Abstract
Breaking of the cell membrane symmetry to form polarized or localized domains/regions of the plasma membrane (PM) is a fundamental cellular process that occurs in essentially all cellular organisms, and is required for a wide variety of cellular functions/behaviors including cell morphogenesis, cell division and cell differentiation. In plants, the development of localized or polarized PM domains has been linked to a vast array of cellular and developmental processes such as polar cell expansion, asymmetric cell division, cell morphogenesis, the polarization of auxin transporters (and thus auxin polar transport), secondary cell wall patterning, cell type specification, and tissue pattern formation. Rho GTPases from plants (ROPs) are known to be involved in many of these processes. Here, we review the current knowledge on ROP involvement in breaking symmetry and propose that ROP-based self-organizing signaling may provide a common mechanism for the spatial control of PM domains required in various cellular and developmental processes in plants.
Introduction
Understanding the mechanisms underlying the formation of polarized/localized PM domains is sorely needed to uncover both developmental mechanisms in plants, and the interaction of plants with many pathogens and symbiotic microorganisms such as rhizobia and mycorrhizae. Thus these processes have received increasing scrutiny in plant biology in recent years. A common mechanism for the generation of localized/polarized PM domain has emerged from recent studies in several model systems such as tip-growing pollen tubes, interdigitated pavement cells, and patterning of secondary cell walls in vessel cells. This design principle centers on the self-organizing regulatory system based on the signaling of ROP GTPases, which belong to the family of Rho small GTPases conserved in eukaryotic kingdoms [1-5]. It is not surprising that Rho-based self-organizing mechanisms also govern cell polarization in fungal and animal cells [6-9]. Several key features of Rho-family GTPases make them central regulators of the polar/local PM domains and these include: 1) binary on/off switch controlled by RhoGEFs and RhoGAPs; 2) reversible regulation of membrane localization by RhoGDIs and other molecules [5]; 3) the cytoskeleton, as a universal Rho GTPase signaling target, which commonly feedback regulates Rho signaling. This review focuses on the recent exciting findings that have shed light on how this conserved mechanism that produces specialized polar/local PM domains in specific biological context is structured.
Self-organization of tip growth domains
Tip growth as found in pollen tubes and root hairs represents an extreme form of polar growth, in which exocytic vesicles are targeted to and fuse with the growing domain of the plasma membrane (PM), termed tip growth domain [10-13]. Pollen tubes were the among first plant cell systems where a polarized PM domain was characterized at the molecular level [14], and consequently the molecular basis for the generation of this polar domain has been most extensively characterized [14-24]. These studies led to the formulation of a model for the self-sustained mechanism of polar cell growth [4, 11, 22]. Active ROP1 forms an apical cap at the PM, which defines the tip growth domain, and the apical cap is formed and sustained by two interlinked feedback mechanisms: a positive feedback regulation to laterally propagate the initially localized active ROP1, and a negative feedback-mediated down regulation of active ROP1 to restrict the active ROP1 to the apical cap [11, 19, 21, 22].
The detailed molecular mechanism underpinning ROP1 positive feedback regulation remains to be elucidated, but it was shown that accumulation of the apical actin microfilaments (F-actin), which is dependent upon RIC4, the ROP1 effector, is required for the positive feedback regulation [17, 18, 25]. Tip-localized F-actin could target ROP1 positive regulators, including RopGEF1 [26], PRK2 [27, 28], and unknown PRK2 ligand/ via a direct or indirect mechanism, through actin-dependent polar exocytosis. In yeast, F-actin also plays a role in the positive feedback regulation of the Rho GTPase CDC42 apical cap, but is not essential [6, 29], illustrating the similar but contrasting mechanisms that regulate the polar PM domains required for tip growth in pollen tubes and yeast cells.
F-actin, through its regulation of exocytosis, appears to also participate in the negative feedback regulation of ROP1. The tip-localized REN1 RhoGAP is an essential regulator to restrict active ROP1 to the apical cap. In the tip, REN1 is localized to exocytic vesicles, whose exocytosis is required for REN1 to suppress ROP1 [20].
The actin/exocytosis-linked tight coupling of the positive and negative feedback regulations of the apical ROP1 domain can explain how this domain can be maintained during rapid tip growth in pollen tubes (up to 1 cm/hr). In pollen tubes, very large amounts of exocytic vesicles fuse very rapidly to the tip growth domain. This could rapidly dilute active ROP1, which defines this domain, resulting in the abolishment of the polar localization of ROP1 and therefore of the apical growth domain, if the feedbacks were not tightly coupled. In contrast, such a tight coupling is not necessary in yeast, where exocytic fusion to the apical domain is much slower. The regulation of the tip growth domain in root hairs, another extensively studied type of tip growing cells in plants, is likely to share common mechanisms with pollen tubes, as the apical ROP cap is also found at the tip of root hairs, and the RLK-RopGEF module also regulates ROP activity [26, 30-33].
ROP-MT crosstalks produce local PM domains defining cell wall patterns and cell shape
The localization and arrangement of cortical microtubules (MT) have long been shown to be critical for cell shape formation. Cortical MTs, which are attached to the inner face of the PM, direct the synthesis of cellulose by guiding the cellulose synthase complex, whose localization and arrangement patterns spatially control cell expansion, and consequently cell morphogenesis and growth. Thus the mechanisms controlling the PM domain containing specific cortical MT arrays underscore cell wall patterning and cell shape formation. Recent studies have begun to uncover these mechanisms through the investigation of ROP-based regulation of localized/polarized PM domains.
The puzzle-piece shaped leaf pavement cells provide a good system to study MT-associated PM domains. These cells contain interdigitated domains enriched in parallel cortical MTs, and domains lacking these MT arrays but enriched in cortical F-actin, which correspond to indentation- and lobe-forming regions of the PM respectively. Consequently, cell wall patterns with interdigitated regions containing thick and thin cellulose microfibrils are generated, leading to the formation of the typical pavement cell wavy cell outline. What is the underlying mechanism for the interdigitated patterning of cortical MT- and actin-associated PM domains? It has been shown that locally activated ROP2 activates its effector RIC4, inducing F-actin accumulation in the lobe-forming domain, while ROP6 is activated in the indenting domain, which in turn activates RIC1 to promote ordered MTs [34, 35] (Figure 1A). Active ROP2 sequesters RIC1 to inhibit the ROP6-MT pathway, while MT suppresses ROP2 activation. Thus the two pathways mutually inhibit each other to generate interdigitated ROP2-actin domains and ROP6-MT domains [4].
Figure 1. The spatial control of PM domains by the mutual inhibition between cortical MTs and ROP signaling.
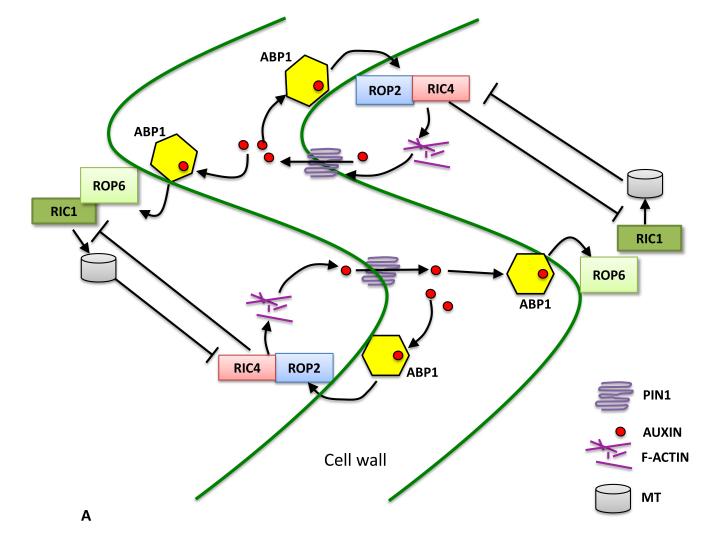
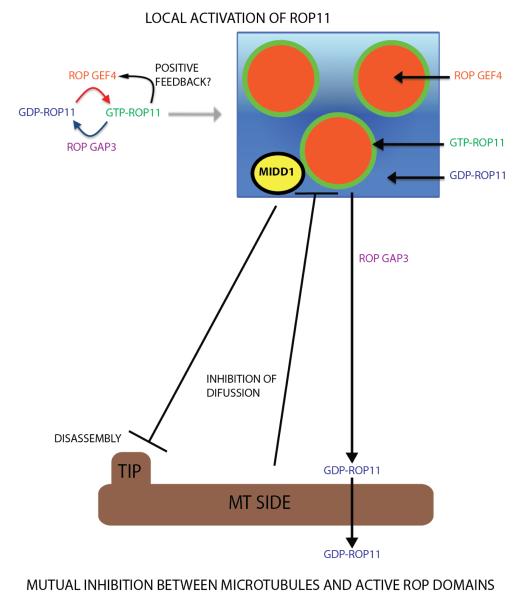
A. Mutual inhibition between the ROP2-actin pathway and the ROP6-MT pathway to maintain the interdigitated PM domains for lobe and indentation formation in pavement cells (Fu, Gu et al., 2005; Fu, Xu et al., 2009; Xu, Wen et al. 2010) ROPs shown in the figure are in their active state and therefore GTP-bound. B. Secondary cell wall patterning in vessel cells is modulated by the mutual inhibition between the ROP11-MIDD1 pathway and cortical MTs (Oda, Iida et al., 2010; Oda, Fukuda. 2012).
Interestingly, an elegant study from Fukuda’s group has now revealed an analogous mutual inhibition between ROPs and MTs that underscores the patterning of the secondary cell wall in xylem cells [36••, 37•]. Xylem vessel cells form patterned thickening of secondary cell walls, such as spiral or pitted patterns, and cortical MTs are arranged in the same pattern to direct the deposition of cellulose in the secondary walls (SW). A MT-associated protein, MIDD1, which belongs to the ICR1/RIP family of novel ROP effector proteins [38, 39], exhibits a patterned PM distribution that mirrors the pitted regions of SW in Arabidopsis xylem vessel cells, promotes the disassembly of MTs in the cortical regions corresponding to SW pits, and is required for the formation of these pits cells [36••, 37•]. The same group has now found that activated ROP11, which exhibits the localization pattern of MIDD1, recruits MIDD1 to the PM in the pit regions. A constitutively active mutant of ROP11 uniformly distributed to the PM, induced uniform distribution of MIDD1 to the PM, and caused the formation of uniform SW, which implies that spatial regulation to generate local active ROP11 domains in the PM pit domains is required for the formation of pitted SW. Interestingly, co-expressing the ROP activator RopGEF4 and the RopGAP3 negative regulator with ROP11, is sufficient to generate active ROP11 domains in non-xylem cells, suggesting a self-organization of active ROP11 domains. RopGEF4 localizes in clusters at the center of active ROP11/MIDD1 domains, and its clustering requires active ROP11, supporting the notion of a positive feedback loop for the activation of ROP11. RopGAP3, which also co-localizes with ROP11/MIDD1 in the pit domain, is required for the formation of local active ROP11. Thus RopGAP3 may be involved in the negative feedback regulation of ROP11 activation. Therefore the formation of local active ROP11 domains in the pit PM in xylem cells appears to bear analogy to the formation of the apical cap of active ROP1 in the apical PM in pollen tubes described above [18, 20, 22, 26].
Oda and Fukuda further provided evidence that the formation of normal pitted SW pattern also involves the interaction of the ROP11/MIDD1 complex with cortical MTs. They observed that cortical MTs restrict ROP11 in the pit PM domain through MT association with MIDD1. Thus MIDD1 has two opposing functions: 1) promoting MT disassembly and 2) restricting ROP11 to the pit PM domain.
Mutual inhibition creates the active ROP11 domain and the MT domain, allowing the formation of pitted SW. This is similar to the mechanism found in PCs, where ROP2-actin and ROP6-MT mutually inhibit each other to generate alternating ROP2-actin domains for lobing and ROP6-MT domains for indenting. This mutual inhibition may provide a more general mechanism for the local patterning of cortical MTs involved in various processes such as tip growing cells (root hairs and pollen tubes). In pollen tube and root hair growth, MTs appear to play a secondary/minor role in the spatial control of the tip growth domain, as depleting these cells of MTs does not eliminate tip growth, but causes reduced polarity and this MT-mediated polarity maintenance interacts with tip-localized ROPs most likely through mutual inhibition [40].
The analogy in the mechanisms regulating polarity in the two systems can be further extended to the action of distinct MT-associated proteins that act as ROP effectors. The SW pattern is established through a ROP driven symmetry breaking system, and a mutual inhibitory interaction between active ROP11 domains and cortical microtubules [36••]. The dual action of MIDD1 is similar to that of RIC1 (the ROP6 effector), which on one hand regulates MT organization, and restricts ROP2 activation on the other [35]. However, in pavement cells, RIC4 in conjunction with ROP2 is responsible for the generation of lobes, whereas in secondary cell wall patterning MIDD1 is proposed to have a dual role: it promotes disassembly at the microtubule tip whilst mediating the elimination of active ROPs at the microtubule sides (Figure 1B).
Last but not the least, the self-organizing regulation of polar or local ROP11/MIDD1 domain resembles the mechanisms for the self-maintenance of lobing and indentations that occurs within each and between two adjacent pavement cells. Just like the ROP11/MIDD1 domain, which is regulated by positive feedback, the ROP2-RIC4 lobing domain is also regulated by a positive feedback through its regulation of PIN1 polarization in pavement cells ([41••]; see below). In pavement cells, self-organization involves cross-talk between the lobe-forming ROP2-RIC4 domain and the indentation-forming ROP6 domain found between neighboring cells. This is mediated by auxin, which is exported by the auxin efflux carrier PIN1 in the lobe-forming domain. PIN1-exported auxin is thought to activate ROP6 in neighboring cells at the steady state [41••] (Figure 1A).
In pavement cells, the ROP6-RIC1 pathway promotes the organization of cortical MTs in the indentation-forming domain, but how MTs adjacent to the ROP11-MIDD1 domain are organized is unknown. Given the extensive similarity between the two systems, it would not be surprising if a ROP pathway were to be involved in the regulation of cortical MTs during the patterning of secondary cell walls. Furthermore, auxin is known to be required for the differentiation of xylem cells. It would be interesting to see whether auxin also does so by activating the ROP signaling pathways during the xylem differentiation.
Auxin regulates the asymmetric distribution of PINs via ROPs
The quintessential small molecule phytohormone auxin coordinates numerous biological processes in development, including patterning, morphogenesis and differential growth. Auxin concentration gradients, that is the differential distribution of auxin within tissues, are often required for auxin to exert its functions. Auxin concentration gradients are created by both locally controlling its biosynthesis, and by regulating its transport between cells in a polar fashion. Polar auxin transport is largely achieved by polar localization of the auxin transporter transmembrane proteins PINs. Polar PIN distribution at the plasma membrane has been shown to be achieved by asymmetric endocytosis and recycling of the PM PINs [42-46]. Early experiments in Arabidopsis roots showed that PIN1 rapidly cycles between the PM and an endosomal compartment via an actin-mediated mechanism [42, 43] [reviewed in [47]]. The phosphorylation status of PIN proteins mediated by the protein kinase PINOID and a phosphatase 2A complex has been shown to regulate the endocytic trafficking of PIN proteins [48, 49] [reviewed in [50]]. Furthermore, phophoinositide metabolism and calcium have also been shown to regulate the polar distribution of PIN proteins at the plasma membrane [51, 52]. However, none of these molecular events have been linked to the regulation of the actin cytoskeleton that affects PIN trafficking.
Recent studies of ROP signaling have helped answering the long-standing question of the actin connection to PIN trafficking and polar distribution. Importantly, these studies have also uncovered a positive feedback mechanism for the polar distribution of PIN proteins at the PM [53••, 54•, 55•]. As discussed above, in Arabidopsis leaf pavement cells, auxin promotes the generation of lobes and indentations that lead to the typical puzzle piece polarity through the activation of ROPs. Nagawa et al. found that the endocytosis of PIN1, which is preferentially localized to the lobe region of the PM, occurs in the indentation region but is inhibited in the lobe region (Nagawa, Xu et al. 2012). The auxin-dependent local activation of ROP2 in the lobe region inhibits PIN1 internalization into the endosomal compartments, leading to higher levels of PIN1 distribution in the lobe region. PIN1 internalization in the lobe region is inhibited by the stabilization of the actin cytoskeleton through the ROP2 effector protein RIC4 [53••]. Thus it was proposed that auxin activation of ROP2, through the stabilization of cortical actin microfilaments, inhibits PIN1 endocytosis, allowing PIN1 polarization to the lobe region (Nagawa, Xu et al. 2012). Polarized PIN1 exports auxin at the lobe region to activate more ROP2 in the same region, providing a positive feedback mechanism for the polar PIN1 distribution.
In Arabidopsis root cells, auxin has been implicated in the polar distribution of PINs, where it inhibits clathrin-dependent PIN endocytosis [56]. Insight into the mechanism through which auxin inhibits PIN endocytosis was recently revealed in Arabidopsis roots with the study of the DHR2-Dock Rho guanine nucleotide exchange factor SPIKE1. SPIKE1 loss of function induced PIN2 internalization that was not suppressed by auxin, as did the loss-of-function mutations for ROP6 or its effector RIC1. These findings established a Rho GTPase-based auxin signaling pathway that maintains PIN2 polar distribution to the PM by inhibiting its internalization [54]. As in pavement cells, ROP-based auxin signaling participates in the positive feedback regulation of PIN polar distributions in root cells. Furthermore, the ROP6-RIC1 pathway appears to promote the stabilization of actin microfilaments to inhibit PIN2 endocytosis [54], as shown for the ROP2-RIC4 pathway in pavement cells.
In Arabidopsis root cells, auxin inhibition of PIN1 and PIN2 endocytosis is mediated by the putative cell surface auxin receptor auxin-binding protein 1 (ABP1) [57••]. It was found that ABP1 promotes PIN1 endocytosis, and that auxin inhibits PIN1 endocytosis by inhibiting ABP1 [57••]. It was recently shown that ABP1 promotes clathrin-mediated endocytosis by inhibiting the ROP6-RIC1 pathway [55]. Secreted ABP1 must interact with other partner(s) to transmit auxin signaling to the intracellular ROP signaling pathways [58]. Because ROP activators RopGEFs are known to interact with receptor-like kinases (RLKs) (Zhang and McCormick. 2007; Duan et al. 2010), it is reasonable to speculate that ABP1 may interact with an RLK to transmit the auxin signal to the intracellular signaling pathways [58].
A possible involvement of ROPs in PIN recycling is suggested by the study of the ROP interactor and polarity regulator scaffold protein ICR1, which was found to be required for the recruitment of PIN proteins to the plasma membrane polar domains. icr1 mutants display a wide range of severe developmental defects caused by a compromised differential auxin distribution. These findings imply that ICR1 is part of an auxin regulated positive feedback loop mediated by ROPs to modulate cell polarity [59•].
Conclusions
Most cells in both animals and plants are polarized and possess distinct PM domains. Creating, maintaining, and modulating these domains is a key process in biology requiring tight regulation and plasticity. The formation of these PM domains results from the polarized trafficking of proteins and lipids. In recent years, Rho-family small GTPases have been shown as central regulators of polarity in different multicellular organisms by modulating polarized trafficking. In plants, a self-organizing (i.e., positive feedback) mechanism based on ROPs (Rho-like small GTPases from plants) has emerged as a common mechanism for symmetry breaking to generate polar or local PM domains. Processes that have been shown to rely on this common mechanism include tip growth, pavement cell morphogenesis, secondary cell wall patterning in xylem cells, and PIN polarization. An important future avenue of research will be to investigate whether ROP-based symmetry breaking provides a universal mechanism for the generation of polarized PM domains required for many other processes to occur such as vascular development (Truernit, Bauby et al. 2012), the formation of Casparian strips from the endodermis [60,60, 61], and asymmetric cell division during the guard cell differentiation [62]. Another critical aspect that should receive attention in the near future includes the elucidation of the processes that allow distinct cell types to use the ROP-based symmetry breaking mechanism to generate distinct polar PM domains with distinct structures and functions. Emerging evidence suggests that functionally distinct ROP downstream effector proteins are critical for the generation and functioning of distinct local PM domains. Future challenges lie in the elucidation of the molecular mechanisms by which ROPs are regulated to generate and maintain these polar PM domains.
Highlights.
Symmetry breaking to generate distinct domains of the plasma membrane (PM) is a fundamental process for many cellular functions
ROP GTPase signaling plays an important role for symmetry breaking to generate polar/local PM domains in plant cells
The tip growth domain is generated and maintained by balancing between the positive feedback-mediated lateral amplification of ROP activation and negative feedback-mediated general deactivation of ROP
Mutual inhibition between cortical microtubules and positive feedback-mediated ROP activation generates polar PM domains for cell wall patterning and cell morphogenesis
Polar distribution of the auxin efflux carrier PIN proteins is modulated a ROP-based auxin signaling to the actin cytoskeleton and a ROP-based positive feedback mechanism
Acknowledgements
The work in the Yang laboratory is supported by grants from National Institute of General Medical Sciences (R01GM081451 and R01GM100130).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279(5350):509–14. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 2.Winge P, Brembu T, Bones AM. Cloning and characterization of rac-like cDNAs from Arabidopsis thaliana. Plant Mol Biol. 1997;35(4):483–95. doi: 10.1023/a:1005804508902. [DOI] [PubMed] [Google Scholar]
- 3.Chant J, Stowers L. GTPase cascades choreographing cellular behavior: movement, morphogenesis, and more. Cell. 1995;81(1):1–4. doi: 10.1016/0092-8674(95)90363-1. [DOI] [PubMed] [Google Scholar]
- 4.Craddock C, Lavagi I, Yang Z. New insights into Rho signaling from plant ROP/Rac GTPases. Trends Cell Biol. 2012;22(9):492–501. doi: 10.1016/j.tcb.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagawa S, Xu T, Yang Z. RHO GTPase in plants: Conservation and invention of regulators and effectors. Small GTPases. 2010;1(2):78–88. doi: 10.4161/sgtp.1.2.14544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wedlich-Soldner R, et al. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science. 2003;299(5610):1231–5. doi: 10.1126/science.1080944. [DOI] [PubMed] [Google Scholar]
- 7.Wedlich-Soldner R, et al. Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling. J Cell Biol. 2004;166(6):889–900. doi: 10.1083/jcb.200405061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izumi G, et al. Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. J Cell Biol. 2004;166(2):237–48. doi: 10.1083/jcb.200401078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris KP, Tepass U. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J Cell Biol. 2008;183(6):1129–43. doi: 10.1083/jcb.200807020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole RA, Fowler JE. Polarized growth: maintaining focus on the tip. Curr Opin Plant Biol. 2006;9(6):579–88. doi: 10.1016/j.pbi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Qin Y, Yang Z. Rapid tip growth: insights from pollen tubes. Semin Cell Dev Biol. 2011;22(8):816–24. doi: 10.1016/j.semcdb.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Z. Cell polarity signaling in Arabidopsis. Annu Rev Cell Dev Biol. 2008;24:551–75. doi: 10.1146/annurev.cellbio.23.090506.123233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YJ, Yang Z. Tip growth: signaling in the apical dome. Curr Opin Plant Biol. 2008;11(6):662–71. doi: 10.1016/j.pbi.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Y, et al. Localization of a Rho GTPase Implies a Role in Tip Growth and Movement of the Generative Cell in Pollen Tubes. Plant Cell. 1996;8(2):293–303. doi: 10.1105/tpc.8.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, et al. Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell. 1999;11(9):1731–42. doi: 10.1105/tpc.11.9.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kost B, et al. Rac homologues and compartmentalized phosphatidylinositol 4, 5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J Cell Biol. 1999;145(2):317–30. doi: 10.1083/jcb.145.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu Y, et al. ROP GTPase regulation of pollen tube growth through the dynamics of tip-localized F-actin. J Exp Bot. 2003;54(380):93–101. doi: 10.1093/jxb/erg035. [DOI] [PubMed] [Google Scholar]
- 18.Gu Y, et al. A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J Cell Biol. 2005;169(1):127–38. doi: 10.1083/jcb.200409140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang JU, et al. Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol Biol Cell. 2005;16(11):5385–99. doi: 10.1091/mbc.E05-05-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang JU, et al. A tip-localized RhoGAP controls cell polarity by globally inhibiting Rho GTPase at the cell apex. Curr Biol. 2008;18(24):1907–16. doi: 10.1016/j.cub.2008.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan A, Xu G, Yang ZB. Calcium participates in feedback regulation of the oscillating ROP1 Rho GTPase in pollen tubes. Proc Natl Acad Sci U S A. 2009;106(51):22002–7. doi: 10.1073/pnas.0910811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang JU, et al. Pollen-tube tip growth requires a balance of lateral propagation and global inhibition of Rho-family GTPase activity. J Cell Sci. 2010;123(Pt 3):340–50. doi: 10.1242/jcs.039180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helling D, et al. Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. Plant Cell. 2006;18(12):3519–34. doi: 10.1105/tpc.106.047373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klahre U, Kost B. Tobacco RhoGTPase ACTIVATING PROTEIN1 spatially restricts signaling of RAC/Rop to the apex of pollen tubes. Plant Cell. 2006;18(11):3033–46. doi: 10.1105/tpc.106.045336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye J, et al. Arabidopsis formin3 directs the formation of actin cables and polarized growth in pollen tubes. Plant Cell. 2009;21(12):3868–84. doi: 10.1105/tpc.109.068700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu Y, et al. Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control Rho GTPase-dependent polar growth. Plant Cell. 2006;18(2):366–81. doi: 10.1105/tpc.105.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, McCormick S. A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2007;104(47):18830–5. doi: 10.1073/pnas.0705874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang F, Gu Y, Yang Z. AtPRK2 promotes ROP1 activation via RopGEFs in the control of polarized pollen tube growth. Molecular Plant. 2012 doi: 10.1093/mp/sss103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Layton AT, et al. Modeling vesicle traffic reveals unexpected consequences for Cdc42p-mediated polarity establishment. Curr Biol. 2011;21(3):184–94. doi: 10.1016/j.cub.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duan Q, et al. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc Natl Acad Sci U S A. 2010;107(41):17821–6. doi: 10.1073/pnas.1005366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheung AY, Wu HM. THESEUS 1, FERONIA and relatives: a family of cell wall-sensing receptor kinases? Curr Opin Plant Biol. 2011;14(6):632–41. doi: 10.1016/j.pbi.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Jones MA, et al. The Arabidopsis Rop2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell. 2002;14(4):763–76. doi: 10.1105/tpc.010359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molendijk AJ, et al. Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J. 2001;20(11):2779–88. doi: 10.1093/emboj/20.11.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu Y, et al. A ROP GTPase signaling pathway controls cortical microtubule ordering and cell expansion in Arabidopsis. Curr Biol. 2009;19(21):1827–32. doi: 10.1016/j.cub.2009.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu Y, et al. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell. 2005;120(5):687–700. doi: 10.1016/j.cell.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 36••.Oda Y, Fukuda H. Initiation of cell wall pattern by a Rho- and microtubule-driven symmetry breaking. Science. 2012;337(6100):1333–6. doi: 10.1126/science.1222597. This is an elegant study that demonstrates that cell wall patterns in xylem cells is established by a plasma membrane symmetry breaking mechanism that invovles mutual inhibition between self-oganizing ROP GTPase activity and cortical MTs.
- 37•.Oda Y, et al. Wood cell-wall structure requires local 2D-microtubule disassembly by a novel plasma membrane-anchored protein. Curr Biol. 2010;20(13):1197–202. doi: 10.1016/j.cub.2010.05.038. This study identified a SCR1-related protein, termed MIDD1, that is localized to the pits of pitted secondary walls and is required for wall patterning by promoting the disassembly of cortical MTs.
- 38.Lavy M, et al. A Novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr Biol. 2007;17(11):947–52. doi: 10.1016/j.cub.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 39.Li S, et al. RIP1 (ROP Interactive Partner 1)/ICR1 marks pollen germination sites and may act in the ROP1 pathway in the control of polarized pollen growth. Mol Plant. 2008;1(6):1021–35. doi: 10.1093/mp/ssn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang G, et al. A mutation in MRH2 kinesin enhances the root hair tip growth defect caused by constitutively activated ROP2 small GTPase in Arabidopsis. PLoS One. 2007;2(10):e1074. doi: 10.1371/journal.pone.0001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Xu T, et al. Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell. 2010;143(1):99–110. doi: 10.1016/j.cell.2010.09.003. The report has established the first cytoplasmic signaling pathways that are dependent on ABP1 and ROP GTPases but independent of the well-known TIR1 pathway. These signaling pathways are shown to promote the generate of multi-polarity in pavement cells.
- 42.Geldner N, et al. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;413(6854):425–8. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- 43.Geldner N, et al. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell. 2003;112(2):219–30. doi: 10.1016/s0092-8674(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 44.Swarup R, et al. Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev. 2001;15(20):2648–53. doi: 10.1101/gad.210501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swarup R, Bennett M. Auxin transport: the fountain of life in plants? Dev Cell. 2003;5(6):824–6. doi: 10.1016/s1534-5807(03)00370-8. [DOI] [PubMed] [Google Scholar]
- 46.Dhonukshe P, et al. Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions. Nature. 2008;456(7224):962–6. doi: 10.1038/nature07409. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Kleine-Vehn J, Friml J. Polar targeting and endocytic recycling in auxin-dependent plant development. Annu Rev Cell Dev Biol. 2008;24:447–73. doi: 10.1146/annurev.cellbio.24.110707.175254. [DOI] [PubMed] [Google Scholar]
- 48.Michniewicz M, et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell. 2007;130(6):1044–56. doi: 10.1016/j.cell.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 49.Friml J, et al. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science. 2004;306(5697):862–5. doi: 10.1126/science.1100618. [DOI] [PubMed] [Google Scholar]
- 50.Gao X, et al. Cell polarity signaling: focus on polar auxin transport. Mol Plant. 2008;1(6):899–909. doi: 10.1093/mp/ssn069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J, et al. Inositol trisphosphate-induced Ca2+ signaling modulates auxin transport and PIN polarity. Dev Cell. 2011;20(6):855–66. doi: 10.1016/j.devcel.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 52.Mei Y, et al. Arabidopsis phosphatidylinositol monophosphate 5-kinase 2 is involved in root gravitropism through regulation of polar auxin transport by affecting the cycling of PIN proteins. Cell Res. 2012;22(3):581–97. doi: 10.1038/cr.2011.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53••.Nagawa S, et al. ROP GTPase-dependent actin microfilaments promote PIN1 polarization by localized inhibition of clathrin-dependent endocytosis. PLoS Biol. 2012;10(4):e1001299. doi: 10.1371/journal.pbio.1001299. The work provides the first direct evidence that ROP GTPase-dependent auxin signaling inhibits PIN1 endocytosis.
- 54•.Lin D, et al. A ROP GTPase-dependent auxin signaling pathway regulates the subcellular distribution of PIN2 in Arabidopsis roots. Curr Biol. 2012;22(14):1319–25. doi: 10.1016/j.cub.2012.05.019. One of the two back-to-back reports showing that ROP GTPase-based auxin signaling inhibits PIN endocytosis in roots, suggesting this signaling provides a common self-organizing mechanism for PIN distribution (see ref 55).
- 55•.Chen X, et al. ABP1 and ROP6 GTPase signaling regulate clathrin-mediated endocytosis in Arabidopsis roots. Curr Biol. 2012;22(14):1326–32. doi: 10.1016/j.cub.2012.05.020. One of the two back-to-back reports showing that ROP GTPase-based auxin signaling inhibits PIN endocytosis in roots, suggesting this signaling provides a common self-organizing mechanism for PIN distribution (see ref 54).
- 56.Kitakura S, et al. Clathrin mediates endocytosis and polar distribution of PIN auxin transporters in Arabidopsis. Plant Cell. 2011;23(5):1920–31. doi: 10.1105/tpc.111.083030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57••.Robert S, et al. ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell. 2010;143(1):111–21. doi: 10.1016/j.cell.2010.09.027. This is the first study to indicate that ABP1-medated perception of auxin regulates endocytosis of PIN proteins.
- 58.Shi JH, Yang ZB. Is ABP1 an auxin receptor yet? Mol Plant. 2011;4(4):635–40. doi: 10.1093/mp/ssr050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Hazak O, et al. A rho scaffold integrates the secretory system with feedback mechanisms in regulation of auxin distribution. PLoS Biol. 2010;8(1):e1000282. doi: 10.1371/journal.pbio.1000282. This paper provides evidence that SCR1 acts as a ROP effector and plays a role in the regulation of PIN1 recycling. This supports a likely link between ROP signaling and PIN1 recycling.
- 60.Alassimone J, Naseer S, Geldner N. A developmental framework for endodermal differentiation and polarity. Proc Natl Acad Sci U S A. 2010;107(11):5214–9. doi: 10.1073/pnas.0910772107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roppolo D, et al. A novel protein family mediates Casparian strip formation in the endodermis. Nature. 2011;473(7347):380–3. doi: 10.1038/nature10070. [DOI] [PubMed] [Google Scholar]
- 62.Dong J, MacAlister CA, Bergmann DC. BASL controls asymmetric cell division in Arabidopsis. Cell. 2009;137(7):1320–30. doi: 10.1016/j.cell.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


