Abstract
Objective
To examine the association of metabolic syndrome (MetS) with objective measures of physical performance.
Design
Cross-sectional analysis of the cohort study, the Osteoporotic Fractures in Men Study.
Setting
Six clinical sites in the US.
Participants
5,457 ambulatory men (mean (±SD), age, 73.6 (5.9) years).
Measurements
Physical performance assessed by grip strength, narrow walk speed, walking speed, and time to complete five repeated chair stands. Individual scores were converted to quintiles (worst [1] to best [5]; unable to complete=0) and summed for an overall score (mean (±SD), 11.6 (4.3), range, 1–20). MetS was defined by World Health Organization criteria that include evidence of glucose dysregulation (insulin resistance, diabetes, or hyperinsulinemia), and at least two additional characteristics: high blood pressure, low high density lipoprotein cholesterol, high triglycerides, or obesity.
Results
26.3% of participants met criteria for MetS. In separate linear regression models, four of five MetS components were related to performance (P<.001); only high blood pressure was unrelated. Men with MetS had a 1.11-point lower performance score (mean (95% confidence interval (CI)) =10.81 (10.61, 11.00)) than men without MetS (mean (95% CI) =11.92 (11.81, 12.03)) (P<.001), adjusting for age, race, education and site. With further covariate adjustment this difference was reduced but remained significant (β=−0.78, P<.001). A graded association was observed between number of MetS components (0, 1, 2, or 3+) and performance (P for trend <.001). Findings were similar excluding men with diabetes or obese men.
Conclusion
Metabolic dysregulation is related to objectively-assessed poorer physical performance among relatively healthy older men.
Keywords: aged, men, metabolic syndrome, physical function, physical performance
INTRODUCTION
Metabolic syndrome (MetS) is defined as a clustering of cardiovascular risk factors, including central adiposity, altered glucose and insulin metabolism, hypertension, and dyslipidemia. The age-adjusted prevalence of MetS is nearly 24% in the US (1) and increases with age (2); among adults age 60 and older, prevalence exceeds 40% (1). MetS increases risk for stroke, cardiovascular diseases (CVD), diabetes, and mortality (3–6). However, there is widespread debate as to whether this particular clustering of risk factors is multiplicative, rather than additive, and whether it improves risk prediction for diabetes, CVD or mortality beyond standard risk factors (7–9). Nonetheless, it is clear that the components of MetS are important vascular risk factors that occur together more commonly than would be expected only by chance (7).
Understanding factors that contribute to increasing disability and functional declines in an aging population has enormous public health value. Studies have linked obesity and diabetes to mobility disability and poorer physical function (10, 11), suggesting that some facets of metabolic dysregulation affect physical performance. The cluster of risk factors known as MetS has been related to cognitive decline (12, 13), declines in self-reported mobility (14) and self-reported incident mobility limitations (15, 16) in the elderly. However, no previous studies in an older cohort have investigated the association of MetS with objective measures of physical performance. Given the high prevalence of MetS among older adults, it is important to examine whether this clustering of risk factors contributes to what are commonly considered age-related functional declines that can lead to disability and impaired quality of life in older adults.
The aim of the present study was to investigate the cross-sectional association between MetS and objective indicators of physical performance among older men. Using data from the Osteoporotic Fractures in Men Study (MrOS), we hypothesized that men with MetS would have poorer physical performance than men without MetS, and that any observed association would be independent of behavioral risk factors (smoking status, alcohol consumption, physical activity) and health status (history of falls/fractures, self-rated health, chronic medical conditions). Given the debate in the literature on the utility of clustering the vascular risk factors that comprise MetS, we evaluated the association of MetS with physical performance in three ways: first, we examined performance scores in relation to individual MetS components; next, we examined performance according to number of MetS components a person had; and then we evaluated performance for men with versus without MetS based on World Health Organization (WHO) criteria (17).
METHODS
Participants
From March 2000 through April 2002, 5,995 community-dwelling, ambulatory men were enrolled in MrOS, a cohort study of healthy aging and fracture risk conducted at six clinical centers in the United States. Eligible men were at least 65 years of age, without bilateral hip replacements, and able to walk without the assistance of another person. Details of the MrOS design and cohort have been reported (18, 19). The Institutional Review Board at each clinical center approved the study protocol, and written informed consent was obtained from all participants. Current analyses were limited to 5,457 men with valid data on all physical performance measures and the components of MetS at the baseline MrOS visit.
Metabolic Syndrome
MetS was defined according to WHO criteria (17), with minor modifications based on data available at the baseline MrOS visit. Men were considered to have MetS if they had evidence of glucose dysregulation or insulin resistance, defined as impaired fasting glucose (IFG) (100 to < 126 mg/dL), diabetes (fasting glucose ≥126 mg/dL or a history of diabetes or use of hypoglycemic medications at baseline), or hyperinsulinemia (top quartile of fasting insulin among non-diabetics within MrOS), and at least two of the following four characteristics: systolic blood pressure (SBP) equal to or greater than 140 mmHg or drug treatment for hypertension (HTN); high density lipoprotein (HDL) cholesterol less than 35 mg/dL; triglycerides (TRIG) equal to or greater than 150 mg/dL; body mass index (BMI) equal to or greater than 30 kg/m2, which was calculated based on standard measures of height and weight, using wall-mounted stadiometers and balance beam or digital scales, respectively. All prescription medication use within the past 30 days was confirmed by medication review at the MrOS baseline visit; data were stored electronically in a medications database at the MrOS Coordinating Center (San Francisco, CA) and each medication was matched to its ingredient(s) based on the Iowa Drug Information Service Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA) (20). WHO criteria also include diastolic blood pressure (DBP) of 90 mmHg or greater and evidence of microalbuminuria; however, these data were not available at the MrOS baseline visit and thus were not part of the definition of MetS used here.
Assays for insulin, glucose, HDL and TRIG were completed using stored serum collected at the MrOS baseline clinic visit, which had been obtained after ≥ 8 hour fast. Stored specimens were thawed, appropriate amounts withdrawn and placed in vials, refrozen, and shipped on dry ice to the Northwest Lipid Metabolism and Diabetes Research Laboratories at the University of Washington (Seattle, WA) for insulin and glucose assays, and to the Oregon Veterans Affairs Clinical Lab (Portland, OR) for lipids and all other assays, completed using a Roche COBAS Integra 800 automated analyzer (Roche Diagnostics Corp., Indianapolis, IN).
Physical Performance
Physical performance was measured at the baseline MrOS study visit with four performance tests that assess upper and lower body strength, gait speed, and balance, and included grip strength, timed walk, narrow walk balance, and repeated chair stands. Grip strength was measured in kilograms (kg) using a Jamar dynamometer (Sammons Preston Rolyan, Bolingbrook, IL, USA) (21). Participants completed two trials for each hand, and the maximum effort across the trials was used for analyses. Gait speed was measured in meters per second (m/s) on a standard, 6-meter walking course. Participants were instructed to walk at their normal pace, and the fastest time of two trials was used for analyses. To test balance, participants were asked to complete a narrow walking course in which they were instructed to walk within a 20-cm wide path that extended over 6 meters, with three attempts to successfully complete two trials, defined as two or fewer deviations from the 20-cm path. A deviation occurred when a participant stepped outside the 20-cm wide path or relied on a wall or the test administrator to maintain his balance. If a participant had three or more deviations, the trial was considered unsuccessful, and a time was not recorded. Narrow walk speed was calculated in m/s, and the fasted time of the successful trial(s) was analyzed. Lower grip strength and slower times on gait speed and narrow walk test reflected worse performance. For the chair stands, each participant was asked to rise from a standard chair without using his arms. If he was able to rise one time successfully, he was then asked to rise from a chair five times without using his arms; the time to complete the five chair stands was recorded, with higher values indicative of worse performance.
Raw scores on each measure were converted to quintiles, based on the distributions of scores, and assigned a score of 1 (worst) to 5 (best), with a “0” assigned if a participant was unable to complete a particular measure. All persons were able to complete the assessment of gait speed, so 0 was only used for the small proportion of participants unable to perform grip strength (1.6%), narrow walk test (9%), or repeated chair stands (3%). An overall performance score then was created by summing across the quintile scores for the four performance measures, with a higher score indicating better overall performance (possible range, 0 to 20; Cronbach’s alpha=0.70). This approach of creating a summary indicator from multiple measures of physical performance is consistent with recommendations from the MacArthur Studies of Successful Aging (22–24) and has been used previously in population-based studies of the elderly (25, 26).
Covariates
Covariates were assessed at the baseline MrOS visit; all participants were interviewed by a trained technician and completed a self-administered questionnaire. Age was self-reported and modeled continuously. Self-identified race/ethnicity was modeled as non-Hispanic white versus not white (>90% of participants were non-Hispanic white). Education was modeled categorically (< high school, high school/some college, completed college/some graduate school, completed graduate school). Self-reported number of alcoholic drinks consumed per week was modeled as none, 1 to <14 drinks per week, and 14 drinks per week or more. Smoking status was modeled as current, past or never smoker. Physical activity was assessed by the Physical Activity Scale for the Elderly (PASE) questionnaire (27) and modeled continuously. Self-reported history of falls or fractures was modeled as a dichotomous variable, and self-reported chronic conditions (stroke, Parkinson’s disease, myocardial infarction, chronic obstructive pulmonary disease, congestive heart failure, arthritis and cancer) were modeled categorically (none, 1 or 2, 3 or more). Self-rated health was modeled dichotomously as fair or poor health versus good, very good or excellent health.
Statistical Analyses
Descriptive statistics were computed to compare men with and without MetS on baseline characteristics; t-tests were used for continuous variables and chi-square tests were used for categorical variables. A series of linear regression models was conducted to assess the association between MetS and physical performance. Model 1 (“minimally-adjusted model”) included covariates for age, race, education, and clinic site. Model 2 (“multivariable-adjusted model”) included additional covariates of smoking status, alcohol consumption, physical activity, history of falls/fractures, self-rated health, and number of chronic medical conditions. In our primary models, the summary physical performance measure was included as the outcome, modeled continuously. To investigate the association of individual MetS components with physical performance, the first set of models evaluated each component separately as a predictor. Next, we evaluated MetS categories, based on the number of MetS components possessed (0 (referent), 1, 2, or 3+). The third set of models evaluated MetS as a binary (yes/no) indicator, based on the WHO definition.
Secondarily, we examined the four individual performance measures as the outcomes, each modeled continuously in separate minimally-adjusted and multivariable-adjusted models, with MetS modeled per the WHO definition. The latter models evaluated whether MetS differentially contributed to poorer functioning in one or more areas (e.g., upper extremity strength, gait, balance). To determine whether any association between MetS and physical performance was largely explained by diabetes or obesity, additional analyses were conducted to examine whether the association of MetS with physical performance was evident in non-diabetic or non-obese men. These included two sets of regression models, one set excluding 840 men with diabetes, and another excluding 1,187 obese men (BMI ≥ 30) but retaining men with diabetes. For these analyses, MetS was modeled as a binary indicator, based on WHO criteria, and the summary performance score was the outcome variable.
RESULTS
Participant Characteristics
Among the 5,457 participants in this study, 60% (n=3,262) had evidence of glucose dysregulation or insulin resistance, including diabetes (840 men), hyperinsulinemia (1,143 men) or IFG (1,279 men); 22% (n=1,187) were obese; 45% (n=2,458) were hypertensive or using anti-hypertensive medications; 13% (n=733) had low HDL, and 36.5% (n=1,992) had high triglycerides. A total of 1,495 men had 3 or more MetS components, 1,447 had 2 components, 1,614 had 1 component, and 901 men had none. A total of 1,437 (26.3%) participants met WHO criteria for MetS (having IFG/diabetes/hyperinsulinemia and at least 2 of the other 4 characteristics). Physical performance scores ranged from 1 to 20; (mean (±SD), for the cohort was 11.62 (4.3), with 20% scoring < 8, indicative of being in the lowest quintile on at least one performance test as well as performing relatively poorly on all four measures.
Table 1 presents mean (±SD), and/or prevalence of baseline participant characteristics and P-values from t-tests or chi-square tests, as appropriate. Men with versus without MetS differed significantly on all characteristics except race/ethnicity and history of falls/fractures.
Table 1.
The Osteoporotic Fractures in Men (MrOS) Study Baseline Participant Characteristics by Presence or Absence of Metabolic Syndrome (MetS)
| Characteristic | MetS (n=1,437) | No MetS (n=4,020) | P-value | ||
|---|---|---|---|---|---|
| Mean (±SD) | % | Mean (±SD) | % | ||
| Age (years) | 72.8 (5.4) | 73.9 (6.0) | <.001 | ||
| Education | <.001 | ||||
| Less than high school | 9% | 6% | |||
| High school/some college | 47% | 38% | |||
| College/some graduate school | 25% | 30% | |||
| Completed graduate school | 19% | 25% | |||
| Non-Hispanic white | 91% | 92% | ns | ||
| SBP (mmHg) | 145 (18) | 136 (18) | <.001 | ||
| BMI (kg/m2) | 30.3 (3.9) | 26.3 (3.2) | <.001 | ||
| Physical Activity Score for the Elderly | 140.1 (67.2) | 149.2 (68.4) | <.001 | ||
| Smoking | <.001 | ||||
| Past | 57% | 65% | |||
| Current | 4% | 3% | |||
| Never | 39% | 32% | |||
| Weekly alcohol consumption | <.001 | ||||
| None | 40% | 34% | |||
| Intermittent to <14 drinks/wk | 49% | 55% | |||
| ≥14 drinks/wk | 11% | 12% | |||
| History of falls/fractures | 64% | 63% | ns | ||
| Chronic medical conditions | <.001 | ||||
| None | 29% | 36% | |||
| 1–2 | 62% | 59% | |||
| 3 or more | 8% | 5% | |||
| Good to excellent self-rated health status | 81% | 87% | <.001 | ||
Note. SBP = systolic blood pressure: BMI = body mass index. MetS was defined by World Health Organization criteria: insulin resistance (evidenced by impaired fasting glucose, diabetes, or hyperinsulinemia) and at least 2 of the following: SBP of 140 mmHg or higher or use of anti-hypertensive medication; BMI of 30 or higher; high density lipoprotein cholesterol < 35 mg/dl; triglycerides of 150 mg/dl or higher. The Physical Activity Scale for the Elderly measured physical activity levels, with higher scores meaning more activity. Chronic medical conditions include history of stroke, Parkinsonism, myocardial infarction, congestive heart failure, lung disease (chronic obstructive pulmonary disease, chronic bronchitis, chronic asthma, emphysema), arthritis, gout, and cancer. P-values are from t-tests or chi-square tests, as appropriate, comparing men with and without MetS on the characteristics listed.
Primary Analyses
Table 2 summarizes the findings from our first set of models evaluating the individual MetS components in relation to physical performance. Four of the five components were significantly associated with poorer performance scores, including IFG/Diabetes/Hyperinsulinemia, High Triglycerides, Low HDL Cholesterol, and High BMI in the minimally adjusted models (Model 1). Following covariate adjustment (Model 2), this pattern was still evident although the relation between Low HDL and performance became marginally significant (P=0.059). Elevated SBP/HTN was unrelated to physical performance in either model.
Table 2.
Association of Individual Components of Metabolic Syndrome With Physical Performance: The Osteoporotic Fractures in Men (MrOS) Study Baseline Examination.
| Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | T-test | p-value | Estimate | SE | T-test | p-value | |
|
| ||||||||
| IFG/Diabetes/Hyperinsulinemia | −0.89 | 0.11 | −8.45 | <.001 | −0.61 | 0.10 | −6.04 | <.001 |
| SBP/HTN | −0.10 | 0.11 | −0.92 | .359 | −0.07 | 0.10 | −0.67 | .501 |
| High Triglycerides | −0.53 | 0.11 | −4.97 | <.001 | −0.33 | 0.10 | −3.2 | .001 |
| Low HDL Cholesterol | −0.64 | 0.15 | −4.22 | <.001 | −0.27 | 0.14 | −1.89 | .059 |
| High BMI | −1.53 | 0.12 | −12.34 | <.001 | −1.18 | 0.12 | −9.95 | <.001 |
Note: IFG = impaired fasting glucose; SBP = systolic blood pressure; HTN = hypertension; HDL = high density lipoprotein; BMI = body mass index; SE = standard error. Values shown are from linear regression models comparing persons with versus without the specified MetS component, as follows: IFG/Diabetes/Hyperinsulinemia compared persons with IFG (100 to < 126 mg/dL), diabetes (fasting glucose ≥126 mg/dL or a history of diabetes or use of hypoglycemic medications at baseline), or hyperinsulinemia (top quartile of fasting insulin among non-diabetics within MrOS) to those meeting none of the criteria; SBP/HTN compared those with high blood pressure, defined as systolic blood pressure of 140 mmHg or higher or using antihypertensive medication to those not meeting these criteria; high triglycerides compared men with triglyceride values of 150 mg/dL or higher to those with values < 150; low HDL cholesterol compared men with values less than 35 mg/dL to those with higher values; High BMI compared men with BMI ≥ 30 kg/m2 to men with BMI < 30. Physical performance was assessed by grip strength, narrow walk speed, walking speed, and time to complete five repeated chair stands; individual scores were converted to quintiles (worst [1] to best [5]; unable to complete=0) and summed for an overall score, which was used in analyses. Model 1 included covariates for age, race, education, and clinic site; Model 2 included additional covariates for smoking, alcohol consumption, physical activity, history of falls/fracture, health status and number of chronic medical conditions. N for Model 1 for all MetS components = 5,457; due to small numbers of missing data on covariates, Ns for Model 2 ranged from 5,443 to 5,446.
The regression models evaluating the number of MetS components revealed a graded association with physical performance. In a minimally-adjusted model, relative to men with no MetS component, men with 3 or more components scored 1.42 points lower (P<.001), and men with 2 components scored 0.70 points lower (P<.001) on the overall performance measure. Men with just 1 MetS component scored lower on the performance measure but did not differ significantly from men with none (P=.19). The trend across component categories was significant (P<.001). Results were similar in the multivariable-adjusted model; adjusted mean overall physical performance scores and 95% confidence intervals (CI) for the four MetS component groups are shown in Figure 1. In a separate analysis, a variable representing the total number of MetS components from 0 through 5 was squared and added to the model to test for a curvilinear relation but none was observed (P=.575; data not shown).
Figure 1.
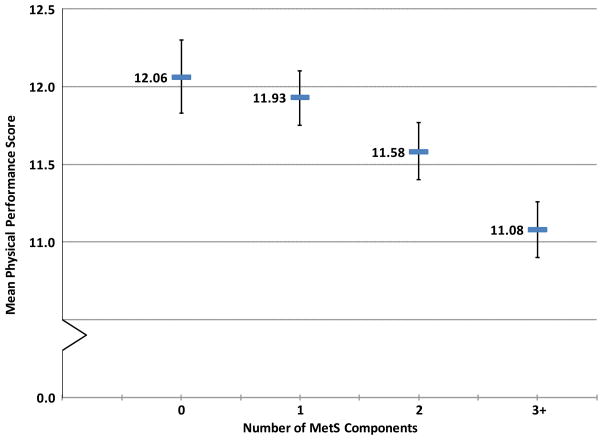
Mean Physical Performance Score By Number of Metabolic Syndrome (MetS) Components. The bars show mean physical performance scores and error bars represent the 95% Confidence Interval (CI) for 5,457 men with 0 (n=901), 1 (n=1,614), 2 (n=1,447) or 3 or more MetS components (n=1,495). MetS components include insulin resistance (impaired fasting glucose (100 to <126 mg/dL), diabetes (fasting glucose 126 mg/dL or greater, a history of diabetes, or use of hypoglycemic medications) or hyperinsulinemia (top quartile of fasting insulin among non-diabetic men) systolic blood pressure of 140 mmHg or higher or use of anti-hypertensive medication; triglycerides of 150 mg/dL or higher; high density lipoprotein cholesterol <35 mg/dL; and body mass index of 30 kg/m2 or greater. Graphed values are least square means and 95% CI from a regression model adjusted for age, race, clinic site, smoking, alcohol consumption, physical activity, history of falls/fractures, self-rated health, and number of chronic conditions. Physical performance scores ranged from 1–20, with higher scores indicating better performance. Means (95% CI) for the four groups were 12.06 (11.83–12.30), 11.93 (11.75–12.10), 11.58 (11.40–11.77), and 11.08 (10.90–11.26), respectively. The trend across categories was significant (P<.001).
Table 3 presents the means and 95% CI for the overall performance scores for men with and without MetS by WHO criteria as well as the mean differences between the groups, and associated 95% CI and P-values, from the analyses evaluating the association between MetS and physical performance. Adjusting for age, race, education, and clinic site, men with MetS had a 1.11-point lower physical performance score than men without MetS (Model 1). Further adjustment for smoking, alcohol consumption, physical activity, history of falls or fractures, self-rated health and number of chronic medical conditions reduced the difference between groups to 0.78 points, but this difference remained highly significant (Model 2). After adjustment, the mean performance score for men with MetS was 7% lower than the mean score of their peers without MetS.
Table 3.
Association of Metabolic Syndrome (MetS) With Physical Performance Scores: The Osteoporotic Fractures in Men (MrOS) Study Baseline Examination.
| MetS | No MetS | Difference between groups | 95% CI | P-value | |||
|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | ||||
| Model 1 | 10.81 | 10.61, 11.00 | 11.92 | 11.80, 12.03 | 1.11 | 0.88, 1.34 | <.001 |
| Model 2 | 11.05 | 10.86, 11.24 | 11.83 | 11.72, 11.94 | 0.78 | 0.56, 1.00 | <.001 |
Note. Values shown are calculated from linear regression models comparing men with and without MetS (referent) as defined by World Health Organization criteria (insulin resistance (evidenced by impaired fasting glucose, diabetes, or hyperinsulinemia) and at least 2 of the following: systolic blood pressure of 140 mmHg or higher or use of anti-hypertensive medication; body mass index of 30 or higher; high density lipoprotein cholesterol < 35 mg/dl; triglycerides of 150 mg/dl or higher). Physical performance was assessed by grip strength, narrow walk speed, walking speed, and time to complete five repeated chair stands; individual scores were converted to quintiles (worst [1] to best [5]; unable to complete=0) and summed for an overall score, which was used in analyses. Model 1 included covariates for age, race, education, and clinic site; Model 2 included additional covariates for smoking, alcohol consumption, physical activity, history of falls/fracture, health status and number of chronic medical conditions. N for Model 1 = 5,457; N for Model 2 = 5,445 due to missing data on some covariates. CI = Confidence interval.
Secondary Analyses
Analyses of the individual physical performance measures showed that men with MetS had significantly slower walking speeds, and poorer performance on the repeated chair stands than men without MetS (all P<.001) but the groups did not differ on grip strength. Table 4 presents the means and 95% CI for participants with and without MetS and the mean differences between the groups, and associated 95% CI and P-values, for the individual performance measures from multivariable-adjusted linear regression models.
Table 4.
Association of Metabolic Syndrome (MetS) With Individual Physical Performance Measures in the Osteoporotic Fractures in Men (MrOS) Study.
| MetS | No MetS | Difference Between Groups | 95% CI | P-value | |||
|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | ||||
| Grip Strength (kg) | 41.67 | 41.27, 42.07 | 41.56 | 41.32, 41.79 | −0.11 | −0.58, 0.35 | 0.635 |
| Narrow Walk Speed (m/s) | 1.12 | 1.10, 1.13 | 1.16 | 1.15, 1.17 | 0.04 | 0.03, 0.06 | <.001 |
| Walking Speed (m/s) | 1.22 | 1.21, 1.23 | 1.26 | 1.25, 1.26 | 0.04 | 0.02, 0.05 | <.001 |
| Repeated Chair Stands (sec) | 11.47 | 11.30, 11.64 | 11.03 | 10.93, 11.13 | −0.44 | −0.63, −0.24 | <.001 |
Note. P-values are from multivariable-adjusted linear regression models comparing men with and without MetS (referent) as defined by World Health Organization criteria (insulin resistance (evidenced by impaired fasting glucose, diabetes, or hyperinsulinemia) and at least 2 of the following: systolic blood pressure of 140 mmHg or higher or use of anti-hypertensive medication; body mass index of 30 or higher; high density lipoprotein cholesterol < 35 mg/dl; triglycerides of 150 mg/dl or higher). Covariates included age, race, education, clinic site, smoking, alcohol consumption, physical activity, history of falls/fracture, health status and number of chronic conditions. CI = Confidence Interval.
Finally, excluding either men with diabetes or a BMI of 30 kg/m2 or higher revealed similar associations as those shown in Table 3. Among non-diabetic men, those otherwise meeting criteria for MetS had > 7% lower performance score than those without MetS (means (95% CI) = 11.22 (10.99–11.45) versus 12.08 (11.96–12.20)), which was unchanged with further covariate adjustment (P<.001). Among non-obese men, those otherwise meeting criteria for MetS had > 4% lower performance score than those without MetS (means (95% CI) = 11.41 (11.12–11.69) versus 11.94 (11.82–12.06)) that was little changed in a multivariable-adjusted model (P<.02).
DISCUSSION
In this study of community-dwelling older men, those with MetS had significantly worse performance on objective indicators of physical function compared to their peers without MetS. The observed association between MetS and physical performance was independent of age, race, and educational attainment as well as health behaviors and health status, and was consistent across measures of upper extremity strength, gait, and balance. A graded association between number of MetS components and performance was observed, such that performance was increasingly worse the more MetS components a man had, and, when evaluated separately, all but one individual MetS component was significantly related to poorer performance. Moreover, analyses that excluded those with either diabetes or obesity – factors known to affect physical function (10, 11) – showed that men who otherwise met criteria for MetS had significantly worse performance than men without MetS. Across all of our analytic models, a consistent pattern of worse performance related to MetS was noted. Thus, this study provides clear evidence that metabolic dysregulation is related to lower levels of objectively-measured physical function in older men.
These results are consistent with previous research showing that metabolic syndrome is related to self-reported mobility limitations and worse self-reported physical functioning in older adults (14–16, 28). However, to our knowledge, this study is among the first to report an association between metabolic syndrome and objective performance-based measures. Such measures are considered more valid than self-report measures, particularly in elderly populations who often are unaware of or under-report limitations because they tend to be relatively sedentary and have inaccurate perceptions of their true physical and functional abilities (29).
Evidence suggests that differences in physical performance of the magnitude observed in this study, likely are clinically meaningful. In the Established Populations for Epidemilogic Studies of the Elderly (EPESE) (22), scores on the Short Physical Performance Battery (SPPB), a widely-used summary measure of 3 performance-based tests (tandem stand, walking speed, and repeated chair stands), were related to significant one-year risk of mobility-related disability. Small, significant annual declines on the SPPB have been reported in the elderly and even a one-point difference in scores has been linked to significant risk of mortality and incidence of severe lower extremity limitations (25,30). On the SPPB (score range, 0–12), estimates of small meaningful change range from 0.27 to 0.55 points; for gait speed, similar estimates are 0.04 to 0.06 m/s (31). Men with MetS in the present study showed an approximately 1-point lower performance score and a .04 m/s slower walking speed than men without MetS. Moreover, even with obese or diabetic men excluded from analyses, a performance decrement of 0.5 to 0.8 points was still observed among men who otherwise met criteria for MetS, relative to men without MetS. Our observed pattern of findings suggests that the metabolic dysregulation evident with MetS contributes to small yet meaningful differences in physical function that likely are clinically relevant, even among relatively healthy older men.
The mechanisms by which metabolic dysregulation might affect physical function are varied. Research suggests that, with age, factors such as increasingly sedentary lifestyles, nutritional deficiencies, exacerbation of chronic diseases, as well as age-related changes in hormonal, immunological and neural mechanisms can all contribute to declines in muscle strength (32). Reduced muscle strength and cardiorespiratory fitness also have been associated with metabolic syndrome (33,34). Recent studies have suggested that the increased pro-inflammatory state seen in aging and obesity likely contributes to strength declines (35). We did not have available data on such mechanisms, although the association of MetS with physical performance was independent of physical activity in this study. Research is needed to better understand the pathways by which metabolic dysregulation in the form of MetS affect physical performance in the elderly.
It has been debated whether the particular clustering of vascular risk factors described as metabolic syndrome is multiplicative versus additive, and whether this particular risk cluster confers excess health risks, above and beyond that of standard risk factors (7–9). The relationship between number of MetS components and performance was mostly graded, as evidenced in Figure 1. Although the performance decrement among men with at least 3 MetS components was twice as large as that seen among men with just 2 components and more than seven times larger than men with just 1 MetS component, we found no evidence of a non-linear association. Our data indicated that metabolic syndrome, regardless of whether it was modeled as a dichotomous variable per WHO criteria or by number of components, was significantly related to an important indicator of physical function in this cohort of older men.
In MrOS, WHO criteria (17) were used to define metabolic syndrome, largely due to the specific data available in MrOS. Other MetS definitions, notably those put forth by the National Cholesterol Education Program Adult Treatment Panel (ATP)-III (36), the International Diabetes Federation (37), and the American Heart Association/National Heart, Lung, and Blood Institute (38), presently are more widely used in the literature. WHO criteria differ from the others by making glucose dysregulation/insulin resistance a defining feature; all definitions have in common criteria related to 5 components: elevated fasting glucose, high triglycerides, elevated blood pressure, reduced HDL-cholesterol, and central adiposity. Several organizations have tried to unify MetS criteria; the consensus reached is that no one component should be obligatory but 3 abnormal findings among the 5 metabolic components would result in a MetS diagnosis, with standard cutpoints for all components except central adiposity (recommendations for waist circumference require further study given known differences by ethnicity, nation and/or geographic region) (39). MrOS did not collect data on waist circumference at the baseline examination and thus could not quantify central adiposity; therefore, BMI was used to identify obese participants and WHO criteria were used to define MetS. However, our analyses based on number of MetS components suggest that using this new consensus definition of MetS, should all the appropriate data be available, would yield largely the same results.
Strengths & Limitations
The present study has several strengths and limitations. Data are from a well-characterized cohort of community-dwelling, older men; however, because participants are predominantly well-educated white men, findings may not generalize to men from other demographic groups. We adapted WHO criteria to define MetS, lacking data on DBP or microalbuminuria, which are part of the full WHO criteria, and using BMI rather than waist circumference. Nonetheless, the adapted criteria are consistent with the five categories of risk factors that other MetS definitions employ and a recent study of more than 50,000 veterans (93% male; mean age, 63 years) using similarly adapted ATP III criteria reported a similar prevalence rate of MetS (40). Physical performance was assessed objectively by four well-validated and widely used performance measures; consistent with current recommendations (22–24), a summary indicator of these measures was created for use in analyses. While this precise summary indicator has not been used in prior research, two of the four measures used are included in the SPPB, a widely used performance battery in studies on aging and all of our measures assess important functional abilities. This study also could not address putative mechanisms by which MetS contributes to poorer performance; future research is warranted to investigate pathways related to inflammation, central adiposity, and fitness and lifestyle factors. Finally, the data presented are cross-sectional only, and thus do not address the temporality of the association between MetS and physical performance.
Longitudinal studies are needed to examine whether the metabolic dysregulation characterized by MetS accelerates declines in physical performance over time. Such information may inform the design of future interventions to prevent or delay development of functional impairment and disability in the elderly, who experience high rates of MetS.
Acknowledgments
Funding Sources and Related Paper Presentation: The Osteoporotic Fractures in Men Study (MrOS) is supported by the National Institutes of Health, with support from: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01 AG027810, and UL1 RR024140. Additional support provided by the American Diabetes Association grant number 1-04-JF-46 to E.S.S. Susan A. Everson Rose (S.A.E-R.) also was supported by funds from the Applied Clinical Research Program and Program in Health Disparities Research at the University of Minnesota. Portions of these findings were presented as a poster at the annual meeting of the Society for Epidemiologic Research in 2009 and published in abstract form (Everson-Rose S, Paudel M, Taylor B, Dam T, Cawthon P, LeBlanc E, Strotmeyer E, Cauley J, Stefanick M, Barrett-Connor E, Ensrud K. Metabolic syndrome and physical performance in elderly men: The Osteoporotic Fractures In Men (MrOS) Study. American Journal of Epidemiology, 2009;169:S93 [abstract #369]).
Sponsor’s Role. None of the sponsors participated in data collection, analysis or interpretation of data, or writing the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs, NIH, NIAMS, NIA or NCRR. This material is the result of work supported with resources and the use of facilities at the Minneapolis VA Medical Center.
Conflict of Interest Disclosures
| Elements of Financial/Personal Conflicts | *Author 1 SAE-R |
Author 2 MP |
Author 3 BT |
Author 4 TD |
||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | x | x | x | x | ||||
| Grants/Funds | x | x | x | x | ||||
| Honoraria | x | x | x | x | ||||
| Speaker Forum | x | x | x | x | ||||
| Consultant | x | x | x | x | ||||
| Stocks | x | x | x | x | ||||
| Royalties | x | x | x | x | ||||
| Expert Testimony | x | x | x | x | ||||
| Board Member | x | x | x | x | ||||
| Patents | x | x | x | x | ||||
| Personal Relationship | x | x | x | x | ||||
| Elements of Financial/Personal Conflicts | Author 5 PMC |
Author 6 EL |
Author 7 ESS |
Author 8 JAC |
||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | x | x | x | x | ||||
| Grants/Funds | x | x | x | x | ||||
| Honoraria | x | x | x | x | ||||
| Speaker Forum | x | x | x | x | ||||
| Consultant | x | x | x | x | ||||
| Stocks | x | x | x | x | ||||
| Royalties | x | x | x | x | ||||
| Expert Testimony | x | x | x | x | ||||
| Board Member | x | x | x | x | ||||
| Patents | x | x | x | x | ||||
| Personal Relationship | x | x | x | x | ||||
| Elements of Financial/Personal Conflicts | Author 9 MS |
Author 10 EB-C |
Author 11 KE |
Etc. | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | x | x | x | x | ||||
| Grants/Funds | x | x | x | x | ||||
| Honoraria | x | x | x | x | ||||
| Speaker Forum | x | x | x | x | ||||
| Consultant | x | x | x | x | ||||
| Stocks | x | x | x | x | ||||
| Royalties | x | x | x | x | ||||
| Expert Testimony | x | x | x | x | ||||
| Board Member | x | x | x | x | ||||
| Patents | x | x | x | x | ||||
| Personal Relationship | x | x | x | x | ||||
Footnotes
Conflicts of Interest. None.
Author Contributions. Dr. Everson-Rose conceived the research questions addressed by this study, directed analyses, wrote the manuscript, and takes full responsibility for this work. Ms. Paudel and Dr. Taylor were responsible for analyses and interpretation of data, helped prepare the Materials and Methods section, and revised the manuscript for important intellectual content. Drs. Dam, Cawthon, LeBlanc and Strotmeyer contributed to interpretation of data, reviewed and revised the manuscript for important intellectual content. Drs. Cauley, Stefanick and Barrett-Connor each are site principal investigators (PIs) for MrOS, responsible for data acquisition at their respective study sites, and each reviewed and critically revised the manuscript for important intellectual content. Dr. Ensrud is a site PI for MrOS, responsible for data acquisition at her study site, and also contributed to conception and design of the study, interpretation of data, and reviewed and critically revised the manuscript for important intellectual content.
References
- 1.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez A, Muller DC, Metter EJ, et al. Aging, androgens, and the metabolic syndrome in a longitudinal study of aging. J Clin Endocrinol Metab. 2007;92:3568–3572. doi: 10.1210/jc.2006-2764. [DOI] [PubMed] [Google Scholar]
- 3.Kurl S, Laukkanen JA, Niskanen L, et al. Metabolic syndrome and the risk of stroke in middle-aged men. Stroke. 2006;37:806–811. doi: 10.1161/01.STR.0000204354.06965.44. [DOI] [PubMed] [Google Scholar]
- 4.McNeill AM, Rosamond WD, Girman CJ, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28:385–390. doi: 10.2337/diacare.28.2.385. [DOI] [PubMed] [Google Scholar]
- 5.Wilson PW, D’Agostino RB, Parise H, et al. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 6.Mozaffarian D, Kamineni A, Prineas RJ, et al. Metabolic syndrome and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med. 2008;168:969–978. doi: 10.1001/archinte.168.9.969. [DOI] [PubMed] [Google Scholar]
- 7.Boden-Albala B. Current understanding of multiple risk factors as the metabolic syndrome: distillation or deconstruction. Semin Neurol. 2006;26:108–116. doi: 10.1055/s-2006-933314. [DOI] [PubMed] [Google Scholar]
- 8.Mozaffarian D, Wilson PW, Kannel WB. Beyond established and novel risk factors: lifestyle risk factors for cardiovascular disease. Circulation. 2008;117:3031–3038. doi: 10.1161/CIRCULATIONAHA.107.738732. [DOI] [PubMed] [Google Scholar]
- 9.Stern MP, Williams K, Gonzalez-Villalpando C, et al. Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care. 2004;27:2676–2681. doi: 10.2337/diacare.27.11.2676. [DOI] [PubMed] [Google Scholar]
- 10.Vincent HK, Vincent KR, Lamb KM. Obesity and mobility disability in the older adult. Obes Rev. 2010;11:568–579. doi: 10.1111/j.1467-789X.2009.00703.x. [DOI] [PubMed] [Google Scholar]
- 11.Strotmeyer ES, de Rekeneire N, Schwartz AV, et al. The relationship of reduced peripheral nerve function and diabetes with physical performance in older white and black adults: the Health, Aging, and Body Composition (Health ABC) study. Diabetes Care. 2008;31:1767–1772. doi: 10.2337/dc08-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaffe K. Metabolic syndrome and cognitive disorders: is the sum greater than its parts? Alzheimer Dis Assoc Disord. 2007;21:167–171. doi: 10.1097/WAD.0b013e318065bfd6. [DOI] [PubMed] [Google Scholar]
- 13.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 14.Blazer DG, Hybels CF, Fillenbaum GG. Metabolic syndrome predicts mobility decline in a community-based sample of older adults. J Am Geriatr Soc. 2006;54:502–506. doi: 10.1111/j.1532-5415.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- 15.Penninx BW, Nicklas BJ, Newman AB, et al. Metabolic syndrome and physical decline in older persons: results from the Health, Aging And Body Composition Study. J Gerontol A Biol Sci Med Sci. 2009;64:96–102. doi: 10.1093/gerona/gln005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stenholm S, Koster A, Alley DE, et al. Joint association of obesity and metabolic syndrome with incident mobility limitation in older men and women--results from the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2009;65:84–92. doi: 10.1093/gerona/glp150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Pahor M, Chrischilles EA, Guralnik JM, et al. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 21.Harkonen R, Harju R, Alaranta H. Accuracy of the Jamar dynamometer. J Hand Ther. 1993;6:259–262. doi: 10.1016/s0894-1130(12)80326-7. [DOI] [PubMed] [Google Scholar]
- 22.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 23.Guralnik JM, Seeman TE, Tinetti ME, et al. Validation and use of performance measures of functioning in a non-disabled older population: MacArthur studies of successful aging. Aging (Milano) 1994;6:410–419. doi: 10.1007/BF03324272. [DOI] [PubMed] [Google Scholar]
- 24.Guralnik JM, Winograd CH. Physical performance measures in the assessment of older persons. Aging (Milano) 1994;6:303–305. doi: 10.1007/BF03324256. [DOI] [PubMed] [Google Scholar]
- 25.Everson-Rose SA, Skarupski KA, Bienias JL, et al. Do depressive symptoms predict declines in physical performance in an elderly, biracial population? Psychosom Med. 2005;67:609–615. doi: 10.1097/01.psy.0000170334.77508.35. [DOI] [PubMed] [Google Scholar]
- 26.Mendes de Leon CF, Barnes LL, Bienias JL, et al. Racial disparities in disability: recent evidence from self-reported and performance-based disability measures in a population-based study of older adults. J Gerontol B Psychol Sci Soc Sci. 2005;60:S263–271. doi: 10.1093/geronb/60.5.s263. [DOI] [PubMed] [Google Scholar]
- 27.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 28.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 29.Simonsick EM, Newman AB, Visser M, et al. Mobility limitation in self-described well-functioning older adults: importance of endurance walk testing. J Gerontol A Biol Sci Med Sci. 2008;63:841–847. doi: 10.1093/gerona/63.8.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cesari M, Kritchevsky SB, Newman AB, et al. Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study. J Am Geriatr Soc. 2009;57:251–259. doi: 10.1111/j.1532-5415.2008.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perera S, Mody SH, Woodman RC, et al. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 32.Rolland Y, Czerwinski S, Abellan Van Kan G, et al. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12:433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LaMonte MJ, Barlow CE, Jurca R, et al. Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: a prospective study of men and women. Circulation. 2005;112:505–512. doi: 10.1161/CIRCULATIONAHA.104.503805. [DOI] [PubMed] [Google Scholar]
- 34.Jurca R, Lamonte MJ, Barlow CE, et al. Association of muscular strength with incidence of metabolic syndrome in men. Med Sci Sports Exerc. 2005;37:1849–1855. doi: 10.1249/01.mss.0000175865.17614.74. [DOI] [PubMed] [Google Scholar]
- 35.Stenholm S, Maggio M, Lauretani F, et al. Anabolic and catabolic biomarkers as predictors of muscle strength decline: The InCHIANTI Study. Rejuvenation Res. 2010;13:3–11. doi: 10.1089/rej.2009.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 37.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 38.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 39.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 40.Keane J, Meier JL, Noth RH, et al. Computer-based screening of veterans for metabolic syndrome. Metab Syndr Relat Disord. 2009;7:557–561. doi: 10.1089/met.2009.0021. [DOI] [PubMed] [Google Scholar]


