Abstract
High-count microelectrode arrays implanted in peripheral nerves could restore motor function after spinal cord injury or sensory function after limb loss. In this study, we implanted Utah Slanted Electrode Arrays (USEAs) intrafascicularly at the elbow or shoulder in arm nerves of rhesus monkeys (n = 4) under isoflurane anesthesia. Input-output curves indicated that pulse-width-modulated single-electrode stimulation in each arm nerve could recruit single muscles with little or no recruitment of other muscles. Stimulus trains evoked specific, natural, hand movements, which could be combined via multielectrode stimulation to elicit coordinated power or pinch grasp. Stimulation also elicited short-latency evoked potentials (EPs) in primary somatosensory cortex, which might be used to provide sensory feedback from a prosthetic limb. These results demonstrate a high-resolution, high-channel-count interface to the peripheral nervous system for restoring hand function after neural injury or disruption or for examining nerve structure.
Keywords: functional electrical stimulation, hand, prosthesis, spinal cord injury, limb loss
disruptions of neural transmission resulting in paralysis—primarily from spinal cord injury (SCI) but also from lesions, stroke, head injuries, and acute nerve injury—leave the patients' limbs and other affected body parts intact but partially or totally unable to move. One emerging treatment for paralyzed individuals is functional electrical stimulation (FES) (e.g., ParaStep I, Freehand, Vocare, and IST-12) (Brissot et al. 2000; Fromm et al. 2001; Kilgore et al. 2008; Martens and Heesakkers 2011). FES-based prostheses can enable paralyzed individuals to grasp objects with a few simple grips, or even enable paraplegic individuals to walk a short distance in conjunction with external support. However, FES systems can be fatiguing and relatively difficult to use because they typically activate near-maximal contractions, preferentially activate fatigable motor units, and provide no somatosensory or proprioceptive sensory feedback (Popovic et al. 1993; Spadone et al. 2003).
The 100-electrode Utah Slanted Electrode Array (USEA) provides a prime candidate for restoring hand function in paralyzed patients by activating motor fibers, and may ameliorate some of the challenges associated with full-muscle FES or extraneural stimulation. The USEA electrodes are arranged in a 10 × 10 configuration, spaced at 400-μm intervals, with electrode lengths ranging from 0.5 to 1.5 mm (Branner and Normann 2000), thereby providing relatively complete coverage of a nerve. Because the electrodes penetrate directly into the nerve fascicles, their tips closely abut different populations of motor or sensory axons, allowing multiple, selective sites for stimulation or recording. The USEA has been used previously to activate cat hindlimb muscles selectively, independently, and in a fatigue-resistant manner via interleaved activation of multiple different motor units for a single muscle, each at a relatively low frequency (Frankel et al. 2011; McDonnall et al. 2004). Thus intrafascicular nerve stimulation with USEAs may also provide an improved level of hand movements, compared with conventional FES. Among other advantages, a USEA may access multiple muscles with a single implant site and independent access to multiple different motor units within the same muscle, thereby also allowing more graded force control and more fatigue-resistant movements via interleaved stimulation (Normann et al. 2012). It may also allow access to intrinsic hand muscles, which is difficult to achieve with conventional extraneural nerve stimulation. Finally, intrafascicular electrodes, such as those of the USEA, can also record single-unit action potentials, opening the possibility of detecting afferent signals from sensory receptors in intact limbs distal to the neural disruption (Branner et al. 2004).
Similarly, amputees also could benefit from the selective stimulation and recording capabilities of intrafascicular electrodes, which would allow the patients' nervous system to communicate with computer-controlled prostheses such as robotic hands or knees. In this instance, implanted electrodes would be used to record from efferent motor fibers to obtain motor command signals and to activate small populations of sensory afferents in order to restore discrete sensations. However, the electrodes' functionality with respect to selective stimulation and recording would remain the same.
Previous studies have shown that, at a gross level, motor fibers do cluster according to their function (Gustafson et al. 2009), and some motor fibers may be part of more than one nerve (Badia et al. 2010). However, these studies do not address the relationship between the sensory and motor fibers within a single fascicle, and it remains unclear whether fibers innervating a given body region tend to cluster together, or whether the nerve fibers organize separately into sensory and motor bundles within the fascicle.
The human hand is a complex mechanical system with 27 degrees of freedom that is difficult to emulate. Monkeys have opposable thumbs, independent finger control (Schieber 1991), and intrinsic and extrinsic muscles controlling the hand and arm similar in number to those in humans (Liu et al. 1996). Monkeys thus provide an attractive model for testing the ability of the USEA to restore human hand function. The muscles used for generating power grip and precision grip are innervated by the median, ulnar, and radial nerves in both humans and monkeys. Selective activation of monkey hand muscles has also been reported with the use of flat interface nerve electrodes (FINEs) (Brill et al. 2009).
In the present study, we examined the feasibility and potential advantages of USEAs for activation of motor and sensory fibers in the median, radial, and ulnar nerves of nonhuman primates, using acute, anesthetized preparations. Although the commercial version of a single Utah Electrode Array (UEA) (with equal-length electrodes) has been previously implanted in the median nerve of one human subject with success (Warwick et al. 2003), the data set from that study was limited. Aside from that somewhat anecdotal report, there have been no previous investigations of USEAs in nonhuman primates, or in any of the forelimb nerves of any species. Here we examined the ability of different USEA electrodes to provide access to different extrinsic and intrinsic hand muscles and the selectivity of that activation. We also examined the ability to activate multiple motor groups via multiple nerves so as to achieve coordinated gripping sequences that could restore clinically useful hand movements after paralysis. In addition to motor responses, we examined stimulation-evoked responses centered around primary somatosensory cortex that could be useful for restoration of cutaneous and proprioceptive sensation in amputees. Finally, the combination of sensory and motor responses was examined to determine whether fibers from a single body region lie together, or whether the nerve fibers organize separately into sensory and motor regions within the fascicle.
MATERIALS AND METHODS
Surgery
These experiments were performed in nonrecovery surgical procedures on four monkeys that were being euthanized after a series of unrelated studies. All procedures were performed under deep surgical levels of anesthesia, with isoflurane gas anesthetic after premedication with buprenorphine, as approved by the Institutional Animal Care and Use Committee of Northwestern University. Experiments lasted ∼30 h. Differences in procedures across animals are summarized in Table 1.
Table 1.
Procedures performed on each monkey
| Name | I/O Curves | Pulse Train | ECoG | Skull Screw |
|---|---|---|---|---|
| NHP1 | x | x | x (lesion) | |
| NHP2 | x | x | ||
| NHP3 | x | x | x | |
| NHP4 | x | x | x |
I/O, input-output; ECoG, electrocorticography.
Skull Screws and Electrocorticography Electrode Grid
The anesthetized monkey was placed in a stereotaxic frame. In three monkeys, skull screws were placed according to steretotaxic coordinates and skull landmarks so as to lie primarily over postcentral cortex for cortical monitoring. The skull screws' positions in relation to the cortex were confirmed posthumously. In the fourth monkey, a craniotomy was performed, and an electrocorticography (ECoG) grid was placed over somatosensory cortex and adjacent cortices.
Electromyography Recording
Fine-wire electromyography (EMG) electrodes were placed in forearm, finger, and wrist muscles, and electrical potentials were recorded on a Cerebus recording system (Blackrock, Salt Lake City, UT) at 10,000 samples/s with a low-pass filter at 7.5 kHz. Bipolar recordings were made with intramuscular electrodes inserted into each muscle, including, in some cases, separate compartments in a single muscle. In all experiments, the main muscles used in grasp were monitored, including flexor carpi radialis (FCR), flexor digitorum superficialis (FDS), flexor carpi ulnaris (FCU), medial head of flexor digitorum profundus (FDPm), ulnar head of flexor digitorum profundus (FDPu), flexor pollicis brevis (FPB), brachioradialis, extensor carpi radialis (ECR), extensor digitorum communis (EDC), extensor carpi ulnaris (ECU), pronator teres (PrT), flexor digitorum profundus (FDP), the dorsal interossicles, and lumbricals. In some monkeys additional electrodes were inserted in triceps lateralis, triceps longus, abductor pollicis brevis (AdP), and palmaris longus. Additionally, separate compartments in EDC and ECR were monitored in two monkeys.
Nerve Exposure
Nerves in the arm were exposed at the elbow and shoulder for subsequent implantation of USEAs. The median nerve was exposed through a longitudinal incision from midhumeral level to beyond the antecubital fossa. The PrT muscle and the brachioradialis muscle were reflected in order to dissect the median nerve free just proximal to its branch point in the proximal forearm. To gain access to the ulnar nerve the medial antebrachial cutaneous nerve was transected at the elbow, and the ulnar nerve was dissected free just proximal to the elbow. The radial nerve was exposed from the volar side of the arm by continuing the dissection of the muscles deep to median and ulnar nerves. Alternatively, the radial nerve was exposed via a second incision on the dorsal aspect of the arm between the brachioradialis and extensor carpi radialis longus (ECRL) muscles, exposing the radial nerve just proximal to its branch points to the brachioradialis and forearm extensor muscles.
All three nerves were also exposed at the brachial plexus to allow implantation of USEAs at a second location in each nerve and to examine the effectiveness of different implant locations. The incision in the arm was extended proximally, and, in order to fully expose the nerves of the brachial plexus, the pectoralis minor and pectoralis major muscles were incised and retracted.
USEA Implantation
USEAs were implanted in nerves just distal to the brachial plexus (Fig. 1A) and near the elbow (Fig. 1B) by means of a high-speed insertion system (Rousche and Normann 1992). Arrays were connected to stimulation and recording systems via a modified Integrated Cable Systems (ICS Mfg., Longmont, CO) or a Tucker-Davis Technologies (TDT, Alachua, FL) 96-pin connector and adapter board.
Fig. 1.
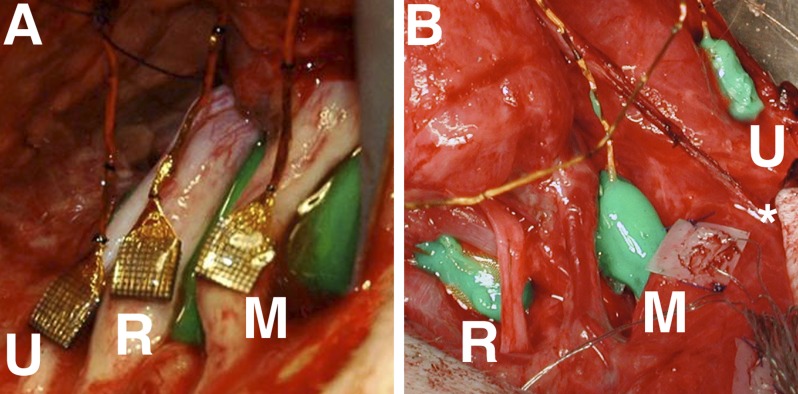
Utah Slanted Electrode Arrays (USEAs) implanted in arm nerves. Surgical access to all 3 target nerves was achieved through a single surgical site at either the elbow or the shoulder. In both images, the more proximal limb is at top and the more distal limb at the bottom, and the volar (palm side) surface of the arm is depicted. R, radial nerve; M, median nerve; U, ulnar nerve; *, olecranon process at the elbow. A: left shoulder-level radial, median, and ulnar nerves, each shown implanted with a 100-electrode USEA. Insertion support (subsequently removed) is seen below the median nerve. B: right elbow-level arm nerves, just proximal to the elbow. USEA implants are shown protected by a custom containment system composed of metal mesh and Kwik-Cast silicone (World Precision Instruments).
USEA-Evoked Motor Responses
Electrical stimulation was delivered through the USEA electrode tips via either a Grass SD-88 stimulator or a custom-built, 300-channel “UINTA” stimulation system (Wilder et al. 2009). We generated EMG stimulus-response curves individually for all 96 electrodes on each of 11 USEAs, using pulse-width-modulated (0.1–1,026 μs), single-pulse, constant-voltage (3 ± 2 V) stimuli controlled by custom software. Stimulation thresholds, plateaus, and intermediate stimulus-response functions were determined through a closed-loop binary search using the evoked EMG signals for feedback.
Individual muscle responses were analyzed to determine which electrodes provided access to appropriate hand muscles. After muscle access had been determined by the delivery of single-pulse stimulation, pulse trains were delivered in an attempt to generate prolonged, useful movements of the hand and wrist. Frequency of stimulation for pulse trains was between 30 and 50 Hz. Cortical activation was monitored during all nerve stimulation. Somatosensory evoked potentials (SSEPs) were computed using 64 averaged trials for each pulse on each electrode.
Before inferential statistical analyses of evoked EMG activity were conducted, EMG values were normalized to the largest response from the maximum of either bipolar stimulation through nerve cuffs or single- or multi-electrode stimulation through the USEA. The EMG values for each run were divided by the maximum evoked EMG to produce a normalized EMG value (nEMG).
A muscle stimulation selectivity index (SI) was calculated for each electrode at a specific nEMG value, by use of the following formula (Dowden et al. 2009):
We analyzed SI values statistically with an overall analysis of variance (ANOVA) with monkey number, nerve implanted (median, radial, or ulnar), and level of implant (elbow or shoulder) as factors, using a hierarchical sum of squares, followed by multiple-comparison tests with a Scheffé correction as appropriate. Unequal group sizes were adjusted via weighted means. Multiple-factor interactions with incomplete terms were not analyzed.
Recording of Cortical Somatosensory Evoked Potentials
Electrical potentials from each screw or grid contact were recorded in relation to a distant reference by a Cerebus recording system as described above.
We also compared selectivity of cortical activation for USEA implants at the elbow and shoulder. Biologically, it is unknown whether the degree of musculotopic organization of motor nerve fibers (i.e., their anatomical arrangement, corresponding to their target muscles) remains constant throughout the nerve length. Thus, from a practical perspective, it was unclear whether both implant sites would work equally well, which was particularly important given that only relatively proximal nerve sites would be available after high-level transhumeral amputations. To address the relationship of motor and sensory fibers within the nerve, we investigated whether different USEA electrodes that activated a given muscle would also evoke responses on a given ECoG electrode, which would imply that sensory and motor fibers travel in the same fascicle in a mixed nerve. We first examined whether the amplitude of the SSEP recorded on a given ECoG electrode was statistically correlated with the pulse width of the stimuli delivered through a given USEA electrode during the recruitment curve that had also been used for muscle activation. For USEA electrodes that could drive cortical activity, we then determined which muscle responded most strongly to that electrode. Finally, for each ECoG electrode, we averaged the correlations across different USEA electrodes that had activated each muscle to determine the mean correlation between muscles activated by USEA electrodes and somatosensory cortical response location.
RESULTS
General Results
Implants in all nerves across all implant levels were capable of evoking muscle contractions in nerve-appropriate muscles that were detectable through EMG or visual inspection. Currents to evoke these contractions were not directly measured (given the use of constant-voltage stimulation at 3 V) but lie below levels that could damage tissue with short-term stimulation sessions, between 5 and 50 μA, as documented in the cat, including for short-term stimulation across multiple sessions (Branner et al. 2004; Frankel et al. 2011; Normann et al. 2012).
Single-Pulse, Single-Electrode Stimulation: Muscle Activation and Selectivity
Recruitment curves.
We first examined the ability to recruit responses in individual muscles by delivering single-pulse stimulation through individual USEA electrodes (typically using a series of varying stimulus-pulse durations) while measuring the evoked EMG responses. As in previous work, the muscle responses to USEA stimulation were graded across the range of pulse widths; perithreshold pulse widths had a mean of 15.4 ± 0.5 μs. Calculated SI values indicated that single-electrode, single-pulse intrafascicular nerve stimulation could often activate individual extrinsic muscles to functionally useful levels without activating other muscles (Fig. 2A) and that different muscles could be recruited selectively by different USEA electrodes (Fig. 2B). Intrinsic muscles could also be activated by USEA stimulation, although they were usually coactivated with other intrinsic muscles.
Fig. 2.
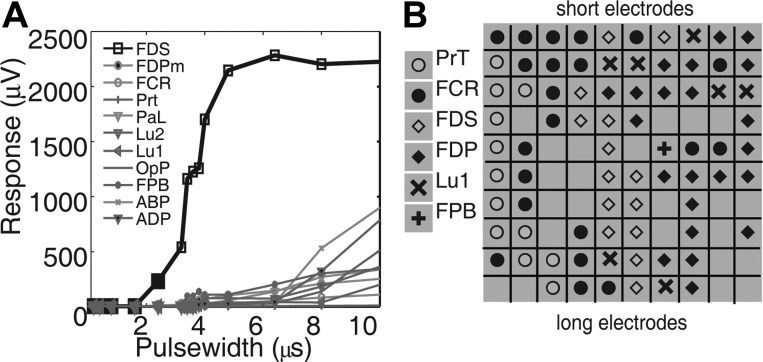
Muscle activation shows selectivity and musculotopy. A: selectivity. Stimuli of increasing pulse width evoked successively larger responses in flexor digitorum superficialis (FDS), with little or no activation of other muscles. This electrode showed a selectivity of 0.85. B: musculotopy. Each tile in the 10-by-10 grid represents an electrode on the USEA, the symbol indicating the muscle most strongly activated by that electrode. Electrodes are shown as in a cross section of the nerve with the most superficial aspect of the nerve at top. Responses in a given muscle tend to be recruited by adjacent USEA electrodes, whereas responses in other muscles are recruited by other USEA electrodes, indicating a musculotopic arrangement of nerve fibers. ABP, abductor pollicis brevis; FDPm, medial head of flexor digitorum profundus (FDP); FCR, flexor carpi radialis; PaL, palmaris longus; PrT, pronator teres; Lu1, 1st lumbrical; Lu2, 2nd lumbrical; OpP, opponens pollicis; FPB, flexor pollicis brevis; ADP, abductor pollicis brevis.
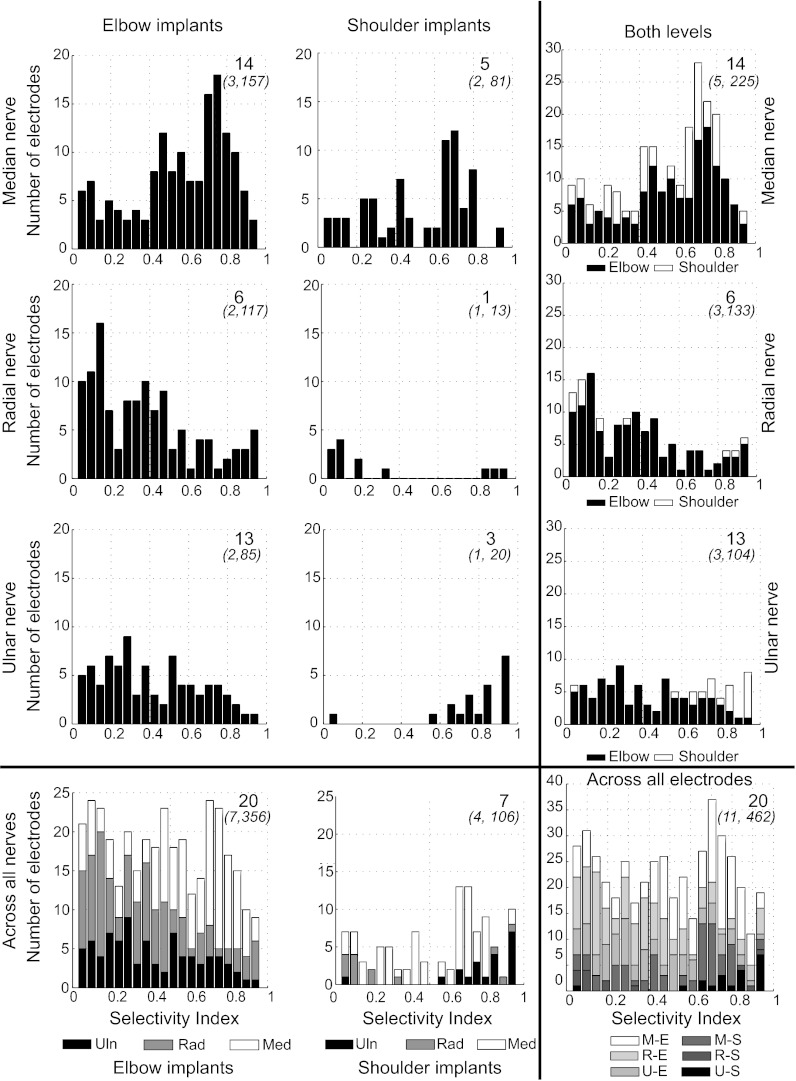
Of a possible 1,056 electrodes across 11 implants, 462 (43%) evoked at least low-level responses (defined as 0.2 nEMG) at pulse widths <512 μs. Many electrodes presumably ended in extrafascicular, nonneuronal tissue, and hence would not have evoked responses except at very strong stimulus levels. In the three monkeys in which input-output curves were generated, the mean SI across all implants at 0.2 nEMG was 0.44 ± 0.01 (mean ± SE reported for all selectivity measures). The mean number of electrodes per array that activated muscles at 0.2 nEMG was 42, and it dropped to 34 at 0.5 nEMG and 18 at 0.9 nEMG. However, some selectivity was maintained at the stronger activation values, 0.5 nEMG (0.43 ± 0.01) and 0.9 nEMG (0.31 ± 0.02). A single USEA thus provided selective activation of multiple muscles innervated by a single nerve, at a variety of activation levels. At the elbow (672 total electrodes, 7 implants) and the shoulder (384 total electrodes, 4 implants) in all three nerves, 382 of the electrodes (36% of all electrodes) elicited strong EMG responses (defined as 0.5 nEMG) in the same muscles in which they elicited weaker responses. At the elbow all implants could reach 0.9 nEMG in some muscles (178 electrodes, 26.5% of elbow electrodes), whereas at the shoulder only the median nerve implants were capable of evoking contractions at 0.9 nEMG (23 electrodes, 5.9%). Because data for values above 0.2 nEMG are incomplete, the selectivity analysis was confined to 0.2 nEMG (Fig. 3); data are summarized for selectivity at higher nEMG values in Table 2.
Fig. 3.
Selectivity of muscle activation for all USEA electrodes and implant sites. The number of electrodes that recruited responses at a given level of selectivity is depicted across all levels and nerves. Left: results for USEA implants near the elbow for median nerve (top), radial nerve (2nd row), and ulnar nerve (3rd row) and across all nerves (bottom). Center: results for USEA implants in nerves near the shoulder. Right: results summated for USEAs at both the elbow and the shoulder. Bottom right: group results across all nerves at both levels. For each panel, the large number at top right indicates how many different muscles could be preferentially activated at that particular level-implant combination across all selectivity indexes (SIs). The smaller numbers in parentheses below the number of muscles indicate the number of implants and the number of electrodes used in the analyses, respectively.
Table 2.
Selectivity of muscle responses at multiple strength levels
| nEMG | Mean SI | SE | Arrays (of 11) | Elbow Electrodes | Shoulder Electrodes | Total (of 1,056) | Mean Pulse Width, μs |
|---|---|---|---|---|---|---|---|
| 0.2 | 0.44 | 0.01 | 11 | 356 | 106 | 462 | 16.7 |
| 0.5 | 0.43 | 0.01 | 11 | 296 | 86 | 382 | 17.0 |
| 0.9 | 0.31 | 0.02 | 6 | 178 | 23* | 201 | 19.0 |
Mean selectivity decreased as muscle activation level increased. At each level of activation [normalized electromyography value (nEMG) 0.2–0.9], the mean selectivity index (SI), SE of the mean SI, and total number of electrodes at each location are shown. Few individual electrodes were capable of eliciting 0.9 nEMG responses, particularly at the shoulder.
Median nerve only.
Muscle selectivity across nerves and implant levels.
An ANOVA of the SI calculated at 0.2 nEMG for the factors of nerve, primate, and implant level indicated that the implant level (elbow or shoulder) was not a significant factor, whereas the individual animal and nerve implanted were significant factors (Table 3). The mean SI calculated at 0.2 nEMG of all elbow implants tended to be lower than the mean of all shoulder implants (0.42 ± 0.01 vs. 0.52 ± 0.03; elbow: 356 electrodes, 7 arrays, shoulder: 106 electrodes, 4 arrays), primarily because of results from the ulnar nerve; however, in the median and radial nerves, this trend was reversed. Specific comparisons regarding implant level for the different nerves were not analyzed for statistical significance because only a single shoulder-level implant was done in the radial and ulnar nerves and because the implant level was not a statistically significant factor. Descriptively, however, within-nerve comparisons of elbow- and shoulder-level SIs in the median nerve (0.54 ± 0.02 vs. 0.47 ± 0.03; 153 and 73 electrodes, 3 and 2 arrays, respectively) and radial nerve (0.32 ± 0.02 vs. 0.26 ± 0.06; 120 and 13 electrodes, 2 and 1 arrays, respectively) showed that selectivity tended to be higher at the elbow than at the shoulder, whereas in the ulnar nerve selectivity at the elbow tended to be lower than at the shoulder (0.26 ± 0.02 vs. 0.78 ± 0.05; 84 and 20 electrodes, 2 and 1 arrays, respectively). Multiple-comparison tests with a Scheffé correction indicated that SI was statistically different across all nerve pairings (all P < 0.05), with population-normalized-mean values as follows: median 0.56 ± 0.02, ulnar 0.44 ± 0.03, and radial 0.36 ± 0.02.
Table 3.
Selectivity of muscle responses at multiple strength levels
| Factor | Sum Sq. | d.f. | Mean Sq. | F | Probability > F |
|---|---|---|---|---|---|
| Implant level | 0.04 | 1 | 0.04 | 0.76 | 3.83E-01 |
| Animal no. | 2.02 | 2 | 1.01 | 17.30 | 5.71E-08 |
| Nerve | 3.15 | 2 | 1.58 | 27.07 | 7.79E-12 |
| Error | 26.57 | 456 | 0.06 | ||
| Total | 32.17 | 461 |
Analysis of variance (ANOVA) of SI, linear model, hierarchical sum of squares. Individual animal and nerve implanted were significant factors. All electrodes capable of eliciting a 0.2 nEMG response or greater were included in the analysis.
Musculotopic arrangement of nerve fibers.
To evaluate the musculotopic arrangement of motor fibers within a nerve, we examined the extent to which neighboring USEA electrodes evoked responses in a common muscle. For all implants, electrode sites that recruited the same muscle or close synergist muscles were usually in close proximity to one another, suggesting a musculotopic arrangement (Fig. 2B). To quantify musculotopy, for each USEA electrode we first calculated the expected number of neighboring (adjacent) electrodes that would activate the same muscle if nerve fibers were randomly distributed, based on the number of responses evoked in each muscle for each given USEA. We then compared the number expected from chance with the number of neighboring electrodes that had actually recruited the same response as the given test electrode at threshold. Significantly more neighboring electrodes recruited the same motor response than expected from chance alone (mean = 0.98 ± 0.07 electrodes, P < 0.05) (Fig. 4), indicating that motor fibers were organized musculotopically within all nerves.
Fig. 4.
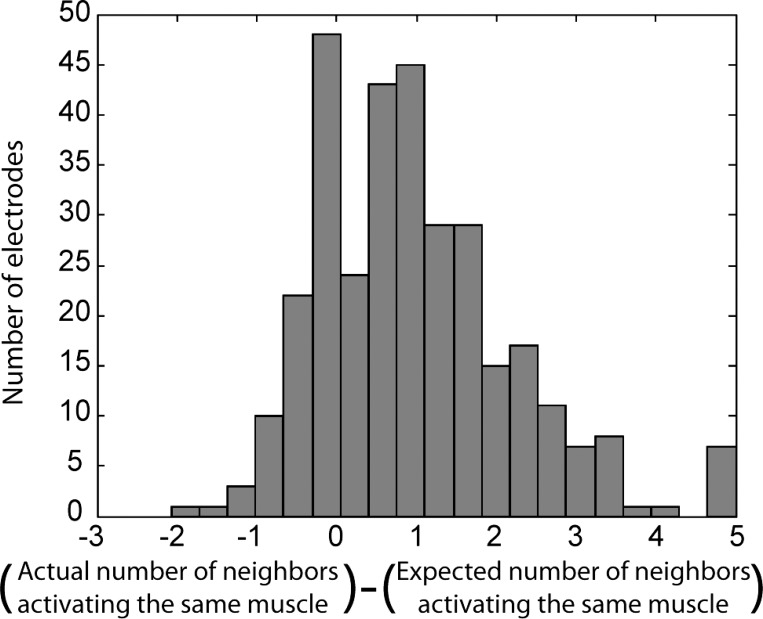
Quantification of musculotopic arrangement of motor fibers. We assessed the musculotopic organization of nerve fibers by comparing the muscle activated by each USEA electrode with the muscles activated by neighboring USEA electrodes. For each electrode capable of activating a muscle, we calculated the probability that a neighboring electrode would activate the same muscle from chance alone. The actual number of neighboring electrodes that preferentially activated the same muscle was consistently higher than the number expected from chance (i.e., the actual − expected difference was >0), indicating a musculotopic arrangement in which motor fibers to a given muscle were close together within the nerve. This pattern held for muscles of all types and each nerve individually.
Single-Electrode Pulse Trains Also Recruited Selective Movements
Functionally useful movements require stimulus trains, rather than single-pulse activation of motor nerve fibers. To test our ability to generate individuated and coordinated movements with the USEA, we applied pulse trains (30–50 Hz, 1.8–3 V) to particular electrodes. Pulse widths used in the functional muscle contraction sequences were higher than perithreshold values. We monitored movements at the hand, elbow, and shoulder, as well as rotation of the forearm. Motions were observed and categorized in terms of the joint at which the movement occurred and its direction, together with the muscles that showed EMG activity. Across all subjects, median nerve stimulation generated six to nine visually different movements across different combinations of joints (Fig. 5 and Supplemental Movie S1).1 These movements approximately corresponded to the activation of individual muscles associated with each movement in various combinations (e.g., FCR for wrist flexion; FDS and FDP for finger flexion; the intrinsic muscles and FPB for small finger and thumb movements; and PrT for arm pronation). The ability of the different USEA electrodes to elicit distinct movements and different EMG responses indicates that selective stimulation was partially maintained during pulse train delivery, such that even with the low-level activation of additional muscles the motions evoked were clearly related to the muscle that was selectively activated through single-pulse stimulation.
Fig. 5.
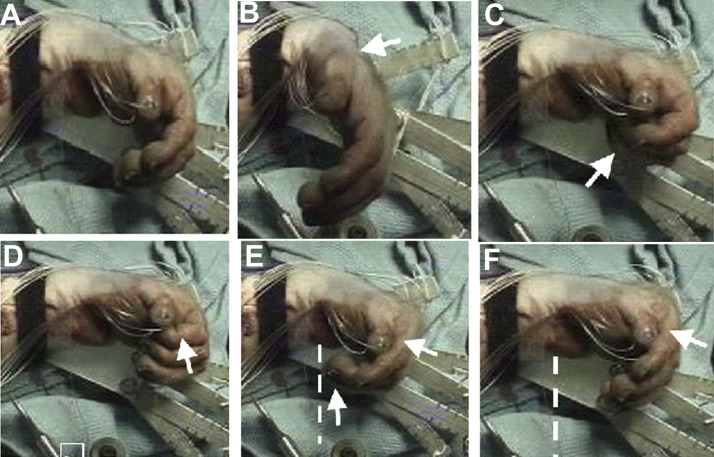
USEA single-electrode pulse-train stimulation of median nerve recruits specific digit and wrist movements (pronation not shown). White arrows indicate fingers/joints in motion. Different USEA electrodes evoked different movements. A: rest. B: wrist flexion. C: digits 3–5, flexion (in shadow). D: digit 2, tip flexion; note the different fingers engaged in C and D. E: digits 2–5, flexion at metacarpophalangeal (MCP) joints. F: digits 2–5, tip extension, with flexion at MCP joints. Note the relative straightening of the fingertips in F compared with the extent of finger flexion in E, demarcated by white lines in E and F.
Multielectrode, Multi-USEA Pulse Trains Evoked Coordinated Grasp
To produce a coordinated grasp, muscles not only must be selectively activated but also must contract and relax in specific patterns (Long et al. 1970; Maier and Hepp-Reymond 1995). To test the ability to evoke these more complex types of movements, between three and nine electrodes across all arrays were selected that activated the muscles necessary for power grip through the UINTA stimulation system custom software. A 2-s movement sequence was programmed consisting of finger extension to open the hand, finger flexion to grasp an object, and, finally, finger extension to release the object. Activation of extrinsic finger flexors that span the wrist typically caused undesired wrist flexion along with flexion of the fingers. In these cases, wrist extensors were also activated to counteract the flexion force, a combination that is necessary under normal conditions as well. A 50-g ball was placed in the animal's palm as it was initially opened. When the hand closed, the ball was held within the hand until the program instructed the fingers to extend (Fig. 6 and Supplemental Movie S2). The shown movement was evoked with six electrodes with pulse widths of 10, 100, 10, 50, 100, and 500 μs (average 128 μs). Once programmed, the control sequence reliably produced the desired movement sequence for the duration of the experiment. Via this technique, the anesthetized monkey's hand also engaged a variation of power grip sometimes called bucket grip. In addition, electrodes associated with intrinsic hand muscles were combined with the extrinsic muscles to generate a pinch grip between the thumb and forefinger (Supplemental Movie S3).
Fig. 6.
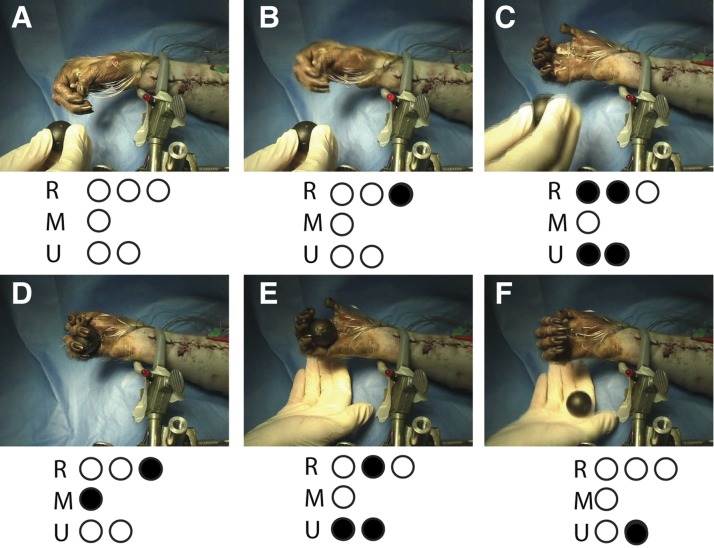
Coordinated, sequential grasp-and-release movements produced by multielectrode, multi-USEA stimulation. USEA stimulation generated grip sufficient to hold a ball. Bottom: electrodes used in the grip sequence for the 3 implanted nerves; filled dots indicate electrodes active at the time of the picture. A: rest position. B: wrist extension. C: 1-s hand opening and forearm supination to accept the ball. The experimenter introduces the ball to the anesthetized primate's hand. D: 1-s power grip. E: wrist and fingers extend again, releasing the ball. F: wrist flexes and forearm pronates to drop the ball.
USEA Activation of Sensory Fibers
To examine our ability to evoke sensory signals, as would be necessary in a limb-loss prosthesis that restores sensation, we monitored SSEPs, using either skull screws (n = 3) or an ECoG grid (n = 1) during USEA stimulation. Stimulation produced short-latency (∼5 ms to onset) SSEPs in and around primary somatosensory cortex on 52% of tested stimulating electrodes. To avoid the possibility of indirect sensory activation (e.g., H or F reflexes), the analysis of SSEP data was limited to the first 20 ms after stimulation (Fig. 7). The short latency of these responses indicates that they are likely due to direct afferent fiber activation, not indirect sensory responses due to movement caused by concurrent muscle activation. In the monkey with the ECoG grid, low-level stimulation applied to USEAs (n = 3 USEAs) recruited cortical responses at a pulse duration that did not activate muscles in 32% of electrodes, providing further evidence that direct sensory fiber activation was achieved.
Fig. 7.
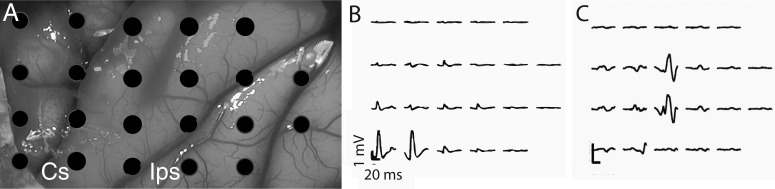
Primary somatosensory cortex was activated through USEA peripheral nerve stimulation of sensory nerve fibers. Anterior to left, medial on top in all panels. Stimuli were delivered at the beginning of each trace. A: electrocorticography (ECoG) electrode positions shown in relation to the cortex. B and C: cortical recording pattern associated with electrodes in the median nerve that activated thumb and index finger intrinsic muscles (B) or electrodes in the radial nerve that activated brachioradialis (C), an elbow flexor. Cs, central sulcus; Ips, intraparietal sulcus.
Relationship Between Somatotopic and Musculotopic Organizations
We next examined whether afferent nerve fibers were organized somatotopically and the relationship between somatotopic and musculotopic organizations.
Different USEA electrodes evoked different cortical responses.
Consistent with a somatotopic organization, different electrodes on the same USEA, or on different USEAs, evoked responses recorded through different cortical electrodes in three monkeys. (Upon postmortem dissection, one primate was found to have a lesion within the somatosensory cortex from previous work that precluded cortical analyses for the present work.) For monkeys with skull screws (n = 2) rather than the ECoG electrode grid, different patterns of cortical activation were discernible only with stimulation via USEAs on different nerves, presumably because of the relatively coarse spatial resolution provided by skull screw recordings. For example, the maximal responses to median nerve stimulation were recorded on electrodes different from the electrodes showing the maximal responses evoked by radial nerve stimulation. Additionally, for the one monkey with the ECoG grid, different USEA electrodes on a single USEA in a given nerve evoked responses in discernibly different cortical regions (i.e., different ECoG electrodes).
Somatotopic and musculotopic maps covary.
Results showed that the amplitude of the SSEP on some cortical electrodes was significantly correlated with stimulation strength on USEA electrodes that activated muscles with similar function (Fig. 8). In addition, adjacent cortical electrodes showed similar correlations, whereas cortical electrodes distant to one another did not. Instead, responses on distal cortical electrodes were correlated with stimulus strength on USEA electrodes that activated other muscles. For example, stimulation strengths on USEA electrodes implanted in the median nerve that activated wrist flexor muscles were correlated (r 0.45 or greater, P < 0.05) with response magnitudes on ECoG electrode 18, whereas stimulus strengths on USEA electrodes that activated finger flexor muscles were correlated (r 0.45 or greater) with response magnitudes on ECoG electrodes 1 and 2 (Fig. 8).
Fig. 8.
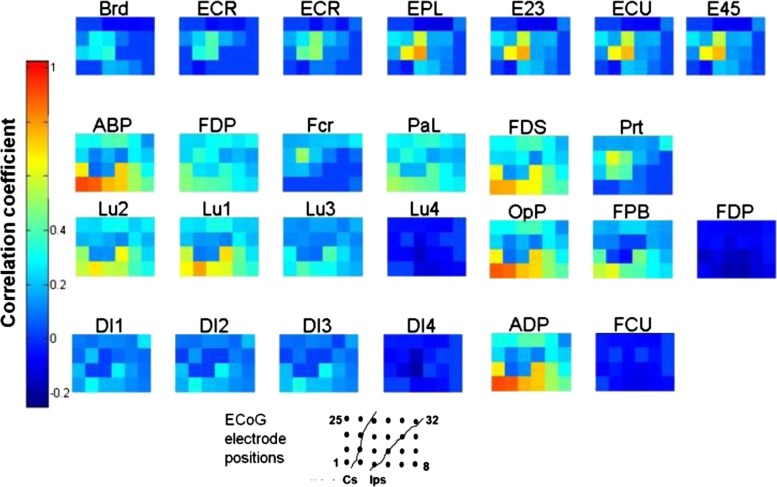
Coregistration of musculotopic and somatotopic maps. Different USEA electrodes that evoked responses in a given muscle via activation of motor nerve fibers also evoked responses on the same cortical ECoG electrodes, via activation of sensory nerve fibers. Each grid displays a color map for the 32 ECoG electrodes for a given muscle, indicated by the label above the grid [e.g, brachioradialis (Brd), extensor carpi radialis (ECR), etc.]. Each electrode was categorized by muscle, requiring an SI of >0.25 calculated at normalized EMG value (nEMG) of 0.2. Colors correspond to the mean Pearson's correlation coefficient (r) between the stimulus pulse width and the amplitude of the evoked cortical response across all electrodes that could activate each muscle (n = 692, P < 0.01 shown). ECoG electrodes within each grid are arbitrarily numbered from 1 to 8 from left to right at bottom, extending through 25–32 at top. USEA electrodes that evoked responses in a given muscle or similar muscles, e.g., wrist extensors, also evoked responses in a similar set of cortical electrodes, whereas USEA electrodes that evoked responses in other muscles activated other cortical areas. For example, USEA electrodes that activated extensor muscles extensor carpi ulnaris (ECU) and ECR also evoked responses on ECoG electrodes 10 and 11, as indicated by the high correlation between the stimulus pulse width and the amplitude of the evoked somatosensory evoked potential (SSEP) on those ECoG electrodes. In contrast, USEA electrodes that activated the flexor muscle FDS evoked responses in more anterior-lateral cortical regions (ECoG electrodes 1 and 2). Muscles are grouped according to their dominant innervation, e.g., radial nerve (top), median nerve (middle 2 rows), and ulnar nerve (bottom).
These results imply that somatosensory fibers and motor fibers for a given body region travel closely together within the nerve. Given that USEA-evoked motor selectivity appears to hold even at the subfascicular level, it is plausible that the motor-sensory coorganization occurs at the subfascicular level as well. These findings complement earlier work demonstrating that somatosensory fibers of the same submodality and receptive field region cluster together within the nerve (Ekedahl et al. 1997; Hallin 1990).
DISCUSSION
General
Here we report the first USEA implantation in the peripheral nerves of a nonhuman primate, the first attempt to quantify the efficacy and selectivity of the USEA in activating extrinsic and intrinsic hand muscles, and the first recordings of cortical sensory responses evoked through USEA stimulation of arm nerves. The results here demonstrate that intrafascicular electrodes can provide excellent access to multiple muscles, including intrinsic hand muscles not typically accessed in conventional FES. The different electrodes of a single USEA could activate multiple different muscles, and the combination of just three USEAs in the median, radial, and ulnar nerves could access nearly all forearm and hand muscles. Although the procedure to implant USEAs for clinical applications would be invasive, it is less invasive and would require less recovery time than, for example, targeted reinnervation approaches presently used successfully for control of prosthetic limbs (Kuiken et al. 2009).
Recruitment of Motor Responses via USEA Stimulation of Motor Fibers
Activation of motor fibers provided fine-resolution control of forearm movements. Selective activation of the muscles used to grip objects was achieved with both the elbow-level and shoulder-level implants, indicating that both locations have potential uses for peripheral nervous system (PNS)-based prostheses. Although shoulder-level implants had a mean SI comparable to elbow-level implants, the small sample size makes determination of the strength of that trend difficult. However, the greater number of usable electrodes suggests that the elbow may be a more desirable implant location when available. Nonetheless, shoulder-level implants would be useful in cases of high-humeral amputation, or for recruiting muscles of the upper arm after SCI, given that some electrodes at the shoulder level were selective (40 electrodes with an SI > 0.5).
Single-pulse activation of individual muscles was often selective, particularly for extrinsic hand muscles. Although the intrinsic muscles with similar functions (such as the lumbricals) were usually recruited together, the intrinsic muscles in different groups (thenar, interossi, and lumbrical) were often recruited separately. On some electrodes (in elbow-level implants in the median nerve), the index lumbrical was recruited alone, without activity on the other lumbricals, further indicating the specificity of muscle stimulation possible through intrafascicular electrodes. From previous work with intrafascicular electrodes, it is known that it is possible to evoke a response from only a portion of a fascicle. In the present study, it was not directly demonstrated whether the selectivity seen is principally due to a similar level of subfascicular selectivity or a more segregated set of fascicular bundles; however, the high impedance of the epineurium surrounding each fascicle substantially limits current spread from one fascicle to another. In either case, under the assumption that the nerve is musculotopically and somatotopically organized, current spread would cause physically close muscles and sensory areas to be activated together. Moreover, current spread cannot fully account for the musculotopy (or somatopy) observed here. Current spread from a given electrode to the neural tissue at an adjacent electrode might indeed active some fibers there, but such current spread could not fully explain why the dominant nEMG response at the given electrode was the same as that at the adjacent electrode. The strongest activation at the given site will reflect activation of the greatest number of nerve fibers, which probabilistically would occur in close proximity to the given electrode tip. Our data indicate that the selectivity of muscle activation was highly variable among different nerves and individuals. However, the overall musculotopic arrangement of fibers across the broad distribution of SIs likely indicates that, independent of the degree to which the selectivity seen in this study is due to fasciculation or instead to subfascicular organization, there is a strong tendency for axons to particular muscles to group together, in agreement with other recent studies of nerve organization (Badia et al. 2010; Brill et al. 2009).
Pulse-train stimulation of selective electrodes generated smooth and distinct movements. Furthermore, different movements evoked by pulse-train stimulation were combined into functional grip-and-release sequences by activating several electrodes simultaneously or in sequence, and multiple types of grip (power, bucket, and pinch) could be reliably generated. These results all indicate the feasibility of using a penetrating electrode in the PNS as a prosthesis for limb reanimation in paralyzed patients. In the cat hindlimb, contractions produced by stimulation through multiple USEA electrodes that activate different motor units of the same muscles can be combined and interleaved to produce fatigue-resistant movements and stable static positions (Normann et al. 2005). So long as stimulation through the USEA electrodes can evoke responses in independent, nonoverlapping motor units, the same approach may work for monkey arm nerves, and presumably for human nerves as well. However, the time constraints of the present acute studies precluded systematic investigations of the overlap of USEA electrode responses and the effects of interleaved stimulation on fatigue resistance (see Normann et al. 2012 for details of the overlap and fatigability tests).
Studies of precision grip indicate that the intrinsic hand muscles, particularly the first dorsal interosseous and the muscles in the thenar group, are important for stabilizing the thumb and finger metacarpophalangeal (MCP) joints (Maier and Hepp-Reymond 1995). Unfortunately, present FES-based solutions do not fully access the hand muscles required for grasp, particularly the intrinsic hand muscles. Although direct stimulation of extrinsic hand muscles does provide functional power grip, the same intramuscular electrodes cannot easily be used for control of intrinsic hand muscles, largely because of their small size and the difficulty of surgical access. Because of these limitations, additional surgeries such as tendon transfers are sometimes necessary to achieve strong, stable grip force (Kilgore et al. 2008). In contrast, our three implanted USEAs allowed access to all the instrumented hand muscles, including all extrinsic and intrinsic muscles implicated in grip (Maier and Hepp-Reymond 1995; Schieber 1995). The activation of intrinsic and extrinsic hand muscles in a coordinated fashion allows for versatile hand posturing and gripping. Thus, for example, we were able to encode a stimulation sequence with four electrodes that brought the thumb and forefinger together (Supplemental Movie S3).
Stimulation of Sensory Fibers
Lack of sensory feedback is also a major challenge for users of a limb-replacement prosthesis. Without normal somatosensory feedback, many patients complain that their prosthetic limb is unwieldy and difficult to use (Biddiss and Chau 2007; Pezzin et al. 2004). Intrafascicular electrode arrays, such as the USEA, should be capable of selectively activating multiple, independent subsets of sensory fibers, just as they can for motor fibers. Motor and sensory nerves remain functional long after limb amputation, and stimulation of sensory fibers can elicit sensation (Anani et al. 1977; Dhillon et al. 2004; Dhillon and Horch 2005; Rossini et al. 2010; Warwick 2005). Hence, it may be possible to stimulate sensory fibers through USEAs and thereby evoke graded and varied sensory responses, including proprioception and pressure, to aid in gripping and reaching tasks.
Here, stimulation through individual USEA electrodes generated a variety of patterns of somatosensory cortical activation. In principle, such differentiable sensory signals could be used to provide cutaneous and proprioceptive sensory feedback from a neuroprosthetic artificial limb. Furthermore, the responses on a given cortical electrode were associated with stimulation on USEA electrodes that were also associated with specific muscles or classes of muscles (e.g., finger flexors). Because motor axons are organized musculotopically, and USEA electrodes that stimulate muscles with similar function are often near one another (e.g., Fig. 2B, FDP and FDS or FCR and PrT), we can conclude that the somatotopic and musculotopic maps in the nerve are in approximate register with one another. Because muscle activity could often be evoked on an electrode that also evoked sensory responses, it is likely that individual fascicles are mixed sensory-motor, consistent with previous studies (Chaudhuri et al. 2011; Schady et al. 1983).
The modality of sensory responses is difficult to determine from recordings from the cortical surface with the electrodes used in this study, especially given that there is some overlap in the representation of body space in the cortex. However, activity from stretch receptors in a given muscle would be expected to lie in close proximity to motor fibers associated with the same muscle, indicating a high likelihood that the evoked potentials could convey some proprioceptive feedback for use in a prosthetic application. Such feedback might provide both intuitive, closed-loop prosthetic control and enhanced integration of the artificial limb with the user's own internal body image.
Considerations for Long-Term Intrafascicular Electrode Implants
In SCI patients, the lower motoneurons remain mostly intact within the spinal cord. However, their chronic deinnervation can cause secondary degeneration, disassembly, or disorganization of the neuromuscular junction, changes in muscle excitability, and muscle atrophy. Thus, in a chronic implantation in a paralyzed individual, the initial conditions of the muscle and neuromuscular junction might be quite different from those in the intact animals in the present study. However, the initial peripheral changes that occur after SCI are largely reversible through FES, which, over time, can restore the neuromuscular junction's natural arborization and can improve the efficacy of muscle activation (Baldi et al. 1998). Indeed, the ability to return the neuromuscular system toward its normal preinjury conditions may constitute an additional benefit of intrafascicular electrode technology. However, without early intervention SCI-induced hypertonia and spasticity can cause permanent changes to the functionality of muscles. All potential therapies, including the proposed USEA, PNS-based prosthesis, thus give the most benefit when provided immediately after injury.
Neurons may undergo important changes at the sites of chronic electrode implants that could affect electrode functionality. Fibrosis around electrodes and a continuing foreign body response can push axons away from the electrode tips, hampering their ability to record and stimulate neurons selectively (Biran et al. 2005). Although all neural implants face the problems associated with tissue response, central nervous system (CNS) implants of UEAs are subjected to less motion than nerve implants, and traditionally have been more reliable (Simeral et al. 2011) than long-term USEA implants in initial studies (Branner et al. 2004). However, recent and ongoing research has demonstrated substantive improvements in both long-term recording and stimulating capabilities of USEAs in cat sciatic nerve (Clark et al. 2011; Frankel et al. 2011; Ledbetter et al. 2011; Normann et al. 2012), which may translate to comparable success for USEAs in monkey arm nerves, and ultimately for clinical applications.
Issues of Muscle Control for the Design of the Motor Program
Strategies for motor restoration that are based on nerve stimulation explicitly involve the activation of lower motor neurons, which can engage spinal reflexes that can operate independently of the brain. For example, Renshaw reflexes involve negative feedback circuits in which a motoneuron inhibits itself (through a Renshaw interneuron). However, synaptic inhibition that occurs at the motoneuron soma many space constants away will have almost no effect on the direct activation of motor fiber axons at the USEA stimulation site.
Because sensory and motor fibers are mixed within the nerve, activation of proprioceptive, cutaneous, or even nociceptive reflex pathways might be engaged coincidently with motor fiber stimulation in the awake animal. In principle, these effects might need to be incorporated into our artificial motor program. However, such considerations have not proven to be problematic in other clinical FES applications with extraneural stimulation. Given the high selectivity and relatively low currents (5–50 μA) associated with intrafascicular stimulation, these concerns also seem unlikely for USEAs.
Brain-Controlled Activation of Motor Nerve Fibers and Behavior
In a closely related project, we have demonstrated that recordings from similar UEAs implanted in the primary motor cortex of monkeys can provide accurate information about muscle activity during normal or intended movement (Pohlmeyer et al. 2007). The information can be used to restore simple voluntary movement to monkeys during peripheral nerve block, used as a temporary paralysis model of SCI.
During this nerve-block paralysis, stimulation through intramuscular electrodes is used to evoke the intended movement, as inferred from the cortical recordings in real time (Ethier et al. 2012; Moritz et al. 2008; Pohlmeyer et al. 2009). Potentially, in future work, USEA-based stimulation of motor fibers could be controlled in a similar manner, providing the monkey—and, ultimately, a paralyzed person—volitional control of more dexterous and coordinated hand movements than can be achieved with intramuscular or extraneural electrodes.
GRANTS
This work was supported by U.S. Army Medical Research and Materiel Command Grant W81XWH-10-1-0931, National Institute of Neurological Disorders and Stroke Grant 01-NS-053603, Northwestern University, and the University of Utah.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.M.L., C.E., L.E.M., and G.A.C. conception and design of research; N.M.L., C.E., E.R.O., S.D.H., A.M.W., J.H.K., S.P.A., L.E.M., and G.A.C. performed experiments; N.M.L. analyzed data; N.M.L., C.E., E.R.O., L.E.M., and G.A.C. interpreted results of experiments; N.M.L. prepared figures; N.M.L. and G.A.C. drafted manuscript; N.M.L., C.E., J.H.K., S.P.A., L.E.M., and G.A.C. edited and revised manuscript; N.M.L. and G.A.C. approved final version of manuscript.
Supplementary Material
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- Anani AB, Ikeda K, Körner L. Human ability to discriminate various parameters in afferent electrical nerve stimulation with particular reference to prostheses sensory feedback. Med Biol Eng Comput 15: 363–373, 1977 [DOI] [PubMed] [Google Scholar]
- Badia J, Pascual-Font A, Vivó M, Udina E, Navarro X. Topographical distribution of motor fascicles in the sciatic-tibial nerve of the rat. Muscle Nerve 42: 192–201, 2010 [DOI] [PubMed] [Google Scholar]
- Baldi JC, Jackson RD, Moraille R, Mysiw WJ. Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord 36: 463–469, 1998 [DOI] [PubMed] [Google Scholar]
- Biddiss E, Chau T. Upper-limb prosthetics: critical factors in device abandonment. Am J Phys Med Rehabil 86: 977–987, 2007 [DOI] [PubMed] [Google Scholar]
- Biran R, Martin DC, Tresco PA. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol 195: 115–126, 2005 [DOI] [PubMed] [Google Scholar]
- Branner A, Normann RA. A multielectrode array for intrafascicular recording and stimulation in sciatic nerve of cats. Brain Res Bull 51: 293–306, 2000 [DOI] [PubMed] [Google Scholar]
- Branner A, Stein RB, Fernandez E, Aoyagi Y, Normann R. Long-term stimulation and recording with a penetrating microelectrode array in cat sciatic nerve. IEEE Trans Biomed Eng 51: 146–157, 2004 [DOI] [PubMed] [Google Scholar]
- Brill N, Polasek K, Oby E, Ethier C, Miller L, Tyler D. Nerve cuff stimulation and the effect of fascicular organization for hand grasp in nonhuman primates. Conf Proc IEEE Eng Med Biol Soc 2009: 1557–1560, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissot R, Gallien P, Le Bot MP, Beaubras A, Laisne D, Beillot J, Dassonville J. Clinical experience with functional electrical stimulation-assisted gait with Parastep in spinal cord-injured patients. Spine (Phila Pa 1976) 25: 501–508, 2000 [DOI] [PubMed] [Google Scholar]
- Chaudhuri D, Borowski P, Zapotocky M. Model of fasciculation and sorting in mixed populations of axons. Phys Rev E Stat Nonlin Soft Matter Phys 84: 021908, 2011 [DOI] [PubMed] [Google Scholar]
- Clark GA, Ledbetter NM, Warren DJ, Harrison RR. Recording sensory and motor information from peripheral nerves with Utah Slanted Electrode Arrays. Conf Proc IEEE Eng Med Biol Soc 2011: 4641–4644, 2011 [DOI] [PubMed] [Google Scholar]
- Dhillon GS, Horch KW. Direct neural sensory feedback and control of a prosthetic arm. IEEE Trans Neural Syst Rehabil Eng 13: 468–472, 2005 [DOI] [PubMed] [Google Scholar]
- Dhillon GS, Lawrence SM, Hutchinson DT, Horch KW. Residual function in peripheral nerve stumps of amputees: implications for neural control of artificial limbs. J Hand Surg Am 29: 605–615, 2004 [DOI] [PubMed] [Google Scholar]
- Dowden BR, Wilder AM, Hiatt SD, Normann RA, Brown NA, Clark GA. Selective and graded recruitment of cat hamstring muscles with intrafascicular stimulation. IEEE Trans Neural Syst Rehabil Eng 17: 545–552, 2009 [DOI] [PubMed] [Google Scholar]
- Ekedahl R, Frank O, Hallin RG. Peripheral afferents with common function cluster in the median nerve and somatotopically innervate the human palm. Brain Res Bull 42: 367–376, 1997 [DOI] [PubMed] [Google Scholar]
- Ethier C, Oby E, Baumann MJ, Miller LE. Restoration of grasp following paralysis through brain-controlled stimulation of muscles. Nature 485: 368–371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel MA, Dowden BR, Mathews VJ, Normann RA, Clark GA, Meek SG. Multiple-input single-output closed-loop isometric force control using asynchronous intrafascicular multi-electrode stimulation. IEEE Trans Neural Syst Rehabil Eng 19: 325–332, 2011 [DOI] [PubMed] [Google Scholar]
- Fromm B, Rupp R, Gerner HJ. [The Freehand System: an implantable neuroprosthesis for functional electrostimulation of the upper extremity.] Handchir Mikrochir Plast Chir 33: 149–152, 2001 [DOI] [PubMed] [Google Scholar]
- Gustafson KJ, Pinault GC, Syed I, Davis JA, Triolo RJ. Fascicular anatomy of human femoral nerve: implications for neural prostheses using nerve cuff electrodes. J Rehabil Res Dev 46: 973–984, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallin RG. Microneurography in relation to intraneural topography: somatotopic organisation of median nerve fascicles in humans. J Neurol Neurosurg Psychiatry 53: 736–744, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore KL, Hoyen HA, Bryden Am, Hart RL, Keith MW, Peckham PH. An implanted upper-extremity neuroprosthesis using myoelectric control. J Hand Surg Am 33: 539–550, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken TA, Li G, Lock BA, Lipschutz RD, Miller LA. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA 301: 619–628, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter NM, Warren DJ, Dowden BR, Frankel M, Normann RA, Harrison RR, Clark GA. Long-term, EMG-free recording and stimulation with Utah Slanted Electrode Arrays in peripheral nerve. Soc Neurosci Abstr 2011: 160–05, 2011 [Google Scholar]
- Liu J, Lau HK, Pereira BP, Kumar VP, Pho RW. Terminal nerve branch entries (motor points) of forearm muscles: a comparative study between monkey and human. Acta Anat (Basel) 155: 41–49, 1996 [DOI] [PubMed] [Google Scholar]
- Long C, 2nd, Conrad PW, Hall EA, Furler SL. Intrinsic-extrinsic muscle control of the hand in power grip and precision handling. An electromyographic study. J Bone Joint Surg Am 52: 853–867, 1970 [PubMed] [Google Scholar]
- Maier MA, Hepp-Reymond MC. EMG activation patterns during force production in precision grip. I. Contribution of 15 finger muscles to isometric force. Exp Brain Res 103: 108–122, 1995 [DOI] [PubMed] [Google Scholar]
- Maier MA, Hepp-Reymond MC. EMG activation patterns during force production in precision grip. II. Muscular synergies in the spatial and temporal domain. Exp Brain Res 103: 123–136, 1995 [DOI] [PubMed] [Google Scholar]
- Martens FM, Heesakkers JP. Clinical results of a Brindley procedure: sacral anterior root stimulation in combination with a rhizotomy of the dorsal roots. Adv Urol 2011: 709708, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnall D, Clark GA, Normann RA. Interleaved, multisite electrical stimulation of cat sciatic nerve produces fatigue-resistant, ripple-free motor responses. IEEE Trans Neural Syst Rehabil Eng 12: 208–215, 2004 [DOI] [PubMed] [Google Scholar]
- Moritz CT, Perlmutter SI, Mavoori J, Lucas TH, Fetz EE. Direct control of paralysed muscles by cortical neurons. Nature 456: 639–642, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann R, McDonnall D, Clark G. Control of skeletal muscle force with currents injected via an intrafascicular, microelectrode array. Conf Proc IEEE Eng Med Biol Soc 7: 7644–7647, 2005 [DOI] [PubMed] [Google Scholar]
- Normann RA, Dowden BR, Frankel MA, Wilder AM, Hiatt SD, Ledbetter NM, Warren DA, Clark GA. Coordinated, multi-joint, fatigue-resistant feline stance produced with intrafascicular hind limb nerve stimulation. J Neural Eng 9: 026019, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzin LE, Dillingham TR, Mackenzie EJ, Ephraim P, Rossbach P. Use and satisfaction with prosthetic limb devices and related services. Arch Phys Med Rehabil 85: 723–729, 2004 [DOI] [PubMed] [Google Scholar]
- Pohlmeyer EA, Oby ER, Perreault EJ, Solla SA, Kilgore KL, Kirsch RF, Miller LE. Toward the restoration of hand use to a paralyzed monkey: brain-controlled functional electrical stimulation of forearm muscles. PLoS One 4: e5924, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmeyer EA, Solla SA, Perreault EJ, Miller LE. Prediction of upper limb muscle activity from motor cortical discharge during reaching. J Neural Eng 4: 369–379, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic DB, Stein RB, Jovanovic K, Dai R, Kostov A, Armstrong WW. Sensory nerve recording for closed-loop control to restore motor functions. IEEE Trans Biomed Eng 40: 1024–1031, 1993 [DOI] [PubMed] [Google Scholar]
- Rossini PM, Micera S, Benvenuto A, Carpaneto J. Double nerve intraneural interface implant on a human amputee for robotic hand control. Clin Neurophysiol 121: 777–783, 2010 [DOI] [PubMed] [Google Scholar]
- Rousche PJ, Normann RA. A method for pneumatically inserting an array of penetrating electrodes into cortical tissue. Ann Biomed Eng 20: 413–422, 1992 [DOI] [PubMed] [Google Scholar]
- Schady W, Ochoa JL, Torebjork HE, Chen LS. Peripheral projections of fascicles in the human median nerve. Brain 106: 745–760, 1983 [DOI] [PubMed] [Google Scholar]
- Schieber MH. Individuated finger movements of rhesus monkeys: a means of quantifying the independence of the digits. J Neurophysiol 65: 1381–1391, 1991 [DOI] [PubMed] [Google Scholar]
- Schieber MH. Muscular production of individuated finger movements: the roles of extrinsic finger muscles. J Neurosci 15: 284–297, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeral JD, Kim SP, Black MJ, Donoghue JP, Hochberg LR. Neural control of cursor trajectory and click by a human with tetraplegia 1000 days after implant of an intracortical microelectrode array. J Neural Eng 8: 025027, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadone R, Merati G, Bertocchi E, Mevio E, Veicsteinas A. Energy consumption of locomotion with orthosis versus Parastep-assisted gait: a single case study. Spinal Cord 41: 97–104, 2003 [DOI] [PubMed] [Google Scholar]
- Warwick K. Future of computer implant technology and intelligent human-machine systems. Stud Health Technol Inform 118: 125–131, 2005 [PubMed] [Google Scholar]
- Warwick K, Gasson M, Hutt B, Goodhew I, Kyberd P, Andrews B, Teddy P, Shad A. The application of implant technology for cybernetic systems. Arch Neurol 60: 1369–1373, 2003 [DOI] [PubMed] [Google Scholar]
- Wilder AM, Hiatt SD, Dowden BR, Brown NA, Normann RA. Automated stimulus-response mapping of high-electrode-count neural implants. IEEE Trans Neural Syst Rehabil Eng 17: 504–511, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.