Abstract
Multisensory superior colliculus neurons in cats were found to retain substantial plasticity to short-term, site-specific experience with cross-modal stimuli well into adulthood. Following cross-modal exposure trials, these neurons substantially increased their sensitivity to the cross-modal stimulus configuration as well as to its individual component stimuli. In many cases, the exposure experience also revealed a previously ineffective or “silent” input channel, rendering it overtly responsive. These experience-induced changes required relatively few exposure trials and could be retained for more than 1 h. However, their induction was generally restricted to experience with cross-modal stimuli. Only rarely were they induced by exposure to a modality-specific stimulus and were never induced by stimulating a previously ineffective input channel. This short-term plasticity likely provides substantial benefits to the organism in dealing with ongoing and sequential events that take place at a given location in space and may reflect the ability of multisensory superior colliculus neurons to rapidly alter their response properties to accommodate to changes in environmental challenges and event probabilities.
Keywords: visual, auditory, multisensory integration, plasticity, sensory exposure
a characteristic feature of superior colliculus (SC) neurons is their ability to synthesize information from different senses. This phenomenon of “multisensory integration” is evident in their marked response enhancement when activated by cross-modal stimuli aligned in space and time (Stein and Meredith 1993). Although not the only form of multisensory integration, multisensory enhancement is the most reliable index of this SC capacity (Kadunce et al. 1997) and reflects an elegant mechanism for selectively increasing the salience of external events.
Spatiotemporally concordant stimuli are generally derived from the same event, and enhancing the event's physiological signature increases its likelihood of initiating SC-mediated orientation responses (Burnett et al. 2004, 2007; Gingras et al. 2009; Jiang et al. 2002; King and Palmer 1985; Stein et al. 1989; Wilkinson et al. 1996; Zahar et al. 2009). The magnitude of this effect is proportionately greatest when individual component stimuli are least effective (Meredith and Stein 1986; Stein and Stanford 2008) and is believed to derive from the conditional independence of sensory reports provided by different sensory channels (Ernst and Banks 2002; Rowland et al. 2007a, 2007c).
The neural representations and processes governing their integration are normally crafted during early postnatal life (Stein et al. 1973b; Wallace and Stein 1997) as the brain accumulates experience with the sensory statistics of the environment (Cuppini et al. 2011; Wallace and Stein 2007; Xu et al. 2012; Yu et al. 2010). Yet recent evidence suggests that even after the response properties of SC neurons are established, they are not immutable. That multisensory neurons retain at least some response plasticity into adulthood is evident in their ability to acquire multisensory integration capabilities after dark rearing, if given sufficient experience with invariant cross-modal stimuli (Yu et al. 2010), and in alterations induced in their unisensory responses by exposure to sequences of modality-specific stimuli (Yu et al. 2009).
These observations raise the possibility that, even after the fundamental properties of multisensory neurons are specified during early life, these neurons retain sufficient plasticity to rapidly alter their responses to certain environmental cues based on their frequency and/or reliability. The objective of the present experiments was to test whether adult SC neurons would alter their responses online as a consequence of exposure to new environmental statistics and, if so, to determine the time course of these changes and the factors predicting their induction. Two populations of visual-auditory neurons were studied: typical multisensory neurons, which overtly respond (i.e., with impulses) to both visual and auditory stimuli, and neurons that respond overtly only to stimuli from one sensory modality but evidence covert (i.e., non-impulse generating) inputs from the second modality by virtue of their ability to significantly modulate overt responses. Both neuronal types are capable of multisensory integration and have been previously noted in SC (Meredith and Stein 1983) and cortex (Allman and Meredith 2007). The results reveal that, following exposure to cross-modal stimuli, both populations of SC neurons increased their responsiveness to the exposure stimuli, suggesting that they retain a functional dynamism whose blueprint is established early in life, but whose features continually adapt to short-term experience even during adulthood.
MATERIALS AND METHODS
All protocols were in accordance with the Guide for the Care and Use of Laboratory Animals, eighth edition (NRC 2011), and were approved by the Animal Care and Use Committee of Wake Forest Medical School, an AAALAC-accredited institution.
Surgical Preparation
Five adult cats (3 females, 2 males) were implanted with chronic stainless-steel recording chambers (McHaffie and Stein 1983). Before surgery, each animal was first rendered tractable with ketamine hydrochloride (20 mg/kg im) and acepromazine maleate (0.1 mg/kg im), intubated, and placed in a stereotaxic head holder. Surgical anesthesia was induced with isoflurane (1.5–3%). A craniotomy was made overlying the SC and covered with a recording chamber that was attached to the skull with stainless steel screws and dental acrylic. The animal then recovered from surgery and completed a course of postsurgical analgesics (buprenorphine, 0.01 mg/kg, prn; ketoprofen, 2 mg/kg sid) and prophylactic antibiotics (cephazolin sodium, 20 mg/kg bid).
Single-neuron Recording
Weekly recording sessions began 7 or more days after surgery. For each session, the animal was anesthetized with ketamine hydrochloride (20 mg/kg im) and acepromazine maleate (0.1 mg/kg im), intubated, and artificially ventilated. Respiratory rate and volume, heart rate, and blood pressure were monitored, and CO2 was maintained at ∼4.0%. An injection of pancuronium bromide (0.1 mg/kg iv) induced paralysis to fix the positions of the eyes and ears. Anesthesia, paralysis, and hydration were maintained by continuous intravenous infusion of ketamine hydrochloride (6–8 mg·kg−1·h−1) and pancuronium bromide (0.05 mg·kg−1·h−1) in 5% dextrose Ringer solution (3–6 ml/h). Body temperature was kept at 37–38°C using a heating pad. The recording chamber was attached to a metal frame in the absence of wounds or pressure points. The frame did not obscure the animal's sight or hearing and allowed unobstructed access to the SC. The pupil of the eye contralateral to the target SC was dilated with 1% atropine sulfate and fitted with a contact lens to correct any refractive error. An opaque lens was placed over the ipsilateral eye. Anesthesia and paralysis were terminated at the end of the experiment, the contact lenses and respiration tube were removed, and the animal was returned to its cage after regaining stable respiration and locomotion.
Conventional methods were used for extracellular single-neuron recordings: an electrode (tip diameter: 1–3 μm, impedance: 1–3 MΩ at 1 kHz) was positioned on a microdrive stage and slowly lowered to the SC. Once at the SC surface, the electrode was advanced by the hydraulic microdrive while visual and auditory search stimuli were presented. Neural activity was recorded, amplified, and routed to an oscilloscope, audio monitor, and computer for online and offline analysis as in the past (Yu et al. 2009, 2010).
Apparatus and Search Stimuli
Sensory neurons were identified using a variety of visual and auditory search stimuli that were chosen based on their effectiveness in activating SC neurons (Stein and Meredith 1993). Visual stimuli were flashes or moving bars of light with different intensities and velocities back-projected from an LC 4445 Philips projector onto a screen located 45 cm from the front of the animal. Auditory stimuli were broadband (20–20,000 Hz) noise bursts, clicks, and taps of a metal object on a table. These stimuli were presented in various orders while the electrode was moved in search of sensory-responsive neurons. When a neuron was found, its modality convergence pattern (e.g., visual, auditory, visual-auditory) was determined using a wide range of stimulus parameters (e.g., intensity, size, location).
An Overt multisensory neuron was operationally defined as one producing impulses that statistically exceeded spontaneous activity to both visual and auditory stimuli independently. These neurons were categorized as capable or not capable of multisensory integration (i.e., integrating or nonintegrating; the latter failed to show a statistically significant increase in responses to the cross-modal stimulus above that to the most effective component stimulus, see Meredith and Stein 1986). A Covert multisensory neuron was operationally defined as one which responded with impulses to only one of these modality-specific stimuli, but whose unisensory (visual or auditory) response was significantly enhanced by a spatiotemporally concordant stimulus from the other modality. The neuron's modality-specific visual and auditory receptive fields (RFs) were mapped as in the past (Jiang et al. 2001, 2006). The visual RF was mapped with moving light bars, and the auditory RF was mapped with broadband noise bursts from any of 16 speakers placed 15° apart and 25 cm from the head. The speakers were mounted on an adjustable hoop.
Test Stimuli
Test stimuli were presented within the neuron's RFs (or presumptive RF in the case of Covert neurons), and, unless otherwise stated, the auditory stimuli consisted of brief (100–200 ms) 60- to 75-dB sound pressure level bursts of broadband noise presented against an ambient background of 48.4- to 52.7-dB sound pressure level. The intensity of the auditory component of the cross-modal pair was chosen to ensure that it would be effective in this stimulus configuration (the highest intensity was used in the case of Covert auditory neurons). This was also true of the visual component of the cross-modal pair. Thus visual stimuli (100- to 200-ms duration) were rectangular bars of light of varying length and diameter (e.g., 6° × 2°, 8° × 2°, 15° × 3°) and intensity (4.8–36.5 cd/m2) presented against a uniform gray background of 0.62 cd/m2 and moved in the preferred direction and speed across the neuron's visual RF. In the case of Covert visual neurons, where stimulus parameters of the visual stimulus could not be tailored for the neuron based on its visual responsiveness, the highest stimulus intensities were used, and the stimulus was moved at velocities and in directions that were effective in neighboring neurons. The visual and auditory stimuli in the cross-modal pair were always in close spatial and temporal register to maximize their excitatory effects (Meredith and Stein 1986; Meredith et al. 1987). All test stimuli were controlled by custom software operating a projector and a NIDAQ digital controller (National Instruments).
Exposure and Testing
Stimulus-driven responses were recorded before and after a series of 80–100 exposures to the same stimulus configuration, with interstimulus intervals (10–12 s) that were chosen to avoid habituation (Oyster and Takahashi 1975). Although every neuron was provided with cross-modal exposure trials, a subset of neurons was also provided with modality-specific exposure trials (the visual and/or auditory component stimulus individually). For Covert multisensory neurons, the presumptive location of the RF for what is operationally referred to here as the “silent” input channel or “silent” modality was estimated using three referents: 1) the visual-auditory RF relationships typical of Overt multisensory neurons (i.e., overlapping in space); 2) the location at which background responses were obtained; and 3) the RFs of overlying neurons in that electrode penetration. After exposure trials, the actual RFs were assessed. In some cases, testing with modality-specific and cross-modal stimuli continued at 10-min intervals to determine the permanence of the induced changes. In some neurons, exposure and test stimuli were presented at different locations to determine whether any induced effects would generalize beyond those noted at the exposure site.
Data Acquisition and Analysis
Custom software acquired raw data waveforms and impulses from single neurons. A threshold criterion of 3 × elevation of the action potential amplitude above background fluctuations was imposed, and the amplitude and wave shape of action potentials were discriminated to ensure that data were being collected from a single neuron. Impulse times were recorded with 1-ms temporal resolution. A geometric algorithm that identifies changes in neural activity based on inflections in the cumulative discharge train was used to determine the onset and offset of the response. This defines the response window, and each response has its own window (Rowland et al. 2007c). The magnitude of each response was identified as the mean number of impulses occurring in the response window minus the expected number within that window given the spontaneous firing rate (calculated in the 500 ms preceding stimulus presentation). The change (Δ) in response magnitude was defined as the value of the mean response magnitude after exposure minus the mean response magnitude before exposure.
The response to the combined cross-modal stimuli was compared with the response evoked by the more effective modality-specific stimulus component to compute the multisensory integration index (MSI): MSI = [(CM − SMmax)/SMmax] × 100%, where CM represents the mean magnitude of multisensory response, and SMmax represents the mean magnitude of the response evoked by the more effective modality-specific stimulus component (Meredith and Stein 1983; Stanford et al. 2005; Stein et al. 2009). Data were compared statistically to determine significant differences using SPSS 11.5 and MATLAB 6.5. In addition, t-tests, Mann-Whitney U-tests, ANOVA, and Pearson product-moment correlation tests were used where appropriate. All of the data were expressed as means ± standard deviation, and the minimum criterion for statistical significance was <0.05.
RESULTS
A total of 222 multisensory SC neurons were studied. All were located in the intermediate and deep layers of the structure, and each was operationally categorized into Overt or Covert categories based on its preexposure response properties. Neurons in the Overt, or typical multisensory group (n = 81; 69 integrating neurons and 12 nonintegrating neurons), are most familiar and have formed the basis for most previous studies (Stein and Stanford 2008). Their response and integrative properties were similar to those detailed earlier (Alvarado et al. 2007b; Jiang et al. 2006; Kadunce et al. 1997; Meredith and Stein 1986). They responded with impulses to visual and auditory stimuli when placed individually within their RFs. Sixty-five of them (53 integrating neurons and 12 nonintegrating neurons) were exposed to the repeated presentation of the visual-auditory cross-modal stimulus, and many showed clear evidence of site-specific unisensory and multisensory response plasticity. The short-term response plasticity in the Overt population suggested a heightened responsiveness to external stimuli and prompted the examination of their Covert multisensory counterparts (n = 141), which are also common in the SC. Impulses from Covert neurons were initially generated by only one of the two stimulus modalities when tested with modality-specific stimuli [i.e., either visual (n = 95) or auditory (n = 46)], and the classification “Covert” acknowledged the presence of what initially seemed like a non-impulse-generating channel, one that is operationally referred to here as “silent,” as it is not informative to downstream neurons when stimulated individually. However, the operation of the silent channel was apparent by virtue of the ability of stimuli specific to it to elevate the neuron's response magnitude when presented together with a stimulus in the active channel (i.e., the cross-modal stimulus combination), changes that are informative to downstream neurons. These Covert neurons also evidenced experience-induced plasticity, in some cases resulting in the appearance of overt responses in the silent channel (i.e., it was “revealed”), and thereby suggesting that previous distinctions between these multisensory neuronal groups might be more apparent than real. These results are described below in detail.
Impact of Sensory Exposure on Overt Multisensory Neurons
Single-neuron example.
The majority of Overt integrating neurons reacted to repeated exposure to identical cross-modal stimuli with enhanced responsiveness to subsequent stimuli (visual, auditory, and/or visual-auditory). This is illustrated in an exemplar neuron in Fig. 1, which also shows the general experimental approach. Before the exposure period, this neuron was most responsive to the visual component stimulus (5.3 impulses/trial), poorly responsive to the auditory component stimulus (0.7 impulses/trial), and exhibited response enhancement to their copresentation (9.3 impulses/trial) (Fig. 1, A and B). The neuron's multisensory response enhancement was significantly greater than the predicted sum of its two unisensory responses (i.e., it was “superadditive,” see Fig. 1C), with an MSI of 75.5% (see materials and methods). After 100 repetitions of the cross-modal stimulus in the exposure series, the mean multisensory and visual responses had more than doubled (to 21.2 impulses/trial and 11.6 impulses/trial, respectively), while the mean auditory response showed an eightfold increase (to 5.6 impulses/trial). However, because the unisensory referent (visual) response and the multisensory response increased in roughly proportionate fashion, the postexposure MSI (83%) was not significantly different from its preexposure level (Fig. 1C). In contrast to the population of Overt integrating neurons to be described below, the Overt nonintegrating population (i.e., those capable of responding to the visual and auditory stimuli independently, but not capable of multisensory enhancement) appeared largely insensitive to the subsequent exposure procedure: none evidenced consistent changes in unisensory and multisensory responses in response to repeated cross-modal stimulus exposure.
Fig. 1.
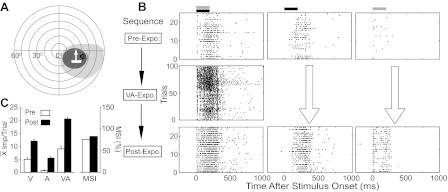
Cross-modal exposure rendered Overt multisensory neurons more responsive to sensory stimuli. A: depicted are the neuron's visual (dark gray; V) and auditory (light gray; A) receptive fields (RFs) and A (broadband noise burst) and V (moving light bar) stimulus locations within a schematic of visual-auditory (VA) space. Each concentric circle represents 10°. B: rasters (trials are ordered from bottom-top) illustrate this neuron's responses to combined VA (left), V (center), and A (right) stimuli before (top row) and after (bottom row) cross-modal exposure. The middle row shows responses during the exposure period (VA-Expo.), which evidenced a large increase in magnitude in later trials. Black and gray bars, respectively, above the rasters represent the V and A stimuli onsets and durations. C: the left portion of the summary bar graph shows the mean response magnitudes, and the right portion the MSI (index of multisensory integration) in the pre- and immediate postexposure periods. Note that the relative increases in unisensory and multisensory responses were balanced so that there were no substantial changes in MSI.
Population: main effect of cross-modal exposure on overt integrating neurons.
The exposure-induced response changes were not limited to the cross-modal stimulus and were most common in response to their modality-specific component stimuli. As noted above, these changes were variable in terms of the modality affected and the magnitude of the induced changes (Fig. 2, A–C). Although there was no single invariant change that was noted across the population of neurons studied, there were some consistencies in the data that generally pointed to a link between excitability and plasticity.
Fig. 2.
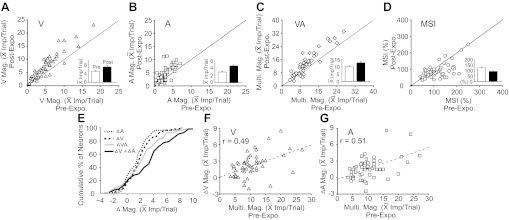
The impact of cross-modal exposure on the neuronal population was characterized by enhanced sensory responses. A–D: shown is the effect of the exposure experience on visual, V (A); auditory, A (B); and multisensory, VA (C) response magnitudes, as well as MSI (D). Solid diagonal lines are line of equality for the population, and inserted bar graphs shows the mean and standard errors of the distributions before (white bar) and after (black bar) the exposure period. Note that each of the activity measures except MSI increased after cross-modal exposure. E: the cumulative distributions of changes (Δ) in A, V, and multisensory responses are shown, as well as the sum of V and A changes (dark solid line). Note that the multisensory response increments were, in the population, greater than that in either of the unisensory responses, but less than their addition. F and G: the data indicate that the greater the preexposure multisensory response magnitude, the more unisensory responses were enhanced. Dashed line = the line of regression.
For example, in the majority of integrating Overt neurons (66%, 35/53), exposure significantly (t-test; P < 0.05) enhanced responses in at least one sensory channel, but this was most often engendered in the auditory channel as it provided the weaker of the two initial responses. The incidence of enhancement for each stimulus configuration was as follows: auditory = 57%, 30/53; visual = 45%, 24/53; multisensory = 38%, 20/53. The proportionate change was also greatest in the weaker channel (auditory = 56.5%; 2.94 ± 1.53 to 4.6 ± 2.72 impulses/trial; visual = 41.9%; 4.94 ± 3.54 to 7.01 ± 4.77 impulses/trial; multisensory = 25%, 10.26 ± 5.28 to 12.82 ± 7.12 impulses/trial). Furthermore, as noted in the exemplar neuron in Fig. 1, because unisensory response changes were either in proportion to the multisensory response changes or larger than them, the majority of neurons (84.9%, 45/53) showed no significant changes in MSI (only 1 showed an increase). In some cases (13.4%, 7/53), MSI actually decreased significantly (Fig. 2D). In a few instances (11%, 6/53), the cross-modal exposure significantly (t-test, P < 0.05) decreased responses in one sensory channel.
Among the response properties, the preexposure multisensory response magnitude was the best ad hoc predictor of the changes in the unisensory responses (visual: r = 0.49, P < 0.001; auditory: r = 0.51, P < 0.001; Pearson product-moment correlation test; see Fig. 2, F and G). In turn, the sum of the changes in the unisensory responses was revealed to be the best post hoc predictor of the change in the multisensory response (Fig. 2E), which it closely matched or exceeded.
Specificity: exposure-induced effects were not noted in all cases.
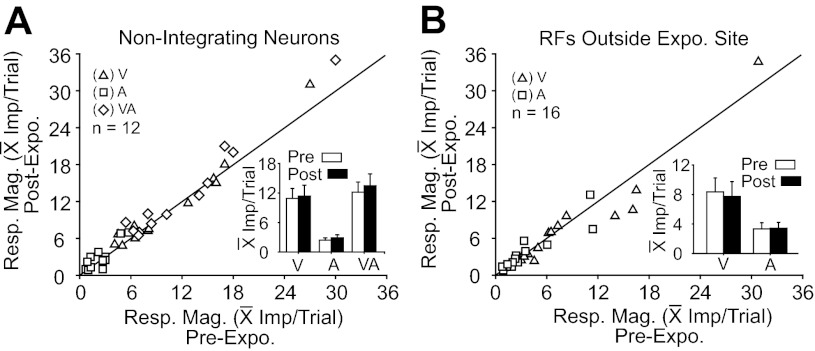
The induced effect did not reflect a general process affecting the entire population of Overt SC neurons. As noted earlier, nonintegrating Overt neurons (n = 12) showed little change in either their unisensory or their multisensory responses as a consequence of cross-modal exposure (Fig. 3A). Similarly, integrating Overt neurons whose RFs did not encompass the exposure site (n = 16, the exposure site was 15° outside the RF) showed no evidence of change in their unisensory response magnitudes, as shown in Fig. 3B (note that postexposure multisensory response changes were not evaluated because the test itself would involve within-field stimuli that could provide the exposure to induce response changes).
Fig. 3.
Cross-modal exposure-induced effects were not evident in some neuronal populations. A: neither unisensory nor multisensory responses were significantly altered by cross-modal exposure in neurons failing to exhibit multisensory integration capabilities. All postexposure response measures fell on or near the line of unity. The inserted bar graph provides pre- and postexposure comparisons of the mean and standard error of the population response magnitudes. B: unisensory responses of neurons with RFs outside the exposure site were also unaffected by cross-modal exposure. Conventions are the same as in previous figures.
As noted above, these observations raised the question of whether the presumptive subthreshold activity taking place within the silent sensory input channels of Covert neurons could be rendered suprathreshold by cross-modal experience so that the channel would become active thereafter. Depending on the nature of the operational mechanism by which exposure affects SC neurons (see discussion), this effect might also be induced by repeated stimulation of the active channel alone, or even by stimulation of the silent channel alone. A detailed examination of these possibilities was therefore conducted in Covert neurons, as described below.
Impact of Exposure on Covert Multisensory Neurons
Covert neurons also proved to be sensitive to this exposure paradigm, and in many cases (32%, 45/141) the silent sensory channel was rendered frankly active. Such neurons would normally have been categorized as unisensory if not tested with a cross-modal stimulus configuration. This effect was not readily induced by repeated exposure to either of the individual modality-specific component stimuli; the paradigm was never effective (0%, 0/141) when the exposure stimulus was matched to the silent channel and was only rarely effective (8%, 8/101) when it was matched to the active channel. However, as described below, when the neurons unaffected by exposure to the active channel stimulus were then exposed to the cross-modal stimulus, they did reveal their silent channel and did so in the same proportion as those exposed only to the cross-modal exposure series.
Single-neuron examples.
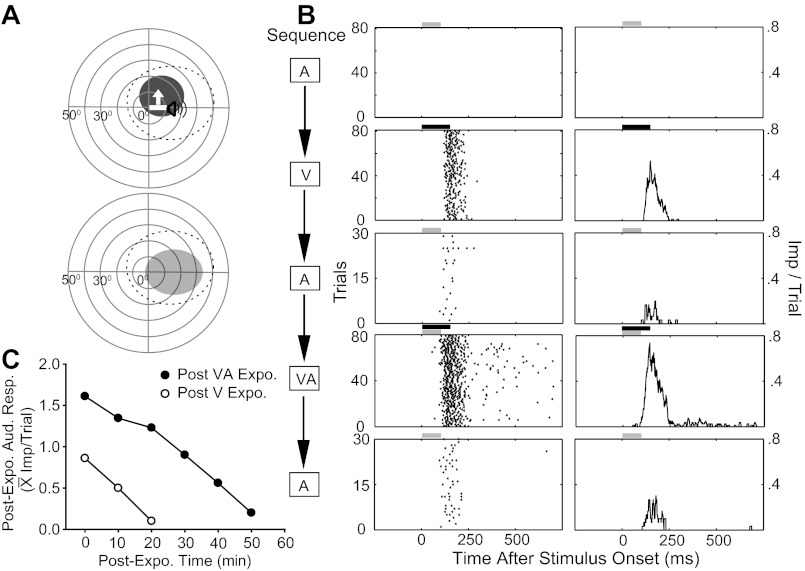
The data from two neurons sampled in preliminary experiments illustrate the inherent plasticity in the Covert neuronal population. The neuron shown in Fig. 4 (top, A–D) initially responded to auditory stimuli, but not to any of the moving or stationary flashed visual stimuli that were presented. These included visual stimuli with various sizes and shapes (8° × 3° – 15° × 3° bars and 3–6° diameter circles), orientations (0–360° tested in 15° increments), velocities (50–100°/s), intensities (4.8–36.5 cd/m2), and locations. Despite the absence of an observable response to a visual stimulus alone, the neuron's auditory response was modulated by a visual stimulus so that the cross-modal stimulus produced a discharge train of significantly more impulses. This particular neuron had a large auditory RF that encroached on the ipsilateral hemifield. An auditory RF of this size generally encompasses the neuron's entire visual RF (Jiang et al. 2006; Kadunce et al. 2001; Meredith and Stein 1996). So, although this neuron had no demonstrable visual responses, a potential visual RF was estimated based on the visual-auditory RF relationships typical of Overt multisensory neurons, the site at which a local visual background response was evoked, and the RFs of overlying visual neurons. All referents converged on a circumscribed region within the auditory RF delimited by a dotted circular line at the top of Fig. 4A. Figure 4B illustrates the sequence of tests and exposures that were conducted on this neuron.
Fig. 4.
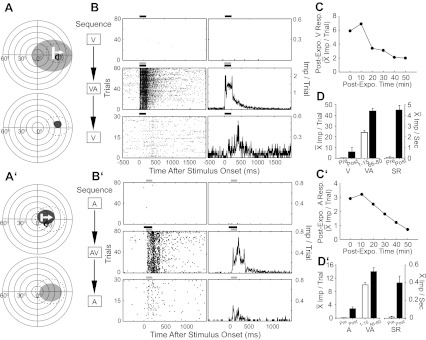
Cross-modal exposure activated the previously silent (V, A–D, top; A, A′–D′, bottom) channel in two Covert multisensory neurons. A (top): the A and predicted V (dotted) RFs are shown, and stimuli are illustrated with icons. A (bottom): the actual V RF (black circle) was mapped following cross-modal exposure, which activated the silent V channel. B: rasters (left) and peristimulus time histograms (right) show preexposure V, cross-modal exposure, and postexposure V responses. Bin width = 10 ms. C: the line graph plots the decline in the mean magnitude of the V response at 10-min intervals following cross-modal exposure (Post-Expo). D: the bar graph summarizes the exposure effects by comparing the mean V responses and spontaneous rates (SR) before (Pre, white bar) and immediately after (Post, black bar) exposure. It also reveals response changes taking place during exposure by illustrating the mean cross-modal responses on the first (1–15) and last (66–80) 15 trials. Conventions are the same as in previous figures.
Repetitions (n = 8) of each of a variety of visual search and test stimuli (i.e., flashing spots and moving light bars of different sizes, directions, and velocities, see above) within the predicted RF failed to evoke impulses, as did 80 repetitions of what is generally a highly effective visual stimulus for SC neurons (a moving bar of light 10° × 2°; luminance: 36.5 cd/m2; duration: 200 ms; speed: 100°/s). However, after an equal number (n = 80) of cross-modal exposure trials, the previously ineffective visual stimulus became reliably effective (Fig. 4C). Its visual RF was then mapped and found to fall within the predicted visual RF location. Responses to the visual stimulus alone were reexamined every 10 min following the cross-modal exposure period and exhibited a progressive decline. After 50 min, responsiveness to this stimulus was barely evident (Fig. 4C).
Among the various sensory inputs to the SC, visual inputs appear to have the highest incidence, broadest distribution, and are generally the most effective in activating multisensory neurons. However, despite this apparent bias, response plasticity in the silent channel was not limited to visual inputs. An example of a Covert multisensory neuron that initially responded to modality-specific visual stimuli, but not to any of the variety of auditory stimuli that were presented (i.e., clicks, hisses, noise bursts), is profiled in Fig. 4 (bottom, A′–D′). This visually responsive neuron showed the complementary cross-modal-induced changes described for the auditory-responsive neuron above.
Population: main effect of repeated cross-modal exposure on the silent sensory channel.
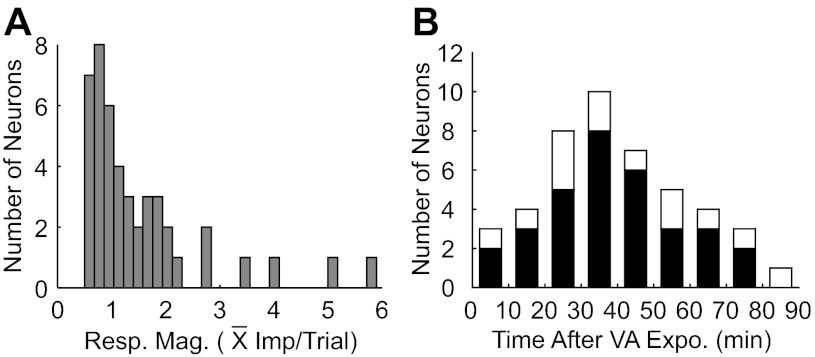
Overall, repeated cross-modal exposure revealed the silent channel in 31% (45/141) of Covert multisensory neurons, rendering them overtly responsive to both tested modalities. The responses to stimuli in the previously silent channel varied in magnitude and persistence, but were often weak and short-lived (Fig. 5), ranging from 0.5 to 5.9 impulses/trial (Fig. 5A), with a mean and standard deviation of 1.52 ± 1.16 impulses/trial. Although it was more common to record neurons with a silent auditory channel (n = 95) than a silent visual channel (n = 46), both showed similar exposure-induced changes (i.e., visual = 2.04 ± 1.41 impulses/trial, auditory = 1.27 ± 0.98 impulses/trial), and their proportions revealed by exposure were similar (33%, 31/95 vs. 30%, 14/46, respectively). Exposure-induced effects appeared to be temporary (Fig. 5B). Although more than one-half (66.7%, 30/45) remained active for more than 30 min, few (n = 8) remained so for more than 60 min.
Fig. 5.
The distributions of mean response magnitudes and of time courses for response decay in the previously silent channels following cross-modal exposure. A: the distribution of response magnitudes for the previously silent modality. B: the postexposure period during which unisensory responses could be elicited in the previously silent channel. Tests of responsiveness began immediately after exposure trials and continued for up to 80 min. In 32 cases, the entire time course of response decay (black bars) could be observed. Only a limited decay period was observed for the 13 other neurons, which are represented by the white bars. The persistence of their responsiveness likely exceeded the values that could be acquired during these periods. See text for details.
Population: effect of repeated modality-specific (active-modality) exposure on the silent sensory channel.
Repeated exposure to stimuli matched to the silent channel never revealed it, but within a subpopulation of 101 Covert neurons tested with repeated stimulation of the active channel, the silent channel was revealed in several cases (8%, 8/101). In each, however, cross-modal exposure was more effective, producing more robust response changes that were more resistant to decay: the mean population response in the channel revealed by active channel stimulation was 0.92 impulses/trial, and 2.15 impulses/trial following cross-modal stimulation. In most of these neurons (7/8), the revealed channel retained some level of overt effectiveness for <20 min (i.e., fewer than three sampling events) following active channel exposure, but did so for >20 min following cross-modal exposure. Figure 6 shows a representative example subjected to both exposure paradigms (Fig. 6, A and B). This neuron did not respond initially to an auditory stimulus, but, after repeated exposure to a visual stimulus, a weak auditory response (0.86 impulse/trial) was reliably elicited. Auditory responsiveness disappeared within 20 min (Fig. 6C, the line of unfilled circles). However, after cross-modal exposure, the neuron's auditory response was more robust (1.61 impulses/trial) and more resistant to decay (50 min to extinction) (Fig. 6C, the line of filled circles).
Fig. 6.
The cross-modal stimulus sensitized the silent sensory channel more than did a modality-specific stimulus. This Covert neuron was visually responsive. A (top): the V (bar) stimulus is shown within its RF, and the A (speaker) stimulus is within its predicted RF. A (bottom): the revealed A RF (gray area) following cross-modal exposure. B: rasters and histograms show responses to the test stimuli in the order in which they were recorded. C: A responses were recorded every 10 min following exposure to the V stimulus (white circles). The process was then repeated after exposure to the cross-modal stimulus (black circles). Responses in the former condition were weaker, and decayed more rapidly, than did those in the latter condition. Conventions are the same as in previous figures.
Population: changes in responsiveness.
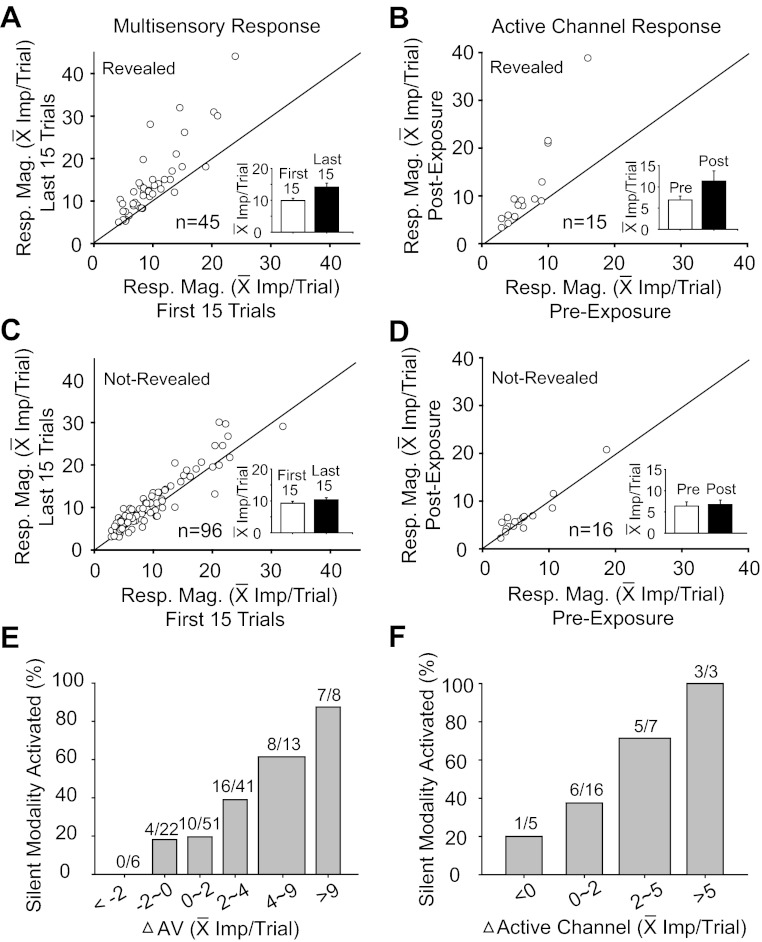
Rendering the silent sensory channel active was believed to reflect an overall increase in the neuron's responsiveness to external stimuli, a hypothesis based on the correlations of exposure-based response enhancement observed across multiple modalities in the Overt population. This possibility was evaluated by comparing responses in a subpopulation of 31 neurons during the first and last 15 cross-modal exposure trials and responses in the active channel before and after the exposure trials. These data were analyzed separately for neurons in which the silent channel was revealed and for neurons in which it was not, and the two groups showed marked differences.
In the former category (Fig. 7A), the average multisensory response was significantly (t-test; P < 0.05) increased in the majority (64.4%, 29/45) of cases, with an average increase of 43% (from 9.9 ± 4.2 to 14.2 ± 8.5 impulses/trial). In the latter category, however (Fig. 7C), the average multisensory response increased significantly (t-test; P < 0.05) in only 29.2% (28/96) of cases, and the average increase was only 12% (from 9.2 ± 5.7 impulses/trial to 10.3 ± 6.2 impulses/trial). Figure 7E summarizes the probability (as a percentage) that the silent channel would be revealed based on the change in the multisensory response between the first 15 and last 15 exposure trials. The silent channel was most likely to be revealed when this change was comparatively large (e.g., 4–9 impulses/trial or more).
Fig. 7.
Neurons whose silent sensory channel was revealed by cross-modal exposure had more robust response changes than did those whose silent channel was not revealed. A and C: comparisons of the mean multisensory response magnitudes in the first 15 (x-axis) and last 15 (y-axis) exposure trials were plotted for neurons in which the silent-channel was (A), or was not (C), revealed. The inserted bar graph in each panel shows the mean population response magnitude in the first (white bar) and last 15 exposure trials (black bar). B and D: response magnitudes in the channel that was active initially were compared before (x-axis) and after (y-axis) exposure for neurons in which the silent channel was (B), or was not (D), revealed. The corresponding inserted bar graphs summarize comparisons. Note that the greatest response enhancement in the active channel was in neurons whose silent input was revealed. E and F: bar graphs show that the probability of exposure-induced activation of the silent channel increased with increasing enhancement in the multisensory response (i.e., differences between mean responses on the first and last 15 exposure trials), and also with the magnitude of the response change in the initially active channel. Numbers above each bar denote the ratio: no. neurons whose silent modality was revealed/total no. neurons whose response change was within the bin (x-axis).
A similar pattern of changes was observed in active channel responses within a subpopulation studied (n = 31). Silent-channel-revealed neurons showed significant (t-test; P < 0.05) increases in their active channel responses in 60% (9/15) of these cases, with an average increase of 60% (from 6.91 ± 3.49 to 11.30 ± 9.30 impulses/trial) (Fig. 7B). In contrast, neurons in which the silent channel was not revealed showed significant (t-test; P < 0.05) increases of active channel responses in only 12.5% (2/16) of cases, and the average increase was only 6% (from 6.35 ± 4.05 to 6.72 ± 4.30 impulses/trial) (Fig. 7D). Figure 7F illustrates that, as in Fig. 7E, the silent modality was most likely to be revealed when the response was relatively large (e.g., 5 impulses/trial or more).
In sum, neurons in which the silent channel was revealed were also likely to evidence changes in their other (i.e., active channel and multisensory) responses, and when multisensory and active channel changes were largest, the probability of revealing the silent channel was highest. Yet, despite these observations, none of these measures (preexposure response levels, exposure-induced multisensory response changes, and active channel response changes) were invariant predictors that a neuron's silent channel would be revealed. There were instances in which comparatively low and comparatively high preexposure response levels were associated with revealing a silent channel, and in some cases, the silent channel was revealed in the absence of overt changes in other sensory responses.
Exposure-induced Changes in the Excitability of Overt and Covert Neurons as Evidenced by Spontaneous Rate
A related issue is whether exposure-induced changes in neuronal excitability, as evidenced by spontaneous activity, would predict changes in sensory responsiveness. This measure is difficult to apply with SC neurons that have low spontaneous rates and is particularly problematic in those with no observable baseline spontaneous activity. Nevertheless, increases in spontaneous activity generally correlated with the exposure-induced changes documented above. Across the Overt group (n = 53), spontaneous rates did significantly change. The mean spontaneous rate increase was 71.5% (from 0.7 ± 1.03 to 1.2 ± 1.71 impulses/s) (Fig. 8A), but this was influenced by a few samples showing dramatic increases. Of the 26.4% (14/53) of cases showing spontaneous rates increase, 86% (12/14) of them also showed a significant increase in at least one of their sensory responses. Put another way, 34% (12/35) of Overt neurons exhibiting response plasticity showed a significant increase in spontaneous rate. Only two neurons showed increases in spontaneous rate without changes in responsiveness.
Fig. 8.
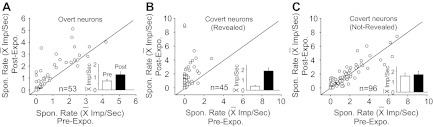
Cross-modal exposure enhanced SRs in Overt and Covert neurons. A–C: comparisons of the SRs before and after cross-modal exposure were plotted respectively for Overt integrating multisensory neurons (A), Covert neurons whose silent channel was revealed (B), and Covert neurons whose silent channel was not revealed (C).
Covert neurons showed a higher correlation between exposure-induced changes in spontaneous rates and responsiveness, correlations evident in the neurons in Fig. 4. In those neurons in which the silent modality was revealed, significant (Mann-Whitney U-test; P < 0.05) increases in spontaneous rate were evident in 66.7% (30/45) of the cases, with an average increase of 374% (from 0.40 ± 0.57 to 1.88 ± 2.00 impulses/s) (Fig. 8B). In the Covert population in which the silent modality was not revealed and which did not show changes in overall responsiveness, significant (Mann-Whitney U-test; P < 0.05) increases in spontaneous rate were evident in only 22% (21/96) of cases, and average increases of only 9.6% (from 1.57 ± 2.91 to 1.72 ± 3.21 impulses/s) (Fig. 8C). Despite these parallels, however, there were many examples in which the silent channel was revealed in the absence of a change in the spontaneous rate (14/45), and in three of these cases (e.g., see Fig. 6), no spontaneous activity was apparent under any circumstance.
DISCUSSION
During early postnatal life, multisensory neurons obtain the sensory experience necessary to contract and align their RFs into multisensory maps, create their response properties, and develop the ability to integrate their different sensory inputs (Stein et al. 1973a, 1973b; Wallace and Stein 1997, 2007). These maturational changes ensure that the SC is well-suited to detect and localize events in that environment (Stein and Stanford 2008).
Many stimulus features and cross-modal relationships remain relatively consistent in very different environmental circumstances, and it is not surprising that the functional characteristics of SC neurons appear relatively stable. However, within the broad categories used to describe these properties, there is the potential for substantial alterations to accommodate short-term changes in stimulus conditions (Leo et al. 2008; Perrault et al. 2011; Reches et al. 2010; Yu et al. 2009). For example, as shown here, the vigor of a multisensory neuron's responses to a particular cross-modal stimulus and its component features can be altered by repeatedly presenting that stimulus. These changes were not due to a systemwide reconfiguration, as they were not induced in neurons whose RFs did not encroach on the exposure site (and were thus not directly privy to the experience) and were not induced by exposure to stimuli to which a neuron was not responsive.
It is noteworthy that changes were induced when the animal was anesthetized and unable to respond to the stimulus or receive an associated reward. They are consistent with the principles of spike timing dependent plasticity and unsupervised Hebbian learning, which predict that the magnitude of potentiation of a connection depends on the reliability with which the presynaptic element's activity precedes postsynaptic activity (Cruikshank and Weinberger 1996; Dan and Poo 2004; Mu and Poo 2006; Schulz et al. 2010; Yu et al. 2009). However, no experimental efforts were made to test this directly.
Presumably, the observed response enhancements selectively heightened the ability of SC neurons to process the initiating event or other events at that location. The transient nature of the changes ensures that, sometime after the actual event, neurons revert back to their prior status. In this respect, the physiological response changes are not unlike those human perceptual changes associated with shifts in the “spotlight of attention” (Posner 1994) or during perceptual learning, when particular representations temporarily achieve prominence (Bolognini et al. 2005; Gilbert et al. 2001; Kim et al. 2008; Shams and Seitz 2008; Shibata et al. 2011). The observation that the spontaneous rate, as a measure of general excitability, sometimes changed along with responsiveness is consistent with this idea, as is the observation that unisensory and multisensory response changes were often correlated. However, the fact that no change or response feature was a definitive predictor of others suggests that the underlying mechanism may not be singular or simple. Furthermore, spontaneous rate is a rather poor metric for excitability in these SC neurons, as their low rates made its exact value difficult to determine given these sample sizes. Thus small changes were more likely to have failed to achieve statistical significance.
Multisensory SC neurons may receive afferents from a large number of cortical and noncortical sites (Edwards et al. 1979; Huerta and Harting 1984; Stein and Meredith 1993) and are embedded in complicated local circuits with multiple nodes (Fuentes-Santamaria et al. 2008). Relevant changes based on short-term experience could be induced at any of these sites to potentiate their responses and/or excitability (Cuppini et al. 2010). Such changes could affect the connection strength of both modalities, and even altering the strength of a single unisensory afferent channel would likely affect the neuron's response to a cross-modal stimulus. Stronger connections are also likely to enhance general excitability in tandem with overt responsiveness. Targeted changes in the kinetics of individual ion channels or other aspects of the postsynaptic membrane resulting from these recent experiences (Aizenman et al. 2003; Frick et al. 2004; Grubb and Burrone 2010; Mozzachiodi and Byrne 2010; Xu and Kang 2005) are other mechanisms that alter a neuron's responsiveness to subsequent inputs. Further research into these possible mechanisms is necessary to better understand both the constraints that govern this phenomenon and whether tailoring the exposure paradigm to each neuron would reveal the silent channel in a far higher percentage of Covert neurons than was possible here.
In the present circumstances, exposure-induced changes in responsiveness were restricted to neurons capable of multisensory integration, suggesting that this change might be mediated via the cortico-SC projections through which SC multisensory integration is expressed. This pathway in cats involves neurons in the anterior ectosylvian and rostral lateral suprasylvian sulci (Alvarado et al. 2007a, 2009; Jiang et al. 2001, 2002; Wallace and Stein 1994; Wilkinson et al. 1996). Presumably, depriving these cortico-SC neurons of the experience obtained during the exposure period (e.g., by reversible deactivation) would prevent the expression of response plasticity on both short and long time scales. Preliminary observations are consistent with this prediction, and ongoing research efforts seek to better characterize these relationships (Rowland et al. 2007b; Stein and Rowland 2007; Yu et al. 2011).
There is an intuitive benefit to these response changes in facilitating the hunting and avoidance strategies of predators and prey. In both cases, it is essential to appreciate all cues relevant to the movement and location of the event of interest (Angelaki et al. 2009; Colonius and Arndt 2001; Corneil and Munoz 1996; Frens et al. 1995), a fundamental role to which the SC and its homologue contribute in all species, including man (Bolognini et al. 2009; Calvert 2001; Leo et al. 2008). But the experience-based enhancement in sensitivity demonstrated here may also be useful in apprehending the specific features of that event. The extent to which event-specific features other than those involved in its movement and location are important to multisensory SC neurons and thus how closely subsequent stimuli must match those in the exposure configuration to take advantage of their changes in sensitivity are not yet known. Nor is it known whether the effect of enhancing neuronal responsiveness to stimuli at one location is accompanied by a corresponding decrease in stimulus responsiveness at other locations, and/or whether response changes could be rendered long-term by prolonged experience, as is presumed to occur in the neonate when constructing the overarching principles of multisensory integration. These issues require further inquiry.
It is interesting to note that the enhancement in responsiveness occurred in both categories of multisensory neurons studied: those that respond overtly to both sensory modalities, and those that respond overtly to only one and covertly to the other. Based on the apparent differences in these populations, it would not be unreasonable to assume that they are different components of ensembles that have different functional roles (Allman and Meredith 2007; Colonius and Diederich 2004). However, by revealing the silent channel in Covert neurons, the exposure paradigm rendered them indistinguishable from Overt neurons, thereby suggesting that categorical distinctions between these groups based on short-term observations may be unwarranted. At any given moment, a neuron's response profile may reflect its recent activation history. Although it has been known for some time that the repetition of modality-specific stimuli at high rates can habituate SC neurons, thereby degrading their responsiveness (Stein and Meredith 1993), the present data show that, under some conditions, the repetition of cross-modal stimuli at slower rates can produce the opposite effect. It should be noted that neurons were identified as “Covert” based on evaluations with a limited set of stimuli. There is no practical way to test the theoretically infinite set of stimuli belonging to the subthreshold modality, and thus no way to obviate the possibility that the neuron would overtly respond to at least one of them initially. However, this possibility does not impact the present findings that cross-modal exposure adapts neuronal sensitivity to the stimuli in the exposure set.
Although the focus in the present study was on exposure-induced changes in the responsiveness of SC neurons, such changes are unlikely to be unique to this population. Covert multisensory neurons have been noted in association areas of cortex (Allman and Meredith 2007; Allman et al. 2009; Bizley and King 2009; Dehner et al. 2004; Mao et al. 2011; Wallace et al. 2004), and they are likely to be subject to these same experience-based changes. Furthermore, similar effects may also occur on multisensory neurons in traditional unisensory areas of cortex. Single neuron studies have found the incidence of such neurons to be low (Brett-Green et al. 2003; Wallace et al. 2004), but far higher incidences are often inferred from event-related potential and functional imaging studies in which high numbers of stimulus repetitions are used (Ghazanfar and Schroeder 2006). If cortical neurons are indeed subject to the same exposure-induced changes, it would underscore the far-reaching effects of stimulus repetition on sensory processing across brain regions that play very differing roles in multisensory information processing (Bolognini et al. 2007; Diaconescu et al. 2011) and that differentially contribute to the behavioral and perceptual functions that are initiated by these stimuli.
GRANTS
This research was supported by National Institutes of Health Grants EY016716 and NS036916.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.Y., B.A.R., and B.E.S. conception and design of research; L.Y. and J.X. performed experiments; L.Y., B.A.R., J.X., and B.E.S. analyzed data; L.Y., B.A.R., J.X., and B.E.S. interpreted results of experiments; L.Y., B.A.R., J.X., and B.E.S. prepared figures; L.Y., B.A.R., and B.E.S. drafted manuscript; L.Y., B.A.R., J.X., and B.E.S. edited and revised manuscript; L.Y., B.A.R., J.X., and B.E.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Nancy London for technical assistance.
REFERENCES
- Aizenman CD, Akerman CJ, Jensen KR, Cline HT. Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron 39: 831–842, 2003 [DOI] [PubMed] [Google Scholar]
- Allman BL, Meredith MA. Multisensory processing in “unimodal” neurons: cross-modal subthreshold auditory effects in cat extrastriate visual cortex. J Neurophysiol 98: 545–549, 2007 [DOI] [PubMed] [Google Scholar]
- Allman BL, Keniston LP, Meredith MA. Not just for bimodal neurons anymore: the contribution of unimodal neurons to cortical multisensory processing. Brain Topogr 21: 157–167, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado JC, Stanford TR, Vaughan JW, Stein BE. Cortex mediates multisensory but not unisensory integration in superior colliculus. J Neurosci 27: 12775–12786, 2007a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado JC, Vaughan JW, Stanford TR, Stein BE. Multisensory versus unisensory integration: contrasting modes in the superior colliculus. J Neurophysiol 97: 3193–3205, 2007b [DOI] [PubMed] [Google Scholar]
- Alvarado JC, Stanford TR, Rowland BA, Vaughan JW, Stein BE. Multisensory integration in the superior colliculus requires synergy among corticocollicular inputs. J Neurosci 29: 6580–6592, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelaki DE, Gu Y, DeAngelis GC. Multisensory integration: psychophysics, neurophysiology, computation. Curr Opin Neurobiol 19: 452–458, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizley JK, King AJ. Visual influences on ferret auditory cortex. Hear Res 258: 55–63, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini N, Rasi F, Coccia M, Ladavas E. Visual search improvement in hemianopic patients after audio-visual stimulation. Brain 128: 2830–2842, 2005 [DOI] [PubMed] [Google Scholar]
- Bolognini N, Leo F, Passamonti C, Stein BE, Ladavas E. Multisensory-mediated auditory localization. Perception 36: 1477–1485, 2007 [DOI] [PubMed] [Google Scholar]
- Bolognini N, Miniussi C, Savazzi S, Bricolo E, Maravita A. TMS modulation of visual and auditory processing in the posterior parietal cortex. Exp Brain Res 195: 509–517, 2009 [DOI] [PubMed] [Google Scholar]
- Brett-Green B, Fifkova E, Larue DT, Winer JA, Barth DS. A multisensory zone in rat parietotemporal cortex: intra- and extracellular physiology and thalamocortical connections. J Comp Neurol 460: 223–237, 2003 [DOI] [PubMed] [Google Scholar]
- Burnett LR, Stein BE, Chaponis D, Wallace MT. Superior colliculus lesions preferentially disrupt multisensory orientation. Neuroscience 124: 535–547, 2004 [DOI] [PubMed] [Google Scholar]
- Burnett LR, Stein BE, Perrault TJ, Jr, Wallace MT. Excitotoxic lesions of the superior colliculus preferentially impact multisensory neurons and multisensory integration. Exp Brain Res 179: 325–338, 2007 [DOI] [PubMed] [Google Scholar]
- Calvert GA. Crossmodal processing in the human brain: insights from functional neuroimaging studies. Cereb Cortex 11: 1110–1123, 2001 [DOI] [PubMed] [Google Scholar]
- Colonius H, Arndt P. A two-stage model for visual-auditory interaction in saccadic latencies. Percept Psychophys 63: 126–147, 2001 [DOI] [PubMed] [Google Scholar]
- Colonius H, Diederich A. Multisensory interaction in saccadic reaction time: a time-window-of-integration model. J Cogn Neurosci 16: 1000–1009, 2004 [DOI] [PubMed] [Google Scholar]
- Corneil BD, Munoz DP. The influence of auditory and visual distractors on human orienting gaze shifts. J Neurosci 16: 8193–8207, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Weinberger NM. Receptive-field plasticity in the adult auditory cortex induced by Hebbian covariance. J Neurosci 16: 861–875, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuppini C, Ursino M, Magosso E, Rowland BA, Stein BE. An emergent model of multisensory integration in superior colliculus neurons. Front Integr Neurosci 4: 6, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuppini C, Stein BE, Rowland BA, Magosso E, Ursino M. A computational study of multisensory maturation in the superior colliculus (SC). Exp Brain Res 213: 341–349, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron 44: 23–30, 2004 [DOI] [PubMed] [Google Scholar]
- Dehner LR, Keniston LP, Clemo HR, Meredith MA. Cross-modal circuitry between auditory and somatosensory areas of the cat anterior ectosylvian sulcal cortex: a ‘new’ inhibitory form of multisensory convergence. Cereb Cortex 14: 387–403, 2004 [DOI] [PubMed] [Google Scholar]
- Diaconescu AO, Alain C, McIntosh AR. The co-occurrence of multisensory facilitation and crossmodal conflict in the human brain. J Neurophysiol 106: 2896–2909, 2011 [DOI] [PubMed] [Google Scholar]
- Edwards SB, Ginsburgh CL, Henkel CK, Stein BE. Sources of subcortical projections to the superior colliculus in the cat. J Comp Neurol 184: 309–329, 1979 [DOI] [PubMed] [Google Scholar]
- Ernst MO, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature 415: 429–433, 2002 [DOI] [PubMed] [Google Scholar]
- Frens MA, Van Opstal AJ, Van der Willigen RF. Spatial and temporal factors determine auditory-visual interactions in human saccadic eye movements. Percept Psychophys 57: 802–816, 1995 [DOI] [PubMed] [Google Scholar]
- Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat Neurosci 7: 126–135, 2004 [DOI] [PubMed] [Google Scholar]
- Fuentes-Santamaria V, Alvarado JC, Stein BE, McHaffie JG. Cortex contacts both output neurons and nitrergic interneurons in the superior colliculus: direct and indirect routes for multisensory integration. Cereb Cortex 18: 1640–1652, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazanfar AA, Schroeder CE. Is neocortex essentially multisensory? Trends Cogn Sci 10: 278–285, 2006 [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M, Crist RE. The neural basis of perceptual learning. Neuron 31: 681–697, 2001 [DOI] [PubMed] [Google Scholar]
- Gingras G, Rowland BA, Stein BE. The differing impact of multisensory and unisensory integration on behavior. J Neurosci 29: 4897–4902, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MS, Burrone J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature 465: 1070–1074, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta MF, Harting JK. The mammalian superior colliculus: studies of its morphology and connections. In: Comparative neurology of the optic tectum, edited by Vanegas H. New York: Plenum, 1984, p. 687–773 [Google Scholar]
- Jiang W, Jiang H, Stein BE. Two corticotectal areas facilitate multisensory orientation behavior. J Cogn Neurosci 14: 1240–1255, 2002 [DOI] [PubMed] [Google Scholar]
- Jiang W, Jiang H, Stein BE. Neonatal cortical ablation disrupts multisensory development in superior colliculus. J Neurophysiol 95: 1380–1396, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Wallace MT, Jiang H, Vaughan JW, Stein BE. Two cortical areas mediate multisensory integration in superior colliculus neurons. J Neurophysiol 85: 506–522, 2001 [DOI] [PubMed] [Google Scholar]
- Kadunce DC, Vaughan JW, Wallace MT, Stein BE. The influence of visual and auditory receptive field organization on multisensory integration in the superior colliculus. Exp Brain Res 139: 303–310, 2001 [DOI] [PubMed] [Google Scholar]
- Kadunce DC, Vaughan JW, Wallace MT, Benedek G, Stein BE. Mechanisms of within- and cross-modality suppression in the superior colliculus. J Neurophysiol 78: 2834–2847, 1997 [DOI] [PubMed] [Google Scholar]
- Kim RS, Seitz AR, Shams L. Benefits of stimulus congruency for multisensory facilitation of visual learning. PLos One 3: e1532, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Palmer AR. Integration of visual and auditory information in bimodal neurones in the guinea-pig superior colliculus. Exp Brain Res 60: 492–500, 1985 [DOI] [PubMed] [Google Scholar]
- Leo F, Bertini C, di Pellegrino G, Ladavas E. Multisensory integration for orienting responses in humans requires the activation of the superior colliculus. Exp Brain Res 186: 67–77, 2008 [DOI] [PubMed] [Google Scholar]
- Mao YT, Hua TM, Pallas SL. Competition and convergence between auditory and cross-modal visual inputs to primary auditory cortical areas. J Neurophysiol 105: 1558–1573, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHaffie JG, Stein BE. A chronic headholder minimizing facial obstructions. Brain Res Bull 10: 859–860, 1983 [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Interactions among converging sensory inputs in the superior colliculus. Science 221: 389–391, 1983 [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. J Neurophysiol 56: 640–662, 1986 [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Spatial determinants of multisensory integration in cat superior colliculus neurons. J Neurophysiol 75: 1843–1857, 1996 [DOI] [PubMed] [Google Scholar]
- Mozzachiodi R, Byrne JH. More than synaptic plasticity: role of nonsynaptic plasticity in learning and memory. Trends Neurosci 33: 17–26, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Poo MM. Spike timing-dependent LTP/LTD mediates visual experience-dependent plasticity in a developing retinotectal system. Neuron 50: 115–125, 2006 [DOI] [PubMed] [Google Scholar]
- Oyster CW, Takahashi ES. Responses of rabbit superior colliculus neurons to repeated visual stimuli. J Neurophysiol 38: 301–312, 1975 [DOI] [PubMed] [Google Scholar]
- Perrault TJ, Jr, Stein BE, Rowland BA. Non-stationarity in multisensory neurons in the superior colliculus. Front Psychol 2: 144, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. Attention: the mechanisms of consciousness. Proc Natl Acad Sci USA 91: 7398–7403, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reches A, Netser S, Gutfreund Y. Interactions between stimulus-specific adaptation and visual auditory integration in the forebrain of the barn owl. J Neurosci 30: 6991–6998, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland BA, Stanford TR, Stein BE. A model of the neural mechanisms underlying multisensory integration in the superior colliculus. Perception 36: 1431–1443, 2007a [DOI] [PubMed] [Google Scholar]
- Rowland BA, Stanford TR, Stein BE. Superior colliculus neurons may use multiple strategies in integrating cross-modal cues. In: 2007 Abstract Viewer/Itinerary Planner, Program No. 614.1. San Diego, CA: Society for Neuroscience, 2007b [Google Scholar]
- Rowland BA, Quessy S, Stanford TR, Stein BE. Multisensory integration shortens physiological response latencies. J Neurosci 27: 5879–5884, 2007c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz JM, Redgrave P, Reynolds JN. Cortico-striatal spike-timing dependent plasticity after activation of subcortical pathways. Front Synaptic Neurosci 2: 23, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams L, Seitz AR. Benefits of multisensory learning. Trends Cogn Sci 12: 411–417, 2008 [DOI] [PubMed] [Google Scholar]
- Shibata K, Watanabe T, Sasaki Y, Kawato M. Perceptual learning incepted by decoded fMRI neurofeedback without stimulus presentation. Science 334: 1413–1415, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford TR, Quessy S, Stein BE. Evaluating the operations underlying multisensory integration in the cat superior colliculus. J Neurosci 25: 6499–6508, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. The Merging of the Senses. Cambridge, MA: MIT, 1993 [Google Scholar]
- Stein BE, Rowland BA. The critical role of cortico-collicular interactions in the development of multisensory integration. In: 2007 Abstract Viewer/Itinerary Planner, Program No. 614.7. San Diego, CA: Society for Neuroscience, 2007 [Google Scholar]
- Stein BE, Stanford TR. Multisensory integration: current issues from the perspective of the single neuron. Nat Rev Neurosci 9: 255–266, 2008 [DOI] [PubMed] [Google Scholar]
- Stein BE, Labos E, Kruger L. Letter: Long-lasting discharge properties of neurons in the kitten midbrain. Vision Res 13: 2615–2619, 1973a [DOI] [PubMed] [Google Scholar]
- Stein BE, Labos E, Kruger L. Sequence of changes in properties of neurons of superior colliculus of the kitten during maturation. J Neurophysiol 36: 667–679, 1973b [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA, Huneycutt WS, McDade L. Behavioral indices of multisensory integration: Orientation to visual cues is affected by auditory stimuli. J Cogn Neurosci 1: 12–24, 1989 [DOI] [PubMed] [Google Scholar]
- Stein BE, Stanford TR, Ramachandran R, Perrault TJ, Jr, Rowland BA. Challenges in quantifying multisensory integration: alternative criteria, models, and inverse effectiveness. Exp Brain Res 198: 113–126, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Cross-modal synthesis in the midbrain depends on input from cortex. J Neurophysiol 71: 429–432, 1994 [DOI] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Development of multisensory neurons and multisensory integration in cat superior colliculus. J Neurosci 17: 2429–2444, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Early experience determines how the senses will interact. J Neurophysiol 97: 921–926, 2007 [DOI] [PubMed] [Google Scholar]
- Wallace MT, Ramachandran R, Stein BE. A revised view of sensory cortical parcellation. Proc Natl Acad Sci USA 101: 2167–2172, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson LK, Meredith MA, Stein BE. The role of anterior ectosylvian cortex in cross-modality orientation and approach behavior. Exp Brain Res 112: 1–10, 1996 [DOI] [PubMed] [Google Scholar]
- Xu J, Kang J. The mechanisms and functions of activity-dependent long-term potentiation of intrinsic excitability. Rev Neurosci 16: 311–323, 2005 [DOI] [PubMed] [Google Scholar]
- Xu J, Yu L, Rowland BA, Stanford TR, Stein BE. Incorporating cross-modal statistics in the development and maintenance of multisensory integration. J Neurosci 32: 2287–2298, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Stein BE, Rowland BA. Adult plasticity in multisensory neurons: short-term experience-dependent changes in the superior colliculus. J Neurosci 29: 15910–15922, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Rowland BA, Stein BE. Initiating the development of multisensory integration by manipulating sensory experience. J Neurosci 30: 4904–4913, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Rowland BA, Stein BE. An active cortex is necessary for the experience-induced acquisition of multisensory integration in the adult superior colliculus. In: 2011 Abstract Viewer/Itinerary Planner, Program No. 481.06. Washington, DC: Society for Neuroscience, 2011 [Google Scholar]
- Zahar Y, Reches A, Gutfreund Y. Multisensory enhancement in the optic tectum of the barn owl: spike count and spike timing. J Neurophysiol 101: 2380–2394, 2009 [DOI] [PubMed] [Google Scholar]