 32.5 (5.7) | 31.4 (4.4) |
| Fantasy (FS) * | 17.3 (3.7) | 12.4 (3.9) |
| Perspective taking (PT) | 15.3 (5.1) | 19.0 (3.2) |
Abbreviations: SANS=Scale for the Assessment of Negative Symptoms; SAPS=Scale for the Assessment of Positive Symptoms.
Significant at p<0.05.
Significant at p<0.01.
Significant at p=0.001.
In summary, SSD and healthy comparison subjects exhibited significant bilateral mu suppression during all hand movement conditions (video hand observation, live hand observation, and self-hand movement)–with the exception of left-sided mu suppression by controls during the live hand observation condition, which only trended toward significance. As a group, the SSD subjects did not exhibit significantly greater mu suppression than the healthy comparison subjects. However, the actively psychotic SSD subgroup showed significantly greater left mu suppression during the live hand observation compared to the residual phase SSD subgroup and the healthy comparison subjects. The actively psychotic participants also showed higher scores on the IRI Fantasy subscale. The SSD residual phase subgroup did not have significantly different mu suppression compared to healthy comparison participants. Within the entire SSD group, greater left-sided mu suppression was significantly correlated with higher global psychosis scores, but not with negative or disorganized symptoms.
4. Discussion
The present research aimed at examining differences in suppression of EEG mu activity (a proxy for mirror neuron functioning) during established motion observation paradigms in individuals with schizophrenia spectrum disorders (SSD) compared to age- and gender-matched healthy comparison subjects. Associations between mu suppression change and empathy and between mu suppression and clinical symptoms of schizophrenia were also examined. Group differences in mu suppression were found, but interestingly were not in the direction that we predicted based upon a previous study of autism spectrum disorders (Oberman et al., 2005). Left-sided mu suppression during observation of live hand movement was greater (rather than lower) in SSD subjects with active or chronic psychotic symptoms relative to healthy comparison participants and SSD patients in the residual phase of their illness. In the healthy comparison group, mu suppression over the left sensorimotor cortex during the observed live hand condition was 53% of that elicited during self-hand movement and was consistent with previous studies (Gastaut and Bert, 1954; Pineda et al., 2000; Muthukumaraswamy et al., 2004; Pfurtscheller et al., 2006). Left-sided mu suppression during the live hand observation among SSD patients in the residual phase of the illness was 61% of that for their own self-hand movement, whereas the SSD group with active psychosis showed mu suppression during the watched live hand condition equal to 71% of their own self-hand movement. Since mu suppression during action observation is considered a measure of mirror neuron activity, this finding translates to nearly 20% greater mirror neuron activity in actively psychotic SSD subjects compared to healthy participants. Importantly, the degree of left-sided mu suppression (i.e., higher mirror neuron activity) was directly correlated with psychotic symptom severity.
Before further discussing the findings in the present study some caveats need acknowledgment. For one, the left-sided mu suppression during the live hand movement observation condition in the healthy comparison group only nearly reached significance (p=.06), which could account for part of the difference observed relative to the actively psychotic SSD subjects. Other limitations of this study include the modest sample size and lack of neuropsychological assessments. Notably, however, the sample size in the present study compares well to the two other electrophysiological studies of mirror neuron function in schizophrenia [15 subjects in each group in Enticott et al. (2008) and 11 in each group for the Schurmann et al. (2007) studies]. In addition, all SSD subjects were prescribed antipsychotics at the time of their EEG examination, and the possible effects of these medications on the findings are largely unknown.
Mu suppression occurs primarily over the sensorimotor cortex and represents a neural basis for both performing and observing hand movement activity in others (Cochin et al., 1998; Babiloni et al., 1999), of which the latter is thought to underlie mirror neuron activity (Pineda et al., 2000; Muthukumaraswamy et al., 2004). Large mu suppression (as shown in the patients with psychosis in this study) may be indicative of increased mirror neuron activity or an increased response to others’ actions as described by Abu-Akel and Bailey (2000), a pattern opposite to findings in autism-spectrum disorders. Patients with autism-spectrum disorder show virtually no mu suppression when observing hand movement in others (Oberman et al., 2005). EEG evidence of increased mu suppression (i.e., greater mirror neuron activity) during observed hand movement parallels the fMRI findings attained during facial affect processing in schizophrenia (Quintana et al., 2001). Similarly, findings in the present study are consistent with the view of Abu-Akel (2003), who suggested that patients with active psychosis have greater empathy and ToM abilities, which tend to normalize with abatement of psychosis (Walston et al., 2000). This view is in part confirmed by the results in the present study in which patients with SSD and active psychosis showed higher IRI Fantasy scores (a measure of cognitive empathy).
Higher than normal levels of Personal Distress on the IRI in people with schizophrenia have been previously reported with some consistency (Montag et al., 2007; Shamay-Tsoory et al., 2007; Achim et al., 2011; Lee et al., 2011). The IRI was designed to be used in healthy populations but has since been used to measure empathy in different clinical populations (Lombardo et al., 2007; Montag et al., 2007; Shamay-Tsoory et al., 2007; Achim et al., 2011; Lee et al., 2011). The Personal Distress category of the IRI is thought to be one of the multi-dimensional components of affective empathy and is described as taking on the vicarious negative arousal of others who are experiencing physical or emotional distress (Davis, 1980). Although the relationship between an overactive mirror neuron system and increased Personal Distress on the IRI needs to be more extensively explored, the finding in the present study is consistent with the view that people with SSD have a breakdown in the boundaries between self and others. To expand, enhanced mirror neuron activity manifested as greater mu suppression during action observation in schizophrenia may be germane to nearly 50 years of literature suggesting that people suffering with schizophrenia have sensory gating deficits that lead to difficulties filtering out extraneous information and/or filtering in more novel, or salient, information (Brockhaus-Dumke, et al., 2008; Brenner et al., 2009). Therefore, high mu suppression, a posited marker of greater mirror neuron activity, may be reflective of a higher level of emotional “resonance with others” in the SSD group with active psychosis. A higher level of emotional resonance may be related to poor filtering of social emotional information, and hence be the underpinning of the breakdown of boundaries between the self and other. If this is the case, the sensory gating hypothesis (Broadbent, 1970; Brockhaus-Dumke, et al., 2008; Brenner et al., 2009) may need to be further characterized by highlighting its relative relevance for social emotional stimuli.
To our knowledge, there are only two other studies that have assessed electrophysiological measures as a biological marker of mirror neuron activity in schizophrenia (Schurmann et al., 2007; Enticott et al., 2008). In the study by Schurmann et al. (2007), median nerve stimulation was used to facilitate measurement of motor cortical excitability at 20 Hz during self and observed movement. In the study by Enticott et al. (2008), transcranial magnetic stimulation was applied to the motor cortex to facilitate measurement of motor cortical excitability in the form of motor evoked potentials during observed movement. The results from both of these studies have suggested that mirror neuron activity may be reduced in schizophrenia. Primary reasons for differences in reported mu suppression activity during action observation between the two earlier studies and ours may be due to the absence of exogenous stimulation in our study, which was modeled after the same procedures used in the Oberman et al. (2005) study. Further research should aim at clarifying mu suppression under differing experimental conditions and in different subtypes of schizophrenia.
5. Summary
The present research focused on potential mechanisms of empathy changes and psychotic symptoms in schizophrenia. EEG was used to assess mu suppression as a non-invasive measure of mirror neuron activity. SSD subjects with active psychosis were found to have greater left-sided mu suppression or greater mirror neuron activity, which correlated to greater psychotic symptoms. SSD subjects also had significantly greater self-reported Personal Distress as measured by the IRI. While lower mirror neuron activity has been reported in autism, the present research showed that mirror neuron activity is elevated among patients with schizophrenia and active psychosis. This phenomenon may be the underpinning of schizophrenia sensory gating deficits and may contribute to sensory misattributions particularly in response to socially relevant stimuli, and be a putative mechanism for delusions and hallucinations.
References
- Abu-Akel A. The neurochemical hypothesis of ‘theory of mind’. Medical Hypotheses. 2003;60:382–386. doi: 10.1016/s0306-9877(02)00406-1. [DOI] [PubMed] [Google Scholar]
- Abu-Akel A, Bailey AL. The possibility of different forms of theory of mind impairment in psychiatric and developmental disorders. Psychological Medicine. 2000;30:735–738. doi: 10.1017/s0033291799002123. [DOI] [PubMed] [Google Scholar]
- Achim AM, Ouellet R, Roy MA, Jackson PL. Assessment of empathy in first-episode psychosis and meta-analytic comparison with previous studies in schizophrenia. Psychiatry Research. 2011;190:3–8. doi: 10.1016/j.psychres.2010.10.030. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th Edition Text Revision American Psychiatric Press, Inc.; Washington, D.C.: 2000. [Google Scholar]
- Andreasen NC. Methods for assessing positive and negative symptoms. Modern Problems of Pharmacopsychiatry. 1990;24:73–88. doi: 10.1159/000418013. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Nasrallah HA, Dunn V, Olson SC, Grove WM, Ehrhardt JC, Coffman JA, Crossett JH. Structural abnormalities in the frontal system in schizophrenia. A magnetic resonance imaging study. Archives of General Psychiatry. 1986;43:136–144. doi: 10.1001/archpsyc.1986.01800020042006. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Calarge CA, O’Leary DS. Theory of mind and schizophrenia: a positron emission tomography study of medication-free patients. Schizophrenia Bulletin. 2008;34:708–719. doi: 10.1093/schbul/sbn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbib MA, Mundhenk TN. Schizophrenia and the mirror system: an essay. Neuropsychologia. 2005;43:268–280. doi: 10.1016/j.neuropsychologia.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Carducci F, Cincotti F, Rossini PM, Neuper C, Pfurtscheller G, Babiloni F. Human movement-related potentials vs desynchronization of EEG alpha rhythm: a high-resolution EEG study. NeuroImage. 1999;10:658–665. doi: 10.1006/nimg.1999.0504. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. The cognitive neuroscience of autism. Journal of Neurology, Neurosurgery, and Psychiatry. 2004;75:945–948. doi: 10.1136/jnnp.2003.018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Bernasconi A, Bosia M, Cavallaro R, Dallaspezia S, Falini A, Poletti S, Radaelli D, Riccaboni R, Scotti G, Smeraldi E. Functional and structural brain correlates of theory of mind and empathy deficits in schizophrenia. Schizophrenia Research. 2009;114:154–160. doi: 10.1016/j.schres.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Bentall RP, Rowse G, Shryane N, Kinderman P, Howard R, Blackwood N, Moore R, Corcoran R. The cognitive and affective structure of paranoid delusions: a transdiagnostic investigation of patients with schizophrenia spectrum disorders and depression. Archives of General Psychiatry. 2009;66:236–247. doi: 10.1001/archgenpsychiatry.2009.1. [DOI] [PubMed] [Google Scholar]
- Bernier R, Dawson G, Webb S, Murias M. EEG mu rhythm and imitation impairments in individuals with autism spectrum disorder. Brain and Cognition. 2007;64:228–237. doi: 10.1016/j.bandc.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand MC, Achim AM, Harvey PO, Sutton H, Malla AK, Lepage M. Structural neural correlates of impairments in social cognition in first episode psychosis. Social Neuroscience. 2008;3:79–88. doi: 10.1080/17470910701563491. [DOI] [PubMed] [Google Scholar]
- Bigelow NO, Paradiso S, Adolphs R, Moser DJ, Arndt S, Heberlein A, Nopoulos P, Andreasen NC. Perception of socially relevant stimuli in schizophrenia. Schizophrenia Research. 2006;83:257–267. doi: 10.1016/j.schres.2005.12.856. [DOI] [PubMed] [Google Scholar]
- Bora E, Gokcen S, Veznedaroglu B. Empathic abilities in people with schizophrenia. Psychiatry Research. 2008;160:23–29. doi: 10.1016/j.psychres.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Kieffaber PD, Clementz BA, Johannesen JK, Shekhar A, O’Donnell BF, Hetrick WP. Event-related potential abnormalities in schizophrenia: a failure to “gate in” salient information? Schizophrenia Research. 2009;113:332–338. doi: 10.1016/j.schres.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Schultze-Lutter F, Mueller R, Tendolkar I, Bechdolf A, Pukrop R, Klosterkoetter J, Ruhrmann S. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biol Psychiatry. 2008;64(5):376–384. doi: 10.1016/j.biopsych.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Brüne M. “Theory of mind” in schizophrenia: a review of the literature. Schizophrenia Bulletin. 2005;31:21–42. doi: 10.1093/schbul/sbi002. [DOI] [PubMed] [Google Scholar]
- Brunet-Gouet E, Decety J. Social brain dysfunctions in schizophrenia: a review of neuroimaging studies. Psychiatry Research. 2006;148:75–92. doi: 10.1016/j.pscychresns.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Burns J. The social brain hypothesis of schizophrenia. World Psychiatry. 2006;5:77–81. [PMC free article] [PubMed] [Google Scholar]
- Cochin S, Barthelemy C, Lejeune B, Roux S, Martineau J. Perception of motion and qEEG activity in human adults. Electroencephalography and Clinical Neurophysiology. 1998;107:287–295. doi: 10.1016/s0013-4694(98)00071-6. [DOI] [PubMed] [Google Scholar]
- Cochin S, Barthelemy C, Roux S, Martineau J. Observation and execution of movement: similarities demonstrated by quantified electroencephalography. The European Journal of Neuroscience. 1999;11:1839–1842. doi: 10.1046/j.1460-9568.1999.00598.x. [DOI] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophrenia Bulletin. 2006;32(Suppl. 1):S44–S63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo Facorro B, Paradiso S, Andreasen NC, O’Leary DS, Hichwa RD. Neural substrates of emotional processing in schizophrenia. A PET study during exposure to pleasant and unpleasant odors. JAMA. 2001;286:427–435. doi: 10.1001/jama.286.4.427. [DOI] [PubMed] [Google Scholar]
- Davis MH. A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology. 1980;10:85. [Google Scholar]
- Davis MH. Measuring individual differences in empathy: evidence for a multi-dimensional approach. Journal of Personality and Social Psychology. 1983;44:113–126. [Google Scholar]
- Dawson G, Warrenburg S, Fuller P. Left hemisphere specialization for facial and manual imitation. Psychophysiology. 1985;22:237–243. doi: 10.1111/j.1469-8986.1985.tb01593.x. [DOI] [PubMed] [Google Scholar]
- Derntl B, Finkelmeyer A, Toygar TK, Hulsmann A, Schneider F, Falkenberg DI, Habel U. Generalized deficit in all core components of empathy in schizophrenia. Schizophrenia Research. 2009;108:197–206. doi: 10.1016/j.schres.2008.11.009. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Experimental Brain Research. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Dziobek I, Rogers K, Fleck S, Bahnemann M, Heekeren HR, Wolf OT, Convit A. Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the Multifaceted Empathy Test (MET) Journal of Autism and Developmental Disorders. 2008;38:464–473. doi: 10.1007/s10803-007-0486-x. [DOI] [PubMed] [Google Scholar]
- Edwards J, Pattison PE, Jackson HJ, Wales RJ. Facial affect and affective prosody recognition in first-episode schizophrenia. Schizophrenia Research. 2001;48:235–253. doi: 10.1016/s0920-9964(00)00099-2. [DOI] [PubMed] [Google Scholar]
- Enticott PG, Hoy KE, Herring SE, Johnston PJ, Daskalakis ZJ, Fitzgerald PB. Reduced motor facilitation during action observation in schizophrenia: a mirror neuron deficit? Schizophrenia Research. 2008;102:116–121. doi: 10.1016/j.schres.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Fahim C, Stip E, Mancini-Marie A, Boualem M, Malaspina D, Beauregard M. Negative socio-emotional resonance in schizophrenia: a functional magnetic resonance imaging hypothesis. Medical Hypotheses. 2004;63:467–475. doi: 10.1016/j.mehy.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Frith CD. The neural basis of hallucinations and delusions. Comptes Rendus Biologies. 2005;328:169–175. doi: 10.1016/j.crvi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Frith CD, Corcoran R. Exploring ’theory of mind’ in people with schizophrenia. Psychological Medicine. 1996;26:521–530. doi: 10.1017/s0033291700035601. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Shimizu M, Hirao K, Miyata J, Namiki C, Sawamoto N, Fukuyama H, Hayashi T, Murai T. Female specific anterior cingulate abnormality and its association with empathic disability in schizophrenia. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32:1728–1734. doi: 10.1016/j.pnpbp.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Gastaut HJ, Bert J. EEG changes during cinematographic presentation; moving picture activation of the EEG. Electroencephalography and Clinical Neurophysiology. 1954;6:433–444. doi: 10.1016/0013-4694(54)90058-9. [DOI] [PubMed] [Google Scholar]
- Herold R, Feldmann A, Simon M, Tenyi T, Kover F, Nagy F, Varga E, Fekete S. Regional gray matter reduction and theory of mind deficit in the early phase of schizophrenia: a voxel-based morphometric study. Acta Psychiatrica Scandinavica. 2009;119:199–208. doi: 10.1111/j.1600-0447.2008.01297.x. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Germine LT, Knight RT, D’Esposito M. Neural activity during social signal perception correlates with self-reported empathy. Brain Research. 2010;1308:100–113. doi: 10.1016/j.brainres.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Mazziotta JC. Mirror neuron system: basic findings and clinical applications. Annals of Neurology. 2007;62:213–218. doi: 10.1002/ana.21198. [DOI] [PubMed] [Google Scholar]
- Järveläinen J, Schurmann M, Avikainen S, Hari R. Stronger reactivity of the human primary motor cortex during observation of live rather than video motor acts. Neuroreport. 2001;12:3493–3495. doi: 10.1097/00001756-200111160-00024. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Towards a unifying neural theory of social cognition. Progress in Brain Research. 2006;156:379–401. doi: 10.1016/S0079-6123(06)56021-2. [DOI] [PubMed] [Google Scholar]
- Langdon R, Ward PB, Coltheart M. Reasoning anomalies associated with delusions in schizophrenia. Schizophrenia Bulletin. 2010;36:321–330. doi: 10.1093/schbul/sbn069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Zaki J, Harvey PO, Ochsner K, Green MF. Schizophrenia patients are impaired in empathic accuracy. Psychological Medicine. 2011:1–8. doi: 10.1017/S0033291711000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Barnes JL, Wheelwright SJ, Baron-Cohen S. Self-referential cognition and empathy in autism. PLoS One. 2007;2:e883. doi: 10.1371/journal.pone.0000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau J, Cochin S, Magne R, Barthelemy C. Impaired cortical activation in autistic children: is the mirror neuron system involved? International Journal of Psychophysiology. 2008;68:35–40. doi: 10.1016/j.ijpsycho.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Martineau J, Andersson F, Barthélémy C, Cottier JP, Destrieux C. Atypical activation of the mirror neuron system during perception of hand motion in autism. Brain Res. 2010;1320:168–175. doi: 10.1016/j.brainres.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Mier D, Sauer C, Lis S, Esslinger C, Wilhelm J, Gallhofer B, Kirsch P. Neuronal correlates of affective theory of mind in schizophrenia out-patients: evidence for a baseline deficit. Psychological Medicine. 2010;40:1607–1617. doi: 10.1017/S0033291709992133. [DOI] [PubMed] [Google Scholar]
- Montag C, Heinz A, Kunz D, Gallinat J. Self-reported empathic abilities in schizophrenia. Schizophrenia Research. 2007;92:85–89. doi: 10.1016/j.schres.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Johnson BW, McNair NA. Mu rhythm modulation during observation of an object-directed grasp. Brain Research. Cognitive Brain Research. 2004;19:195–201. doi: 10.1016/j.cogbrainres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Brain Research. Cognitive Brain Research. 2005;24:190–198. doi: 10.1016/j.cogbrainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Ramachandran VS, Pineda JA. Modulation of mu suppression in children with autism spectrum disorders in response to familiar or unfamiliar stimuli: the mirror neuron hypothesis. Neuropsychologia. 2008;46:1558–1565. doi: 10.1016/j.neuropsychologia.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Andreasen NC, Crespo Facorro B, O’Leary DS, Hichwa RD. Emotion in unmedicated patients with schizophrenia evaluated with Positron Emission Tomography. Am J Psychiatry. 2003;160:1775–1783. doi: 10.1176/appi.ajp.160.10.1775. [DOI] [PubMed] [Google Scholar]
- Park KM, Kim JJ, Ku J, Kim SY, Lee HR, Kim SI, Yoon KJ. Neural basis of attributional style in schizophrenia. Neuroscience Letters. 2009;459:35–40. doi: 10.1016/j.neulet.2009.04.059. [DOI] [PubMed] [Google Scholar]
- Park IH, Ku J, Lee H, Kim SY, Kim SI, Yoon KJ, Kim JJ. Disrupted theory of mind network processing in response to idea of reference evocation in schizophrenia. Acta Psychiatrica Scandinavica. 2011;123:43–54. doi: 10.1111/j.1600-0447.2010.01597.x. [DOI] [PubMed] [Google Scholar]
- Perkins T, Stokes M, McGillivray J, Bittar R. Mirror neuron dysfunction in autism spectrum disorders. Journal of Clinical Neuroscience. 2010;17:1239–1243. doi: 10.1016/j.jocn.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Brunner C, Schlogl A, Lopes da Silva FH. Mu rhythm (de)synchronization and EEG single-trial classification of different motor imagery tasks. NeuroImage. 2006;31:153–159. doi: 10.1016/j.neuroimage.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Pineda JA, Allison BZ, Vankov A. The effects of self-movement, observation, and imagination on mu rhythms and readiness potentials (RP’s): toward a braincomputer interface (BCI) IEEE Transactions on Rehabilitation Engineering. 2000;8:219–222. doi: 10.1109/86.847822. [DOI] [PubMed] [Google Scholar]
- Preston SD, de Waal FB. Empathy: Its ultimate and proximate bases. The Behavioral and Brain Sciences. 2002;25:1–20. doi: 10.1017/s0140525x02000018. discussion 20–71. [DOI] [PubMed] [Google Scholar]
- Quintana J, Davidson T, Kovalik E, Marder SR, Mazziotta JC. A compensatory mirror cortical mechanism for facial affect processing in schizophrenia. Neuropsychopharmacology. 2001;25:915–924. doi: 10.1016/S0893-133X(01)00304-9. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Salvatore G, Dimaggio G, Lysaker PH. An intersubjective perspective on negative symptoms of schizophrenia: implications of simulation theory. Cognitive Neuropsychiatry. 2007;12:144–164. doi: 10.1080/13546800600819921. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Jarvelainen J, Avikainen S, Cannon TD, Lonnqvist J, Huttunen M, Hari R. Manifest disease and motor cortex reactivity in twins discordant for schizophrenia. The British Journal of Psychiatry. 2007;191:178–179. doi: 10.1192/bjp.bp.106.024604. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Shur S, Harari H, Levkovitz Y. Neurocognitive basis of impaired empathy in schizophrenia. Neuropsychology. 2007;21:431–438. doi: 10.1037/0894-4105.21.4.431. [DOI] [PubMed] [Google Scholar]
- Thatcher RW, Biver CJ, North DM. Quantitative EEG and the Frye and Daubert standards of admissibility. Clinical Electroencephalography. 2003;34:39–53. doi: 10.1177/155005940303400203. [DOI] [PubMed] [Google Scholar]
- Varcin KJ, Bailey PE, Henry JD. Empathic deficits in schizophrenia: the potential role of rapid facial mimicry. Journal of the International Neuropsychological Society. 2010;16:621–629. doi: 10.1017/S1355617710000329. [DOI] [PubMed] [Google Scholar]
- Walston F, Blennerhassett RC, Charlton BG. “Theory of mind”, persecutory delusions and the somatic marker mechanism. Cognitive Neuropsychiatry. 2000;5:161–174. [Google Scholar]
- Zaki J, Weber J, Bolger N, Ochsner K. The neural bases of empathic accuracy. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11382–11387. doi: 10.1073/pnas.0902666106. [DOI] [PMC free article] [PubMed] [Google Scholar]


