Abstract
The proper development of the nervous system requires precise spatial and temporal control of gene expression at both the transcriptional and translational levels. In different experimental model systems, microRNAs (miRNAs) – a class of small, endogenous, noncoding RNAs that control the translation and stability of many mRNAs – are emerging as important regulators of various aspects of neuronal development. Further dissection of the in vivo physiological functions of individual miRNAs promises to offer novel mechanistic insights into the gene regulatory networks that ensure the precise assembly of a functional nervous system.
Introduction
Recent progress in understanding the different classes of small, noncoding RNAs has led to the discovery of novel gene regulatory mechanisms. This knowledge greatly enhances our ability to decipher the complex molecular networks that control the precise spatial and temporal patterns of gene expression crucial for various aspects of animal development. One class of regulatory RNAs is microRNAs (miRNAs), small RNAs of ~22 nucleotides (nt) processed from genome-encoded transcripts of 70–80 nt [1–5]. The first two miRNAs shown to be involved in the control of developmental timing in Caenorhabditis elegans were lin-4, discovered in 1993 [6], and the evolutionarily conserved let-7, discovered in 2000 [7]. Since then, hundreds of miRNAs have been cloned from different species, and a dozen or so have been found to regulate several developmental processes by inhibiting translation or destabilizing target mRNAs [8–11]. Moreover, each miRNA is predicted to target hundreds of mRNAs, suggesting that many protein-coding genes are potentially regulated by this pathway [8–11].
Many miRNAs are developmentally regulated and show tissue-specific expression patterns, including dozens expressed only in the nervous system [12–15]. These miRNAs may play important roles in neuronal development, neuronal function, or both [16,17]. This review summarizes recent exciting findings about the roles of miRNAs and some miRNA pathway proteins in neuronal development, from early neurogenesis and cell-fate specification to neuronal differentiation and synaptic development of postmitotic neurons. The potential link between the miRNA pathway and human neurological disorders is also discussed.
Dicer in neuronal development
One of the key enzymes in miRNA biogenesis is Dicer, a member of the RNase III family of nucleases that cleave double-stranded RNAs [18]. In the canonical miRNA pathway, primary miRNAs (pri-miRNAs) are mostly transcribed by RNA polymerase II and contain 5′ cap structures and poly(A) tails. Pri-miRNAs are processed by the RNase III Drosha in the nucleus to produce ~70 nt precursor miRNAs (pre-miRNAs) with a hairpin structure [19] which are transported to the cytoplasm by exportin-5 in a Ran-GTP-dependent manner [20]. Dicer is required to cleave pre-miRNAs and generate an ~22 nt miRNA duplex that is incorporated into the RNA-induced silencing complex (RISC) [18,21,22]. In the absence of Dicer, most, if not all, miRNAs are not produced properly. Besides its functions in many other developmental processes [23–26], Dicer is essential for proper brain morphogenesis: maternal-zygotic dicer zebrafish mutant embryos fail to produce mature miRNAs and exhibit gross morphological defects in the nervous system. Remarkably, these defects were largely rescued by injection of microRNA-430 (miR-430) alone [27]. Although this study did not detect any dramatic disruption of major signaling pathways involved in early patterning, subtle defects remain to be examined in detail.
A more informative approach is to remove Dicer activity in specific neuronal cell types. In mice, for instance, deletion of Dicer in postmitotic midbrain dopamine neurons causes a progressive loss of those cells, suggesting an essential role of miRNAs in the differentiation or maintenance of dopamine neurons [28]. A good candidate for this process is miR-133b, which is enriched in midbrain and deficient in patients with Parkinson's disease and in animal models of that disorder. Loss of miR-133b activity seems to increase dopamine release slightly in cell cultures [28], suggesting a subtle role in dopamine neuron maturation and function.
The essential role of Dicer in the maintenance of the mature nervous system is also demonstrated by the finding that loss of Dicer-1 in Drosophila dramatically enhances neurodegeneration caused by a mutant form of the spinocerebellar ataxia type 3 (SCA3) protein; this effect may be explained, at least in part, by the absence of bantam, an miRNA that prevents apoptosis during development by suppressing the proapoptotic gene hid [29]. Consistent with this notion, selective genetic ablation of Dicer in Purkinje cells leads to cerebellar degeneration and ataxia [30]. Moreover, in miR-8 mutant flies, the increased expression of the transcriptional regulator Atrophin increases apoptosis in the nervous system [31]. Thus, miRNAs are important in maintaining the structural integrity of mature neurons through fine-tuning the expression levels of key target genes.
Dendritic spines and postsynaptic densities are enriched in Dicer, raising the possibility that it also participates in synaptic development and plasticity [32]. However, the underlying mechanisms may be complicated. Besides the canonical miRNA pathway, Dicer is also required to generate mirtrons—novel ~22 nt small RNAs processed from short intronic hairpins [33,34]. Unlike miRNAs processed by the canonical miRNA pathway, mirtrons are generated independently of Drosha. The involvement of Dicer in other processes, such as heterochromatin assembly and processing of other endogenous double-stranded RNAs, also remains to be further elucidated in various model systems [35]. Therefore, neurodevelopmental defects as a result of the global loss of Dicer activity need to be interpreted with caution. For this reason, using loss-of-function approaches to understand the functions of specific miRNAs in different aspects of neuronal development may prove to be more informative.
Fragile X mental retardation protein 1
Another protein implicated in miRNA biogenesis is the fragile X mental retardation protein 1 (FMRP). Loss of FMRP function causes fragile X syndrome, the most common form of inherited mental retardation in humans [36]. FMRP is an evolutionarily conserved RNA-binding protein with two ribonucleoprotein K homology (KH) domains and an arginine- and glycine-rich domain (RGG box) that preferentially bind in vitro to tertiary RNA structures named the ‘kissing complex’ [37] and the ‘G quartet’ [38], respectively. Although hundreds of mRNAs associate preferentially with FMRP-containing complexes [39,40], systematic identification of the RNA species that directly bind to FMRP in the native environment in neurons remains a major challenge.
Although the exact function of FMRP at the molecular level still remains unknown, it appears to be associated with the miRNA pathway. In concurrent attempts to identify new components in purified RISC [41] and in a complex associated with the Drosophila homolog of the FMRP (dFMR1) [42], it was found that dFMR1 and the RISC subunit Argonaute 2 (Ago2) form a complex in Drosophila S2 cells. Ago2- and dFMR1-associated complexes also contain Dicer and miR-2b [41,42]. Moreover, FMRP, the mammalian Argonaute family protein elF2C2 and 20 nt small RNAs with unknown identity were found in the immunoprecipitated mRNP complexes from human tumor cell lines [43]. These findings suggest a role for dFMR1 in the miRNA pathway but dFMR1 does not appear to be an essential component in RISC [41,42].
Mutant mice or flies lacking FMRP or dFMR1 are viable and have a grossly normal nervous system. Nonetheless, the loss of these proteins causes various defects in spine and synapse formation [44–47], axonal and dendritic growth and branching [48–51] and neurogenesis [52]. Although FMRP is a multifunctional protein involved in several different stages of RNA metabolism, its effects on neuronal development are probably mediated at least in part through the miRNA pathway. Consistent with this notion, dFMR1 interacts with Mel31B, a P-body protein required for miRNA-mediated repression of translation [53]. Further dissection of the exact role of dFMR1 and FMRP in RISC will help explain the link between the miRNA pathway and human mental disorders.
miRNAs in early neurogenesis
Genetic analysis of individual miRNAs has begun to shed light on their specific functions in different aspects of neuronal development, from early neurogenesis to synaptic formation. The Drosophila peripheral nervous system is an excellent model system for dissecting genetic programs underlying early neurogenesis. Clusters of ectodermal cells that express proneural genes give rise to sensory organ precursors (SOPs) through lateral inhibition, a process that requires the actions of the Notch signaling pathway (Figure 1) [54]. Enhancer of Split complex (E[spl]-C) and the Bearded complex (Brd-C) are major Notch target genes whose 3′ UTRs contain potential target recognition sequences for several miRNAs [55]. Indeed, ectopic expression of miR-7 and a few other miRNAs increases SOP production, likely through downregulation of Notch target genes, although the effects of these miRNAs on SOP specification have not been examined by loss-of-function approaches [56].
Figure 1.
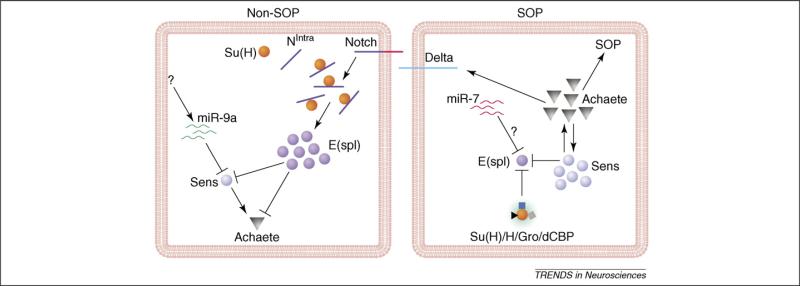
The roles of miRNAs in the specification of SOPs. In non-SOP cells in the proneural cluster, enhanced Notch signaling leads to the association between Su(H) and Notch intracellular domain (NIntra), which in turn activates the transcription of E(spl). E(spl) suppresses the expression of Sens and proneural genes. To ensure a low level of Sens expression in non-SOP cells, miR-9a suppresses Sens through its 3′ UTR. In SOPs, the lack of Notch signaling leads to the formation of a repressor complex containing Su(H), which inhibits E(spl) expression. Sens expression is high and maintains proneural gene expression that endows the SOP fate. The absence of miR-9a in SOPs is partially responsible for the high level of Sens expression. miR-7 and other miRNAs may be involved in the suppression of E(spl).
Drosophila miR-9a is 100% conserved at the nucleotide level with vertebrate miR-9a, which is specifically expressed in the brain [12–14], raising the possibility that it may play an important role in brain development. The physiological function of miR-9a in neuronal development was revealed in Drosophila by both loss- and gain-of-function analyses [57]. miR-9a mutant flies are viable and fertile, but a small number of mutant embryos or adults show extra sensory neurons and sensory bristles as a result of increased production of SOPs during early neurogenesis. Conversely, ectopic expression of the miR-9a precursor suppressed SOP specification [57]. miR-9a is highly expressed in epithelial cells, including those adjacent to SOPs in proneural clusters, indicating that miR-9a inhibits neuronal fate in non-SOP cells to ensure the precise specification of neuronal precursors during development (Figure 1).
During SOP specification, expression of the zinc finger transcription factor Senseless (Sens) must be downregulated in non-SOP cells in the proneural cluster [58]. The sens 3′ UTR contains three computationally predicted miR-9a binding sites [59]. Indeed, in transfected HEK 293 cells, reporter gene expression was suppressed by miR-9a through the sens 3′ UTR [57]. More importantly, miR-9a and sens showed strong genetic interactions. For instance, loss of one copy of sens significantly rescued the SOP defects in miR-9a mutants, indicating that the sens 3′ UTR is a key, physiologically relevant in vivo target of miR-9a in non-SOP cells during early neurogenesis [57]. Although each miRNA is predicted to target hundreds of mRNAs, it is possible that only the alterations in the expression levels of a few key target mRNAs are relevant to a specific biological process.
It is likely that miR-9a also regulates early neurogenesis in vertebrates, as it is specifically expressed in proliferating neural precursors in zebrafish [14] and in mouse embryos and adult mice [60,61]. In fact, miR-9a appears to contribute to the in vitro differentiation of embryonic stem cells [62]. Whether miR-9a also functions to suppress the random activation of neurogenic genes during mammalian neurogenesis is unknown. Loss-of-function studies in genetically altered mice should offer novel insights into this important question. miR-9a is 100% conserved at the nucleotide level from flies to humans, but the mechanism of its action and its targets may not be evolutionarily conserved. Indeed, the microRNA let-7 regulates different genetic pathways in different organisms, probably through distinct downstream target mRNAs [63]. The key target mRNAs of miR-9a in mammalian neurogenesis are determined.
Lsy-6 and miR-273 in cell-fate specification
A remarkable feature of the nervous system is the diversity of its neurons, which differ in dendritic morphologies, axonal targeting specificities, neurotransmitters and other cell-fate-specific characteristics. Although transcriptional control of neuronal cell identity is well established, miRNAs are also involved in this important step of neuronal development.
In C. elegans, for example, two morphologically similar chemosensory neurons – ASE left (ASEL) and ASE right (ASER) – express different chemoreceptors that correlate with the functional differences between the two neurons [64,65]. lsy-6, the first miRNA shown to be involved in neuronal development, is expressed in ASEL but not in ASER (Figure 2). Genetic analysis showed that lsy-6 is required to specify ASEL identity: loss of lsy-6 leads to loss of the ASEL-specific chemoreceptor Gcy-7 and ectopic expression of the ASER-specific chemoreceptor Gcy-5 in ASEL neurons [66]. lsy-6 exerts its effects by binding to the 3′ UTR of cog-1, an Nkx-type homeobox gene, which leads to downregulation of Cog-1 expression [66]. The specific expression of lsy-6 in ASEL but not in ASER neurons is controlled by the zinc finger transcription factors Lsy-2 [67] and Die-1 [68]. Interestingly, miR-273 is expressed at a much higher level in ASER than in ASEL neurons, and overexpression of miR-273 in ASEL suppresses Die-1 expression, although the effects of loss of miR-273 have not been examined yet [68]. Thus, downregulation of Die-1 in ASER is probably a result of the action of miR-273. Moreover, miR-273 expression is activated by Cog-1. Suppression of Cog-1 expression in ASEL by lsy-6 accounts for the lower expression of miR-273 in those neurons than in ASER neurons [69]. These findings indicate that specific miRNAs and transcription factors form a double-negative feedback loop to maintain the cellular identities of ASEL and ASER neurons (Figure 2) [70]. In this case, the direct effect of miRNAs on target gene expression does not have to be dramatic, but they can still function as developmental switches as a result of their involvement in feedback loops that can amplify and maintain different expression levels of key transcription factors in these two types of neurons.
Figure 2.
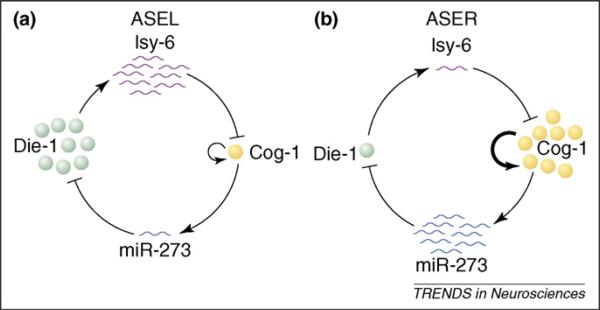
Schematic representation of the double negative feedback loops between miRNAs and transcription factors. (a) In ASEL sensory neurons in C. elegans, a high level of lsy-6 suppresses Cog-1, which controls the expression of miR-273. (b) In ASER, a high level of miR-273 suppresses Die-1, a transcription factor required for lsy-6 expression.
miR-124a in neuronal differentiation
miR-124a is 100% conserved at the nucleotide level from worms to humans and is expressed throughout the embryonic and adult central nervous systems of different species [12–15]. It is estimated that miR-124a is the most abundant miR in the brain, accounting for 25%–48% of all brain miRNAs [12]. Thus, it may play an important role in neuronal differentiation or function.
In mouse brain, miR-124a seems to be largely restricted to differentiating and mature neurons, with much less expression in neural progenitors [60]. This expression profile seems to be controlled by RE1 silencing transcription factor (REST), a transcription repressor that inhibits miR-124a expression in nonneuronal cells and neural progenitors but is absent from the miR-124a locus in mature neurons [71].
Ectopic expression of miR-124a in HeLa cells leads to the suppression of a large number of nonneuronal transcripts [72]. Some of these transcripts are elevated in cortical neurons treated with antisense 2′-O-methyl oligonucleotides complementary to miR-124a [71], suggesting that these mRNAs are endogenous targets of miR-124a. Therefore, neuronal differentiation may require both derepression of REST and downregulation of some mRNAs by miR-124a. As the most abundant miRNA in the brain, miR-124a likely regulates many target mRNAs and therefore may even play a role in maintaining the homeostasis of differentiated neurons. For instance, target mRNAs encoding small C-terminal domain phosphatase 1 (SCP1) [73], laminin γ1 and integrin β1 [74] are downregulated in differentiated neurons (Figure 3). Moreover, downregulation of the RNA-binding protein PTBP1 by miR-124a during neuronal differentiation leads to a global neuron-specific alternative splicing pattern [75].
Figure 3.
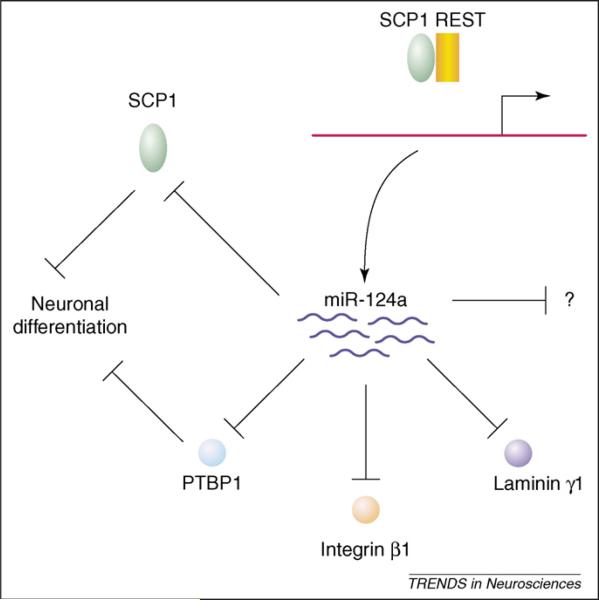
The role of miR-124a in neuronal development and its regulation by REST. The upregulation of miR-124a expression during neuronal differentiation requires the derepression by the REST-SCP1 complex. As the most abundant miRNA in the brain, miR-124a regulates the expression of many target mRNAs. Yet, the developmental consequences of lack of miR-124a in vivo remain to be further examined.
The exact developmental consequences of the loss of endogenous miR-124a in differentiating neurons are unclear. In the chick neural tube, neither inhibition nor overexpression of miR-124a altered neuronal fate, as assessed with cell-specific markers [74]. However, another study reported a seemingly opposite result from the same assay system [73]. The reason for this discrepancy is unknown. Further investigation will be required to determine whether endogenous miR-124a affects other aspects of neuronal differentiation, such as dendritic/axonal growth as in the case of miR-132 [76], or synaptogenesis, as in the case of miR-134 [77].
miR-7 in photoreceptor differentiation
Another well-established system for studying cell differentiation is the Drosophila eye, which consists of ~800 ommatidia. In each ommatidium, the R8 photoreceptor neuron differentiates first and recruits other progenitor cells to differentiate into seven other photoreceptor neurons and support cells. A key factor that ensures the timely differentiation of retinal cells is the ETS-domain transcription repressor Yan, which is expressed in progenitor cells and suppresses their differentiation into photoreceptors [78]. Signaling through the epidermal growth factor leads to activation of the RAS-ERK pathway and rapid degradation of Yan [78]. The absence of Yan in differentiating cells is essential for the specification of photoreceptor neuronal fate.
Yan controls miR-7 transcription, and high-level Yan expression in progenitor cells represses miR-7 expression. In differentiating cells, miR-7 expression is elevated as a consequence of Yan degradation, and miR-7 further represses Yan expression by binding to sequences in its mRNA 3′ UTR. Thus, miR-7 and Yan form a reciprocal negative feedback loop and show a mutually exclusive expression pattern (Figure 4). Indeed, ectopic expression of miR-7 promotes photoreceptor neuron differentiation [79]. Interestingly, miR-7 loss-of-function mutants have no obvious defects in eye development [79], suggesting that in this particular case, miR-7 does not function as an absolute switch in the feedback loop. ERK-mediated phosphorylation and degradation likely play a major role in downregulating Yan in differentiating photoreceptor neurons, whereas miR-7 ensures its complete depletion. Such a negative feedback loop also operates between miR-133b and the paired-like homeodomain transcription factor Pitx3 during the maturation of midbrain dopamine neurons [28], raising the possibility that it is a common module in gene regulatory networks.
Figure 4.
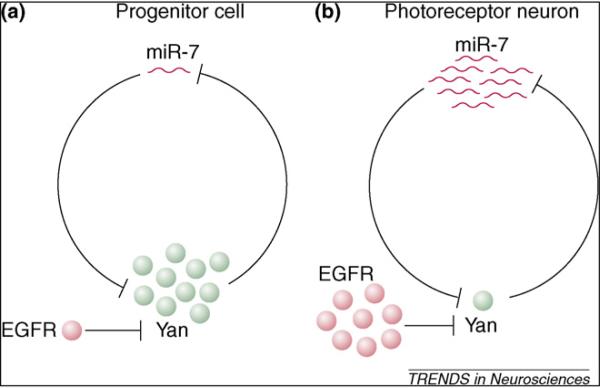
A negative feedback loop between an miRNA and a transcription factor in Drosophila. (a) In progenitor cells in the Drosophila eye, high-level expression of the transcription factor Yan suppresses miR-7 expression. (b) During photoreceptor differentiation, transient activation of the epidermal growth factor receptor (EGFR) signaling pathway leads to the degradation of Yan and the expression of miR-7, which further downregulates the level of Yan through binding to its 3′ UTRs.
miR-134 in synaptogenesis
Since the discovery of polyribosomes near spines in distal dendrites of dentate granule neurons in 1982 [80], a large number of mRNAs have been found in dendrites [81]. The functional significance of local protein synthesis in dendrites was demonstrated by its requirement in brain-derived neurotrophic factor (BDNF)-induced synaptic plasticity in the hippocampus and by experiments in different experimental systems [81]. Because miRNAs, as well as several protein factors that either positively or negatively influence mRNA translation and stability, are often associated with active polyribosomes, some miRNAs are expected to be present in dendrites and help control local protein synthesis. Therefore, these miRNAs may contribute to synapse formation and synaptic function by regulating the local translation of their target mRNAs.
Indeed, miR-134, a brain-specific miRNA, localizes near synaptic sites in dendrites of hippocampal neurons and regulates the size of dendritic spines [77]. miR-134, but not let-7c, negatively regulates the width of dendritic spines but not their density or dendritic branching. Reduced miR-134 activity with 2′-O-methylated antisense oligonucleotide decreased spine width by 7.6%. Considering the heterogeneity and the dynamic nature of dendritic spines on cultured neurons, this phenotype is relatively subtle and suggests a modulatory role for miR-134 in spine formation. Future genetic knockout of miR-134 will no doubt reveal the full extent of miR-134 function in this important process.
How does miR-134 exert its effect on spine shape? The 3′ UTR of Limk1, one of the BDNF-induced genes that regulate actin polymerization and microtubule disassembly [82], contains one miR-134 binding site and acts as the major downstream mediator of miR-134 function. miR-134 negatively regulates the translation of Limk1 mRNA in dendrites through its 3′ UTR in a manner that is dependent on the miR-134 binding site. Moreover, the effect of miR-134 overexpression on spine shape can be rescued by overexpression of Limk1, whose mRNA is not regulated by miR-134. Interestingly, BDNF treatment relieves miR-134-dependent inhibition of Limk1 translation, which seems to be mediated by the mammalian target of rapamycin (mTOR) pathway [77]. Exactly how BDNF does so and how miRNAs regulate local translation in dendrites remain to be determined [83]. It is largely unknown what regulates the association and dissociation of miRNAs and their target 3′ UTRs in neurons. However, recent advances in our understanding of the actions of miRNAs in other cell types [84–87] raise the possibility that regulation of translation initiation, elongation, polyadenylation or mRNA stability by different miRNAs in response to extrinsic factors or neuronal activity may operate locally near synapses as well.
Concluding remarks
It has become increasingly clear that miRNAs modulate gene expression levels during multiple steps of neuronal development in diverse organisms, from early neurogenesis to synaptogenesis. In a few cases, miRNAs are involved in feedback loops with some key transcription factors and seem to function as molecular switches in neuronal development. In many other cases, the effects of a specific miRNA are relatively subtle, suggesting that miRNAs ensure the precision of gene expression and the accuracy of these neurodevelopmental events. This unique function is no less important than other molecular regulators whose misexpression often leads to robust developmental defects. However, our current understanding of miRNA function in the nervous system is still in its infancy, and the number of miRNAs that have been analyzed by loss-of-function approaches remains very small (Table 1). Additional genetic analysis with more sensitive functional assays will undoubtedly reveal the full extent of miRNA function in neuronal development and may offer novel insights into human neurological disorders as well.
Table 1.
The functions of different miRNAs in the development and maintenance of the nervous system
| miRNAs | Species | Approaches | Functions | Targets | Refs |
|---|---|---|---|---|---|
| lsy-6 | C. elegans | LOF and GOF in vivo | Required to specify ASEL sensory neuron identity | Cog-1 | [66,70] |
| miR-273 | C. elegans | GOF in vivo | Expressed in ASER and suppresses ASEL identity | Die-1 | [68–70] |
| miR-7 | Drosophila | LOF and GOF in vivo | Ensures complete depletion of Yan photoreceptor differentiation | Yan | [79] |
| miR-430 | Zebrafish | Genetic rescue | Required for clearance of maternal mRNAs and brain morphogenesis | ?? | [27] |
| miR-134 | Rodent | LOF and GOF in culture | Modulates the size of dendritic spines in cultured neurons | LimK1 | [77] |
| miR-9a | Drosophila | LOF and GOF in vivo | Ensures the precise specification of SOPs in Drosophila | Senseless | [57] |
| miR-124a | Vertebrates | LOF and GOF in vivoa and in culture | Promotes neuronal differentiation (?) | Laminin γ1, integrin β1, SCP1, PTBP1 | [71–75] |
| miR-132 | Rodent | LOF and GOF in culture | Regulates neuronal morphogenesis and circadian clock | P250GAP, etc. | [76,88] |
| miR-9a | Rodent | LOF and GOF in culture | Involved in neural lineage differentiation from embryonic stem cells | ?? | [62] |
| miR-133b | Rodent | LOF and GOF in culture | Regulates the maturation/function of midbrain dopamine neurons | Pitx3 | [28] |
| Bantam | Drosophila | LOF and GOF in vivo | Prevents neurodegeneration in a Drosophila model of SCA3 | ?? | [29] |
| miR-8 | Drosophila | LOF and GOF in vivo | Required for neuronal survival | Atrophin | [31] |
| miR-219 | Rodent | LOF in vivob | Regulates circadian period length in mice | SCOP, etc. | [88] |
Abbreviations: GOF, gain of function; LOF, loss of function; SCA3, spinocerebellar ataxia type 3.
Antisense 2′-O-methyl oligonucleotides were used to inhibit the activity of endogenous miR-124a in the developing chick neural tube.
Cholesterol-modified oligonucleotides (antagomirs) were used to repress miR-219 in vivo.
Acknowledgements
I thank J. Carroll for help with graphics, S. Ordway for editorial assistance, E. Pierce for administrative assistance, and laboratory members for discussions over the years. This work is supported by the FRAXA Foundation and the National Institutes of Health (F-B.G.).
References
- 1.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 3.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 4.Du T, Zamore PD. microPrimer: the biogenesis and function of microRNA. Development. 2005;132:4645–4652. doi: 10.1242/dev.02070. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y, et al. Drosha in primary microRNA processing. Cold Spring Harb. Symp. Quant. Biol. 2006;71:51–57. doi: 10.1101/sqb.2006.71.041. [DOI] [PubMed] [Google Scholar]
- 6.Lee RC, et al. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 7.Reinhart BJ, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 8.Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev. Cell. 2006;11:441–450. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, Srivastava D. A developmental view of microRNA function. Trends Biochem. Sci. 2007;32:189–197. doi: 10.1016/j.tibs.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Bushati N, Cohen SM. microRNA functions. Annu. Rev. Cell Dev. Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 11.Chang TC, Mendell JT. The roles of microRNAs in vertebrate physiology and human disease. Annu. Rev. Genomics Hum. Genet. 2007;8:215–239. doi: 10.1146/annurev.genom.8.080706.092351. [DOI] [PubMed] [Google Scholar]
- 12.Lagos-Quintana M, et al. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 13.Kim J, et al. Identification of many microRNAs that copurify with polyribosomes in mammalian neurons. Proc. Natl. Acad. Sci. U. S. A. 2004;101:360–365. doi: 10.1073/pnas.2333854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wienholds E, et al. MicroRNA expression in zebrafish embryonic development. Science. 2005;309:310–311. doi: 10.1126/science.1114519. [DOI] [PubMed] [Google Scholar]
- 15.Aboobaker AA, et al. Drosophila microRNAs exhibit diverse spatial expression patterns during embryonic development. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18017–18022. doi: 10.1073/pnas.0508823102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao X, et al. Noncoding RNAs in the mammalian central nervous system. Annu. Rev. Neurosci. 2006;29:77–103. doi: 10.1146/annurev.neuro.29.051605.112839. [DOI] [PubMed] [Google Scholar]
- 17.Kosik KS. The neuronal microRNA system. Nat. Rev. Neurosci. 2006;7:911–920. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- 18.Bernstein E, et al. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 20.Yi R, et al. Exportin-5 mediates the nuclear export of premicroRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutvágner G, et al. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 22.Grishok A, et al. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell. 2001;106:23–34. doi: 10.1016/s0092-8674(01)00431-7. [DOI] [PubMed] [Google Scholar]
- 23.Wienholds E, et al. The microRNA-producing enzyme Dicer1 is essential for zebrafish development. Nat. Genet. 2003;35:217–218. doi: 10.1038/ng1251. [DOI] [PubMed] [Google Scholar]
- 24.Hatfield SD, et al. Stem cell division is regulated by the microRNA pathway. Nature. 2005;435:974–978. doi: 10.1038/nature03816. [DOI] [PubMed] [Google Scholar]
- 25.Harfe BD, et al. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc. Natl. Acad. Sci. U. S. A. 2005;102:10898–10903. doi: 10.1073/pnas.0504834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murchison EP, et al. Critical roles for Dicer in the female germline. Genes Dev. 2007;21:682–693. doi: 10.1101/gad.1521307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giraldez AJ, et al. MicroRNAs regulate brain morphogenesis in zebrafish. Science. 2005;308:833–838. doi: 10.1126/science.1109020. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, et al. A microRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317:1220–1224. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bilen J, et al. MicroRNA pathways modulate polyglutamine-induced neurodegeneration. Mol. Cell. 2006;24:157–163. doi: 10.1016/j.molcel.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 30.Schaefer A, et al. Cerebellar neurodegeneration in the absence of microRNAs. J. Exp. Med. 2007;204:1553–1558. doi: 10.1084/jem.20070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karres JS, et al. The conserved microRNA miR-8 tunes atrophin levels to prevent neurodegeneration in Drosophila. Cell. 2007;131:136–145. doi: 10.1016/j.cell.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 32.Lugli G, et al. Dicer and eIF2c are enriched at postsynaptic densities in adult mouse brain and are modified by neuronal activity in a calpain-dependent manner. J. Neurochem. 2005;94:896–905. doi: 10.1111/j.1471-4159.2005.03224.x. [DOI] [PubMed] [Google Scholar]
- 33.Okamura K, et al. The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell. 2007;130:89–100. doi: 10.1016/j.cell.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruby JG, et al. Intronic microRNA precursors that bypass Drosha processing. Nature. 2007;448:83–86. doi: 10.1038/nature05983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zofall M, Grewal SI. RNAi-mediated heterochromatin assembly in fission yeast. Cold Spring Harb. Symp. Quant. Biol. 2006;71:487–496. doi: 10.1101/sqb.2006.71.059. [DOI] [PubMed] [Google Scholar]
- 36.Penagarikano O, et al. The pathophysiology of fragile X syndrome. Annu. Rev. Genomics Hum. Genet. 2007;8:109–129. doi: 10.1146/annurev.genom.8.080706.092249. [DOI] [PubMed] [Google Scholar]
- 37.Darnell JC, et al. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–499. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- 38.Darnell JC, et al. Kissing complex RNAs mediate interaction between the fragile-X mental retardation protein KH2 domain and brain polyribosomes. Genes Dev. 2005;19:903–918. doi: 10.1101/gad.1276805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown V, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 40.Miyashiro KY, et al. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron. 2003;37:417–431. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 41.Caudy AA, et al. Fragile X related protein and VIG associate with the RNA interference machinery. Genes Dev. 2002;16:2491–2496. doi: 10.1101/gad.1025202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishizuka A, et al. A Drosophila fragile X protein interacts with components of RNAi and ribosomal proteins. Genes Dev. 2002;16:2497–2508. doi: 10.1101/gad.1022002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin P, et al. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat. Neurosci. 2004;7:113–117. doi: 10.1038/nn1174. [DOI] [PubMed] [Google Scholar]
- 44.Nimchinsky EA, et al. Abnormal development of dendritic spines in FMR1 knock-out mice. J. Neurosci. 2001;21:5139–5146. doi: 10.1523/JNEUROSCI.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, et al. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001;107:591–603. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]
- 46.Grossman AW, et al. Hippocampal pyramidal cells in adult Fmr1 knockout mice exhibit an immature-appearing profile of dendritic spines. Brain Res. 2006;1084:158–164. doi: 10.1016/j.brainres.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 47.Pfeiffer BE, Huber KM. Fragile X mental retardation protein induces synapse loss through acute postsynaptic translational regulation. J. Neurosci. 2007;27:3120–3130. doi: 10.1523/JNEUROSCI.0054-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dockendorff TC, et al. Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron. 2002;34:973–984. doi: 10.1016/s0896-6273(02)00724-9. [DOI] [PubMed] [Google Scholar]
- 49.Morales J, et al. Drosophila fragile X protein, dFXR, regulates neuronal morphology and function in the brain. Neuron. 2002;34:961–972. doi: 10.1016/s0896-6273(02)00731-6. [DOI] [PubMed] [Google Scholar]
- 50.Lee A, et al. Control of dendritic development by the Drosophila fragile X-related gene involves the small GTPase Rac1. Development. 2003;130:5543–5552. doi: 10.1242/dev.00792. [DOI] [PubMed] [Google Scholar]
- 51.Michel CI, et al. Defective neuronal development in the mushroom bodies of Drosophila fragile X mental retardation 1 mutants. J. Neurosci. 2004;24:5798–5809. doi: 10.1523/JNEUROSCI.1102-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castren M, et al. Altered differentiation of neural stem cells in fragile X syndrome. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17834–17839. doi: 10.1073/pnas.0508995102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barbee SA, et al. Staufen- and FMRP-containing neuronal RNPs are structurally and functionally related to somatic P bodies. Neuron. 2006;52:997–1009. doi: 10.1016/j.neuron.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Modolell J. Patterning of the adult peripheral nervous system of Drosophila. Perspect. Dev. Neurobiol. 1997;4:285–296. [PubMed] [Google Scholar]
- 55.Lai EC. Micro RNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat. Genet. 2002;30:363–364. doi: 10.1038/ng865. [DOI] [PubMed] [Google Scholar]
- 56.Lai EC, et al. Pervasive regulation of Drosophila Notch target genes by GY-box-, Brd-box-, and K-box-class microRNAs. Genes Dev. 2005;19:1067–1080. doi: 10.1101/gad.1291905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, et al. MicroRNA-9a ensures the precise specification of sensory organ precursors in Drosophila. Genes Dev. 2006;20:2793–2805. doi: 10.1101/gad.1466306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nolo R, et al. Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell. 2000;102:349–362. doi: 10.1016/s0092-8674(00)00040-4. [DOI] [PubMed] [Google Scholar]
- 59.Stark A, et al. Animal microRNAs confer robustness to gene expression and have a significant impact on 3′UTR evolution. Cell. 2005;123:1133–1146. doi: 10.1016/j.cell.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 60.Deo M, et al. Detection of mammalian microRNA expression by in situ hybridization with RNA oligonucleotides. Dev. Dyn. 2006;235:2538–2548. doi: 10.1002/dvdy.20847. [DOI] [PubMed] [Google Scholar]
- 61.Kloosterman WP, et al. In situ detection of miRNAs in animal embryos using LNA-modified oligonucleotide probes. Nat. Methods. 2006;3:27–29. doi: 10.1038/nmeth843. [DOI] [PubMed] [Google Scholar]
- 62.Krichevsky AM, et al. Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells. 2006;24:857–864. doi: 10.1634/stemcells.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ambros V, Chen X. The regulation of genes and genomes by small RNAs. Development. 2007;134:1635–1641. doi: 10.1242/dev.002006. [DOI] [PubMed] [Google Scholar]
- 64.Bargmann CI, Horvitz HR. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991;7:729–742. doi: 10.1016/0896-6273(91)90276-6. [DOI] [PubMed] [Google Scholar]
- 65.Yu S, et al. Guanylyl cyclase expression in specific sensory neurons: a new family of chemosensory receptors. Proc. Natl. Acad. Sci. U. S. A. 1997;94:3384–3387. doi: 10.1073/pnas.94.7.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnston RJ, Hobert O. A microRNA controlling left/right neuronal asymmetry in Caenorhabditis elegans. Nature. 2003;426:845–849. doi: 10.1038/nature02255. [DOI] [PubMed] [Google Scholar]
- 67.Johnston RJ, Hobert O. A novel C. elegans zinc finger transcription factor, lsy-2, required for the cell type-specific expression of the lsy-6 microRNA. Development. 2005;132:5451–5460. doi: 10.1242/dev.02163. [DOI] [PubMed] [Google Scholar]
- 68.Chang S, et al. MicroRNAs act sequentially and asymmetrically to control chemosensory laterality in the nematode. Nature. 2004;430:785–789. doi: 10.1038/nature02752. [DOI] [PubMed] [Google Scholar]
- 69.Johnston RJ, et al. MicroRNAs acting in a double-negative feedback loop to control a neuronal cell fate decision. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12449–12454. doi: 10.1073/pnas.0505530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hobert O. Architecture of a microRNA-controlled gene regulatory network that diversifies neuronal cell fates. Cold Spring Harb. Symp. Quant. Biol. 2006;71:181–188. doi: 10.1101/sqb.2006.71.006. [DOI] [PubMed] [Google Scholar]
- 71.Conaco C, et al. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2422–2427. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lim LP, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 73.Visvanathan J, et al. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 2007;21:744–749. doi: 10.1101/gad.1519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cao X, et al. A functional study of miR-124 in the developing neural tube. Genes Dev. 2007;21:531–536. doi: 10.1101/gad.1519207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Makeyev EV, et al. The microRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol. Cell. 2007;27:435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vo N, et al. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc. Natl. Acad. Sci. U. S. A. 2005;102:16426–16431. doi: 10.1073/pnas.0508448102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schratt GM, et al. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- 78.Voas MG, Rebay I. Signal integration during development: insights from the Drosophila eye. Dev. Dyn. 2004;229:162–175. doi: 10.1002/dvdy.10449. [DOI] [PubMed] [Google Scholar]
- 79.Li X, Carthew RW. A microRNA mediates EGF receptor signaling and promotes photoreceptor differentiation in the Drosophila eye. Cell. 2005;123:1267–1277. doi: 10.1016/j.cell.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 80.Steward O, Levy WB. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J. Neurosci. 1982;2:284–291. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 82.Bernard O. Lim kinases, regulators of actin dynamics. Int. J. Biochem. Cell Biol. 2007;39:1071–1076. doi: 10.1016/j.biocel.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 83.Tai HC, Schuman EM. MicroRNA: microRNAs reach out into dendrites. Curr. Biol. 2006;16:R121–R123. doi: 10.1016/j.cub.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 84.Suvendra N, et al. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111–1124. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 85.Lytle JR, et al. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′UTR as in the 3′UTR. Proc. Natl. Acad. Sci. U. S. A. 2007;104:9667–9672. doi: 10.1073/pnas.0703820104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chendrimada TP, et al. MicroRNA silencing through RISC recruitment of eIF6. Nature. 2007;447:823–828. doi: 10.1038/nature05841. [DOI] [PubMed] [Google Scholar]
- 87.Mathonnet G, et al. MicroRNA inhibition of translation initiation in vitro by targeting the cap-binding complex eIF4F. Science. 2007;317:1764–1767. doi: 10.1126/science.1146067. [DOI] [PubMed] [Google Scholar]
- 88.Cheng HY, et al. MicroRNA modulation of circadian-clock period and entrainment. Neuron. 2007;54:813–829. doi: 10.1016/j.neuron.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


