Abstract
Cranial primary afferents from the viscera enter the brain at the solitary tract nucleus (NTS), where their information is integrated for homeostatic reflexes. The organization of sensory inputs is poorly understood, despite its critical impact on overall reflex performance characteristics. Single afferents from the solitary tract (ST) branch within NTS and make multiple contacts onto individual neurons. Many neurons receive more than one ST input. To assess the potential interaction between converging afferents and proximal branching near to second-order neurons, we probed near the recorded soma in horizontal slices from rats with focal electrodes and minimal shocks. Remote ST shocks evoked monosynaptic excitatory postsynaptic currents (EPSCs), and nearby focal shocks also activated monosynaptic EPSCs. We tested the timing and order of stimulation to determine whether focal shocks influenced ST responses and vice versa in single neurons. Focal-evoked EPSC response profiles closely resembled ST-EPSC characteristics. Mean synaptic jitters, failure rates, depression, and phenotypic segregation by capsaicin responsiveness were indistinguishable between focal and ST-evoked EPSCs. ST-EPSCs failed to affect focal-EPSCs within neurons, indicating that release sites and synaptic terminals were functionally independent and isolated from cross talk or neurotransmitter overflow. In only one instance, focal shocks intercepted and depleted the ST axon generating evoked EPSCs. Despite large numbers of functional contacts, multiple afferents do not appear to interact, and ST axon branches may be limited to close to the soma. Thus single or multiple primary afferents and their presynaptic active release sites act independently when they contact single second-order NTS neurons.
Keywords: A-fiber, C-fiber, spillover, microstimulation, vagus
optimized homeostatic life support relies on the constant central processing of information arising from multiple organs and autonomic regulation. One important station for integrating this information resides at the nucleus of the solitary tract (NTS) at the earliest stage of these reflex pathways (Andresen and Paton 2011; Loewy 1990). Visceral information constantly arises from pools of organ-specific afferents and is sent directly to the brain stem, where the nerves branch to form terminal clusters within caudal NTS. Single pulmonary afferent axons, for example, branch to make varicosities, which are thought to be ∼40 synapses on single NTS neurons (Anders et al. 1993; Kalia and Richter 1985a, 1988; Kubin et al. 2006). Functional studies estimate that this afferent synaptic structure gives rise to powerful excitatory postsynaptic currents (EPSCs) composed of an average of 20 active release sites per solitary tract (ST) axon (Andresen and Peters 2008; Bailey et al. 2006b; Peters et al. 2008). Dye tracer studies suggest that afferent terminals commonly localize on the proximal somatodendritic tree, and ST stimulation generates shock synchronized monosynaptic EPSCs (Andresen and Peters 2008; Doyle and Andresen 2001; Kline et al. 2002; Sekizawa et al. 2003). Remarkably, peripheral cranial visceral afferent nerve trunks are organized into discrete subbundles containing one or two myelinated afferent axons, together with five or more unmyelinated, C-type axons (Doan et al. 2004; Krauhs 1979, 1984), although it is not known whether this intimate coassociation of myelinated and unmyelinated afferents persists centrally. Most medial second-order neurons in caudal NTS receive input from one primary afferent axon, but just over one-third receive multiple primary afferent inputs, i.e., convergent sensory inputs (McDougall et al. 2009). In addition, primary afferents are segregated into transient receptor potential vanilloid subtype 1 (TRPV1+) and TRPV1−, regardless of the number of afferents converging at individual NTS neurons (Peters et al. 2011). The presence of multiple afferent sources, afferent branching, and multiple contacts that are concentrated near the cell soma suggested a structure with great potential for afferent interaction that, importantly, may impact information processing at these NTS neurons and their reflex paths beyond.
ST primary afferents are well known to generate EPSCs through a distinctly reliable and surprisingly homogeneous glutamate release mechanism (Doyle and Andresen 2001; Liu et al. 2000). Heterosynaptic interaction suggested that visceral ST afferents interacted with GABA release so that, even during block of ionotropic glutamate transmission, ST activation released glutamate that altered GABA transmission, an interaction mediated by activation of metabotropic glutamate receptors (mGluRs) (Jin et al. 2004). This raised the question of whether this interacting glutamate was delivered by direct presynaptic contact on GABA terminals or indirectly via spillover. In a case supporting spillover, the anatomical proximity of high probability of release terminals in the lateral geniculate nucleus at retinal ganglion cell terminals fosters intersynaptic spillover and homosynaptic interactions (Budisantoso et al. 2012). The dense and compact structure of cranial visceral afferents raises the possibility that afferent presynaptic interaction might be prominent within NTS.
To test for interactions, we cut horizontal brain stem slices that contained medial NTS neurons and used an experimental approach to independently activate two cranial visceral afferent axons to single second-order neurons. This was accomplished by locating neurons in which distal shocks delivered through a remote electrode on the ST activated EPSCs and then searched for locations close to the neurons at which an independent focal electrode activated EPSCs to the same neuron. This strategy allowed us to activate two separate inputs to the same NTS neuron and manipulate the timing of these events. The focal electrode intercepted convergent axon branches in the immediate vicinity of the soma. Our findings indicate that focally evoked EPSCs in all respects closely resembled ST-EPSCs. ST and focal-EPSCs were either TRPV1+ or TRPV1−, but never a mixture of the two on the same neuron. Changes in activation timing indicated that ST and focal events were fully independent, since activating focal-EPSCs failed to affect the amplitude or timing of ST-EPSCs and vice versa. In one case (1 of 19 focal-ST paired events), the focal electrode activated the same axon as the ST shocks. Thus convergent primary afferent axons were completely segregated phenotypically, and two converging inputs acted as fully independent excitatory sources without interaction. So despite the close anatomical proximity of large numbers of sensory glutamate terminals, we found no evidence for functional axo-axonic or synaptic coupling by glutamate spillover in NTS.
METHODS AND MATERIALS
All animal procedures were performed with the approval and supervision of the Institutional Animal Care and Use Committee at Oregon Health and Science University and conform to the guidelines of the National Institutes of Health publication “Guide for the Care and Use of Laboratory Animals.”
Horizontal brain stem slices.
Brain stem slices were prepared from adult (>180 g, average weight 320 ± 11 g, n = 45) Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), as described previously (Doyle and Andresen 2001). Briefly, rats were deeply anesthetized with isoflurane, and the medulla was removed, rapidly cooled, and trimmed to yield a brain stem block centered on obex. A rostral-caudal wedge of ventral brain stem was removed to orient the remaining tissue to yield a single, 250-μm-thick, horizontal slice, which contained the greatest length of ST axons, together with the medial NTS. Slices were cut with a sapphire knife (Delaware Diamond Knives, Wilmington, DE) mounted in a vibrating microtome (VT1000S; Leica Microsystems, Bannockburn, IL). The external solution was an artificial cerebrospinal fluid containing the following (mM): 125 NaCl, 3 KCl, 1.2 KH2PO4, 1.2 MgSO4, 25 NaHCO3, 10 dextrose, and 2 CaCl2. Slices were secured with a nylon mesh in a tissue chamber and perfused with artificial cerebrospinal fluid at 34°C, 300 mosM, bubbled with 95% O2-5% CO2.
Whole cell recordings.
Recording pipettes (2.5–3.5 MΩ) were guided to neurons in the medial subnucleus within the intermediate region of the caudal NTS. Neurons were visualized for patching (Doyle et al. 2004) using infrared illumination with differential interference contrast optics (×40 water immersion lens) on an Axioskop-2 FS plus fixed-stage microscope (Zeiss, Thornwood, NJ) with digital camera (Hamamatsu Photonic Systems, Bridgewater, NJ). Pipettes were filled with an intracellular solution containing the following (mM): 10 NaCl, 40 KCl, 90 K-gluconate, 11 EGTA, 1 CaCl2, 1 MgCl2, 10 HEPES, 2 Na2ATP, and 0.2 Na2GTP (pH 7.3 and 296 mosM). In these solutions, EPSCs and inhibitory postsynaptic currents (IPSCs) (chloride equilibrium potential = −25 mV) were inward at holding potential = −60 mV, but IPSCs were readily discerned by their slow kinetics and selective blockers (Jin et al. 2004). All recordings were made in open, whole cell patch configuration under voltage clamp using a Multiclamp 700B (Molecular Devices, Sunnyvale, CA). Signals were sampled at 30 kHz and filtered at 10 kHz using p-Clamp software (version 9.2, Molecular Devices, Sunnyvale, CA). All figure traces were filtered with a low-pass Bessel (8-pole) to 3 kHz for display purposes, except for Fig. 8A. Liquid junction potentials were not corrected (estimated as 9.5 mV at 34°C).
Fig. 8.

In one case, activation of ST substantially depressed the response to focal activation and vice versa. A: in this neuron, leading bursts of ST activation evoked typical frequency depressing EPSCs, but focal shocks immediately following these responses resulted in greatly depressed or absent EPSCs (failures: for example, single red trace), and a similar pattern was found when the order was reversed (5 traces overlaid, shock artifacts blanked). The focal electrode was ∼65 μm from the cell soma in this instance. B: ST (black) and focally (gray) evoked EPSC1 events were indistinguishable (average trace of 10 trials, aligned onsets). C: averaging across 30 trials of responses in this single neuron, frequency-dependent depression was induced in the leading EPSCs, and amplitudes remained depressed in the following train, regardless of ST or focal order. *P < 0.05 vs. corresponding following EPSC, two-way ANOVA. D: the results suggest that the focal electrode intercepted the same ST axon to the recorded neuron as the axon activated by the remote electrode placed on the ST.
Evoked synaptic currents: ST and focal afferent activation.
In principle, low-intensity shocks likely activate axons nearest to the stimulating electrode and will be all or none if a single axon is excited (Andresen and Yang 1995; Bailey et al. 2008; Doyle and Andresen 2001). To activate cranial visceral afferent axons, a concentric bipolar stimulating electrode (CB-BRF50; Frederick Haer, Bowdoinham, ME) was placed in a fixed position on the visible ST ∼1–3 mm rostral of recorded neuron cell bodies. The remote placement of the electrode minimized the likelihood that electrical shocks would activate non-ST axons or local neurons directly (Bailey et al. 2008; Doyle et al. 2004). For the focal activation of axons, a fine glass pipette (1.0–1.5 MΩ) was filled with bath solution and placed at various positions 50–400 μm from the recorded neuron cell body. Stimulus shocks (ST and focal) were 0.1 ms in duration. The focal pipette was moved, and, at each position, 10 trials were tested as part of an initial characterization of focal evoked events. To provide for more detailed analysis, increased numbers of shocks, different shock intensities, and different patterns were tested (see below).
Shock intensity-recruitment profiles.
To isolate the activation of individual primary afferents, we closely monitored evoked synaptic responses in relation to graded shock intensity. Once synaptic responses were detected, a series of finely graded intensities defined the minimum shock intensity (recruitment threshold) for each event. Events were discriminated by arrival time and waveform. To create recruitment profiles, event amplitudes or integrated currents were plotted against shock intensity and compared across events. The sharp threshold and lack of amplitude change with increased intensity of all stimulus recruitment profiles suggest that each synaptic event reflects the all-or-none activation of a single initiating axon. Shock parameters and timing were controlled with a Master-8 isolated programmable stimulator (A.M.P.I., Jerusalem, Israel) (McDougall et al. 2009). The remote current return path for the focal pipette produced highly localized stimuli. At each intensity, single to five shock bursts (50 Hz) were repeated every 6 s for a minimum of 10 consecutive trials for both the ST and focal electrodes. Neurons were tested with a range of stimulus shock intensities (10–800 μA, ST; 10–200 μA, focal). At some sites, focal shocks directly activated the recorded neuron with a near-zero latency, large, inward action current, which prevented further study at greater intensities at those sites.
Discriminating synaptic interactions between ST and focal evoked events.
Analysis of latency was based on responses to the first shock delivered [postsynaptic current 1 (PSC1)] from either the ST or focal electrode. Synaptic jitter for PSC1 was calculated over 40 trials as the standard deviation (SD) of latency and served as the critical index of synaptic order. Synaptic jitter of <200 μs was considered monosynaptic, and jitters >200 μs indicated a polysynaptic connection from the ST or focal shock site (Bailey et al. 2006a; Doyle and Andresen 2001). Polysynaptic pathways activated by shocks sometimes failed at PSC1, even with infrequent stimuli (Andresen and Peters 2008; Bailey et al. 2006a). When these suprathreshold shocks failed to evoke the characteristic PSC at otherwise successful sites, these trials were counted as synaptic failures. To be quantitatively rigorous, failure rates were calculated over 40 trials and expressed as a percentage of total shocks delivered for a constant, suprathreshold intensity. Only second-order NTS neurons were included in our studies, so that recordings from NTS neurons that exhibited only polysynaptic or no EPSCs in response to ST shocks were discarded (McDougall et al. 2009). Likewise, if focal shocks evoked no response, the focal electrode was moved to a different location. NBQX, SR-95531, and capsaicin (CAP) were purchased from Tocris (Ellisville, MO).
Data analysis and statistics.
Analytic variables for afferent-evoked PSC included latency, jitter, amplitude, and failure rates for PSC1, and these were not normally distributed so that comparisons between groups used rank values and a Kruskal-Wallis one-way ANOVA on ranks with a Student-Newman-Keuls post hoc test (SigmaStat, San Jose, CA). Paired pulse ratio was based on single shocks from the ST and focal electrodes and associated decay time constants obtained using Minianalysis (Synaptosoft) based on a single 10–90% exponential fit per event. All data represented as means ± SE were considered statistically significant if P < 0.05.
RESULTS
Minimal focal shocks recruit single excitatory inputs.
Despite phenotypic heterogeneity and the presence of neurons not coupled to the ST (Bailey et al. 2006a), medial NTS neurons are overwhelmingly second order, defined by direct ST visceral primary afferent contacts (McDougall et al. 2009). In the present studies, ST shocks activated low-jitter EPSCs, which indicated functioning, monosynaptic ST sensory contacts at each second-order neuron studied (Fig. 1, A and B). In each case of a second-order neuron, a second stimulating electrode was placed at different sites close to these neurons (focal, 50–400 μm), and focal shocks most often activated low-jitter EPSCs. IPSCs with characteristically slow relaxation kinetics (Jin et al. 2004) were activated by focal shocks near NTS neurons (Fig. 2A), estimated as 9% of focal sites (McDougall and Andresen 2012), but were not studied further. The focal pipette was generally placed rostral and/or medial to the neuron cell body in an attempt to intersect axons arriving from the ST (Fig. 1A). The intensity-response profiles for both ST and focal shocks were consistent with activation of single axons. Thus these EPSC responses to graded shock intensities had sharp minimum thresholds with distinct and consistent amplitudes that did not increase over an interval of intensities above threshold (Fig. 1B, insets). In addition, neither ST nor focal synaptic events failed with repeated shocks. Application of the non-N-methyl-d-aspartate (non-NMDA) blocker NBQX rapidly and completely blocked ST and focal synaptic events, confirming that they were glutamate mediated (Fig. 1C). These characteristics suggest that focal shocks near minimal intensity are highly localized and selectively activated single-axon responses. Thus ST and focal shocks activated axons eliciting similar non-NMDA EPSCs (Andresen and Yang 1990) that converged at these single NTS neurons (Fig. 1D).
Fig. 1.
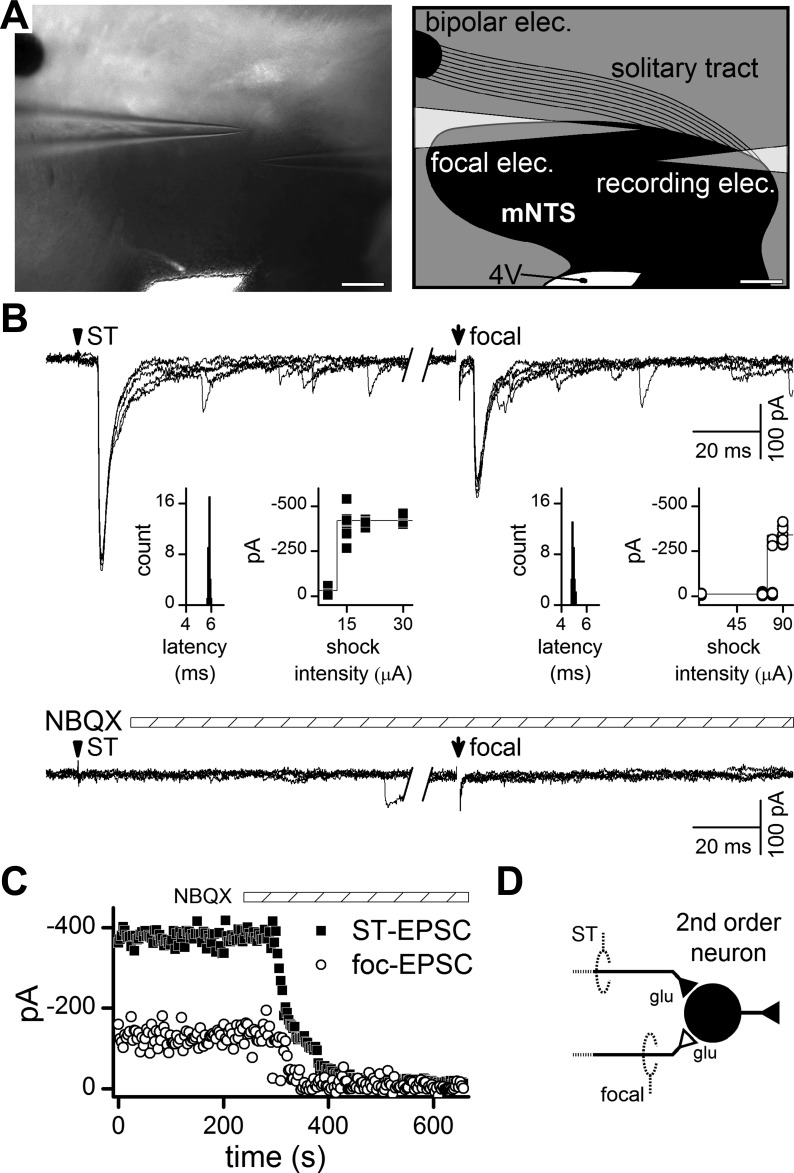
Focal (foc) and solitary tract (ST) shocks activate similar unitary excitatory postsynaptic currents (EPSCs) at second-order neurons within the medial subnucleus of caudal ST nucleus (mNTS). A: photomicrograph of the horizontal slice, and a schematic representation of ST and mNTS region with placement of distal (bipolar, ST) and focal electrodes for stimulation. The recording electrode exits the setup to the right. Scale bar = 200 μm. 4V, fourth ventricle. B: in a representative neuron, remote shocks to ST activated time-invariant EPSCs (left, 5 traces overlaid with shock artifacts blanked, arrowhead). The invariant latency (inset) showed a narrow distribution with no failures. The ST stimulus-recruitment profile (inset, left) indicates an all-or-none threshold, consistent with activation of a single axon. In the same neuron, minimal focal shocks (right, arrow) delivered 200 μm from the cell body recruited an EPSC resembling the ST-EPSC (left). Note 800-ms delay from ST shock to focal shock (break between traces). Calculated jitters were 51 and 78 μs for ST- and focal-EPSCs, respectively, and met the monosynaptic criterion. Both responses were blocked by the non-N-methyl-d-aspartate (non-NMDA) receptor antagonist NBQX (20 μM). C: NBQX acted rapidly and nearly simultaneously to completely block both ST- and focal-EPSCs. D: combined, these results suggest that ST and focal stimulating electrodes activated different afferent axons which converged onto this single second-order neuron. glu, Glutamate.
Fig. 2.
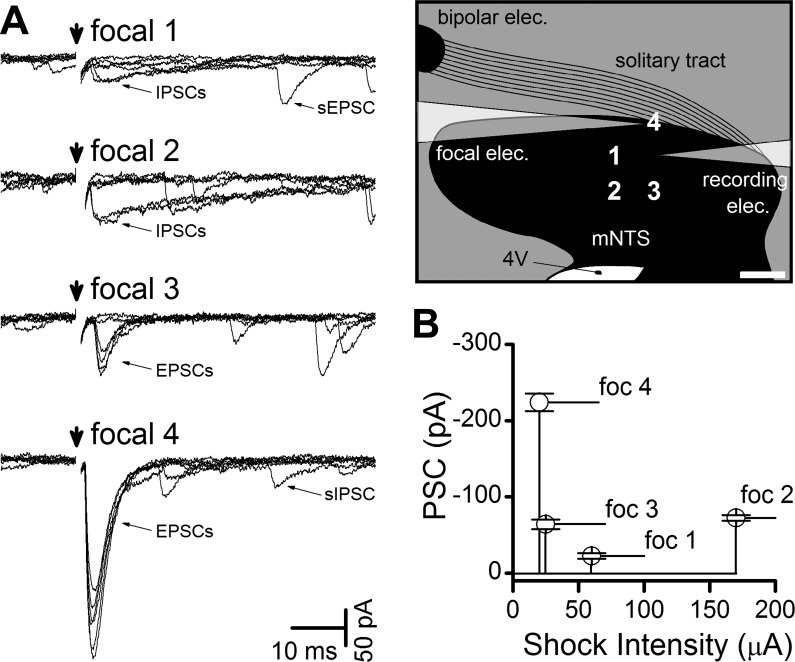
Minimal focal shocks evoked different postsynaptic currents (PSCs) from each shock site around the same neuron. A: focal sites around this neuron were tested (schematic, scale bar = 200 μm) with focal shocks (arrow). Threshold intensity focal shocks evoked consistent PSCs on repeated trials (6 traces overlaid with shock artifacts blanked) from each site with different latencies, synaptic jitters, failure rates, and decay dynamics, and on this basis classed as inhibitory or excitatory. In focal position 1, focal shocks activated slow relaxing events at fixed latency classed as inhibitory PSCs (IPSCs), but note the failures of some shock trials and the spontaneous EPSC (sEPSC) trailing in one sweep. The stimulation site of focal 2 excited only IPSCs with failures and some spontaneous IPSCs (sIPSC) trailed. In focal 3, small EPSCs were triggered, and much larger EPSCs were triggered at site focal 4, followed by a sIPSC in one sweep. Scale bar in sketch equals 200 μm. B: the summary of trials with minimal shock intensity at each site also varied and indicated each site had a unique activation threshold intensity. Combined, these data indicate focal sites activated unique afferent inputs to the same neuron. Focal EPSCs (e.g., site 4) represented the most common response observed across neurons. Not shown, but relatively common, were sites at which no PSC was evoked, despite high-intensity (200 μA) focal shocks. Note that the high-intensity shocks at focal 3 did not trigger the nearby focal IPSC, indicating spatial isolation between sites.
To test for different input axons converging onto a single neuron, the focal pipette was maneuvered to deliver shocks to test several points around the recorded cell body (Fig. 2). At each site, unique threshold shock intensities were determined. At most neurons, shocks in the immediate vicinity of the cells triggered low-jitter EPSCs or IPSCs, but some locations triggered higher jitter synaptic responses. The present study focused on monosynaptic EPSCs that also received a focally activated monosynaptic EPSC. The first focal sites tested were 400 μm from the soma and usually resulted in no responses (94% of neurons). Following this, 50-μm sites were tested if no response could be evoked further away (those shown in the grid, Fig. 2A). Overall, the most common (71%) position for successfully evoking monosynaptic EPSCs was with the focal electrode placed 200–235 μm from the recorded neuron (e.g., positions 1 and 2 in Fig. 2A). In rare cases, closer sites yielded focal-EPSCs with 13% at 95 μm (positions 3 or 4 on Fig. 2A) or closer at 50 μm (10%). In general, therefore, greater distances from the cell body decreased the likelihood of activating a convergent axon, despite using much larger focal shock intensities. Focally activated synaptic events generally had quite short latencies compared with ST-EPSCs, as would be expected from the relative difference in distances from stimulation site to soma (Fig. 3). Detailed mapping tests were not performed in every neuron. Across the group (n = 48 neurons), monosynaptic ST-evoked EPSCs arrived significantly later than focally activated monosynaptic EPSCs (Fig. 3). In all other respects beyond latency, monosynaptic (i.e., low jitter) EPSCs did not differ between ST and focal activation in their synaptic jitter, amplitude variability, or their near-zero failure rates (Fig. 3). Across all pairs of synaptic inputs to single neurons (n = 48), there was no systematic difference between EPSCs activated with the remote ST electrode from those activated focally, including their synaptic jitters [P = 0.37, one-way repeated-measures (RM) ANOVA], their amplitudes (P = 1.0, one-way RM ANOVA), or their amplitude variations (P = 0.49, one-way RM ANOVA), results consistent with all of these EPSCs arising from peripheral primary afferent neurons. Such analysis alone does not rule out the possibility that focal shocks intercepted central glutamatergic axons. To test this, additional lines of evidence were sought to better characterize focal axon properties.
Fig. 3.
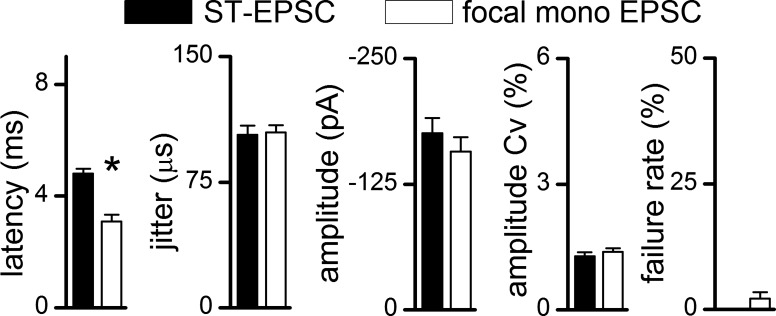
Focally activated monosynaptic EPSCs had characteristics which closely resembled ST-EPSCs. On average across neurons (n = 48), focal EPSCs were classified as either mono- or polysynaptic using a synaptic jitter cutoff of <200 μs. Monosynaptic focal EPSCs (open bars) had shorter mean latencies than ST-EPSCs (filled bars, *P < 0.05, one-way ANOVA). Synaptic jitter, amplitude coefficients of amplitude variation (Cv), and failure rates were similar for focal monosynaptic and ST-EPSCs. Inhibitory PSCs and polysynaptic EPSCs with higher jitter were encountered but not presented here.
Focal shocks recruit primary afferents: TRPV1 evidence.
Electrical shocks to axons within NTS poorly differentiate across axons, e.g., those arising from myelinated or unmyelinated or peripheral sensory vs. central sources (Bailey et al. 2002). However, TRPV1 receptors serve well as a key molecular discriminator of ST afferent axons and the sensitivity of these terminals to the vanilloid agonist, CAP (TRPV1+ neurons) (Doyle et al. 2002). Since focally activated, low-jitter EPSCs closely resembled ST-EPSCs, we tested their sensitivity to CAP in a subset of neurons (n = 8). In most of these neurons (n = 6), 100 nM CAP blocked their ST-evoked EPSCs, and, in every one of these cases, CAP also eliminated the focal-EPSCs, indicating that both of these glutamate inputs were TRPV1+ (Fig. 4, A and B). Note that, in the presence of CAP, the rate of spontaneous synaptic events in these TRPV1+ neurons increased greatly. In contrast, neurons in which the ST-EPSC was unaffected by CAP also had focal responses that were CAP resistant (n = 2, TRPV1−, Fig. 4B). In the case of TRPV1+ neurons, transmission from the focal and ST shocks most often failed at different durations of CAP exposure, suggesting that they were independent inputs on which CAP acted uniquely. Overall, no neurons in which CAP only partially reduced the EPSC amplitude were identified for the ST and focally evoked monosynaptic EPSCs (Fig. 4C). Thus responses to CAP were all or none for CAP-sensitive (TRPV1+, n = 6) or CAP-insensitive neurons (TRPV1−, n = 2, Fig. 4C). As expected, TRPV1+ terminals were more prevalent, but ST- and focal-EPSCs were generally similar in amplitude (ST-EPSC vs. focal-EPSC; P = 0.064, one-way ANOVA). Nonetheless, the similar TRPV1+ profiles suggested that focal monosynaptic EPSCs likely arise from peripheral primary afferent neurons (Fig. 4D).
Fig. 4.
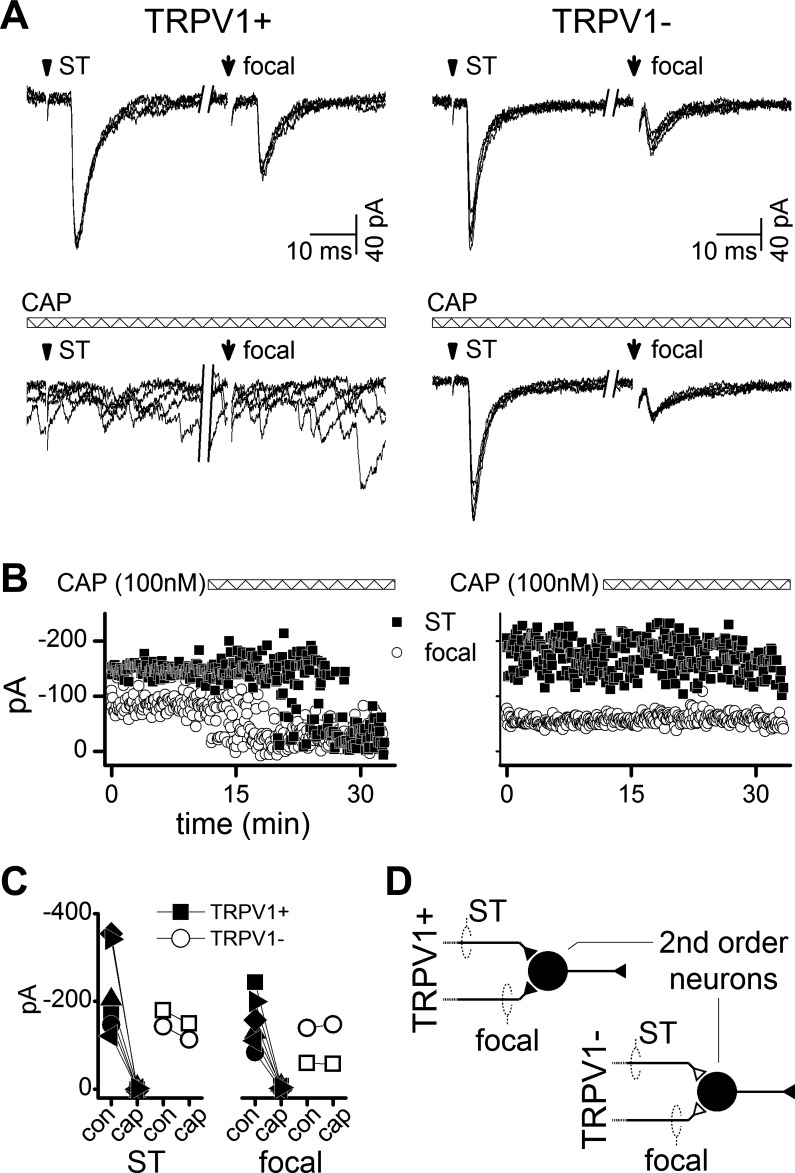
EPSCs from ST and focal activation were either capsaicin (CAP) sensitive [transient receptor potential vanilloid subtype 1 (TRPV1+)] or not (TRPV1−) and never mixed. A: minimal shocks recruited unitary ST and focal EPSCs with low jitter as in Fig. 1. Addition of 100 nM CAP blocked EPSCs synchronized to ST and focal shocks in TRPV1+ neurons (left panels), but increased the rate of sEPSCs. In TRPV1− neurons, EPSCs were resistant and unaltered by CAP (right panels). Traces are from two representative neurons (5 overlaid traces each, 800-ms break between ST and focal shocks, shock artifacts blanked). B: the time course of CAP action was not always identical for ST and focal-evoked EPSC amplitudes (same neurons as in A). Note that, in this case, CAP blocked the focal EPSC several minutes before the ST-EPSC (TRPV1+), which is consistent with two independent TRPV1+ inputs. Neither input to the TRPV1− neuron (right) was altered by CAP. C: across neurons (n = 8), ST and focal evoked EPSCs were either both blocked by CAP or unaffected by CAP. TRPV1+ inputs were never mixed with TRPV1− inputs and indicated cranial afferent axon segregation. con, Control. D: thus focal shocks intercepted the final segment of a TRPV1+ sensory afferent as it approached the recorded NTS neuron. The diagram illustrates that primary afferent axons are segregated based on TRPV1 phenotype at second-order sensory neurons.
Sensory afferents terminating at common neurons do not interact.
Testing focal shocks delivered close to the soma of the recorded neuron should increase the likelihood of encountering a proximal portion or perhaps even a partial branch of the same visceral primary afferent axon that was activated by the remote ST electrode. Second, if the focally recruited axon is different from the ST axon, then properly timed release of glutamate from one axon might affect the response of the other axon. To test these two possibilities, we paired activations using both electrodes and timed evoked events to intersect in arrival times. In the case of a single axon, activation with one stimulus might occlude or collide with the activation from the other electrode. Delivering focal shocks at ever shorter intervals following the ST shocks failed to alter the success or the amplitude of evoked focal EPSCs (Fig. 5A). Note that reversing the order so that focal shocks preceded the ST shocks (a −5-ms “delay”) failed to alter or interact with ST-EPSCs (Fig. 5A). The separation times of these shock pairings were incremented by 1 ms to span a range from 5 ms preceding to 5 ms following the anticipated event arrival. When the time interval was equal to the difference in ST and focal EPSC latency (1 ms in Fig. 5A), then the events arrived effectively simultaneously and resulted in a fused EPSC that was equal to the sum of the temporally isolated events (Fig. 5B). With decreasing interval periods between ST and focal shocks, we recorded little change in ST and focal-evoked EPSC amplitude, indicating no interaction between the terminal sources (Fig. 5B). On average (n = 10, Fig. 5C), the ratios of amplitudes of focal- and ST-derived low-jitter EPSCs showed no interaction (depression or facilitation) across all stimulus separation intervals tested. With a shock interval of 800 ms set as control, the paired pulse ratio of ST to focal ESPC amplitudes with shorter shock intervals remained constant near 1.0 (Fig. 5C, left graph), indicating neither a positive nor a negative effect of ST upon focal-evoked glutamate release. Likewise, the waveform of the focal-EPSCs as measured in the decay time constant was unaffected by varying intervals after ST-EPSCs (Fig. 5C, right graph). Thus focal- and ST-EPSC responses were consistently independent of each other. To better illustrate this lack of interaction, please view the Supplemental Movie Clip to see an example of variations in timing between focal- and ST-evoked EPSCs. (The online version of this article contains supplemental data.).
Fig. 5.

Whether remotely or focally activated, primary afferent axons were independent and added strictly linearly. Given that many medial NTS neurons receive a single ST afferent, we tested whether the focal and ST electrodes activated the same or different axons. A: progressively shorter intervals between ST and focal shocks resulted in a summation of EPSCs (5 traces overlaid, shock artifacts blanked). Neither ST nor focal-evoked EPSCs was affected by the preceding event at any of the intervals tested. During a narrow time window of coincidence, activation of both ST and focal afferents evoked precisely summed EPSCs (i.e., ST pre-con. 1 ms). See detailed animation in Supplemental Fig. S1. B: analysis of responses for neuron in A show that both focal (open circles) EPSC amplitudes and ST amplitudes (filled squares) are constant over a broad range of preconditioning intervals, except at 1 ms when amplitudes simply summed. C: across neurons (n = 10), the amplitude ratio was calculated as a paired pulse ratio (PP) of focal to ST-EPSC amplitude. PP ratios were not different from 1.0 (at 800-ms interval), and there was no correlation to shock interval. Similarly, the decay time constant of focal EPSCs following ST-EPSCs did not change with shock interval, suggesting no interaction. Note that shock intervals were performed at 200, 400, and 600 ms (not shown for clarity), but included for statistical analysis. Thus the activity of one input had no effect on responses to the other.
Another signature of the glutamate release process is frequency-dependent depression (FDD). FDD differs somewhat across individual neurons and has been suggested to represent depletion of neurotransmitter (Andresen and Yang 1995; Peters et al. 2010; Schild et al. 1995). Thus, if focal and ST shocks activated different locations along the same axon, shocks delivered first might attenuate (depress) subsequent evoked release at common terminals. In a separate group of neurons (n = 8), we tested this issue with a second approach (Fig. 6). Bursts of five ST shocks (50 Hz) produced strongly depressing trains of EPSCs, and, in this test, focal shocks commenced immediately (Fig. 6A). Responses were similar whether ST shocks or focal shocks were presented first. Together, irrespective of the order of presentation (ST first or focal first), the FDD was unchanged, and the first EPSC was always the highest in amplitude. The shock order (focal or ST leading) had no effect on EPSC amplitudes. Note that, when the ST response was reduced to 10–20% of EPSC1, i.e., substantial FDD, then immediate following focal shocks evoked an initial focal-EPSC that was full size (Fig. 6A). Thus, across neurons, FDD averaged 70–90% depression (Fig. 6B), but the amplitudes of following response were not different whether this followed or preceded preconditioning. ST amplitudes were unchanged when preceded by the focally activated axon or vice versa (ST leads vs. ST follows, P = 0.639; focal leads vs. focal follows, P = 0.204, two-way ANOVA, n = 8, Fig. 6B). These two interaction protocols (Figs. 5 and 6) provide evidence of completely independent events responsible for focal- and ST-evoked EPSCs within single neurons (Fig. 6C). The results suggest that, despite releasing glutamate onto the same neurons, release from one set of terminals did not modulate the other.
Fig. 6.
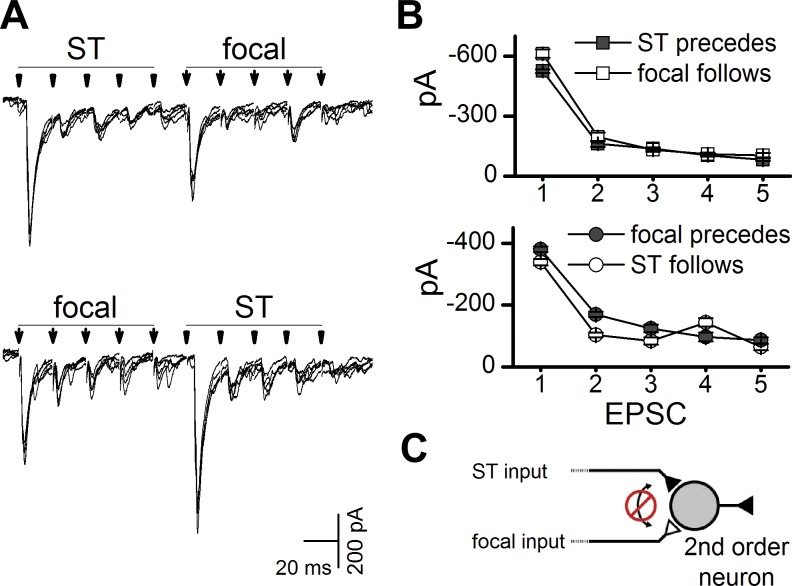
Despite frequency-dependent depression, burst activation of the remote ST-EPSCs did not alter the amplitude of the focal EPSCs. A: trains of five shocks (50 Hz) depressed EPSC amplitudes similarly for both ST and focal EPSCs. Focal shocks timed to evoke EPSCs during ST depression were similar, irrespective of focal/ST order of presentation. In each case, 5 traces were overlaid (shock artifacts blanked). B: across neurons (n = 8), frequency-dependent depression was unaltered by the order of shock presentation (ST lead vs. follow, P = 0.639; focal lead vs. follow, P = 0.204; two-way ANOVA). C: schematic of results depicts independent glutamatergic inputs. The lack of occlusive interaction in these same neurons suggests that focal and ST shocks activated two separate axons, and that the terminal fields were effectively independent. In one case, the neuron showed substantial interaction (see Fig. 8).
Focal activation triggers asynchronous release.
The FDD characteristics of focal-evoked EPSCs and matched CAP sensitivity suggest that monosynaptic focal-EPSCs arise from peripheral primary afferent axons. In TRPV1+ neurons, our previous work (Peters et al. 2010) suggests that EPSCs are generated from two pools of glutamate vesicles. A burst of shocks to depolarize primary afferent terminals triggers a synchronous release of glutamate underlying the low-jitter EPSCs, but this is followed by a period of asynchronous release evident in elevated frequency of stochastic EPSCs for several seconds. We found that, in TRPV1+ neurons, both ST and focal shock trains evoked asynchronous release for up to 4 s (Fig. 7, A and B). Thus combined activation of ST and focal shock trains triggered a combined increase in asynchronous release that was significantly greater than ST or focal alone (Fig. 7C). This increase in asynchronous release (15 ± 4 Hz) was proportional to the sum of ST and focal induced asynchronous releases alone (14 ± 4 Hz). Thus focally activated EPSCs generated asynchronous release activity that closely resembled ST responses in TRPV1+ neurons.
Fig. 7.

In TRPV1+ neurons, ST and focal shocks evoked both synchronous EPSCs, as well as asynchronous EPSCs in proportion to afferent activation. A: the basal level of sEPSCs was defined as the 1-s period before stimulation. ST and focal shocks (50 Hz, arrowheads) evoked large synchronous EPSCs. Gabazine (3 μM) was present throughout. The rate of asynchronous EPSCs was calculated as the EPSC frequency greater than basal following shocks. Asynchronous release increased after activation of both ST and focal shocks (6 traces overlaid, shock artifacts blanked). B: analysis of A showed that asynchronous EPSC frequency following shock bursts (arrowheads) and combined shocks increased release rates over ST and or focal shocks alone (100-ms bins). C: across cells (n = 4), basal EPSC frequency remained constant between tests (hashed bars). Asynchronous release (net EPSC frequency greater than basal for 1 s following shocks) with ST and focal shock bursts was greater than ST or focal shocks alone. *P < 0.05, one-way ANOVA with repeated measures.
ST and focal shocks rarely trigger a common afferent axon.
To detect interaction or a common axon of origin, our experimental strategy independently activated two inputs to the same neurons using focal and ST stimulation. In one neuron (1 of 19 neurons fully tested), we found evidence consistent with activation of a common axon (Fig. 8). First, the amplitudes and kinetic waveforms of focally evoked EPSCs were equal to the amplitudes of the ST events in this neuron (Fig. 8, A and B). Second, activation of focal-EPSCs in an order following the five-shock ST trains produced responses which simply continued the prototypical FDD pattern. If the order was reversed and the focal train preceded the ST testing, then ST-EPSCs began with a depressed amplitude, which continued the pattern of a prototypical FDD established by the focal shock train (Fig. 8C). Thus, unlike all of the other recordings, focal shocks never summed to activate a high-amplitude event during coactivation with ST. Third, the presence of an ST train of shocks induced synaptic failures in responses to the first focal shock in the following train (Fig. 8A, red). Normally, the failure rates after first shocks were <1% whether ST or focal (see Figs. 1 and 6), something that rarely occurred with EPSCs activated by focal shocks alone. Last, similar response patterns were found, regardless of whether ST or focal shocks were delivered first. These ST-focal interactions were consistent with coactivation of a single-afferent axon (Fig. 8D). Such findings suggest that leading shocks effectively depressed subsequent glutamate release to following shocks. This suggests that ST- or focal-EPSCs arrived at the same cohort of depressed terminals and effectively extended the ongoing FDD. In this single neuron, the ST- and focal-EPSCs exhibited indistinguishable jitters (96 and 107 μs), amplitudes (72 and 75 pA, Fig. 8B), and amplitude variation (1.2 and 1.3%), as determined over 40 trials (ST vs. focal amplitude, P = 0.28, t-test). Without leading shocks, the waveforms of the ST-EPSC and focal-EPSCs were identical and followed a strict, order-dependent FDD (EPSC1–5, Fig. 8C). Combined, the evidence indicates that the focal electrode intercepted the same primary afferent axon that was activated by the ST electrode located >2 mm from the focal electrode site (Fig. 8D).
DISCUSSION
Visceral afferent axons are densely bundled together as they enter the brain stem as the visible ST. However, very little is known about the relationship between synaptic contacts arising from ST primary afferent axons and their organization at second-order neurons in the caudal NTS. Clear evidence identifies a another class of different glutamatergic EPSCs: synapses that are activated indirectly by cranial afferent stimulation, either ST or peripheral nerve trunks, but which must arise from local (i.e., central) glutamatergic neurons (Bailey et al. 2006a, 2008; Doyle and Andresen 2001; McDougall et al. 2009; Scheuer et al. 1996; Zhang and Mifflin 1997). Little direct information is available about these non-ST axons within the visceral afferent portions of caudal NTS. To more directly address these issues, we activated axons very close to second-order NTS neurons in horizontal slices by using weak, graded focal shocks and compared these focal-EPSCs with EPSCs activated by remote ST shocks. Our focus was on the functional characteristics of these EPSCs and their interaction when coactivating these glutamatergic inputs. Our main findings include the following. 1) Focally activated excitatory fibers overwhelmingly resembled ST-EPSCs with indistinguishable synaptic jitter, low failure rates, substantial depression, and phenotypic cosegregation by TRPV1 expression. 2) Minimal focal shocks failed to activate EPSCs with characteristics different than ST afferents, which would be expected of non-ST sourced, central excitatory inputs. 3) Monosynaptic, focal-EPSCs failed to interact with ST-EPSCs converging on the same neurons. Combined, these findings suggest a synaptic organization for NTS second-order neurons in which powerful ST excitatory fibers act independently.
Branching of ST afferent axons within NTS.
Neuroanatomical evidence indicates substantial branching of afferent axons as they approach presumed second-order NTS neurons (Kubin et al. 2006). Intracellular fills of single ST axons of pulmonary afferents show branched arbors that stretch rostro-caudally over 1.7–2.1 mm with hundreds of varicosities thought to correspond to terminal boutons en passant (Anders et al. 1993; Kalia and Richter 1985a). Electrophysiological maps of retrograde activation patterns sketched largely similar branched arbors within NTS from single nodose neurons of pulmonary receptors, as well as carotid chemoreceptors and baroreceptor afferents (Donoghue et al. 1982, 1984). Thus a broadly consistent structure that appears to hold for diverse ST afferents is one in which the single-afferent axon divides over a wide region and raises the potential for wide broadcast of sensory afferent influence. Despite the ultrastructural evidence of pre- and postsynaptic specializations consistent with synaptic transmission (Anders et al. 1993; Kalia and Richter 1985b), the functional characteristics of ST afferents are incompletely understood. Perhaps surprisingly in the face of this potentially rich innervation structure, in vivo recordings suggest that in most cases (85% of neurons) NTS neurons are contacted by a single primary afferent axon, and this finding includes tests with maximal shocks across multiple peripheral nerve trunks (Bonham and Hasser 1993; Donoghue et al. 1985; Mifflin 1996; Ootani et al. 1995). Similarly cardiac mechanoreceptors rarely (<13%) converge to excite the same NTS neurons as arterial baroreceptors using pharmacological or physiological activation (Paton 1998; Seagard et al. 1999). The experimental focus in our studies was restricted to activation of single axons with the goal of detecting interaction, a much finer resolution than is possible in the in vivo experimental context. Despite these intrinsic differences in approach, the general conclusion from our synaptic studies indicates full independence of action between synapses formed from separate afferent axons. Likewise in our studies, focally activated EPSCs were indistinguishable from distally evoked ST-EPSCs, and this suggests that our focal electrodes activated similar cohorts of primary afferent-derived terminals. We found no evidence to support activation of subarbors of ST afferents. Thus weak focal shocks yielded EPSC responses with all-or-none amplitudes. Instances in which higher intensity (supra threshold) shocks modified the EPSC dynamics resulted from separately recruited convergent inputs that were themselves all or none with unique latency characteristics.
Minimal focal shock activation and synaptic discrimination.
Focal shocks proved to be highly localized, since moving the focal pipette even 25 μm resulted in loss of evoked responses or recruitment of new events with different threshold and waveform characteristics. This suggests that the focal pipette tip needed to be quite close to the stimulated axon at minimal intensities. However, even at >10 times higher intensities, many focal sites were unresponsive, and yet slight relocation of the focal pipette successfully recruited synaptic inputs. Together, our findings suggest that the unipolar stimulus pipette approach afforded the advantages of both sufficient current densities to activate axons and yet a relatively limited spatial reach to recruit additional axons. Brief electrical shocks hold additional analytic advantages of crisp latencies (μs jitters) and high resolution of graded intensity-response profiling used to discriminate unitary from fused compound event complexes. Alternative approaches to detecting converging axons often lack this precise temporal information, especially the sharply defined, iterative activations that provide quantitative synaptic performance assessment. This strategy of focal activation effectively detected and isolated unitary GABAergic IPSCs that contacted second-order NTS neurons and provided evidence of a low density of fibers near these neurons and a very low probability of GABA release (McDougall and Andresen 2012). In the case of GABA axon activation in NTS, effective minimal shocks were highly localized, and this approach was essential to detection of unitary, rather than fused, compound events (McDougall and Andresen 2012). Caged glutamate release or optogenetic challenges have intrinsically more variable onsets and more diffuse activation zones (Brill and Huguenard 2009; Katz and Dalva 1994). Disadvantages of our focal approach include the nonspecificity of electrical shocks for axons only, the necessity to physically move the focal electrode to new locations, poor access to fibers obstructed by the recording pipette, and the small fraction of the total number of axons that can be detected and characterized in a limited experimental period.
Focal activation of ST afferents at second-order neurons.
Second-order neurons likely account for 75% of medial NTS neurons (McDougall et al. 2009), but multiple primary afferent convergence is limited (Bailey et al. 2006a; Donoghue et al. 1981, 1985; McDougall et al. 2009). We anticipated that focal shocks close to the cell body of these neurons would encounter the same afferent axon activated by ST shocks, but this happened only once in our studies. All focal-EPSCs, however, closely resembled ST-EPSCs in every respect, including the predominance of TRPV1+ axons (Andresen and Peters 2008; Histed et al. 2009). Furthermore, our focal data reinforce the concept of an obligate segregation of TRPV1+ inputs, in this case both ST and focally evoked. This offers additional direct evidence that afferent axons are segregated fully to individual second-order neurons in medial NTS. These findings further indicate that reflex pathways begin with afferent subclass dedicated to second-order neurons, although a small proportion of cells receive more than two ST inputs (Peters et al. 2011). Likewise, this CAP sensitivity confirmed that most focal-EPSCs originate from cranial visceral afferents, since TRPV1 is rarely present in adult central neurons, including NTS (Cavanaugh et al. 2011). TRPV1 receptors within NTS disappear following removal of nodose ganglion neurons and therefore are dependent on the presence of ST sensory afferent axons (Patterson et al. 2003). Remarkably, however, focal shocks failed to detect EPSCs with distinctly non-ST characteristics. Likewise, blockade of TRPV1+ EPSCs with CAP failed to reveal any remaining (i.e., not blocked) non-TRPV1 EPSCs to the same neurons that might represent central glutamatergic inputs. We did not detect focally evoked EPSCs with characteristics more typical of other central neurons, including low quantal content, high-amplitude variability, high failure rates, and frequency-dependent facilitation (Allen and Stevens 1994; Christie and Jahr 2006). Clearly, intermediary non-ST glutamatergic neurons must be present in NTS to mediate the observed focally evoked polysynaptic EPSCs and polysynaptic NBQX-sensitive IPSCs (Bailey et al. 2008; Fernandes et al. 2011; McDougall et al. 2009). However, more direct evidence remains to be found, such as the glutamate release characteristics from such central afferent axons, whether interneuron or descending in origin. It is possible that central excitatory afferents might be included in our focal-EPSC data. The focal-EPSCs observed in the present study share the extraordinarily high release probability of cranial visceral afferents and substantial paired pulse depression. These characteristics would be highly unusual for central neuron glutamate release. More commonly, lower release probability and substantial paired pulse facilitation are observed in the hypothalamus (Stern et al. 2000), the cerebellum (Bender et al. 2009), or the hippocampus (Christie and Jahr 2006). ST-evoked polysynaptic EPSCs are generally characterized by high synaptic jitter, high failure rates, no FDD, and high-amplitude variability (McDougall et al. 2009). If NTS excitatory interneurons had a high probability mechanism of glutamate release that closely resembled primary afferents, then one might expect more reliable polysynaptic transmission. It is possible that we may have focally activated central excitatory afferent axons and dismissed these responses as polysynaptic, if their jitter exceeded 200 μs. This seems unlikely since even low-release monosynaptic GABA transmission exhibits synaptic jitters below 200 μs (McDougall and Andresen 2012). Focal evoked IPSCs were blocked by glutamatergic antagonists and thus were polysynaptic with synaptic jitters over 200 μs (McDougall and Andresen 2012). Note that synaptic jitter is strongly temperature dependent, and a different criterion would apply at lower recording temperatures (Peters et al. 2010). Thus, based on negative results, we cannot conclude with absolute certainty that CAP-resistant EPSCs were not of central origin, but the weight of the data suggests that they most likely arose from ST primary afferent axons.
Functionally independent afferent synaptic transmission.
Generally, ST and focal-EPSCs arose from separate primary afferent axons to the same neuron. Since each ST axon makes multiple contacts, the overlap of convergent synaptic contacts might favor interterminal interactions. Thus our finding that their coactivation failed to reveal any influence of one primary afferent input upon the other EPSC seems quite remarkable. TRPV1-mediated asynchronous release due to both ST and focal bursts were found to equal the sum of ST and focal shocks alone within the same neurons. This proportional increase in release of glutamate in the seconds after evoked release also failed to interact at neighboring primary afferent terminals. This finding conforms to the strictly arithmetic summation of asynchronous release rate increases with coactivation of multiple, converging primary afferents (Peters et al. 2011). Given that each ST afferent makes an estimated ∼20 active synaptic contacts per ST axon (Andresen and Peters 2008; Bailey et al. 2006b; Peters et al. 2008), the absence of synaptic cross talk indicates important constraints on glutamate diffusion and signaling at these neurons and contrasts to highly dense and interconnected networks found elsewhere in the central nervous system, such as in the neocortex (Fino and Yuste 2011). Likewise, we failed to detect any evoked activation of prominent mGluR mechanisms extant on ST terminals (Chen et al. 2002; Fernandes et al. 2011). Our results indicate that at primary afferent terminals there is no functionally important glutamate overflow. Structural studies describe isolated synaptic contacts at NTS neurons and high astrocyte coverage (Chounlamountry and Kessler 2011; Lachamp et al. 2002), structures that might limit glutamate diffusion and synaptic interactions. Previously, we reported mGluR modulation of GABA release by ST shocks (Fernandes et al. 2011; Jin et al. 2004). Thus the overall results may be more consistent with specific axo-axonic primary terminals as responsible for actions of glutamate at these GABA terminals. Likewise, it seems unlikely that ST glutamate spillover would reach only GABA terminals and not glutamate terminals of central origin. Thus our present studies indicate an absence of functional spillover of glutamate between ST terminals, even with physiologically relevant frequencies of activation. Thus focal- and ST-EPSCs were fully additive and independent.
Synaptic network organization at second-order medial NTS neurons.
Our previous investigations indicate that large-amplitude EPSCs in second-order NTS neurons arise from single ST axons with ∼20 active contacts (Andresen and Peters 2008). These multiple contacts likely represent the functioning of clusters of primary afferent ST terminals evident in histology of single-afferent axons within NTS (Kubin et al. 2006). The present data demonstrate that convergent primary afferent axons, despite their multiple active contacts, operate independently and did not influence the release of glutamate from a nearby cohort of afferent terminals on the same neurons when coactivated. All afferent terminations were strictly sorted by TRPV1-based phenotype. No laminar or subregional framework exists paralleling the structural organization of the dorsal horn of the spinal cord (Andresen and Paton 2011). Despite the apparent overlap within NTS implied by common regional maps of afferent tracers from various organs (Corbett et al. 2005; Mendelowitz et al. 1992; Shin et al. 2009; Shin and Loewy 2009), the function of those synaptic branches is quite discrete and focused which should make second-order neurons substantially organ specific. The implications of this afferent-NTS organization suggest that second-order NTS neurons are quite focused on limited afferent information. This degree of synaptic isolation is consistent with our recent report of the absence of shared inputs of near neighbor, NTS second-order neuron pairs (McDougall et al. 2009).
The present findings indicate highly restricted and independent primary afferent synaptic inputs that act exclusively by postsynaptic mechanism. This contrasts to the very substantial evidence that many centrally sourced inputs to NTS act through presynaptic molecular mechanisms that are heterogeneous across ST terminals and act through modulating glutamate release from ST terminals, albeit via peptidergic G-protein-coupled receptors (GPCRs) (Appleyard et al. 2005, 2007; Bailey et al. 2006b; Barnes et al. 2003; Cui et al. 2011; Peters et al. 2008). The continuing themes are that glutamate mechanisms are highly conserved across ST terminals, despite differences between TRPV1+ and TRPV1− afferents with basic excitation conveyed by released glutamate and non-NMDA receptors. However, integrative modulation is strongly presynaptic and highly differentiated through GPCRs. An important ramification of TRPV1-based afferent segregation is that physiologically these information paths will be driven by the very different discharge characteristics of A or C sensory neurons (Schild et al. 1994). Thus for aortic baroreceptors that terminate predominantly in medial NTS, the C-type are often silent and activated only at elevated pressures compared with A-type (Thoren et al. 1999). Although our studies here focused on presynaptic properties, TRPV1+ second-order NTS neurons tend to have stronger 4-aminopyridine-sensitive potassium currents which act to dampen transmission of primary afferent activity into conducted action potentials (Bailey et al. 2002, 2007). Together, high physiological thresholds for C-type afferent discharge may collude with potassium currents to dampen activation of TRPV1+ circuits early in their pathway by postsynaptic as well as presynaptic mechanisms. Our results suggest that A/C signals will be communicated by separate, dedicated information systems at second-order sensory neurons within NTS. Perhaps the biggest puzzle is the precise location of descending contacts from other central neurons within this network structure. Central descending inputs might preferentially target presynaptic ST sites and/or higher order neurons. Some hypothalamic inputs likely contact ST presynaptic locations via GPCRs, and this positioning makes them prime locales for modulating afferent-centric processing within NTS, affecting both acute and chronic reflex adaptations (Michelini and Stern 2009). The NTS network organization of higher order neurons may well be the site of afferent A/C fiber subclass convergence, visceral cross-organ signal integration, and specific efferent targeting of brain areas (Bailey et al. 2006a).
In summary, gradations of weak focal shocks within the NTS can recruit single cranio-sensory afferent inputs to second-order NTS neurons. Intercepting these axons with an electrode separated from the ST offers the added control of independent timing of separate synaptic events. This separate control strengthens confidence in negative test results for interactions between primary afferent terminals contacting second-order NTS neurons. The lone example where both distal and focal activation of a single axon produced fully duplicating EPSCs further bolsters confidence by demonstrating that measurement discrimination was not an issue. The results provide the first explicit tests at high resolution that the medial region of NTS contains an overwhelmingly sensory afferent network of axons at high density, and that those afferents show surprising independence of action, despite that high afferent density-low convergence and no interaction. Thus sensory afferent glutamate must diffuse only limited distances and does not overflow the cleft to influence extrasynaptic sites either pre- or postsynaptically. This afferent glutamate system supports discrete actions within highly compact networks of interlaced mode-specific reflex pathways terminating at neighboring second-order neurons within NTS.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-41119 (M. C. Andresen) and HL-105703 (M. C. Andresen), and National Health and Medical Research Council of Australia, Overseas Training C J Martin Fellowship no. 400405 (S. J. McDougall).
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.J.M. and M.C.A. conception and design of research; S.J.M. performed experiments; S.J.M. analyzed data; S.J.M. and M.C.A. interpreted results of experiments; S.J.M. prepared figures; S.J.M. and M.C.A. drafted manuscript; S.J.M. and M.C.A. edited and revised manuscript; S.J.M. and M.C.A. approved final version of manuscript.
Supplementary Material
REFERENCES
- Allen C, Stevens CF. An evaluation of causes for unreliability of synaptic transmission. Proc Natl Acad Sci U S A 91: 10380–10383, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders K, Ohndorf W, Dermietzel R, Richter DW. Synapses between slowly adapting lung stretch receptor afferents and inspiratory beta-neurons in the nucleus of the solitary tract of cats: A light and electron microscopic analysis. J Comp Neurol 335: 163–172, 1993 [DOI] [PubMed] [Google Scholar]
- Andresen MC, Paton JF. The nucleus of the solitary tract: processing information from viscerosensory afferents. In: Central Regulation of Autonomic Functions , edited by Llewellyn-Smith IJ, Verberne AJ. London: Oxford University Press, 2011, p. 23–46 [Google Scholar]
- Andresen MC, Peters JH. Comparison of baroreceptive to other afferent synaptic transmission to the solitary tract nucleus. Am J Physiol Heart Circ Physiol 295: H2032–H2042, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol Heart Circ Physiol 259: H1307–H1311, 1990 [DOI] [PubMed] [Google Scholar]
- Andresen MC, Yang M. Dynamics of sensory afferent synaptic transmission in aortic baroreceptor regions of nucleus tractus solitarius. J Neurophysiol 74: 1518–1528, 1995 [DOI] [PubMed] [Google Scholar]
- Appleyard SM, Bailey TW, Doyle MW, Jin YH, Smart JL, Low MJ, Andresen MC. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J Neurosci 25: 3578–3585, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard SM, Marks D, Kobayashi K, Okano H, Low MJ, Andresen MC. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J Neurosci 27: 13292–13302, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TW, Appleyard SM, Jin YH, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS). J Neurophysiol 99: 1712–1722, 2008 [DOI] [PubMed] [Google Scholar]
- Bailey TW, Hermes SM, Aicher SA, Andresen MC. Target-specific, dynamic pathway tuning by A-type potassium channels in solitary tract nucleus: cranial visceral afferent pathways to caudal ventrolateral medulla or paraventricular hypothalamus. J Physiol 582: 613–628, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TW, Hermes SM, Andresen MC, Aicher SA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns. J Neurosci 26: 11893–11902, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TW, Jin YH, Doyle MW, Andresen MC. Vanilloid sensitive afferents activate neurons with prominent A-type potassium currents in nucleus tractus solitarius. J Neurosci 22: 8230–8237, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TW, Jin YH, Doyle MW, Smith SM, Andresen MC. Vasopressin inhibits glutamate release via two distinct modes in the brainstem. J Neurosci 26: 6131–6142, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes KL, DeWeese DM, Andresen MC. Angiotensin potentiates excitatory synaptic transmission to medial solitary tract nucleus neurons. Am J Physiol Regul Integr Comp Physiol 284: R1340–R1353, 2003 [DOI] [PubMed] [Google Scholar]
- Bender VA, Pugh JR, Jahr CE. Presynaptically expressed long-term potentiation increases multivesicular release at parallel fiber synapses. J Neurosci 29: 10974–10978, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham AC, Hasser EM. Area postrema and aortic or vagal afferents converge to excite cells in nucleus tractus solitarius. Am J Physiol Heart Circ Physiol 264: H1674–H1685, 1993 [DOI] [PubMed] [Google Scholar]
- Brill J, Huguenard JR. Robust short-latency perisomatic inhibition onto neocortical pyramidal cells detected by laser-scanning photostimulation. J Neurosci 29: 7413–7423, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budisantoso T, Matsui K, Kamasawa N, Fukazawa Y, Shigemoto R. Mechanisms underlying signal filtering at a multisynapse contact. J Neurosci 32: 2357–2376, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O'Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci 31: 5067–5077, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Ling Eh EH, Horowitz JM, Bonham AC. Synaptic transmission in nucleus tractus solitarius is depressed by Group II and III but not Group I presynaptic metabotropic glutamate receptors in rats. J Physiol 538: 773–786, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chounlamountry K, Kessler JP. The ultrastructure of perisynaptic glia in the nucleus tractus solitarii of the adult rat: comparison between single synapses and multisynaptic arrangements. Glia 59: 655–663, 2011 [DOI] [PubMed] [Google Scholar]
- Christie MJ, Jahr CE. Multivesicular release at Schaffer collateral-CA1 hippocampal synapses. J Neurosci 26: 210–216, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett EK, Sinfield JK, McWilliam PN, Deuchars J, Batten TF. Differential expression of vesicular glutamate transporters by vagal afferent terminals in rat nucleus of the solitary tract: projections from the heart preferentially express vesicular glutamate transporter 1. Neuroscience 135: 133–145, 2005 [DOI] [PubMed] [Google Scholar]
- Cui RJ, Li X, Appleyard SM. Ghrelin inhibits visceral afferent activation of catecholamine neurons in the solitary tract nucleus. J Neurosci 31: 3484–3492, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan TN, Stephans K, Ramirez AN, Glazebrook PA, Andresen MC, Kunze DL. Differential distribution and function of hyperpolarization-activated channels in sensory neurons and mechanosensitive fibers. J Neurosci 24: 3335–3343, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue S, Felder RB, Gilbey MP, Jordan D, Spyer KM. Post-synaptic activity evoked in the nucleus tractus solitarius by carotid sinus and aortic nerve afferents in the cat. J Physiol 360: 261–273, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue S, Felder RB, Jordan D, Spyer KM. The central projections of carotid baroreceptors and chemoreceptors in the cat: a neurophysiological study. J Physiol 347: 397–409, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue S, Fox RE, Kidd C, Koley BN. The distribution in the cat brain stem of neurones activated by vagal non-myelinated fibres from the heart and lungs. Q J Exp Physiol Cogn Med Sci 66: 391–404, 1981 [DOI] [PubMed] [Google Scholar]
- Donoghue S, Garcia M, Jordan D, Spyer KM. The brain-stem projections of pulmonary stretch afferent neurones in cats and rabbits. J Physiol 322: 353–363, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle MW, Andresen MC. Reliability of monosynaptic transmission in brain stem neurons in vitro. J Neurophysiol 85: 2213–2223, 2001 [DOI] [PubMed] [Google Scholar]
- Doyle MW, Bailey TW, Jin YH, Andresen MC. Vanilloid receptors presynaptically modulate visceral afferent synaptic transmission in nucleus tractus solitarius. J Neurosci 22: 8222–8229, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle MW, Bailey TW, Jin YH, Appleyard SM, Low MJ, Andresen MC. Strategies for cellular identification in nucleus tractus solitarius slices. J Neurosci Methods 37: 37–48, 2004 [DOI] [PubMed] [Google Scholar]
- Fernandes LG, Jin YH, Andresen MC. Heterosynaptic crosstalk: GABA-glutamate metabotropic receptors interactively control glutamate release in solitary tract nucleus. Neuroscience 174: 1–9, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron 69: 1188–1203, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Histed MH, Bonin V, Reid RC. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron 63: 508–522, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YH, Bailey TW, Andresen MC. Cranial afferent glutamate heterosynaptically modulates GABA release onto second order neurons via distinctly segregated mGluRs. J Neurosci 24: 9332–9340, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia M, Richter DW. Morphology of physiologically identified slowly adapting lung stretch receptor afferents stained with intra-axonal horseradish peroxidase in the nucleus of the tractus solitarius of the cat. I. A light microscopic analysis. J Comp Neurol 241: 503–520, 1985a [DOI] [PubMed] [Google Scholar]
- Kalia M, Richter DW. Morphology of physiologically identified slowly adapting lung stretch receptor afferents stained with intra-axonal horseradish peroxidase in the nucleus of the tractus solitarius of the cat. II. An ultrastructural analysis. J Comp Neurol 241: 521–535, 1985b [DOI] [PubMed] [Google Scholar]
- Kalia M, Richter DW. Rapidly adapting pulmonary receptor afferents. I. Arborization in the nucleus of the tractus solitarius. J Comp Neurol 274: 560–573, 1988 [DOI] [PubMed] [Google Scholar]
- Katz LC, Dalva MB. Scanning laser photostimulation: a new approach for analyzing brain circuits. J Neurosci Methods 54: 205–218, 1994 [DOI] [PubMed] [Google Scholar]
- Kline DD, Takacs KN, Ficker E, Kunze DL. Dopamine modulates synaptic transmission in the nucleus of the solitary tract. J Neurophysiol 88: 2736–2744, 2002 [DOI] [PubMed] [Google Scholar]
- Krauhs JM. Structure of rat aortic baroreceptors and their relationship to connective tissue. J Neurocytol 8: 401–414, 1979 [DOI] [PubMed] [Google Scholar]
- Krauhs JM. Morphology of presumptive slowly adapting receptors in dog trachea. Anat Rec 210: 73–85, 1984 [DOI] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol 101: 618–627, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachamp P, Tell F, Kessler JP. Successive episodes of synapses production in the developing rat nucleus tractus solitarii. J Neurobiol 52: 336–342, 2002 [DOI] [PubMed] [Google Scholar]
- Liu Z, Chen CY, Bonham AC. Frequency limits on aortic baroreceptor input to nucleus tractus solitarii. Am J Physiol Heart Circ Physiol 278: H577–H585, 2000 [DOI] [PubMed] [Google Scholar]
- Loewy AD. Central autonomic pathways. In: Central Regulation of Autonomic Functions , edited by Loewy AD, Spyer KM. New York: Oxford University Press, 1990, p. 88–103 [Google Scholar]
- McDougall SJ, Andresen MC. Low fidelity GABA transmission within a dense excitatory network of the solitary tract nucleus. J Physiol 590: 5677–5689, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SJ, Peters JH, Andresen MC. Convergence of cranial visceral afferents within the solitary tract nucleus. J Neurosci 29: 12886–12895, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelowitz D, Yang M, Andresen MC, Kunze DL. Localization and retention in vitro of fluorescently labeled aortic baroreceptor terminals on neurons from the nucleus tractus solitarius. Brain Res 581: 339–343, 1992 [DOI] [PubMed] [Google Scholar]
- Michelini LC, Stern JE. Exercise-induced neuronal plasticity in central autonomic networks: role in cardiovascular control. Exp Physiol 94: 947–960, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifflin SW. Convergent carotid sinus nerve and superior laryngeal nerve afferent inputs to neurons in the NTS. Am J Physiol Regul Integr Comp Physiol 271: R870–R880, 1996 [DOI] [PubMed] [Google Scholar]
- Ootani S, Umezaki T, Shin T, Murata Y. Convergence of afferents from the SLN and GPN in cat medullary swallowing neurons. Brain Res Bull 37: 397–404, 1995 [DOI] [PubMed] [Google Scholar]
- Paton JFR. Pattern of cardiorespiratory afferent convergence to solitary tract neurons driven by pulmonary vagal C-fiber stimulation in the mouse. J Neurophysiol 79: 2365–2373, 1998 [DOI] [PubMed] [Google Scholar]
- Patterson LM, Zheng H, Ward SM, Berthoud HR. Vanilloid receptor (VR1) expression in vagal afferent neurons innervating the gastrointestinal tract. Cell Tissue Res 311: 277–287, 2003 [DOI] [PubMed] [Google Scholar]
- Peters JH, McDougall SJ, Fawley JA, Andresen MC. TRPV1 marks synaptic segregation of multiple convergent afferents at the rat medial solitary tract nucleus. PLos One 6: e25015, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JH, McDougall SJ, Fawley JA, Smith SM, Andresen MC. Primary afferent activation of thermosensitive TRPV1 triggers asynchronous glutamate release at central neurons. Neuron 65: 657–669, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JH, McDougall SJ, Kellett DO, Jordan D, Llewellyn-Smith IJ, Andresen MC. Oxytocin enhances cranial visceral afferent synaptic transmission to the solitary tract nucleus. J Neurosci 28: 11731–11740, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuer DA, Zhang J, Toney GM, Mifflin SW. Temporal processing of aortic nerve evoked activity in the nucleus of the solitary tract. J Neurophysiol 76: 3750–3757, 1996 [DOI] [PubMed] [Google Scholar]
- Schild JH, Clark JW, Canavier CC, Kunze DL, Andresen MC. Afferent synaptic drive of rat medial nucleus tractus solitarius neurons: dynamic simulation of graded vesicular mobilization, release, and non-NMDA receptor kinetics. J Neurophysiol 74: 1529–1548, 1995 [DOI] [PubMed] [Google Scholar]
- Schild JH, Clark JW, Hay M, Mendelowitz D, Andresen MC, Kunze DL. A- and C-type nodose sensory neurons: model interpretations of dynamic discharge characteristics. J Neurophysiol 71: 2338–2358, 1994 [DOI] [PubMed] [Google Scholar]
- Seagard JL, Dean C, Hopp FA. Role of glutamate receptors in transmission of vagal cardiac input to neurones in the nucleus tractus solitarii in dogs. J Physiol 520: 243–253, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekizawa S, Joad JP, Bonham AC. Substance P presynaptically depresses the transmission of sensory input to bronchopulmonary neurons in the guinea pig nucleus tractus solitarii. J Physiol 552: 547–559, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JW, Geerling JC, Loewy AD. Vagal innervation of the aldosterone-sensitive HSD2 neurons in the NTS. Brain Res 1249: 135–147, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JW, Loewy AD. Gastric afferents project to the aldosterone-sensitive HSD2 neurons of the NTS. Brain Res 1301: 34–43, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Hestrin S, Armstrong WE. Enhanced neurotransmitter release at glutamatergic synapses on oxytocin neurones during lactation in the rat. J Physiol 526: 109–114, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoren PN, Munch PA, Brown AM. Mechanisms for activation of aortic baroreceptor C-fibres in rabbits and rats. Acta Physiol Scand 166: 167–174, 1999 [DOI] [PubMed] [Google Scholar]
- Zhang J, Mifflin SW. Influences of excitatory amino acid receptor agonists on nucleus of the solitary tract neurons receiving aortic depressor nerve inputs. J Pharmacol Exp Ther 282: 639–647, 1997 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


