Abstract
Objective
Develop a novel approach of optimizing continuous flow left ventricular assist device (CF-LVAD) function and diagnosing device malfunctions.
Background
In CF-LVAD patients, the dynamic interaction of device speed, left and right ventricular decompression, and valve function can be assessed during an echocardiography-monitored speed ramp-test.
Methods
We devised a unique ramp-test protocol to be routinely done at the time of discharge for speed optimization and/or if device malfunction was suspected. The patient’s left ventricular end diastolic dimension (LVEDD), frequency of aortic valve (AV) opening, valvular insufficiency, blood pressure, and CF-LVAD parameters were recorded at increments of 400 rpm from 8,000 rpm to 12,000 rpm. The results of the speed designations were plotted, and linear function slopes for LVEDD, PI, and power were calculated.
Results
Fifty-two ramp-tests from 39 patients were prospectively collected and analyzed. Twenty-eight ramp-tests were performed for speed optimization, and speed was changed in 17 (61%) with a mean absolute value adjustment of 424±211 rpm. Seventeen patients had ramp-tests performed for suspected device thrombosis and 10 tests were suspicious for device thrombosis; these patients were then treated with intensified anticoagulation and/or device exchange/emergent transplant. Device thrombosis was confirmed in 8/10 cases at the time of emergent device exchange or transplant. All patients with device thrombosis, but none of the remaining patients, had a LVEDD slope > −0.16.
Conclusion
Ramp-tests facilitated optimal speed changes and device malfunction detection, and may be used to monitor the effects of therapeutic interventions and need for surgical intervention in CF-LVAD patients.
Keywords: LVAD, Ramp test, LVEDD, Device thrombosis
Introduction
Continuous flow left ventricular assist devices (CF-LVAD) are an important therapeutic option for advanced congestive heart failure patients1–3. LVADs have traditionally been implanted as a bridge-to-transplantation (BTT) 2, 3 with average support times of 6–12 months. However, LVADs are increasingly used for the purpose of destination therapy (DT) 1, where support duration averages 2–4 years and is approaching a decade in individual patients. One year survival in patients awaiting transplantation while on LVAD support has increased from 55% to 85%4 and is approaching 80% in DT patients in the most recent US experience5. Cumulative global experience with the currently FDA approved HeartMate II device (Thoratec Cooperation, Pleasanton, CA) exceeds 8,000 patient-years, and it has become clear that management of the patient-device interface (e.g. speed optimization and anticoagulation) is at the epicenter of further improvement possibilities.
Currently, recommendations for device speed adjustment include target measures of mean arterial pressure above 65 mmHg, middle interventricular septum position, and intermittent aortic valve (AV) opening while maintaining no more than mild mitral regurgitation (MR) to ensure appropriate unloading of the left ventricle6. Optimal speed settings may reduce the frequency of several of the key complications related to chronic LVAD support. The importance of ensuring middle septal position for optimal right ventricular function is now well established6, but our understanding of speed optimization continues to evolve beyond acute hemodynamic effects. For example, we recently reported the development of de novo aortic insufficiency (AI) in 25% of patients remaining on continuous flow LVADs for at least one year7. Interestingly, AI occurred in the majority of patients (66%) whose aortic valves remained closed during support, but rarely (8%) in those whose AV opened regularly; a near identical prevalence of AI and association with AV opening has been reported by others8, 9. It is therefore conceivable, although unproven, that proactively maintaining intermittent opening of the AV during support may delay or prevent the development of AI. Intermittent AV opening also results in a more pulsatile flow pattern, and it has been hypothesized that increased pulsatility may attenuate the development of von Willebrand Factor deficiency.10
The dynamic assessment of device speed, left ventricular decompression, and valvular function during an echocardiographically-monitored ramp study may not only allow device speed optimization in individual patients, but abnormalities in this interaction may also aid in the diagnosis of device malfunction. Although the utilization of ramp studies for CF-LVAD management is recommended in the literature, no specific protocol has been reported or endorsed.
In the current study, we aimed to develop a systematic approach to perform and analyze ramp tests in order to optimize device function and diagnose device malfunctions, specifically device thrombosis, an uncommon but potentially catastrophic complication of CF-LVADs.
Methods
A prospective study of all ramp tests performed at Columbia University Medical Center-New York Presbyterian Hospital from June 1, 2011 until April 5, 2012 was conducted. The Columbia IRB approved this study and all patients signed informed consent.
After devising a standardized Ramp Test Protocol for Heartmate II (Table 1) in early 2011, Ramp Tests have been performed at our institution routinely for speed optimization or when device thrombosis is suspected. Protocol for patients supported by the Heartware device is attached in the appendix.
Table I.
Ramp Test Protocol (for HeartMate II)
| Speed | PI | Power | Flow | BP | HR | LVEDD | LVESD | AV opening | AI | MR | RVSP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 8000 | |||||||||||
| 8400 | |||||||||||
| 8800 | |||||||||||
| 9200 | |||||||||||
| 9600 | |||||||||||
| 10000 | |||||||||||
| 10400 | |||||||||||
| 10800 | |||||||||||
| 11200 | |||||||||||
| 11600 | |||||||||||
| 12000 |
Similar Ramp Test Protocol was developed for Heartware Device.
PI-pulsatility index, BP-blood pressure, HR-heart rate, LVEDD-left ventricular end diastolic diameter, LVESD-left ventricular end systolic diameter, AV opening-aortic valve opening, AI-aortic insufficiency, MR-mitral regurgitation, RVSP-right ventricular systolic pressure
With regard to speed optimization, we followed the current recommendations to ensure middle interventricular septum position, and intermittent AV opening while maintaining no more than mild MR.
Device thrombosis was suspected clinically based upon at least one the following:
Transient increases in pump power (power spikes) more than 14 days after device implantation, or gradually increasing power requirements of at least 2 watts;
Lactate dehydrogenase (LDH) > 1000 or a persistently rising LDH in repeated blood work in conjunction with low haptoglobin and/or high plasma free hemoglobin in the absence of other causes of hemolysis (Our outpatient LVAD follow-up protocol consists of routine LDH level checks once per month). At Columbia University Medical Center, the upper limit of normal for LDH is 221 U/L;
Development of left sided heart failure without apparent other causes such as severe AI.
The final diagnosis of device thrombosis required direct visualization of clot in the pump upon device explant and/or disassembly by the manufacturer.
Ramp Test Protocol (Figure 1, Table 1)
Figure 1.
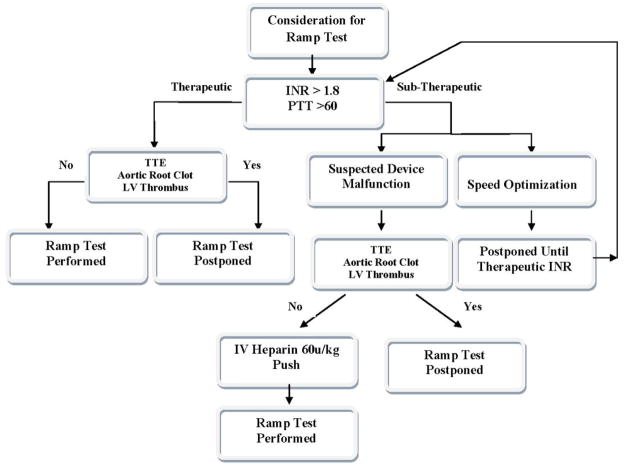
Consideration for the Ramp Test Algorithm
Algorithm for consideration of ramp studies based on anticoagulation studies and presence of intracardiac thrombus.
First, baseline demographics as well as surgical history, current medications, and laboratory parameters, including anticoagulation, platelets count, LDH, bilirubin, haptoglobin, and plasma free hemoglobin, where applicable, were collected.
Second, baseline parameters were reviewed to ensure safety. Specifically:
Appropriate anticoagulation (INR >1.8 or PTT >60) was confirmed. If adequate anticoagulation was not demonstrated, a routine ramp test for speed adjustment was postponed until therapeutic anticoagulation was reached. In cases of suspected device thrombosis, Heparin 60u/kg was given intravenously and the ramp test was subsequently performed.
Baseline trans-thoracic echocardiography (TTE) was performed; if it revealed an intraventricular or aortic root thrombus, the ramp study was not performed due to the possibility of thrombus dislodgement.
Third, baseline echocardiographic and device parameters (see below for details) were recorded. Pulsatility Index (PI) - Contraction of the left ventricle increases the flow of blood into the pump. The magnitude of these flow pulses is measured by the pump and averaged over a 15-second interval to produce the displayed PI value.
Suction events - Suction events occur when the pump speed is set high enough to cause partial collapse of the left ventricle resulting in obstruction of the inflow cannula by adjacent myocardium. When suction events occur, the automatically reduces its speed to the low speed setting (typically set 400 to 800 rpm below the fixed speed setting).
Fourth, the LVAD back-up speed was set to 8,000 rpm to allow the actual device speed to be set as low as 8,000 rpm without causing low-flow speed alarms.
Fifth, the patient’s device speed was lowered to 8,000 rpm. After 2 minutes, TTE images were obtained, and the following parameters were recorded: left ventricular end diastolic dimension (LVEDD), left ventricular end systolic diameter (LVESD), frequency of AV opening, degree of AI, degree of MR, right ventricular systolic pressure (RVSP), Doppler blood pressure, and heart rate. In addition the following pump parameters were recorded: power, pulsatility index (PI), and flow.
Specifically, TTE parameters were measured as follows (Figure 2):
Figure 2.
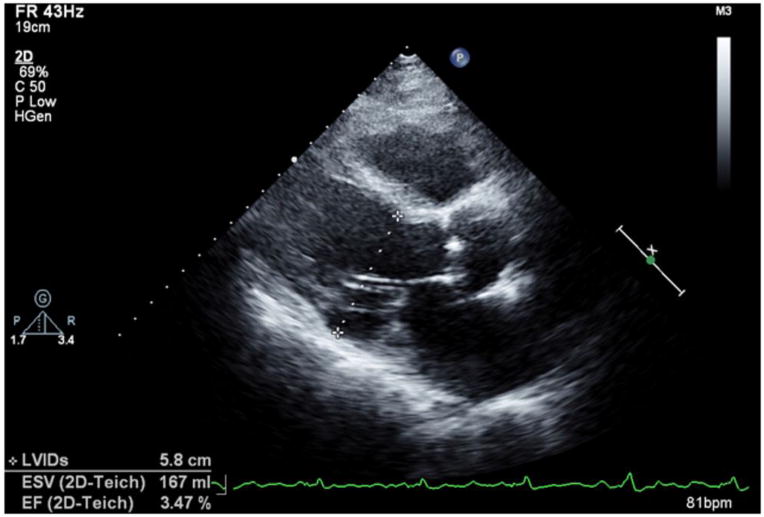
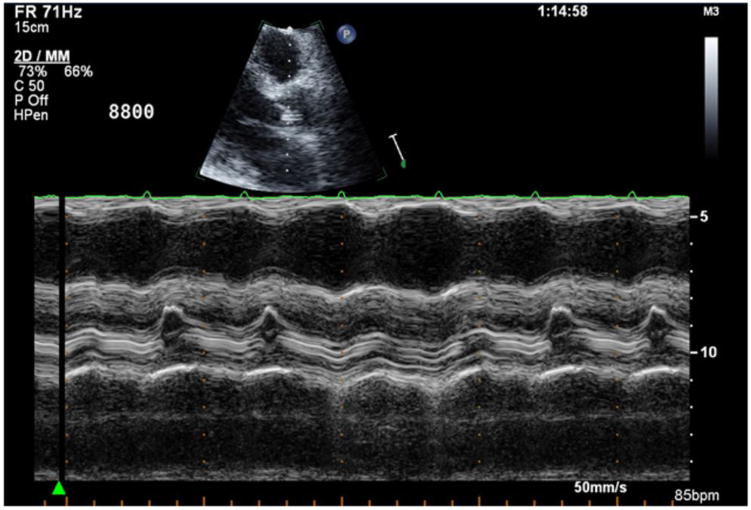
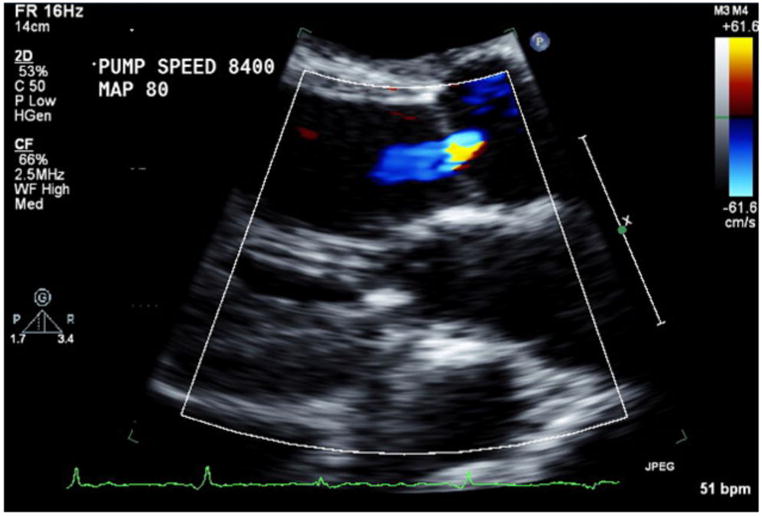
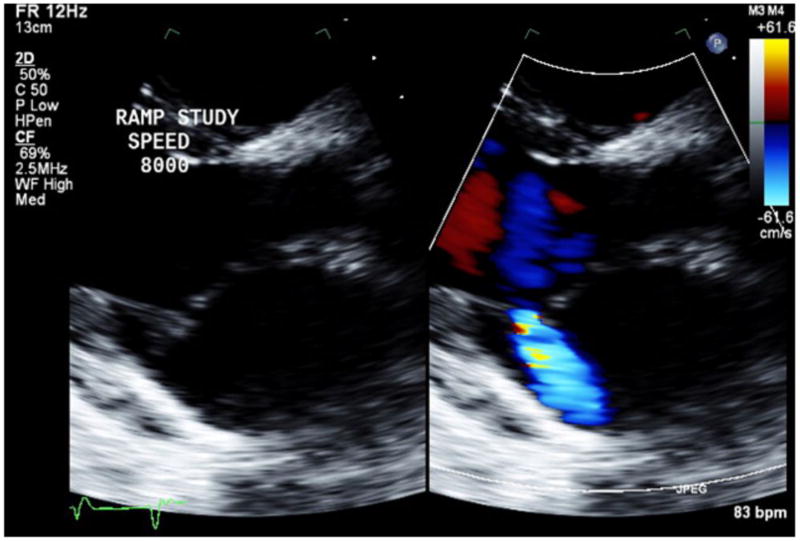
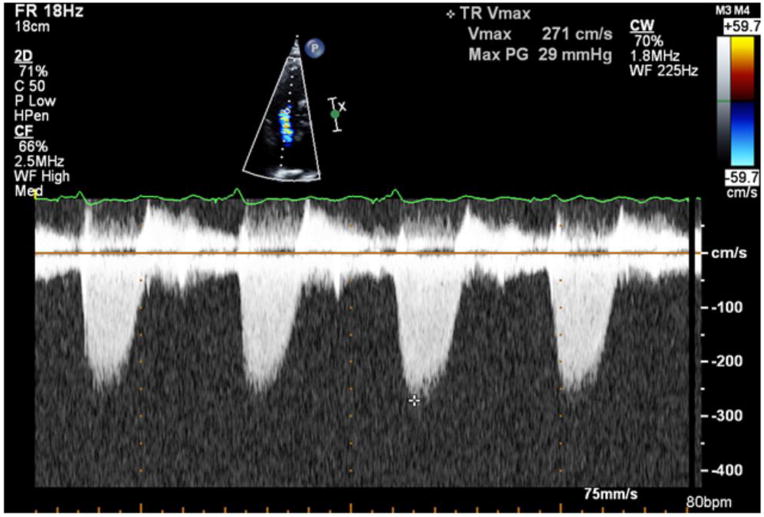
Images for Obtaining TTE Parameters
2a: LVEDD assessment in the parasternal long axis view.
2b: Aortic valve opening assessment by M-mode.
2c: Aortic valve insufficiency assessment by color Doppler.
2d: Mitral valve insufficiency assessment by color Doppler.
2e: Right ventricular systolic pressure assessment by Doppler.
LVEDD and LVESD were measured from the parasternal long axis view (Figure 2a).
AV opening was assessed using M-mode over the AV in the parasternal long axis view. At least 10 consecutive cardiac cycles were reviewed, and the frequency of AV opening was recorded as the percentage of cycles with AV opening (Figure 2b).
Visual estimation of severity of AI and MR was performed in the parasternal long axis view using Colored Doppler technique (Figure 2c, 2d). For assessment of AI and MR, the degree of regurgitation was graded from 0 to 6 (0 = none; 1 = trace; 2 = mild; 3 = mild-to-moderate; 4 = moderate; 5 = moderate-to-severe; 6 = severe). Taking into account that AI during CF-LVAD support is generally both systolic and diastolic, AI was deemed significant if graded 3 (mild to moderate) or greater7.
Right Ventricular Systolic Pressure (RVSP) was estimated from peak TR velocity using modified Bernoulli’s equation (Figure 2e).
Next, the device speed was subsequently increased by 400 rpm at 2 minute intervals with repeated acquisition of all echocardiographic and device parameters at each speed step. The 400 rpm increments continued from 8,000 rpm to 12,000 rpm. The ramp test was stopped if any suction events occurred, and/or if the LVEDD decreased to less than 3.0 cm. At the test’s conclusion, the attending cardiologist reviewed the recordings from the test while at the patient’s bedside. Device speed was then set to allow intermittent AV opening while maintaining Doppler blood pressure > 65 mmHg and avoiding more than mild MR.
Finally, the recordings of the ramp test parameter results at the respective 11 speed points were plotted in Excel Software (2007 Microsoft Corp., Redmond, Washington). Linear function slopes for LVEDD, PI, and power were calculated (Figure 3a) using Excel Software.
Figure 3.
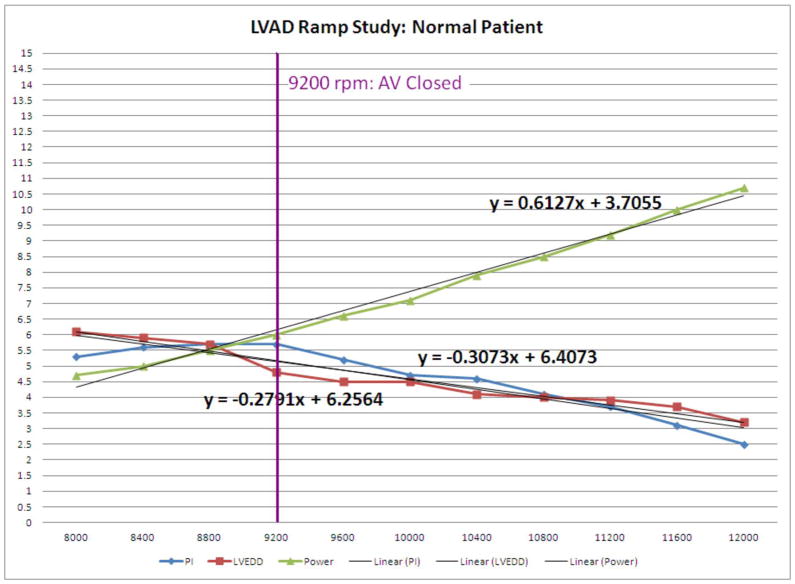
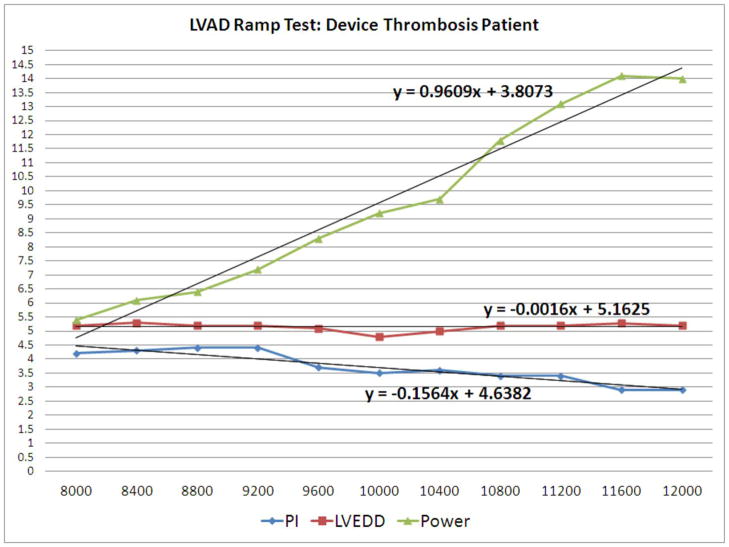
Normal Example vs. Device Thrombosis Example of LVEDD, PI, and Power Graphs
3a: LVEDD slope, PI slope, and power slope of a patient with normal device function.
3b: LVEDD slope, PI slope, and power slope of a patient with device thrombosis
Statistical Analysis
Data was collected using Excel software (2007 Microsoft Corp., Redmond, Washington). All data was analyzed using R version 2.15.0 (2012). Categorical variables were summarized by frequencies and percentages, and were analyzed using Fisher’s Exact. Student’s t-test for independent samples was used to determine differences in normally distributed data. Wilcoxon’s rank sum test was used to determine differences in non-normal distributions. Normality was assessed using the Shapiro-Wilk normality test. ROC curve analysis was done using the pROC package11. Each patient’s LVEDD, PI, and power were plotted against device speed, ranging in value from 8,000 rpm to 12,000 RPM in 400 rpm increments. The slopes for the lines were generated by fitting a linear function to each of the respected parameters using Microsoft Office Excel 2007 (2007). The fit of the linear model was assessed using correlation between LVEDD, PI and Power slopes and ramp speed.
Results
During the study period, 39 HeartMate II patients had a total of 52 ramp tests.
Demographics for the 39 patients are shown in Table 2. The mean age was 57±14 years, 85% were men, 51% had dilated cardiomyopathy, 36% had diabetes mellitus, and 33% had a history of hypertension. Nine (23%) patients had undergone mitral valve repair, 8 (21%) aortic valve repair, 1 (3%) aortic valve closure, 4 (10%) tricuspid valve repair, and 1 (3%) PFO closure at the time of HMII surgical implantation.
Table II.
Baseline Characteristics & Results: Device Thrombosis Pts (n=8) vs. No Thrombosis Pts (n=29) vs. All Patients (n=39)
| Baseline Characteristics | Confirmed Thrombosis Pts (n=8) | No Thrombosis Pts (n=29) | P Value | All Patients (n=39*) |
|---|---|---|---|---|
| Age | 53±20 years | 59±14 years | 0.47 | 57±14 years |
| Sex, Males | 5 (62%) | 26 (90%) | 0.10 | 33 (85%) |
| Race | 2 (25%) AA 6 (75%) Other |
3 (12%) AA 22 (88%) Other |
1 | 6 (15%) AA 33 (85%) Other |
| Heart Failure Etiology, Dilated Cardiomyopathy | 4 (50%) | 12 (48%) | 0.70 | 20 (51%) |
| Hypertension | 3 (38%) | 7 (28%) | 1 | 13 (33%) |
| Diabetes Mellitus | 3 (38%) | 7 (28%) | 0.66 | 14 (36%) |
| PMH Smoker | 5 (62%) | 14 (56%) | 0.70 | 21 (54%) |
| IVS, Mean | 1.0±0.2 cm | 1.1±0.2 cm | 0.06 | |
| LVAD Surgery combined with: | ||||
| Mitral Valve Repair | 1 (13%) | 7 (28%) | 0.65 | 9 (23%) |
| Aortic Valve Repair/Closure | 0 (0%) | 7 (28%) | 0.16 | 8 (21%) |
| Tricuspid Valve Repair | 1 (13%) | 2 (8%) | 1 | 4 (10%) |
| PFO Closure | 1 (13%) | 0.24 | 1 (3%) | |
| Ramp Test Results | ||||
| LVEDD Slope, Mean | −0.08±0.04 | −0.29±0.11 | <0.001 | N/A |
| PI Slope, Mean | −0.16±0.04 | −0.46±0.20 | <0.001 | N/A |
| Power Slope, Mean | 0.74±0.15 | 0.62±0.17 | 0.03 | N/A |
| Speed for complete AV Closure, Mean | 11,100±1,146 | 9,124±1,222 | <0.001 | |
| LDH Value, Mean | 1737±684 | 454±263 | <0.001 | N/A |
| Low Haptoglobin, Mean | <7 | N/A | N/A | N/A |
| High Plasma Free Hemoglobin, Mean | 19.1±14.0 | N/A | N/A | N/A |
| Follow Up Days Post-Ramp Test, Mean | 171±111 days | 148±89 days | 0.6 | N/A |
Note: Two patients, one with a gross bend relief disconnect and one who is stable and being followed on a maximally intensified anticoagulation regimen are included in the all patient cohort, but they were excluded in the confirmed device thrombosis vs. no thrombosis patient chart.
PMH-past medical history, IVS-interventricular septum, PFO-patent foramen ovale, LVEDD-left ventricular end diastolic diameter, LDH-lactate dehydrogenase, AA-African American
Speed Optimization
Twenty-eight ramp tests were performed for speed optimization. Device speed was changed in 17 (61%) of these tests with a mean absolute value speed adjustment of 424±211 rpm in order to achieve optimal LVEDD, AV opening, and MR as described above. Eight tests resulted in increased speeds for patients in order to allow for improved decompression of the LVEDD and a better interventricular septum position; the mean increased speed change was 450±177 rpm. Nine tests resulted in decreased speeds for patients in order to allow at least intermittent opening of the AV and to prevent leftward interventricular septum deviation; the mean decreased speed change was −400±245 rpm. In eleven tests, the speed was not changed, and the programmed clinical speed was therefore validated as the optimal speed. The speed optimization results are summarized in Table 3.
Table III.
Summary of Speed Optimization Ramp Tests (n=28 Tests)
| Parameter | Set Clinical Speed Pre- Ramp Test | New Speed Post-Ramp Test |
|---|---|---|
|
| ||
| Average Set Speed (rpm) | 8,850±431 rpm | 8,850±470 rpm |
|
| ||
| Delta Change in Speed Pre- to Post-Test | N/A | Absolute Speed Change: 424±211 rpm |
| Speed Change in 17/28 Tests (61%) | ||
| Increased Speed: 8 Tests | ||
| Mean Increase Speed Change: 450±177 rpm | ||
| Decreased Speed: 9 Tests | ||
| Mean Decrease Speed Change: -400±245 | ||
| No Speed Change: 11 Tests | ||
|
| ||
| LVEDD, Mean | 5.7±1.2 cm | 5.7±1.2 cm |
|
| ||
| Blood Pressure (MAP), Mean | 85.3±9.7 mmHg | 86.1±11.3 mmHg |
|
| ||
| Frequency of Aortic Valve Opening | Closed: 13 Open Intermittently: 3 Open Every Beat: 6 Excluded for AV Repair/Closure at HMII Implant: 6 |
Closed: 9 Open Intermittently: 10 Open Every Beat: 3 Excluded for AV Repair/Closure at HMII Implant: 6 |
The aortic valves in 6 of the 28 patients who underwent speed optimization tests were repaired and/or closed at the time of HMII surgical implantation, and these patients were excluded from the AV optimization analysis. In the remaining 22 patients, AV opening occurred in 9 (41%) patients before and 13 (59%) patients after speed optimization. The aortic valves of the remaining 9 patients did not open at the lowest pump speed that maintained adequate blood pressure with no more than mild MR.
Suspected Device Thrombosis
Ramp tests were performed in 17 patients for suspected device thrombosis. In ten patients, the LVEDD changed only minimally with increasing speeds (see Figure 3b) whereas it changed more substantially in the remaining 7(see Figure 3a). Because of heightened concern over device thrombosis based on this finding, these ten patients had their anticoagulation regimen maximally intensified, and were observed for worsening hemolysis and/or evidence of end organ dysfunction. The latter occurred in 9 of the 10 patients, and they underwent device exchange (n=7), device explant (n=1), or urgent transplant (n=1). For these 9 patients, the mean time to surgery post-ramp test was 9.0±9.0 days. One patient of the ten remains stable on intensified anticoagulation.
Devices of all 9 patients were examined post explant and thrombus was found in 8. One patient did not have a thrombus, but instead had a grossly disconnected bend relief, which was functioning as a major obstruction to flow at the time of explant. All patients who underwent device exchange or heart transplant are alive without having suffered permanent end organ damage. For the 8 patients with confirmed device thrombosis, the mean age was 53±20 years, 62% were males, 50% had dilated cardiomyopathy, and 25% were African American (Table 2). The seven patients whose ramp test demonstrated significant changes in LVEDD with increasing pump speed were monitored on their original anti-coagulations regimens. None of them developed end organ dysfunction and none experienced thromboembolic events.
Ramp Study Results
LVEDD Slope
The LVEDD slope reflects reduction in LV size throughout the ramp study; thus, LVEDD slope is usually negative and the larger the absolute value of the slope, the steeper the slope. The mean LVEDD slope was −0.08±0.04 in confirmed device thrombosis patients, and −0.29±0.11 in the remaining patients (p < 0.001). The histogram of the LVEDD slopes is shown in Figure 4a. All patients with confirmed device thrombosis or disconnected outflow bend relief, but none of the remaining patients had a LVEDD slope > −0.16. Thus, a LVEDD slope of ≥ −0.16 was diagnostic for obstruction to flow, usually due to device thrombosis, but in one case due to outflow bend relief disconnect.
Figure 4.
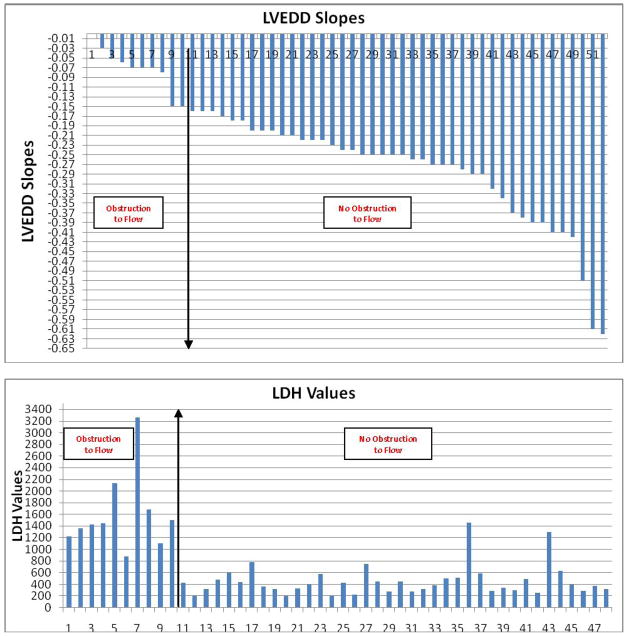
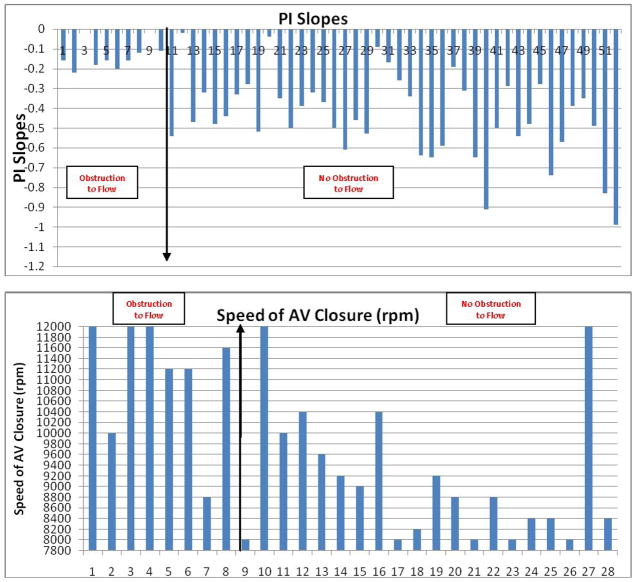
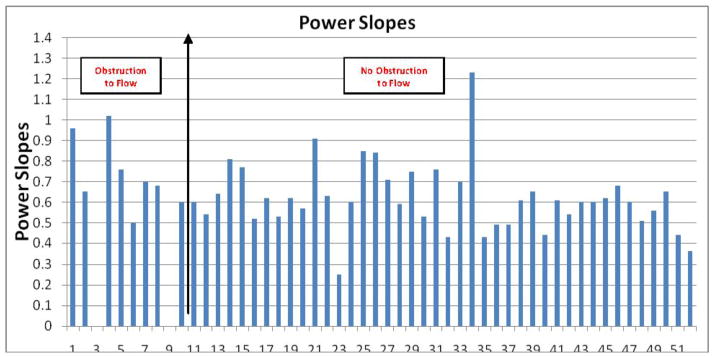
Histograms of LVEDD, LDH, AV Closure Speeds, PI, and Power Slopes
PI slope
The mean PI slope was −0.16±0.04 in confirmed device thrombosis patients, and −0.46±0.20 in the remaining patients (p <0.001). All patients (100%) with confirmed device thrombosis and a recorded PI had a PI slope in the lower quartile (>−0.28), while only 5 patients (17.2%) with normal device function had a PI slope in the lower quartile. The histogram of the PI slopes is shown in Figure 4c.
Speed for complete closure of the aortic valve
The mean speed at which the AV closed was 9,124±1,222 rpm in the patients with confirmed thrombosis and 11,100±1,146 rpm in those without confirmed thrombosis. The histograms of aortic valve closure speed (Figure 4d) shows that the aortic valves of confirmed clot patients closed at a much higher speed on average than the rest of the patient cohort without clot.
Power slope
The mean power slope was 0.74±0.15 in confirmed device thrombosis patients, and 0.62±0.17 in remaining patients (p = 0.03). The histogram of the power slopes is shown in Figure 4e.
Of 52 tests, the speed was increased to 12,000 rpm in 15 tests without meeting safety endpoints outlined above. The ramp was terminated prior to reaching 12,000 rpm in 37 patients; the mean speed at termination of the test in these patients was 10,573 + 620 rpm. There were a total of 22 suction events during ramp tests. The mean speed at which there was a suction event was 10,991±856 rpm. There were no adverse events associated with any ramp tests. None of the tests resulted in sustained ventricular tachyarrhythmias. No suction events occurred in the cohort of patients with confirmed device thrombosis.
Ramp Test results for one normal patient and for one patient with device thrombosis are presented in Figures 3a and 3b, respectively. A flat LVEDD slope, an only slightly negative PI slope, and a dramatically high Power slope characterize a ramp test suggestive of device thrombosis. A photo of a device thrombosis visualized in the device impeller upon explant of the device is shown in Figure 5.
Figure 5.
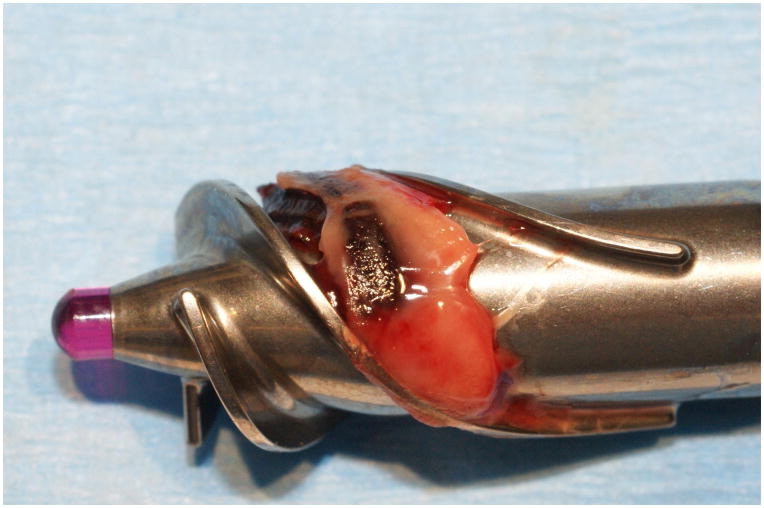
Photo of Confirmed Device Thrombosis upon Explant
LDH
LDH levels were elevated in all patients with confirmed device thrombosis with a mean LDH of 1737±684. In addition, all had a haptoglobin < 7 and the mean plasma free hemoglobin was elevated at 19.1±14.0. Two patients had an elevated LDH and signs of hemolysis but with the LVEDD slope > |−0.16| threshold. These two patients were subsequently followed for 287 and 105 days, without signs of left heart failure or any systemic embolization and their devices were found to be without thrombus when explanted. The histogram of the LDH values is shown in Figure 4b. LDH levels were much higher in thrombosis patients compared to the remainder of patients with a mean LDH of 454±263. ROC curve analysis for LDH as an indicator of thrombosis found that a LDH above 1103 (5x the upper limit of normal) has a sensitivity of 100.0% and a specificity of 92.5% (Figure 6).
Figure 6.
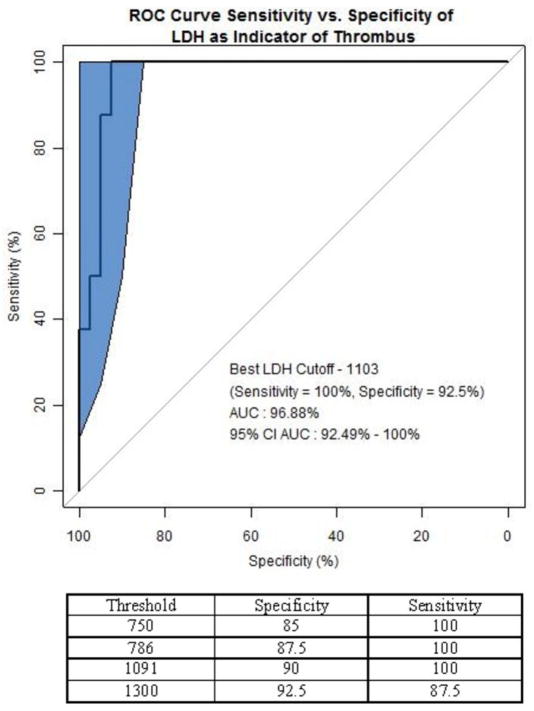
Roc Curve Analysis of LDH as Indicator of Device Thrombosis
The specificity and sensitivity values for LDH as an indicator for thrombosis were identified from the ROC curve. An LDH value above 750 u/mL has a specificity of 85% and should trigger further evaluation for device thrombosis diagnosis. The optimal results were achieved with an LDH cutoff of 1103 u/mL.
The blue region of the graph represents a 95% confidence interval for the ROC curve using the bootstrap method in the pROC package11 in R version 2.15.0.
Discussion
In this current study, we devised a unique, standardized clinical Ramp Test protocol to be routinely done at the time of hospital discharge for speed optimization or if device malfunction is suspected. We developed a standardized methodology to analyze the ramp test results using the slopes (change in x/y on a graph) of the LVEDD, PI, and power combined with clinical parameters, such as LDH. Our principal findings are as follows: First, the performance of a standardized ramp test was both safe and feasible in patients with CF-LVADs. Second, speed changes were made in 61% of patients when the test was performed for speed optimization; and third, the test proved to be highly sensitive and specific in the detection of obstruction to flow, namely device thrombosis when used in conjunction with LDH.
Echocardiography has rapidly assumed a central role in understanding the complex patient-device interface. Topilsky et al.6 reviewed the use of echocardiography for speed adjustment, and elegantly characterized the relationship between LVEDD, optimal septum position, and severity of MR for optimal device support benefit. However, in their study, repeated echocardiography imaging needed to be completed in order to achieve the stated goals. Slaughter et al.12 recommended the performance of ramp tests on every patient after device implantation: these authors advised that tests be done in the operating room at the end of device implantation surgery with TEE and hemodynamic monitoring. There is no doubt that this initial ramp test is important in the early post operative care of patients with LVADs; however, immediate post-operative conditions will almost certainly be different from the conditions in which stable LVAD patients are discharged from the hospital. It is reasonable to assume that following optimization of volume status and weaning of both inotropic and vasoactive medications12 prior to discharge the relationship between device speed and optimal hemodynamics will be different than it had been in the immediate post-operative period. Furthermore, the immediate post-operative goals for the device-patient interface may be different than those of the long-term care. For example, in the initial phase, speed adjustment in order to allow for aortic valve opening is not crucial to patient care. However, during the chronic phase of device treatment, there is a clear association of the aortic valve remaining closed and the development of aortic insufficiency in patients supported by continuous flow pumps7.
Though advocated in the care of CF-LVAD patients, currently no standardized clinical protocol for performance of a ramp study exists. Consequently, each medical center uses its own discretion for performing speed adjustments and diagnosing device malfunctions. Here we present the Columbia Ramp Study (CORS), which enumerates a standardized echocardiographically-guided assessment of the relationship between the device and the patient, including study indications, contraindications, speed change intervals, echocardiographic parameters to measure, and a novel methodology for analyzing the data obtained with mathematical inference.
With a single study, the clinician gains an accurate understanding of the optimal device settings to preserve left ventricular geometry, reduce mitral regurgitation severity, and allow the aortic valve to open intermittently. Routine application of this test allows for an overall characterization of the interface between the patient’s native heart and the device. Importantly, each patient’s baseline ramp test also serves as an important reference with which to compare future tests which may be done in the setting of suspected device thrombosis or device malfunction, much like a BNP level drawn during euvolemia.
Perhaps even more important than its use for speed optimization is the test’s ability to aid in the diagnosis of pump thrombosis. Previous literature on CF-LVADs reported that the frequency of device thrombosis is in the range of 2% in the BTT population2 and 4% in the DT population1. Of note, a strict definition for device thrombosis was applied in the clinical trials:
“Any obstructive thrombus in the device or its conduits associated with clinical symptoms of impaired pump performance (e.g. decreased pump flow, need to increase pump speed, increased power, hemolysis) or the need for thrombolytic or surgical intervention. In addition, pumps will be analyzed at Thoratec. Any severe thrombus scored as a level 3 thrombus (>50% obstruction) will be captured as an event1.”
The true incidence of clinically relevant device thrombosis may be higher. The problem of device thrombosis will surely remain an important one as newer CF-VADs like the Heartware device (Heartware Corporation, Framingham, MA) are introduced into clinical practice. Risk factors for device thrombosis have been reported and include less aggressive anticoagulation, infection, and hypercoaguability syndromes (i.e. Systemic Lupus Erythematosus). One barrier in the treatment of patients with device thrombosis stems from the difficulty in making this diagnosis prior to catastrophic thromboembolic events. Currently, suspicion for thrombosis arises when there are signs of hemolysis, (elevated LDH, high plasma free hemoglobin, and low haptoglobin), transient increases in device power more than 14 days post-implantation, or reoccurrence of congestive heart failure1. However, these criteria lack both sensitivity and specificity for the diagnosis of device thrombosis. Regular echocardiography typically fails to diagnose the majority of device thromboses13, 14. CT scan with contrast media has been proposed as a diagnostic option for inflow and outflow cannulas thrombosis15, 16, but is a severely limited approach as thrombus within the device cannot be detected.
In the current study, we observed that the ramp test can identify a perturbation of the relationship between the patient’s native heart and the device. We have demonstrated that LVEDD slope correlates with device thrombosis and/or severe outflow obstruction due to a disconnected bend relief. This finding is explained by the fact that the impediment to flow caused by device thrombosis leads to an uncoupling of the relationship between the device speed and LVEDD. In other words, blunted reductions in LVEDD in response to increase in pump speed indicate an obstruction to flow through the device. Not surprisingly, LVEDD slopes were the most accurate measure in the diagnosis of thrombosis. The combination of clinical suspicion for thrombosis based on heart failure symptoms, LDH levels, and LVEDD, PI and power slopes led us to intensify anticoagulation regimens and/or perform device exchange in 8 patients, 7 (88%) of which were found to have device thrombosis with the eighth patient having an obstruction to flow due to a gross bend relief disconnect.
Our study has several limitations. First, this is a single-center prospective analysis of a relatively small cohort of patients. Second, our methods for definitive diagnosis of pump thrombosis was to send suspected thrombosis pumps to Thoratec Corporation upon explant at Columbia for further inspection of the pump housing and final validation of device clot. This may introduce selection bias. Our current practice is to send all explanted pumps, including both “normal” and malfunctioning pumps to the manufacturer for disassembly and inspection. Third, the Ramp Test protocol is time-consuming and cumbersome in that it requires strict adherence to the two-minute time interval between speed designations. This Ramp Test protocol may be able to be abbreviated once a larger data set is reviewed. We have designed a protocol to be used with the Heartware device, and preliminary results are indicative of the Ramp Test being a beneficial tool for speed adjustment and device malfunction diagnosis.
Further follow-up is needed on the patients underwent ramp test for speed optimization for outcome evaluation; the development of AI and frequency of device related complication.
Recommendation
Based on our experience at Columbia, we developed a working algorithm to facilitate the diagnosis of device malfunction (Figure 7).
Figure 7.
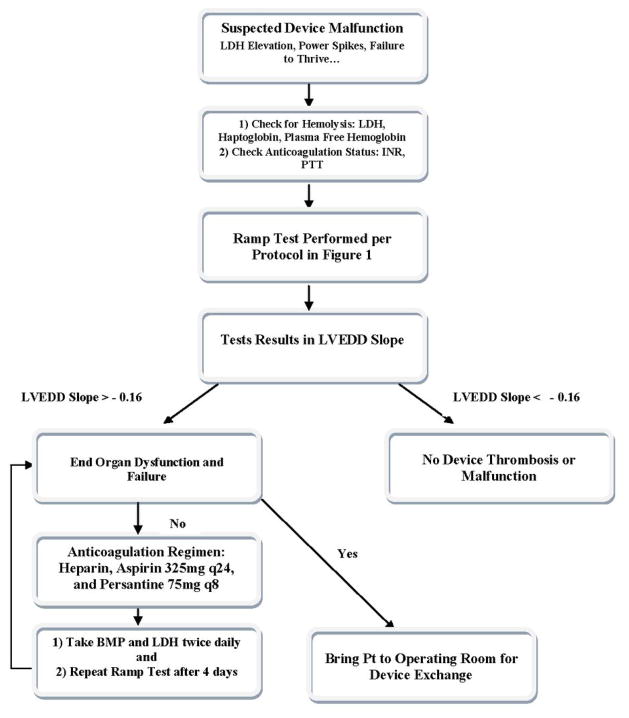
Suspected Device Malfunction Algorithm
Suggested management algorithm for patients with suspected device thrombosis.
In conclusion, Ramp Tests done to monitor appropriateness of CF-LVAD programmed settings very frequently led to changes in device speed, and thereby, should be done routinely in CF-LVAD recipients. Ramp Tests allowed for device malfunction detection in the setting of suspected device thrombosis and, pending validation in larger studies, they may be used to monitor the effects of therapeutic interventions and to determine the need for surgical intervention.
Supplementary Material
Acknowledgments
This study was supported by a gift from Thoratec Cooperation to the Trustees of Columbia University supporting a mentored (Dr. Ulrich Jorde) MCSD fellowship at Columbia University. Dr. Jorde is supported by NIH R01-HL 868845-04. Dr. Nir Uriel is supported by NIH 1 K23 HL 103795-01A1.
Appendix I
Heartware Ramp Test
| Speed | LF | UF | Power | Flow | BP | HR | LVEDD | LVESD | AV opening | AI | MR | RVSP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2400 | ||||||||||||
| 2460 | ||||||||||||
| 2520 | ||||||||||||
| 2580 | ||||||||||||
| 2640 | ||||||||||||
| 2700 | ||||||||||||
| 2760 | ||||||||||||
| 2820 | ||||||||||||
| 2880 | ||||||||||||
| 2940 | ||||||||||||
| 3000 | ||||||||||||
| 3060 | ||||||||||||
| 3120 |
LF – lower flow, UF – upper flow, BP-blood pressure, HR-heart rate, LVEDD-left ventricular end diastolic diameter, LVESD-left ventricular end systolic diameter, AV opening-aortic valve opening, AI-aortic insufficiency, MR-mitral regurgitation, RVSP-right ventricular systolic pressure
Appendix II
Assessing the error of fit LVEDD, PI and Power slopes to the linear function model.
Slopes can be expected to vary from the true curves when the linear fit is poor, however, the correlations were consistently satisfactory in our study.
For the group of thrombus-positive ramp tests, the mean correlation between LVEDD and speed was −0.7824 ± 0.1603. The thrombus-negative ramp tests had mean correlation of −0.9312 ± 0.04925.
The mean correlation between PI and speed for thrombus-positive ramp tests was −0.8477 ± 0.1140. Thrombus-negative ramp tests had mean correlation of −0.9064 ± 0.1309.
The mean correlation between Power and speed for thrombus-positive ramp tests was −0.9779 ± 0.0253. Thrombus-negative ramp tests had mean correlation of −0.9693 ± 0.0812.
Footnotes
Relationship with Industry:
Ulrich P. Jorde, MD: Honorarium, Thoratec Cooperation (Pleasanton, CA)
Yoshifumi Naka: Honorarium, Thoratec Cooperation (Pleasanton, CA)
Ranjit John: Honorarium, Thoratec Cooperation (Pleasanton, CA)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009 Dec 3;361(23):2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 2.Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007 Aug 30;357(9):885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 3.Pagani FD, Miller LW, Russell SD, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009 Jul 21;54(4):312–321. doi: 10.1016/j.jacc.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 4.John R, Naka Y, Smedira NG, et al. Continuous flow left ventricular assist device outcomes in commercial use compared with the prior clinical trial. Ann Thorac Surg. Oct;92(4):1406–1413. doi: 10.1016/j.athoracsur.2011.05.080. discussion 1413. [DOI] [PubMed] [Google Scholar]
- 5.Park SJ, Milano CA, Tatooles AJ, et al. Outcomes in advanced heart failure patients with left ventricular assist devices for destination therapy. Circ Heart Fail. Mar 1;5(2):241–248. doi: 10.1161/CIRCHEARTFAILURE.111.963991. [DOI] [PubMed] [Google Scholar]
- 6.Topilsky Y, Hasin T, Oh JK, et al. Echocardiographic variables after left ventricular assist device implantation associated with adverse outcome. Circ Cardiovasc Imaging. Nov;4(6):648–661. doi: 10.1161/CIRCIMAGING.111.965335. [DOI] [PubMed] [Google Scholar]
- 7.Pak SW, Uriel N, Takayama H, et al. Prevalence of de novo aortic insufficiency during long-term support with left ventricular assist devices. J Heart Lung Transplant. Oct;29(10):1172–1176. doi: 10.1016/j.healun.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Cowger J, Pagani FD, Haft JW, Romano MA, Aaronson KD, Kolias TJ. The development of aortic insufficiency in left ventricular assist device-supported patients. Circ Heart Fail. Nov;3(6):668–674. doi: 10.1161/CIRCHEARTFAILURE.109.917765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatano M, Kinugawa K, Shiga T, et al. Less frequent opening of the aortic valve and a continuous flow pump are risk factors for postoperative onset of aortic insufficiency in patients with a left ventricular assist device. Circ J. 75(5):1147–1155. doi: 10.1253/circj.cj-10-1106. [DOI] [PubMed] [Google Scholar]
- 10.Uriel N, Pak SW, Jorde UP, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. Oct 5;56(15):1207–1213. doi: 10.1016/j.jacc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. Apr;29(4 Suppl):S1–39. doi: 10.1016/j.healun.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Estep JD, Stainback RF, Little SH, Torre G, Zoghbi WA. The role of echocardiography and other imaging modalities in patients with left ventricular assist devices. JACC Cardiovasc Imaging. Oct;3(10):1049–1064. doi: 10.1016/j.jcmg.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Aissaoui N, Paluszkiewicz L, Koertke HH, Morshuis M, Gummert J. Echocardiography can be more sensitive than thoracic computed tomography in detecting a thrombus in the inflow cannula of a continuous left ventricular assist device. Echocardiography. Nov;28(10):E215–216. doi: 10.1111/j.1540-8175.2011.01496.x. [DOI] [PubMed] [Google Scholar]
- 15.Mishkin JD, Enriquez JR, Meyer DM, et al. Utilization of cardiac computed tomography angiography for the diagnosis of left ventricular assist device thrombosis. Circ Heart Fail. Mar 1;5(2):e27–29. doi: 10.1161/CIRCHEARTFAILURE.111.966119. [DOI] [PubMed] [Google Scholar]
- 16.Mellnick VM, Raptis DA, Raptis C, Bhalla S. Imaging of Left Ventricular Device Complications. J Thorac Imaging. Dec 21; doi: 10.1097/RTI.0b013e318232e5e4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


