Abstract
DAX-1 [dosage-sensitive sex reversal, adrenal hypoplasia congenita (AHC) critical region on the X chromosome, gene 1] is an orphan nuclear receptor that represses transcription by steroidogenic factor-1 (SF-1), a factor that regulates expression of multiple steroidogenic enzymes and other genes involved in reproduction. Mutations in the human DAX1 gene (also known as AHC) cause the X-linked syndrome AHC, a disorder that is associated with hypogonadotropic hypogonadism also. Characterization of Dax1-deficient male mice revealed primary testicular defects that included Leydig cell hyperplasia (LCH) and progressive degeneration of the germinal epithelium, leading to infertility. In this study, we investigated the effect of Dax1 disruption on the expression profile of various steroidogenic enzyme genes in Leydig cells isolated from Dax1-deficient male mice. Expression of the aromatase (Cyp19) gene, which encodes the enzyme that converts testosterone to estradiol, was increased significantly in the Leydig cells isolated from mutant mice, whereas the expression of other proteins (e.g., StAR and Cyp11a) was not altered. In in vitro transfection studies, DAX-1 repressed the SF-1-mediated transactivation of the Cyp19 promoter but did not inhibit the StAR or Cyp11a promoters. Elevated Cyp19 expression was accompanied by increased intratesticular levels of estradiol. Administration of tamoxifen, a selective estrogen-receptor modulator, restored fertility to the Dax1-deficient male mice and partially corrected LCH, suggesting that estrogen excess contributes to LCH and infertility. Based on these in vivo and in vitro analyses, aromatase seems to be a physiologic target of Dax-1 in Leydig cells, and increased Cyp19 expression may account, in part, for the infertility and LCH in Dax1-deficient mice.
DAX-1 [dosage-sensitive sex reversal, adrenal hypoplasia congenita (AHC) critical region on the X chromosome, gene 1] is an orphan member of the nuclear hormone receptor superfamily of transcription factors (1–3). Duplication of the region on the X chromosome containing the DAX1 gene is associated with male-to-female sex reversal in XY individuals (1). Loss-of-function mutations in DAX1 are responsible for X-linked AHC, a disorder characterized by primary adrenal insufficiency. Affected individuals lack the permanent zone of the adrenal cortex and have low serum concentrations of mineralocorticoids and glucocorticoids. Hypogonadotropic hypogonadism is also a feature of the syndrome and reflects combined defects in the production of hypothalamic gonadotropin-releasing hormones and pituitary gonadotropins (4–6).
Consistent with the clinical features of AHC, Dax1 transcripts are expressed in the hypothalamus, pituitary gonadotrope cells, adrenal glands, and gonads (6, 7). This pattern of expression is strikingly similar to that of another orphan nuclear receptor, steroidogenic factor-1 (SF-1; ref. 7). SF-1 orchestrates the expression of several steroidogenic enzyme genes and genes that govern sex differentiation and reproduction (8–10). Sf1 knockout mice lack adrenal glands and gonads and exhibit impaired pituitary gonadotrope cell function (11–14). In addition, male knockout mice have XY sex reversal and persistent Müllerian structures (11–14). The colocalization of Sf1 and Dax1 led to the suggestion of a functional interaction between these two orphan nuclear receptors (6, 7). Consistent with this idea, DAX-1 interacts directly with SF-1 and inhibits SF-1-mediated transactivation (15, 16).
Testicular Leydig cells express both Dax1 and Sf1 (7, 17) and constitute the major site of testosterone production in males (17). Testosterone biosynthesis requires five steroidogenic proteins: steroidogenic acute regulatory protein (StAR), cholesterol side-chain cleavage enzyme (CYP11A), 3β-hydroxysteroid dehydrogenase (3β-HSD type II), 17α-hydroxylase (CYP17), and 17β-hydroxysteroid dehydrogenase (17β-HSD type III). Testosterone can be converted to estradiol by means of the nonreversible action of aromatase (CYP19; ref. 17). Therefore, the relative amount of testosterone and estrogen in males is determined largely by the level and activity of aromatase in the testis and other peripheral tissues such as adipose tissue.
Dax1-deficient male mice are infertile and have small testes (18). Serum levels of testosterone, gonadotropins, and adrenal steroids are normal but have been examined only in a limited number of physiologic states (18). In addition to the progressive degeneration of seminiferous tubules, the Dax1-deficient mice exhibit Leydig cell hyperplasia (LCH; ref. 18). Although DAX-1 has been shown to repress SF-1-mediated actions in vitro (15, 16), its functional role in vivo remains poorly understood. In this report, we examined the expression level of steroidogenic enzyme genes in Leydig cells purified from the testes of wild-type and Dax1-deficient mice. These analyses indicate that Dax-1 represses only a subset of SF-1-regulated genes, and that Cyp19 is a physiologic target gene for Dax-1 in Leydig cells. The overexpression of Cyp19 may account, in part, for the infertility and LCH that occurs in Dax1-deficient mice, and potentially in humans with AHC.
Materials and Methods
Animals.
The generation of Dax1 (Ahch)-deficient males has been described (18). All mice were housed under controlled conditions of temperature (21–24°C) and light (12-h light/dark cycle; 7 a.m.–7 p.m.) and maintained on normal mouse chow and water ad libitum. All animal procedures were approved and performed in accordance with the policies of Northwestern University's Animal Care and Use Committee.
Isolation and Culture of Leydig Cells.
The method of Leydig cell isolation from mouse testis was adapted from Hales et al. (19). Twelve-week-old Dax1-deficient male mice and wild-type littermates were killed by cervical dislocation and their testes were removed immediately. After decapsulation, each testis was digested in M199 medium (GIBCO/BRL) containing 0.75 mg/ml collagenase (Worthington) for 10 min at 37°C under conditions of vigorous shaking. Interstitial cells were collected by centrifugation at 600 × g for 20 min and resuspended in M199 medium supplemented with 2.2 g/liter sodium bicarbonate/10 mM Hepes, pH 7.4/500 ng/ml insulin/100 units/ml penicillin/100 μg/ml streptomycin/1 mg/ml BSA. Macrophages were removed by adherence to culture dishes for 25 min in a humidified atmosphere of 5% CO2 at 32°C. Leydig cells were purified from the nonadherent crude interstitial cells by centrifugation (Sorvall HB-4 rotor, 57,000 rpm × 5 min) through an 11–23% metrizamide density gradient and resuspended in serum-free DMEM/F12 medium supplemented with 2.2 g/liter sodium bicarbonate/10 mM Hepes, pH 7.4/500 ng/ml insulin/100 units/ml penicillin/100 μg/ml streptomycin/1 mg/ml BSA. Cells were plated at a density of 1 × 105 cells per cm2 and incubated in a humidified atmosphere of 5% CO2 at 32°C. The Leydig cell preparations were determined to be ≈90% pure by using immunohistochemical staining for 3β-HSD (20).
Reverse Transcription (RT)-PCR.
Total RNA was extracted from purified Leydig cells by using TRIzol reagent (GIBCO/BRL). The RT reaction was performed as described (21). Expression levels of Dax1, StAR, Cyp11a, Cyp17, Cyp19, and Gapdh mRNAs were measured by using primers summarized in Table 1. Primers specific for the testicular isoforms of 3β-HSD (type II) and 17β-HSD (type III) were based on studies by O'Shaughnessy et al. (ref. 22; Table 1). PCR conditions were 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min and 15 seconds, for 24–33 cycles. PCR products were analyzed on 1% agarose gels. For semiquantitative PCR, [32P]dCTP was added to the reaction and PCR products were separated on 6% nondenaturing polyacrylamide gels, visualized by autoradiography, and quantitated by using a phosphor imager (Bio-Rad).
Table 1.
Primer sequences used in PCR
| Target mRNA | Sequence of Primer (5′→3′)
|
|
|---|---|---|
| Sense | Antisense | |
| Dax1 | CACTTGCTCCCAGCTGCTGC | TTGATGAATCTCAGCAGGAA |
| StAR | CGCTCAGGACCTTGAAAGGC | TACAGCGCACGCTCACGAAG |
| Cyp11a | AAGTGGCAGTCGTGGGGACA | ACCCCAATGGGCCTCTGATA |
| Cyp17 | GCCTGACAGACATTCTG | TCGTGATGCAGTGCCCAG |
| 3β-HSD type II | ACTGCAGGAGGTCAGAGCT | GCCAGTAACACACAGAATACC |
| 17β-HSD type III | ATTTTACCAGAGAAGACATCT | GGGGTCAGCACCTGAATAATG |
| Cyp19 | CACCCTTCCAAGTGACAGGA | AAAAAAGTAAAGTTCTATGGGAA |
| Gapdh | CCCTTCATTGACCTCAACTA | CCAAAGTTGTCATGGATGAC |
Western Blot Analyses.
Whole cell protein extracts were prepared from purified Leydig cells, and the protein concentration was determined by using the Bradford assay (23). Proteins were separated on 10% SDS/polyacrylamide gels and electroblotted onto nitrocellulose membranes. Blots were probed with a rabbit polyclonal antiserum directed against human aromatase at a dilution of 1:4,000 (provided by Y. Osawa, Hauptman–Woodward Medical Research Institute, Buffalo, NY; ref. 24). Goat anti-rabbit IgG coupled to peroxidase (Promega) was used as a secondary antibody at a dilution of 1:5,000. Proteins were detected by using an enhanced chemiluminescence detection system (Amersham Pharmacia).
Measurement of Aromatase Activity.
Aromatase activity was determined by using a described tritiated water method (25) that measured the 3H2O released from [1β-3H]androstenedione (36 pmol/μl; New England Nuclear). Briefly, freshly isolated Leydig cells from wild-type or mutant mice testes were plated in 12-well culture dishes in serum-free DMEM/F12 medium. The final concentration of androstenedione was 150 pmol/ml, consisting of 50% [1β-3H]androstenedione and 50% unlabeled androstenedione. Cells were incubated for 12, 24, or 36 h at 32°C. Incubations were conducted in an identical fashion in the absence of cells to establish background values. The incubation was terminated by transferring 1 ml of medium to tubes containing 1 ml of ice-cold 30% (vol/vol) trichloracetic acid. The mixture was vortexed vigorously for 30 sec, and centrifuged at 2,000 × g for 5 min at 4°C to remove precipitated protein. The medium was extracted with 5 ml of chloroform. The aqueous layer (1 ml) was removed and mixed with 1 ml of activated charcoal suspension (5%) containing Dextran (0.5%). The mixture was vortexed well and centrifuged at 2,000 × g for 20 min at 4°C to remove the charcoal. An aliquot (1 ml) of the supernatant was added to 15 ml of Scintisafe fluid (Research Products International) in scintillation vials, vortexed, and assayed for radioactivity. Blank values (incubation of dishes containing medium without cells) were subtracted, and aromatase activity was expressed as fmol 3H2O released per 106 cells per h.
Hormone Assays.
Testicular homogenates were prepared following the method of Matsumiya et al. (26) to obtain intratesticular measurements of estradiol. The left testis was weighed, homogenized in 0.5 ml of water, and the sample was centrifuged at 10,000 rpm for 10 min. The supernatant was removed and used for RIA. Serum and intratesticular estradiol RIAs were performed according to the manufacturer's protocols (Diagnostic Products, Los Angeles). The sensitivity for estradiol measurement was 2.0 pg/ml, with intraassay variance of 7.3%, and interassay variance of 9.5%. Serum and intratesticular testosterone RIAs were performed according to the manufacturer's protocols (ICN). The sensitivity for testosterone measurement was 0.01 ng/ml with intraassay variance of 3.5%, and interassay variance of 8.2%. Luteinizing hormone, follicle-stimulating hormone, and prolactin RIAs were performed by using antibodies and reference preparations from the National Hormone Pituitary Program (provided by A. Parlow, Harbor–UCLA Medical Center, Torrance, CA).
Tamoxifen Administration and Assessment of Fertility.
Tamoxifen (0.25 mg) or placebo 90-day release pellets (Innovative Research of America) were implanted s.c. in 7-week-old Dax1-deficient male mice. After 3 weeks of treatment, each male was housed with 2 8-week-old wild-type females, and fecundity was determined as the average number of pups weaned (21 days old) from each cage. Males were killed 90 days after pellet implantation by cervical dislocation, and sperm count and motility were determined as described (27).
Plasmid Construction.
Mammalian expression vectors for murine SF-1, human DAX-1, and the Δ448-470 DAX-1 mutant were described (15, 28). The promoter regions of the rat Cyp19 (−294–+20), murine StAR (−289–+18), and murine Cyp11a (−290–+42) genes were amplified by PCR and cloned into the pA3 luciferase reporter vector.
Cell Culture, Transfections, and Luciferase Assays.
Human embryonic kidney tsa201 cells were grown in DMEM supplemented with 10% (vol/vol) FBS. The cells were incubated in a 5% CO2 atmosphere at 37°C. All transfections were performed in triplicate, using the calcium phosphate method as described (29). Luciferase assays (30) were performed 48 h after transfection and are reported as the mean ± SEM in relative light units.
Statistical Analysis.
All data are expressed as mean ± SEM and were analyzed by Student's t test by using GraphPad (San Diego) prism 2.0b for the PowerPC Macintosh. Differences were considered significant when P < 0.05.
Results
Purification of Leydig Cells from Testes.
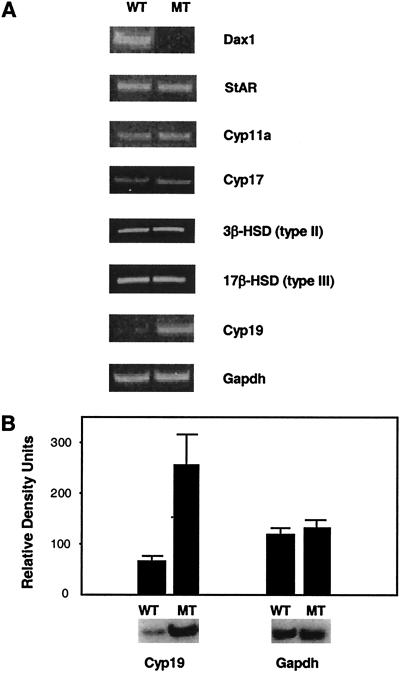
Dax1-deficient males had a significantly greater number of Leydig cells per gram of testis weight (5.91 × 106 ± 0.3) compared with wild-type mice (4.72 × 106 ± 0.2, P < 0.05), reflecting the presence of LCH. The Leydig cell preparation was ≈90% pure based on 3β-HSD immunohistochemical staining (data not shown). Expression of Müllerian-inhibiting substance (MIS) mRNA, a Sertoli cell marker, was used to assess contamination by Sertoli cells. The MIS transcript was detected readily in the whole testis but not in the Leydig cell preparation (data not shown), consistent with relatively pure Leydig cell isolation. Expression of the SF-1-regulated Leydig insulin-like gene (Insl3) mRNA, a marker specific for Leydig cells (31, 32), was similar in wild-type and mutant mice after correcting for Leydig cell number (data not shown). The Dax1 transcript was present in Leydig cells isolated from wild-type mice but was absent from cells prepared from Dax1-deficient mice (Fig. 1A).
Figure 1.
RT-PCR analysis of purified Leydig cells. (A) Leydig cells were purified from testes of wild-type (WT) and mutant (MT) mice. Total RNA extracted from the purified Leydig cells (1 μg) was subjected to RT reaction (20 μl). By using 3 μl of the RT reaction, PCR was performed for detection of Dax1 and the steroidogenic enzyme genes listed in Table 1 (24–33 cycles). Gapdh serves as a control. (B) PCR for Cyp19 and Gapdh was performed in the presence of [32P]dCTP (30 cycles for Cyp19 and 22 cycles for Gapdh). Densitometric analysis was performed by using a phospho imager (P < 0.05).
Expression of Steroidogenic Enzyme Genes in Purified Leydig Cells.
The expression levels of genes involved in testosterone synthesis were analyzed to assess the effect of Dax-1 deficiency on Leydig cell steroidogenesis. Leydig cells isolated from wild-type and Dax1-deficient mice were equalized before RNA extraction to correct for the presence of LCH in the Dax1-deficient male mice. Leydig cells from 10 Dax1-deficient mice (12 weeks old) and their wild-type littermates were pooled from 3 independent preparations. The expression levels of StAR, Cyp11a, Cyp17, 3β-HSD type II, and 17β-HSD type III mRNAs were not altered significantly in Dax1-deficient Leydig cells compared with wild-type (Fig. 1A). In contrast, the expression of Cyp19 mRNA was increased 4-fold in Dax1-deficient Leydig cells in three independent experiments (Fig. 1B). Levels of SF-1 and MIS receptor mRNAs were unchanged (data not shown), confirming relatively selective induction of Cyp19 mRNA. These data were replicated in 5-week-old and 1-year-old Dax1-deficient male mice. Cyp19 mRNA levels were not altered in the ovaries of homozygous Dax1-deficient females (data not shown).
Aromatase Activity in Purified Leydig Cells.
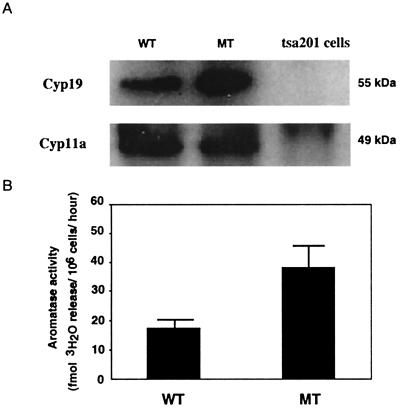
Western blot analysis, using an antibody raised against human placental aromatase, confirmed that aromatase protein (55 kDa) levels were increased in 12-week-old Dax1-deficient Leydig cells compared with wild-type (Fig. 2A). Cyp11a protein levels were unchanged, consistent with the RT-PCR results. An aromatase activity assay was performed to assess directly the enzyme activity in Dax1-deficient and wild-type Leydig cells. By using a saturating substrate concentration, the aromatization reaction rate was linear during a 36-h incubation (data not shown). Aromatase enzymatic activity was increased ≈2-fold in Dax1-deficient Leydig cells (38 fmol 3H2O/106 cells per h) compared with wild-type cells (18 fmol 3H2O/106 cells per h; Fig. 2B).
Figure 2.
Cyp19 expression and activity in purified Leydig cells. (A) Whole cell extracts were prepared from purified Leydig cells of wild-type (WT) and mutant (MT) mice. Extracts (20 μg) were subjected to Western blot analysis, using anti-Cyp19 and anti-Cyp11a antibodies. (B) The aromatase activity in purified Leydig cells from WT and MT mice was assessed by using the modified tritiated water method. The activity is expressed as fmol 3H2O released per 106 cells per h (P < 0.05).
Serum and Intratesticular Hormone Measurements.
Intratesticular levels of estradiol were 40-fold greater in Dax1-deficient male mice compared with wild-type mice (Table 2). There was also a trend, although nonsignificant, for increased serum estradiol in Dax1-deficient males (Table 2). Consistent with chronic overproduction of estradiol, serum levels of prolactin were increased significantly in mutant mice (Table 2), whereas luteinizing hormone, follicle-stimulating hormone, and testosterone levels did not differ from wild-type, as described (18).
Table 2.
Hormone measurements in 12-week-old Dax1-deficient male mice
| Serum estradiol, pg/ml | Intratesticular estradiol, pg/g of testes | Serum prolactin, ng/ml | Serum LH, ng/ml | Serum FSH, ng/ml | Serum testosterone, ng/ml | |
|---|---|---|---|---|---|---|
| Dax1 knockout (n = 16) | 10.2 ± 3.8 | 747.4 ± 357.6 | 105.6 ± 16.7 | 0.17 ± 0.1 | 11.5 ± 1.7 | 2.7 ± 1.1 |
| Wild type (n = 15) | 2.0 ± 1.5 | 18.8 ± 1.0 | 27.6 ± 11.3 | 0.20 ± 0.1 | 12.9 ± 1.2 | 3.4 ± 1.8 |
| P value | 0.06 | 0.01* | 0.01* | 0.16 | 0.07 | 0.34 |
Data are presented as the mean ± SEM and differences considered significant (*), where P < 0.05. FSH, follicle-stimulating hormone.
Tamoxifen Restores Fertility and Reduces Leydig Cell Hyperplasia.
The markedly raised intratesticular estradiol levels led us to investigate the gonadal effects of estrogen inhibition. The selective estrogen receptor modulator, tamoxifen, was used to block estrogen action in adult Dax1-deficient male mice. The majority (n = 7) of placebo-treated Dax1-deficient male mice was completely infertile (Table 3). One placebo-treated Dax1-deficient male sired a single litter (n = 10 pups). By comparison, all tamoxifen-treated mutant mice produced numerous healthy offspring over the 90-day treatment period (Table 3). The fertility (6.1 litters per male) and fecundity (16.7 offspring per male) did not differ from wild-type male mice in this colony (27).
Table 3.
Fertility and testicular morphology in tamoxifen-treated Dax1-deficient male mice
| Tamoxifen (n = 9) | Placebo (n = 8) | P value | |
|---|---|---|---|
| Offspring sired | 150 | 10* | NA |
| Litters sired | 55 | 1 | NA |
| Testis weight, mg | 140.0 ± 5.9 | 103.8 ± 8.9 | 0.003* |
| Sperm count, sperm per mg of testes | 9.7 × 103 ± 1.6 | 6.7 × 104 ± 1.0 | 0.0001* |
| Sperm motility, % | 53.2 ± 0.9 | 42.6 ± 1.6 | 0.0001* |
| Leydig cell count, cells per g of testes | 5.13 × 106 ± 0.2 | 6.01 × 106 ± 0.4 | 0.039* |
| Intratesticular estradiol, pg per g of testes | 640.15 ± 280.1 | 716.0 ± 310.4 | 0.12 |
| Serum LH, ng/ml | 0.40 ± 0.07 | 0.21 ± 0.02 | 0.018* |
Data are presented as the mean ± SEM and differences considered significant (*), where P < 0.05. NA, not applicable.
The complete rescue of fertility by tamoxifen was accompanied by significant improvements in several parameters of sperm production and function (Table 3). Testis weight increased significantly in tamoxifen-treated Dax1-deficient mice (104 mg in controls vs. 140 mg in tamoxifen-treated), but did not fully reach normal size (wild-type value 210 ± 9.6 mg). Sperm count and motility also were improved significantly in tamoxifen-treated mice (Table 3). Serum levels of luteinizing hormone increased 2-fold after tamoxifen treatment, but intratesticular estradiol levels were unaltered, consistent with an antagonistic action of tamoxifen on the estrogen receptor. The number of Leydig cells isolated from tamoxifen-treated testes was reduced significantly (Table 3) with levels approaching those in wild-type mice (4.72 × 106 ± 0.2), suggesting a decrease in LCH.
DAX-1 Selectively Represses the Promoter Activity of Steroidogenic Enzyme Genes.
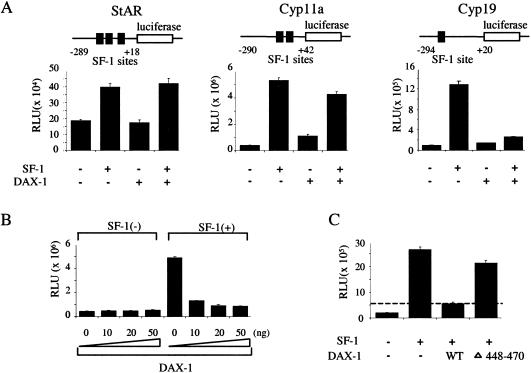
The effect of DAX-1 on SF-1-induced transactivation of the StAR, Cyp11a, and Cyp19 promoters was studied in human embryonic kidney tsa201 cells, which lack endogenous SF-1 and DAX-1 (data not shown). The Cyp19 promoter was stimulated 13-fold by SF-1 (10 ng) but was not altered by DAX-1 (Fig. 3A). When cotransfected, DAX-1 (50 ng) repressed SF-1-mediated transactivation of the Cyp19 promoter by more than 80%. In contrast, under comparable conditions, DAX-1 did not significantly alter SF-1-mediated transactivation of the Cyp11a or StAR promoters. Increasing amounts of DAX-1 expression plasmids (0, 10, 20, and 50 ng), cotransfected with a constant amount of SF-1 plasmid (10 ng), progressively suppressed SF-1-mediated transactivation of the Cyp19 promoter (Fig. 3B). The DAX-1 C-terminal truncation mutant (Δ448-470), shown previously to impair DAX-1-mediated repression (15), exhibited reduced Cyp19 repression (Fig. 3C).
Figure 3.
Effect of DAX-1 on promoter activity of steroidogenic enzyme genes. (A) Reporter constructs containing the promoter region of the StAR, Cyp11a, and Cyp19 genes linked to luciferase (0.5 μg) were transfected into tsa201 cells with or without SF-1 (10 ng) and DAX-1 (50 ng) expression vectors. The total amount of transfected plasmid was adjusted with empty vector. Luciferase assays were performed 48 h after transfection. Results are the mean ± SEM of triplicate transfections. The location of SF-1 response elements in the promoters of each gene is shown above. (B) The Cyp19 reporter (0.5 μg) was cotransfected into tsa201 cells with a constant amount of empty or SF-1 expression vector (10 ng) and increasing amounts of DAX-1 expression vectors (0, 10, 20, and 50 ng). (C) The Cyp19 reporter (0.5 μg) was cotransfected into tsa201 cells with or without SF-1 expression vector (10 ng) and wild-type (WT) or Δ448-470 mutant DAX-1 expression vectors (50 ng). RLU, relative light units.
Discussion
The primary aim of this study was to examine the consequences of DAX-1 deficiency on Leydig cell steroidogenesis in vivo. Unexpectedly, there was no alteration in the expression of the five steroidogenic genes required for testosterone biosynthesis. In contrast, the mRNA, protein, and enzymatic activity of aromatase (Cyp19), the enzyme responsible for the conversion of testosterone to estradiol, was increased significantly in Dax1-deficient Leydig cells. Increased aromatase expression was accompanied by a 40-fold increase in intratesticular estradiol. The antiestrogenic compound tamoxifen reduced LCH and restored fertility to Dax1-deficient mice, suggesting that increased estrogen levels play an important role in the infertility observed in these mice. These in vivo findings are consistent with the observed repression of SF-1-mediated transactivation of the Cyp19 promoter by DAX-1 in transient transfection studies in vitro.
Previous in vitro studies indicate that DAX-1 is a repressor of several steroidogenic enzymes. Zazopoulos et al. (16) showed that DAX-1 represses StAR expression in Y-1 adrenocortical cells, thereby blocking the rate-limiting step in steroid synthesis. In addition, DAX-1 inhibited both Cyp11a and 3β-HSD expression in Y-1 cells (33). In the present study, we did not find significant alterations in the levels of these, or several other SF-1 target genes, when examined in Dax1-deficient male mice. The relatively large amounts of DAX-1 expressed in transfection studies likely exceed physiologic levels, perhaps accounting for the observed repression of multiple steroidogenic enzyme promoters (33). For this reason, we attempted to use lower amounts of the DAX-1 expression vector in transfection studies. Under these conditions, only Cyp19 expression was repressed, consistent with the situation observed in vivo. The issue of transcription-factor expression level may be particularly relevant for DAX-1 in view of its proposed role as a dosage-sensitive gene (1). It is also notable that aromatase expression was not altered in the ovaries of Dax1-deficient female mice (data not shown). The apparent discrepancy in aromatase expression in males and females may reflect sexually dimorphic actions of Dax-1 or the fact that aromatase expression is induced transiently during follicular development.
Several mechanisms have been proposed to account for DAX-1 repression of SF-1-mediated transactivation. DAX-1 binds directly to SF-1 in vitro (15). The inhibitory features of DAX-1 require the extreme C-terminal region of the protein, which is deleted in some patients with AHC, and corresponds to the AF-2 domain in other nuclear receptors (15). DAX-1 also has been shown to recruit the corepressor, NCoR, to SF-1-responsive promoters (34), and to bind hairpin-loop structures of DNA (16). Recently, DAX-1 was suggested to exert inhibitory effects at both transcriptional and posttranscriptional levels (35), as a significant proportion of DAX-1 is complexed with polyadenylated RNA in polyribosomes. Mutations found in patients with AHC significantly impair RNA binding. Additional studies are required to further elucidate mechanisms of transcriptional repression by DAX-1.
It has been suggested that Sertoli cells produce one or more paracrine factors that regulate the pathway of Leydig cell development (36–39). For example, transgenic mice deficient in MIS, a Sertoli cell product, develop LCH and they occasionally develop Leydig cell tumors (40). There was no significant alteration in MIS expression in testes from the Dax1-deficient male mice, and MIS receptor mRNA also was unchanged in Leydig cells isolated from the mutant mice (data not shown). In addition, we have shown that the Sertoli cell-specific rescue of Dax1 expression in Dax1-deficient male mice did not diminish LCH (27). Rather, we postulate that the elevated expression of Cyp19 in the Dax1-deficient mice, and consequent increase in estrogen production, stimulates LCH. Acute treatment with estradiol has been shown to stimulate DNA synthesis and to cause LCH in murine Leydig cells (41). In addition, transgenic mice that overexpress aromatase exhibit LCH and an increased incidence of Leydig cell tumors (42). The finding that tamoxifen administration reduced the number of Leydig cells in the testes of Dax1-deficient mice is consistent with this mechanism of estrogen-induced LCH. Two patients with X-linked AHC have been reported to have LCH (43, 44). As yet, there are no reports of CYP19 activity or estrogen levels in these individuals.
This study adds to a growing body of evidence that estrogen plays an important role in normal male reproductive development and function. Targeted disruption of the genes encoding the estrogen receptor-α or Cyp19 indicates that estrogen is essential for normal male fertility (45–49). On the other hand, estrogen excess stimulates LCH in rodents and has been associated with cryptorchidism, testicular cancers, and impaired spermatogenesis (50). The finding that DAX-1 selectively represses aromatase expression in Leydig cells underscores the importance of examining the effects of transcription factors in vivo as well as in vitro.
Acknowledgments
We thank J. McAllister (Pennsylvania State University) for advice on aromatase activity assays; Y. Osawa for the antiserum against human placental aromatase; J. Weiss and J. Meeks for helpful discussions; F. Martinson, T. Russell, and T. Kotlar for excellent technical assistance; B. Mann for performing the hormone RIAs; and A. Parlow for the prolactin, luteinizing hormone, and follicle-stimulating hormone RIA reagents. This work was performed as part of the National Cooperative Program for Infertility Research and was supported by National Institutes of Health Grants U54-HD-29164 and PO1 HD-21921. Z.J.W. is a recipient of a Howard Hughes Medical Institute Medical Student Research Training Fellowship. B.J. holds a Wellcome Trust International Prize Traveling Research Fellowship Grant 056375.
Abbreviations
- SF-1
steroidogenic factor-1
- AHC
adrenal hypoplasia congenita
- LCH
Leydig cell hyperplasia
- MIS
Müllerian-inhibiting substance
- RT
reverse transcription
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bardoni B, Zanaria E, Guioli S, Floridia G, Worley K C, Tonini G, Ferrante E, Chiumello G, McCabe E R, Fraccaro M, et al. Nat Genet. 1994;7:497–501. doi: 10.1038/ng0894-497. [DOI] [PubMed] [Google Scholar]
- 2.Swain A, Zanaria E, Hacker A, Lovell-Badge R, Camerino G. Nat Genet. 1996;12:404–409. doi: 10.1038/ng0496-404. [DOI] [PubMed] [Google Scholar]
- 3.Swain A, Lovell-Badge R. Acta Paediatr. 1997;423,Suppl.:46–49. doi: 10.1111/j.1651-2227.1997.tb18368.x. [DOI] [PubMed] [Google Scholar]
- 4.Habiby R L, Boepple P, Nachtigall L, Sluss P M, Crowley W F, Jr, Jameson J L. J Clin Invest. 1996;98:1055–1062. doi: 10.1172/JCI118866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prader A, Zachmann M, Illig R. J Pediatr. 1975;86:421–422. doi: 10.1016/s0022-3476(75)80978-4. [DOI] [PubMed] [Google Scholar]
- 6.Guo W, Burris T P, McCabe E R. Biochem Mol Med. 1995;56:8–13. doi: 10.1006/bmme.1995.1049. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda Y, Swain A, Weber T J, Hentges K E, Zanaria E, Lalli E, Tamai K T, Sassone-Corsi P, Lovell-Badge R, Camerino G, Parker K L. Mol Endocrinol. 1996;10:1261–1272. doi: 10.1210/mend.10.10.9121493. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda Y, Shen W H, Ingraham H A, Parker K L. Mol Endocrinol. 1994;8:654–662. doi: 10.1210/mend.8.5.8058073. [DOI] [PubMed] [Google Scholar]
- 9.Shen W H, Moore C C, Ikeda Y, Parker K L, Ingraham H A. Cell. 1994;77:651–661. doi: 10.1016/0092-8674(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 10.Parker K L, Ikeda Y, Luo X. Steroids. 1996;61:161–165. doi: 10.1016/0039-128x(96)00006-2. [DOI] [PubMed] [Google Scholar]
- 11.Luo X, Ikeda Y, Parker K L. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 12.Luo X, Ikeda Y, Schlosser D A, Parker K L. Mol Endocrinol. 1995;9:1233–1239. doi: 10.1210/mend.9.9.7491115. [DOI] [PubMed] [Google Scholar]
- 13.Shinoda K, Lei H, Yoshii H, Nomura M, Nagano M, Shiba H, Sasaki H, Osawa Y, Ninomiya Y, Niwa O, et al. Dev Dyn. 1995;204:22–29. doi: 10.1002/aja.1002040104. [DOI] [PubMed] [Google Scholar]
- 14.Sadovsky Y, Crawford P A, Woodson K G, Polish J A, Clements M A, Tourtellotte L M, Simburger K, Milbrandt J. Proc Natl Acad Sci USA. 1995;92:10939–10943. doi: 10.1073/pnas.92.24.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito M, Yu R, Jameson J L. Mol Cell Biol. 1997;17:1476–1483. doi: 10.1128/mcb.17.3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zazopoulos E, Lalli E, Stocco D M, Sassone-Corsi P. Nature (London) 1997;390:311–315. doi: 10.1038/36899. [DOI] [PubMed] [Google Scholar]
- 17.Sundaram K, Kumar N. In: The Leydig Cell. Payne A, Hardy M, Russell L, editors. Vienna, IL: Cache River Press; 1996. pp. 287–306. [Google Scholar]
- 18.Yu R N, Ito M, Saunders T L, Camper S A, Jameson J L. Nat Genet. 1998;20:353–357. doi: 10.1038/3822. [DOI] [PubMed] [Google Scholar]
- 19.Hales D B, Sha L L, Payne A H. J Biol Chem. 1987;262:11200–11206. [PubMed] [Google Scholar]
- 20.Wiebe J P. Endocrinology. 1976;98:505–513. doi: 10.1210/endo-98-2-505. [DOI] [PubMed] [Google Scholar]
- 21.Ito M, Yu R N, Jameson J L. Mol Endocrinol. 1998;12:290–301. doi: 10.1210/mend.12.2.0059. [DOI] [PubMed] [Google Scholar]
- 22.O'Shaughnessy P J, Baker P J, Heikkila M, Vainio S, McMahon A P. Endocrinology. 2000;141:2631–2637. doi: 10.1210/endo.141.7.7545. [DOI] [PubMed] [Google Scholar]
- 23.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida N, Osawa Y. Biochemistry. 1991;30:3003–3010. doi: 10.1021/bi00226a004. [DOI] [PubMed] [Google Scholar]
- 25.Ackerman G E, Smith M E, Mendelson C R, MacDonald P C, Simpson E R. J Clin Endocrinol Metab. 1981;53:412–417. doi: 10.1210/jcem-53-2-412. [DOI] [PubMed] [Google Scholar]
- 26.Matsumiya K, Meistrich M L, Shetty G, Dohmae K, Tohda A, Okuyama A, Nishimune Y. Endocrinology. 1999;140:4912–4915. doi: 10.1210/endo.140.10.7026. [DOI] [PubMed] [Google Scholar]
- 27.Jeffs B, Ito M, Yu R N, Martinson F A, Wang Z J, Doglio L T, Jameson J L. Endocrinology. 2001;142:2481–2488. doi: 10.1210/endo.142.6.8187. [DOI] [PubMed] [Google Scholar]
- 28.Tabarin A, Achermann J C, Recan D, Bex V, Bertagna X, Christin-Maitre S, Ito M, Jameson J L, Bouchard P. J Clin Invest. 2000;105:321–328. doi: 10.1172/JCI7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham F L, van der Eb A J. Virology. 1973;52:456–487. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 30.de Wet J R, Wood K V, DeLuca M, Helinski D R, Subramani S. Mol Cell Biol. 1987;7:725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adham I M, Burkhardt E, Benahmed M, Engel W. J Biol Chem. 1993;268:26668–26672. [PubMed] [Google Scholar]
- 32.Zimmermann S, Schwarzler A, Buth S, Engel W, Adham I M. Mol Endocrinol. 1998;12:706–713. doi: 10.1210/mend.12.5.0107. [DOI] [PubMed] [Google Scholar]
- 33.Lalli E, Melner M H, Stocco D M, Sassone-Corsi P. Endocrinology. 1998;139:4237–4243. doi: 10.1210/endo.139.10.6217. [DOI] [PubMed] [Google Scholar]
- 34.Crawford P A, Dorn C, Sadovsky Y, Milbrandt J. Mol Cell Biol. 1998;18:2949–2956. doi: 10.1128/mcb.18.5.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lalli E, Ohe K, Hindelang C, Sassone-Corsi P. Mol Cell Biol. 2000;20:4910–4921. doi: 10.1128/mcb.20.13.4910-4921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernier M, Chatelain P, Mather J P, Saez J M. J Cell Physiol. 1986;129:257–263. doi: 10.1002/jcp.1041290218. [DOI] [PubMed] [Google Scholar]
- 37.Khan S, Teerds K, Dorrington J. Biol Reprod. 1992;46:335–341. doi: 10.1095/biolreprod46.3.335. [DOI] [PubMed] [Google Scholar]
- 38.Ojeifo J O, Byers S W, Papadopoulos V, Dym M. J Reprod Fertil. 1990;90:93–108. doi: 10.1530/jrf.0.0900093. [DOI] [PubMed] [Google Scholar]
- 39.Wu N, Murono E P. Mol Cell Endocrinol. 1994;106:99–109. doi: 10.1016/0303-7207(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 40.Behringer R R, Finegold M J, Cate R L. Cell. 1994;79:415–425. doi: 10.1016/0092-8674(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 41.Juriansz R L, Huseby R A, Wilcox R B. Cancer Res. 1988;48:14–18. [PubMed] [Google Scholar]
- 42.Fowler K A, Gill K, Kirma N, Dillehay D L, Tekmal R R. Am J Pathol. 2000;156:347–353. doi: 10.1016/S0002-9440(10)64736-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajpert-De Meyts E, Schwartz M, Skakkebaek N E. Horm Res. 1999;51, Suppl. 2:54–74. [Google Scholar]
- 44.Seminara S B, Achermann J C, Genel M, Jameson J L, Crowley W F., Jr J Clin Endocrinol Metab. 1999;84:4501–4509. doi: 10.1210/jcem.84.12.6172. [DOI] [PubMed] [Google Scholar]
- 45.Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach K S, Simpson E R. N Engl J Med. 1997;337:91–95. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 46.Morishima A, Grumbach M M, Simpson E R, Fisher C, Qin K. J Clin Endocrinol Metab. 1995;80:3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 47.Smith E P, Boyd J, Frank G R, Takahashi H, Cohen R M, Specker B, Williams T C, Lubahn D B, Korach K S. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 48.Robertson K M, O'Donnell L, Jones M E, Meachem S J, Boon W C, Fisher C R, Graves K H, McLachlan R I, Simpson E R. Proc Natl Acad Sci USA. 1999;96:7986–7991. doi: 10.1073/pnas.96.14.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hess R A, Bunick D, Lee K H, Bahr J, Taylor J A, Korach K S, Lubahn D B. Nature (London) 1997;390:509–512. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abney T O. Steroids. 1999;64:610–617. doi: 10.1016/s0039-128x(99)00041-0. [DOI] [PubMed] [Google Scholar]