Abstract
Acid-sensing ion channels (ASICs) are expressed in skeletal muscle afferents, in which they sense extracellular acidosis and other metabolites released during ischemia and exercise. ASICs are formed as homotrimers or heterotrimers of several isoforms (ASIC1a, ASIC1b, ASIC2a, ASIC2b, and ASIC3), with each channel displaying distinct properties. To dissect the ASIC composition in muscle afferents, we used whole-cell patch-clamp recordings to study the properties of acid-evoked currents (amplitude, pH sensitivity, the kinetics of desensitization and recovery from desensitization, and pharmacological modulation) in isolated, labeled mouse muscle afferents from wild-type (C57BL/6J) and specific ASIC−/− mice. We found that ASIC-like currents in wild-type muscle afferents displayed fast desensitization, indicating that they are carried by heteromeric channels. Currents from ASIC1a−/− muscle afferents were less pH-sensitive and displayed faster recovery, currents from ASIC2−/− mice showed diminished potentiation by zinc, and currents from ASIC3−/− mice displayed slower desensitization than those from wild-type mice. Finally, ASIC-like currents were absent from triple-null mice lacking ASIC1a, ASIC2a, and ASIC3. We conclude that ASIC1a, ASIC2a, and ASIC3 heteromers are the principle channels in skeletal muscle afferents. These results will help us understand the role of ASICs in exercise physiology and provide a molecular target for potential drug therapies to treat muscle pain.—Gautam, M., Benson, C. J. Acid-sensing ion channels (ASICs) in mouse skeletal muscle afferents are heteromers composed of ASIC1a, ASIC2, and ASIC3 subunits.
Keywords: sensory neurons, muscle pain, exercise
Skeletal muscle has the capacity for high metabolic activity and is susceptible to rapid drops in pH during ischemia and/or maximal exercise. During rest or light exercise, blood flow is adequate to supply oxygen necessary to generate ATP via the respiratory cycle. However, with insufficient blood supply and oxygen, which occurs during maximal isotonic exercise or isometric contraction or ischemia induced by peripheral vascular disease, myocytes will attempt to maintain contractile function by switching to anaerobic glycolysis. Consequently, lactic acid is generated and, along with ATP hydrolysis, reduces intracellular pH, which in turn leads to acidification of the interstitium. With extreme exercise, the extracellular pH in human skeletal muscle can drop to the 6.7−7.0 range (1, 2), and lactate levels can rise from a resting level of ∼1 to 15–30 mM (3). These metabolic changes, as well as mechanical perturbations, are sensed by sensory nerves (muscle afferents) that richly innervate muscle tissue. Within these muscle afferents, increasing evidence suggests that acid-sensing ion channels (ASICs) are important molecular sensors.
ASICs are H+-gated channels of the degenerin (DEG)/epithelial sodium (ENaC) family, expressed principally in the central nervous system (CNS) and in peripheral sensory neurons. In general, they seem to be highly expressed in organs of high metabolic activity, including the brain, and sensory nerves that innervate the heart and skeletal muscle (4–7). Several observations highlight the importance of ASICs in skeletal muscle afferents. First, ASIC expression in muscle afferents is higher than that in cutaneous afferents, and they are activated in the narrow range of extracellular pH (pH 7.0–6.8) that occurs during muscle ischemia (5, 8–10). Second, ASICs are required for the development of normal muscle pain. Both inflammation-inducing muscle insults and direct acid injection into muscle cause an increase in pain in mice that is dependent on ASICs. Either genetic deletion of specific ASIC subunits, knockdown of ASICs by RNA interference in muscle, or pharmacological inhibition of ASICs attenuates hyperalgesia in these mouse models of muscle pain (11–14). Third, ASICs are required for normal exercise-mediated reflexes. Activation of muscle afferents during exercise evokes reflexes that increase blood pressure, heart rate, and ventilation (termed the “exercise pressor reflex”; refs. 15–17). In several recent studies, ASIC antagonists have been shown to attenuate the exercise pressor reflex (18–20). Hayes et al. (21) recently showed that an ASIC blocker, A-317567, inhibited the pressor response to lactic acid injection by 75% and to static muscle contraction by 60% and yet had no effect on the pressor responses to passive stretch or capsaicin injection. Last, in an effort to identify metaboreceptive muscle afferents, Light et al. (10) used calcium imaging to identify a population of skeletal muscle afferents that were maximally activated by a combination of protons, ATP, and lactate at physiological concentrations. Besides being activated by protons, ASICs are potentiated by both ATP and lactate (22, 23), and pharmacological block of ASICs completely inhibited the response to the combination of agonists (10).
In rodents, ASICs include 4 genes that encode ≥6 subunits (ASIC1a, -1b, -2a, -2b, -3, and -4; ASIC1 and ASIC2 have alternate splice transcripts). Functional ASICs consist of a complex of 3 subunits; individual subunits form homotrimers, whereas 2 or more subunits can assemble to generate heterotrimers. There is strong evidence that ASIC1a, ASIC2a, and ASIC3 each contribute to the formation of either homomeric or heteromeric ASICs in peripheral sensory neurons (24, 25). The role of ASIC1b subunits in the periphery is less understood, perhaps because a specific knockout model is lacking. ASIC2b does not form H+-gated channels by itself but may influence the channel properties if it heteromultimerizes with other subunits (26, 27). ASIC4 is not know to contribute to H+-gated channels but may affect trafficking of other subunits (28). Notably, each of the various homomeric and heteromeric ASICs displays unique biophysical and pharmacological properties. Moreover, nature seems to have used these diverse properties by varying the composition of ASICs in different populations of neurons. For example, neurons in the CNS are composed primarily of a combination of ASIC1a homomers and ASIC1a/2 heteromeric channels (29–31), whereas in cardiac dorsal root ganglion (DRG) sensory neurons, the channels principally consist of ASIC2a/3 heteromers (32). Whereas ASICs are highly expressed in skeletal muscle afferents, the subunit composition of the channels in these cells is unknown. By comparing the ASIC currents from labeled skeletal muscle afferents from mice with genetic deletion of selective ASIC subunits with those from wild-type mice and comparing their properties with those of heterologously expressed ASICs, we were able to define the subunit composition of ASICs in skeletal muscle afferents.
MATERIALS AND METHODS
Generation of ASIC-knockout mice
All animal procedures were followed in accordance with and were approved by the Institutional Animal Care and Use Committee of the University of Iowa. The generation of ASIC1-, ASIC2-, and ASIC3-null mice has been reported previously (6, 11, 33). Subsequently, these mice were backcrossed for 10 generations onto a C57BL/6J background to generate congenic lines of each. These congenic lines were crossed to one another to generate a congenic C57BL/6J line with the simultaneous disruption of ASIC subunits.
Labeling of muscle sensory neurons
Sensory neurons innervating muscle were fluorescently labeled using the retrograde tracer 1,1-dioctadecyl-3,3,3,3 tetramethylindocarbocyanine perchlorate (DiI; 17 mg/ml dissolved in 20% v/v ethanol and suspended in 80% v/v sterile saline; Invitrogen, Eugene, OR, USA). Animals were anesthetized with 2–5% isoflurane; a small incision was made in skin over the left gastrocnemius muscle, and 10 μl of DiI solution was injected into the muscle as described previously (34). After injection, saline-soaked sterile gauze was placed on the open incision for 10 min to prevent the dye from leaking to the overlying skin. The skin was then sutured closed, and mice were allowed to recover for 2 wk.
Culture of DRG neurons
At 2 wk after DiI injection, the mice were euthanized, and the ipsilateral DRGs (L4–L6) were collected and dissociated as described previously (24). DRGs were successively treated with papain and collagenase-dispase and then were gently triturated to isolate neurons. Neuron suspensions were then plated on 35-mm Petri dishes coated with poly-d-lysine and laminin. Cells were cultured in F12 medium supplemented with 10% heat-inactivated serum, penicillin-streptomycin, and 50 ng/ml nerve growth factor. Muscle afferents were identified by fluorescence microscopy (see Fig. 1A) and were studied 18–48 h after plating.
Figure 1.
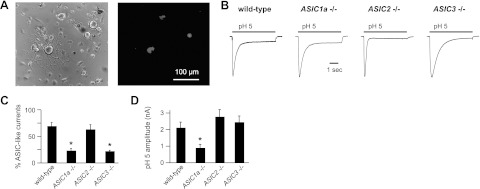
ASICs in skeletal muscle afferents are composed of multiple subunits. A) Corresponding phase (left panel) and fluorescence (right panel) micrographs of 3 labeled skeletal muscle afferents in primary dissociated culture of DRG neurons collected 2 wk after injection of DiI into the mouse gastrocnemius muscle. B) Representative currents evoked by application of pH 5 solution to muscle afferents from mice of the indicated genotype. Currents are normalized to demonstrate differences in kinetics (current amplitudes: wild-type, 1.04 nA; ASIC1a−/−, 2.85 nA; ASIC2−/−, 3.55 nA; and ASIC3−/−, 5.46 nA). C) Percentages of muscle afferents from each genotype that responded to pH 5 with an evoked current > 60 pA. Three wild-type mice (n=11–12 neurons/mouse), 6 ASIC1a−/− mice (n=5–15 neurons/mouse), 3 ASIC2−/− mice (n=8–9 neurons/mouse), and 7 ASIC3−/− mice (n=5–16 neurons/mouse) were studied. *P < 0.02 vs. wild-type. D) Mean peak pH 5-evoked current amplitude of the responding neurons. *P < 0.01 vs. wild-type.
Electrophysiology
Whole-cell patch-clamp recordings (at −70 mV) of DiI-labeled muscle sensory neurons were performed at room temperature with an Axopatch 200B amplifier (Axon Instruments, Foster City, CA, USA) and were acquired and analyzed with Clampex 8.2 (Axon Instruments) and IGOR Pro 6.01 (WaveMetrics, Lake Oswego, OR, USA) software. Currents were filtered at 1 kHz and sampled at 2 kHz. Micropipettes (3-5 MΩ) were filled with internal solution: 100 mM KCl, 10 mM EGTA, 40 mM HEPES, and 5 mM MgCl2, pH 7.4 with KOH. External solution contained 120 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, and 10 mM 2-(N-morpholino)ethane sulfonic acid. pH was adjusted with tetramethylammonium hydroxide, and osmolarity was adjusted with tetramethylammonium chloride. Rapid extracellular solution exchanges were made using a computer-driven BPS 8 system (ALA Scientific, Westbury, NY, USA). Kinetics of desensitization were fit with single exponential equations, and time constants (τ) are reported. pH activation curves were fit using the Hill equation: fraction of open channels = 1/[1 + (pH10)/pH5010)n], where pH50 is the pH at which half of the channels are opened. Data are means ± se. Statistical significance was assessed using an unpaired Student's t test.
RESULTS
ASICs in muscle afferents are formed by multiple different subunits
To understand the composition of ASICs in skeletal muscle afferents, we studied acid-evoked currents in isolated mouse muscle afferents that had been labeled by injection of a retrograde tracer dye (DiI) into the gastrocnemius muscle (Fig. 1A). We studied muscle afferents from wild-type mice, as well as those from mice that had undergone targeted deletion of specific ASIC subunits. Figure 1B shows typical ASIC-like currents evoked by pH 5 application to DiI-labeled muscle afferents from the indicated genotypes; the currents rapidly activate and then desensitize in the continued presence of acidic solution (transient current), followed by variable persistent activation (sustained current). The transient component of the currents was inhibited by the ASIC blocker amiloride (data not shown). These currents were elicited from a high percentage (68%) of muscle afferents (Fig. 1C), which is in agreement with previous work showing that ASIC currents are more consistently evoked in muscle afferents compared with cutaneous afferents (5). Notably, ASIC-like currents were not eliminated in any of the single-ASIC-knockout mice, which indicates that the channels are not composed of any single subunit. However, the percentage of muscle afferents that displayed ASIC-like currents was significantly less in ASIC1a−/− and ASIC3−/− mice compared with wild-type mice (Fig. 1C), and current amplitudes were smaller in the ASIC1a−/− mice (Fig. 1D). These initial data suggest that ASICs are expressed in a majority of mouse muscle afferents, the channels consist of >1 subunit, and most likely ASIC1a and ASIC3 are contributors.
ASIC1a contributes to ASICs in muscle afferents
To further determine the composition of the ASICs in muscle afferents, we took advantage of the fact that each of the different homomeric and heteromeric ASICs possesses unique biophysical properties (Table 1). We hypothesized that if a particular subunit contributes to the composition of the channels, then targeted deletion of that subunit would change the properties of the residual currents in a predictable manner. We first compared the properties of muscle afferent currents from ASIC1a−/− mice with those of wild-type mice. Figure 2A shows representative currents evoked by various pH solutions in skeletal afferents from the two genotypes. By normalizing current amplitudes evoked at various pH solutions to that evoked by pH 5 (generally the maximal current), we plotted pH dose responses and found that the pH sensitivity of activation of muscle afferents from ASIC1a−/− mice was shifted to the right compared with that of wild-type mice (Fig. 2B). This result indicates that loss of ASIC1a reduces the pH sensitivity of ASICs in muscle afferents. Loss of ASIC1a could cause a shift in the mean values by either significantly altering the properties of only a few cells or altering the properties of all or most cells. To test these two possibilities, we plotted individual data points and found very little overlap between the ranges of ASIC1a−/− and wild-type data (Fig. 2B, right panel), suggesting that ASIC1a is a major constituent of ASICs in most muscle afferents.
Table 1.
Biophysical properties of ASICs in skeletal muscle afferents from wild-type and the indicated ASIC−/− mice and heterologously expressed ASIC subunits
| Afferent or subunit | Amplitude (nA) | pH50 | τ Desensitization (s) |
τ Recovery (s) | ||
|---|---|---|---|---|---|---|
| pH 6.0 | pH 5.0 | pH 4.0 | ||||
| Muscle afferent | ||||||
| Wild-type | 2.1 ± 0.3 | 6.4 ± 0.04 | 0.17 ± 0.02 | 0.21 ± 0.02 | 0.23 ± 0.04 | 0.48 ± 0.05 |
| ASIC1a−/− | 0.9 ± 0.2** | 6.0 ± 0.07** | 0.18 ± 0.02 | 0.31 ± 0.04* | Sustained | 0.11 ± 0.03** |
| ASIC2−/− | 2.7 ± 0.4 | 6.4 ± 0.04 | 0.14 ± 0.01 | 0.18 ± 0.01 | 0.30 ± 0.06 | 0.53 ± 0.04 |
| ASIC3−/− | 2.4 ± 0.4 | 6.3 ± 0.07 | 1.75 ± 0.20** | 0.64 ± 0.04** | 0.34 ± 0.08 | 0.89 ± 0.05** |
| ASIC1a/3−/− | 0.7 ± 0.2** | 4.1 ± 0.10** | 2.52 ± 0.50** | |||
| ASIC subunit expressed in CHO cells | ||||||
| ASIC1a | 6.6a | 1.60a | 0.80a | 0.45a | 11b | |
| ASIC2a | 3.8a | 2.80b | 0.60b | |||
| ASIC3 | 6.8a | 0.29a | 0.41a | 0.47a | 0.40b | |
| ASIC1a + ASIC2a | 6.1b | 0.90b | 0.65a | 0.86a | 0.63b | |
| ASIC1a + ASIC3 | 6.6b | 0.14b | 0.15a | 0.32a | 0.64b | |
| ASIC2a + ASIC3 | 5.9b | 0.14a | 0.34a | Sustainedc | 0.08b | |
| ASIC1a + ASIC2a + ASIC3 | 6.4b | 0.13a | 0.28a | 0.31a | 0.32b | |
Maximal amplitudes were evoked by pH 3.5 in ASIC1a/3−/− mice and by pH 5 for other genotypes. Values for pH50 and τ of desensitization and recovery from desensitization are derived from the fits of data from individual cells.
P < 0.05,
P < 0.01 vs. wild-type muscle afferent.
Data of heterologously expressed ASICs from Hattori et al. (32).
Data from Benson et al. (24).
Data from Hesselager et al. (26).
Figure 2.
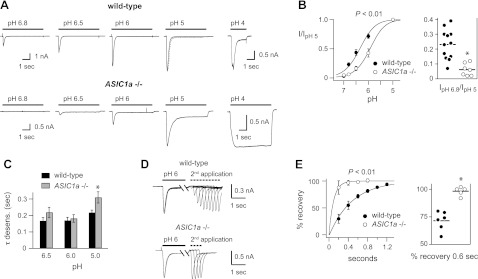
Properties of acid-evoked currents in skeletal muscle afferents from wild-type and ASIC1a−/− mice. A) Representative currents evoked by the indicated pH solutions from a control solution of pH 7.4 in muscle afferents from wild-type and ASIC1a−/− mice. The pH 4-evoked currents were from separate cells. B) pH dose-response data for pH-evoked currents in wild-type and ASIC1a−/− muscle afferents. Data were normalized to the peak currents evoked by pH 5. Lines are fits of the Hill equation of the means (n=5–13). P < 0.01 for pH50 values calculated from the fits of the Hill equation. Right graph, normalized individual data points and means at pH 6.8. *P < 0.01 vs. wild-type. C) Mean τ of desensitization as measured from single exponential fits to the falling phase of the transient currents (dashed line over pH 5-evoked current from wild-type neuron in panel A) evoked by the indicated pH solutions in wild-type and ASIC1a−/− muscle afferents (n=6–24). Rates were not calculated for pH 4-evoked currents from ASIC1a−/− muscle afferents because these currents were mostly sustained. *P < 0.03 vs. wild-type pH 5-evoked currents. D) Overlay of current traces showing recovery from desensitization of a wild-type and an ASIC1a−/− muscle afferent. Current was desensitized with a 7-s application of pH 6 (only the first 1.25 s is shown). Cells were then exposed to pH 7.4 solution for the indicated times (see x axis in panel E) before they were stimulated again with pH 6. Recovery is the percentage of current evoked by the second pH 6 application compared with the first. E) Mean recovery data as collected in panel D for wild-type and ASIC1a−/− muscle afferents (n≥5). Lines are fits of single exponentials of the means. P < 0.01 for τ was calculated from the fits of individual cells. Right graph, individual data points and means for recovery at 0.6 s. *P < 0.01 vs. wild-type.
By fitting the desensitizing phase of the currents to a single exponential (Fig. 2A, dashed line overlying the pH 5-evoked current from the wild-type afferent) and plotting τ, we found that ASIC-like currents from muscle afferents desensitize very fast (Fig. 2C). In fact, pH 6- and pH 5-evoked currents desensitized twice as fast as homomeric ASIC3 channels, which have the fastest desensitization of any of the homomeric ASICs (Table 1). Only heteromeric channels that contain ASIC3 as one of the subunits possess such fast desensitization kinetics for currents evoked in this pH range (Table 1). By comparing the kinetics of desensitization of ASIC1a−/− mice with those from wild-type mice, several observations can be gleaned. First, pH 5-evoked currents from ASIC1a−/− mice desensitized slower than those from wild-type mice (Fig. 2C). Second, the current traces in Fig. 2A demonstrate that the sustained current amplitudes evoked by pH 5 as a proportion of the peak amplitude were significantly larger in ASIC1a−/− mice (0.45±0.09, n=8, P<0.05) than those in wild-type mice (0.22±0.04, n=22). Third, the currents evoked by pH 4 did not desensitize (Fig. 2A) or underwent very slow desensitization such that it could not be fit in the majority of muscle afferents from ASIC1a−/− mice (7 of 9 cells), compared with wild-type mice (only 2 of 7 cells).
Last, we measured the rate at which the channels recover from desensitization. After entering into a desensitized state, ASICs require exposure to a more alkaline pH before they then can again be activated by acidic pH (Fig. 2D, E). Currents from ASIC1a−/− mice recovered significantly faster than those from wild-type mice, and plotting the data from individual cells at one time point indicates a shift in all cells (Fig. 2E, right panel). In summary, these data provide significant insight into the composition of ASICs in muscle afferents. First, the majority of channels are heteromers, because only heteromeric channels possess such fast desensitization kinetics. In fact, the channels in ASIC1a−/− mice also appear to be heteromers. because the pH 6- and pH 5-evoked currents from ASIC1a−/− mice also desensitized faster than any of the homomeric ASICs (Table 1). Second, ASIC1a is a major constituent of the channels in muscle afferents: acid-evoked currents from ASIC1a−/− mice were of smaller amplitude, had larger relative sustained currents, had slower kinetics of desensitization at pH 5, and had faster recovery from desensitization. Moreover, the range of data from ASIC1a−/− cells was shifted beyond the range of data from wild-type cells, suggesting that ASIC1a is a constituent in a majority of, if not all, ASICs in muscle afferents. Third, the properties of the ASICs in ASIC1a−/− mice are most consistent with those of heteromeric channels formed by ASIC2a and ASIC3 subunits: the large sustained currents evoked at pH 4 and the very fast recovery from desensitization are both characteristic properties of ASIC2a/3 heteromers (refs, 24, 26, 32 and Table 1).
ASIC2 subunits contribute to ASICs in muscle afferents
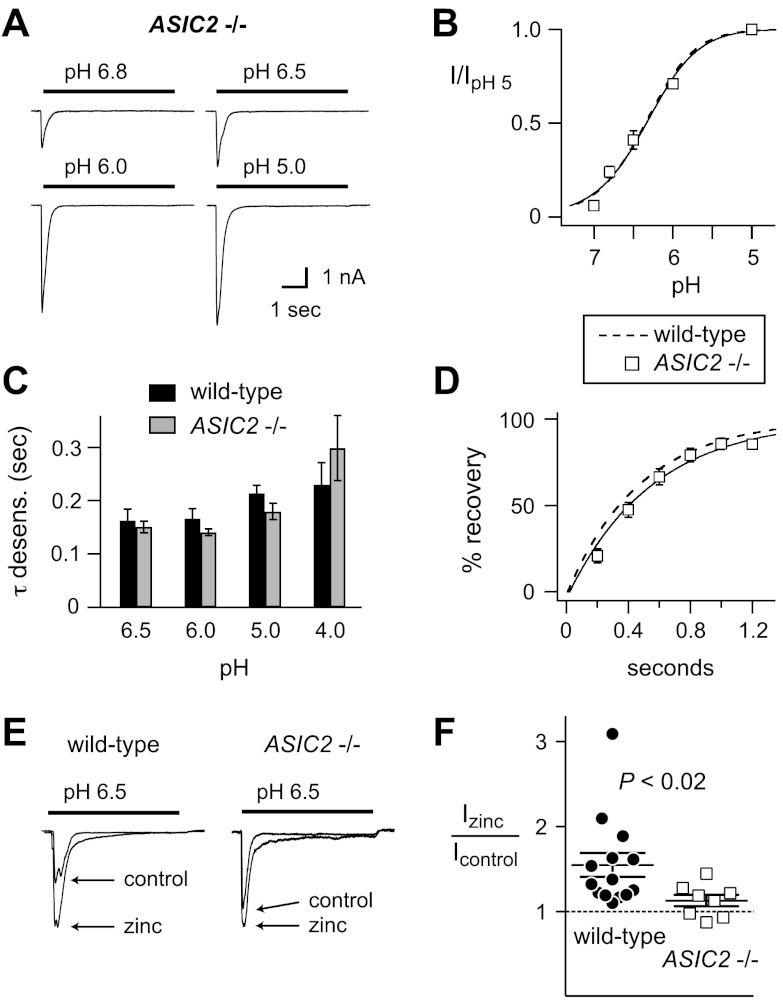
To further test whether ASIC2 subunits contribute to ASICs in muscle afferents, we studied ASIC-like currents from mice that lack ASIC2 subunits. Notably, the pH sensitivity of activation (Fig. 3A, B), as well as the kinetics of both desensitization (Fig. 3C) and recovery from desensitization (Fig. 3D), were not significantly different than data from wild-type muscle afferents. This result suggests that ASIC2 subunits are not part of the channels. However, whereas heteromeric channels formed by the coexpression of two subunits all possess distinguishing properties, when ASIC2 subunits are coexpressed with both ASIC1a and ASIC3 subunits, the resultant pH sensitivity and kinetics are practically indistinguishable from currents formed by ASIC1a and ASIC3 subunits alone (Table 1). Because our data from wild-type and ASIC1a−/− mice suggest that ASICs in muscle afferents are heteromeric channels composed of at least ASIC1a and ASIC3 subunits, the loss of ASIC2 might not be expected to result in a change in the measured biophysical properties. Thus, to test for the presence of ASIC2 subunits, we studied acid-evoked currents in wild-type and ASIC2−/− muscle afferents in the presence of zinc. Zinc can have complex modulating effects on ASICs, depending on its concentration and the subunit composition of the channels. However, whereas zinc inhibits currents via other ASIC subunits (35–37), only ASIC2a homomeric and ASIC2a-containing heteromeric channels are potentiated by zinc (38). Therefore, we tested for the presence of ASIC2a in wild-type muscle afferents by studying the effect of 300 μM zinc on pH 6.5-evoked current amplitude, conditions at which zinc potentiation is greatest (38). We found that zinc potentiated current in a majority of wild-type muscle afferents (Fig. 3E, F). In comparison, zinc potentiation was abolished in ASIC2−/− muscle afferents, demonstrating that the effect was dependent on ASIC2 subunits (Fig. 3F).
Figure 3.
Properties of acid-evoked currents in skeletal muscle afferents from ASIC2−/− mice. A) Representative currents evoked by the indicated pH solutions in muscle afferents from ASIC2−/− mice. B) pH dose-response data of currents evoked from ASIC2−/− muscle afferents normalized to the currents evoked by pH 5 (n≥12). Line is fit of the Hill equation. Dashed line is fit of data from wild-type afferents in Fig. 2B. C) Mean τ of desensitization of the transient currents evoked by the indicated pH solutions from ASIC2−/− muscle afferents compared with wild-type data from Fig. 2C (n≥11). D) Recovery from desensitization data for ASIC2−/− muscle afferents (n≥5). Line is fit of single exponential of the means. Dashed line is fit of data from wild-type afferents in Fig. 2E. E) Representative pH 6.5-evoked currents in the presence and absence of 300 μM zinc in wild-type and ASIC2−/− muscle afferents. Zinc was present only in the pH 6.5 solution and not in the bathing solution. Zinc potentiated currents in wild-type (n=14; P<0.01 vs. control using paired Student's t test) but not in ASIC2−/− muscle afferents (n=8; P=0.16 vs. control). F) Relative change in pH 6.5-evoked currents in presence of zinc. Data are individual data points as well as means ± se. *P < 0.02 vs. wild-type.
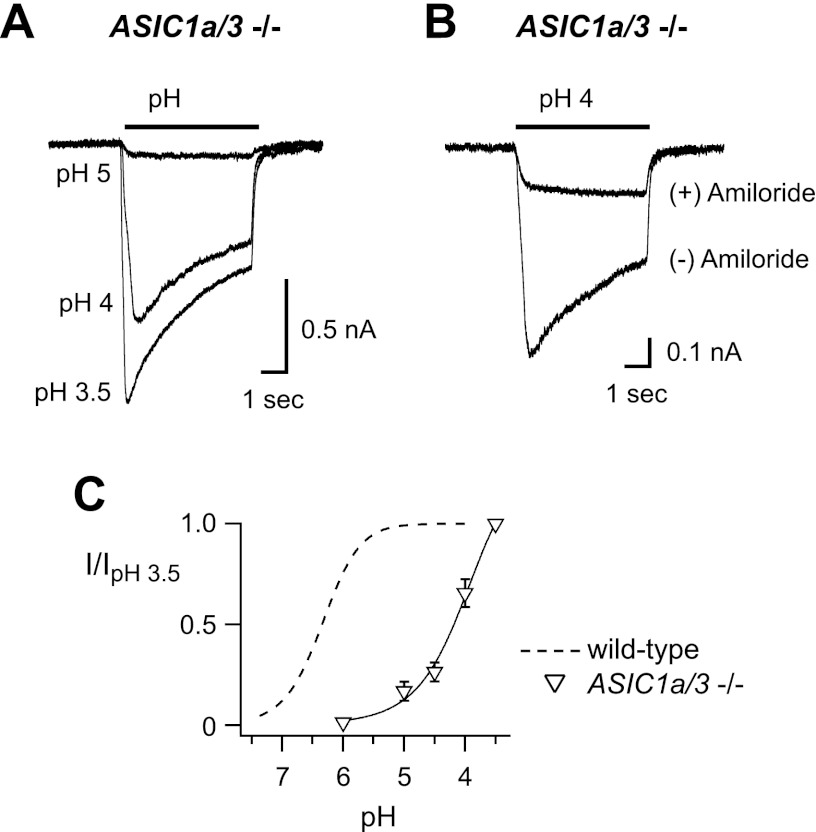
As a further test of the contribution of ASIC2 subunits in muscle afferents, we studied mice that lacked both ASIC1a and ASIC3 genes. We found transient acid-evoked currents in 7 of 17 (41%) muscle afferents studied (Fig. 4A), and the mean maximal current amplitude was 792 ± 178 pA. These transient currents were blocked by 300 μM amiloride (Fig. 4B). Low pH (pH 4–3.5) otherwise activated smaller sustained currents in all other cells studied; however, these currents were not blocked by amiloride (data not shown). Figure 4A, C shows that it took much more acidic pH to activate transient currents from ASIC1a/3−/− muscle afferents than those from other genotypes, and this low-pH sensitivity is consistent with ASIC2a channels (Table 1). In addition, the kinetics of desensitization matched the properties of ASIC2a homomers (Table 1). Together, these data suggest that ASIC2a is a component of the H+-gated channels in a large percentage of muscle afferents.
Figure 4.
Properties of acid-evoked currents in skeletal muscle afferents from ASIC1a/3 double-null mice. A) Superimposed currents evoked by the indicated pH solutions in a muscle afferent from ASIC1a/3−/− mice. B) Overlay of pH 4-evoked currents in the presence and absence of 300 μM amiloride. Amiloride was only present in the pH 4 test solution. All of the transient components of the currents were blocked, whereas the sustained components were not inhibited. C) pH dose-response data from ASIC1a/3−/− muscle afferents normalized to the currents evoked by pH 3.5 (n≥4). Line is fit of the Hill equation. Dashed line is fit of data from wild-type afferents in Fig. 2B.
ASIC3 contributes to ASICs in muscle afferents
Given the fast desensitization kinetics of currents evoked in the pH 6.5-5 range, we predicted that ASIC3 is a constituent of ASICs in wild-type muscle afferents. To confirm this hypothesis, we studied muscle afferents from ASIC3−/− mice. Loss of ASIC3 did not result in a shift in pH sensitivity of activation compared with wild-type data (Fig. 5A, B). On the other hand, loss of ASIC3 resulted in acid-evoked currents that desensitized 10-fold slower than those from wild-type muscle afferents (Fig. 5A, D). In addition, the currents recovered from desensitization at a significantly slower rate than those from wild-type mice (Fig. 5C). As with the data from ASIC1a−/− mice, the desensitization time constants from each ASIC3−/− muscle afferent do not overlap with wild-type data (Fig. 5D), suggesting that ASIC3 is a constituent of ASICs in all muscle afferents that express ASICs. In addition, the properties of the currents from ASIC3−/− muscle afferents are best matched by heteromeric channels. For example, the pH50 of activation (pH 6.3) from ASIC3−/− mice rules out the possibility that they are generated by ASIC2a homomers (pH50=3.8; Table 1), and the rate of recovery from desensitization (τ=0.9 s) means it is highly unlikely that ASIC1a homomers (recovery τ=11 s) underlie the currents. Thus, consistent with our other data, the ASICs in ASIC3−/− muscle afferents are most likely heteromeric channels composed of ASIC1a and ASIC2 subunits.
Figure 5.
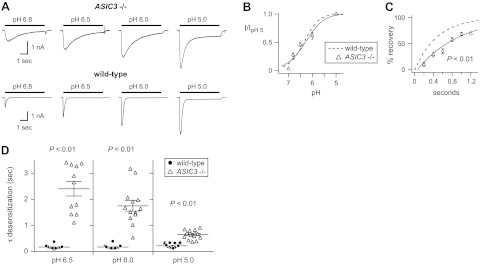
Properties of acid-evoked currents in skeletal muscle afferents from ASIC3−/− mice. A) Representative currents evoked by the indicated pH solutions in muscle afferents from ASIC3−/− mice compared with those from wild-type mice. B) pH dose-response data from ASIC3−/− muscle afferents normalized to the currents evoked by pH 5 (n≥12). Line is fit of the Hill equation. Dashed line is fit of data from wild-type afferents in Fig. 2B. C) Recovery from desensitization data for ASIC3−/− muscle afferents (n≥5). Line is fit of single exponential of the means. Dashed line is fit of data from wild-type afferents in Fig. 2E. D) Mean ± se τ of desensitization as well as individual data points of the transient currents evoked by the indicated pH solutions from ASIC3−/− muscle afferents compared with wild-type data from Fig. 2C (n≥11). P < 0.01 for data at each pH compared with wild-type.
ASIC-like currents in muscle afferents are absent in ASIC1a/2/3−/− mice
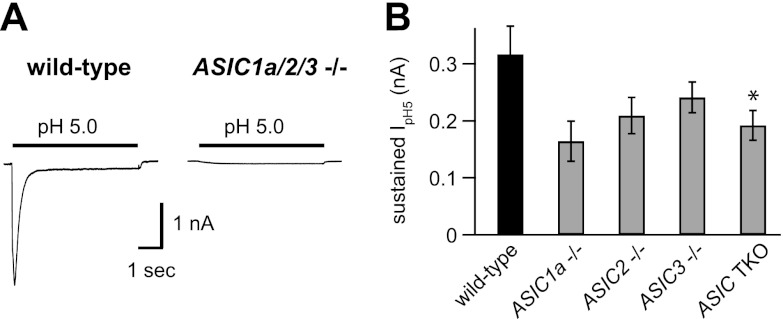
Our data thus far suggested that ASIC1a, ASIC2, and ASIC3 contribute to ASICs in muscle afferents. As a further test of this possibility, we studied pH-evoked currents in muscle afferents from mice that lacked ASIC1a, ASIC2, and ASIC3 genes. As illustrated in Fig. 6A, no transient H+-gated currents were evoked in 22 muscle afferents studied from triple-knockout (TKO) mice. In addition, we measured and compared the amplitudes of the sustained currents evoked by pH 5 in the various genotypes (Fig. 6B). Sustained currents were smaller in ASIC TKO than in wild-type muscle afferents but were not eliminated. This result suggests that ASICs conduct a part of the sustained currents evoked at low pH in muscle afferents; however, other channels also contribute.
Figure 6.
ASIC-like currents are absent in skeletal muscle afferents from ASIC1a/2/3 TKO mice. A) Representative currents evoked by pH 5 solution from wild-type and TKO muscle afferents. Transient ASIC-like currents were absent in all 22 TKO muscle afferents studied. B) Mean pH 5-evoked sustained current amplitudes from wild-type and the indicated ASIC−/− muscle afferents (n≥8). *P = 0.03 vs. wild-type.
DISCUSSION
ASICs are highly expressed in sensory nerves innervating skeletal muscle, and recent evidence suggests that they are important mediators of muscle pain and autonomic reflexes triggered during exercise. Our goal was to define the molecular composition of ASICs in muscle afferents. To study the detailed electrophysiological properties of the channels, we labeled and isolated mouse muscle afferents using a retrograde tracer dye for study by the patch-clamp technique. We compared data from mice that had undergone targeted deletion of specific ASIC subunits with those from wild-type mice. We found that deletion of any one subunit did not abolish ASIC-like currents. Instead, the residual current properties from each ASIC−/− mouse matched the properties of heteromeric channels formed by the other subunits. On the whole, our data suggested that native ASICs in muscle afferents are predominantly heteromeric channels comprising ASIC1a, ASIC2, and ASIC3 subunits. Consistent with this idea, ASIC-like currents were absent in muscle afferents from mice lacking ASIC1a, ASIC2, and ASIC3.
As with our previous studies in other populations of sensory neurons (24, 32), we found that most ASICs in mouse skeletal muscle afferents are formed by heteromers of multiple different subunits. This result is best appreciated by our data measuring the kinetics of desensitization. For example, with the exception of one cell, the time constants of all pH 6-evoked currents were faster than 300 ms (Fig. 5D); only heteromeric channels that contain ASIC3 as one of the constitutive subunits can reproduce such fast kinetics (24, 26). As would then be expected, genetic deletion of ASIC3 caused a 10-fold slowing of the desensitization kinetics of pH 6-evoked currents in all muscle afferents studied. On the other hand, not all of our findings are consistent with the idea that all ASICs in muscle afferents are heteromers. Specifically, a smaller percentage of cells expressed ASIC-like currents in ASIC1a−/− and ASIC3−/− mice, and current amplitudes were smaller in ASIC1a−/− mice, suggesting that some percentage of wild-type muscle afferents expressed primarily ASIC1a and/or ASIC3 homomeric channels. This might be a reasonable conclusion, except that we found the properties of ASIC-like currents from all muscle afferents from wild-type mice to be consistent with heteromeric channels. Our studies do not address this paradox; however, we have previously shown that genetic deletion of ASIC2 or ASIC3 does not significantly alter the mRNA expression of the remaining ASIC subunits (32, 39). Thus, we speculate that alterations in ASIC subunit composition might affect channel assembly and/or trafficking to and from the cell surface. In addition, there probably is some degree of heterogeneity in the composition of ASICs in different cells and within cells. Nevertheless, on the whole our data suggest that, as for other populations of mouse DRG neurons, ASICs in skeletal muscle afferents are heteromeric channels, and ASIC3 is a necessary component.
In other respects, we found the composition of ASICs in muscle afferents to be unique compared with that of other sensory neurons. Our data demonstrate that ASIC1a is a major constituent of the channels in muscle afferents; compared with data from wild-type mice, acid-evoked currents from ASIC1a−/− mice displayed a decrease in pH sensitivity, a slowing of desensitization, and a faster recovery from desensitization. This is in contrast to data from DRG neurons that innervate the heart, in which ASIC-like currents were completely unaltered in ASIC1a−/− mice (32). Moreover, our data here differ from previous recordings from unlabeled DRG neurons. In unlabeled DRG neurons, the pH50 was unaltered in ASIC1a−/− compared with those in wild-type mice(24); however, in skeletal muscle afferents we measured a significant shift in the pH sensitivity of ASIC1a−/− (pH50=6.0) compared with that in wild-type mice (pH50=6.4). Consistent with our data that ASIC1a and -3 are important components in skeletal muscle afferents, ASIC1a−/− and ASIC3−/− mice do not develop normal pain responses after acid injection into the muscle or muscle inflammation (12, 14, 40). Our data from ASIC2−/− mice also suggest that the composition of ASICs in muscle afferents is unique compared with that for other populations of sensory nerves. Loss of ASIC2 did not alter the pH sensitivity or the kinetics of the currents. In contrast, labeled cardiac afferents from ASIC2−/− mice showed a significant shift in pH sensitivity and the kinetics of desensitization compared with those in wild-type mice(32). Similarly, loss of ASIC2 altered the pH sensitivity and the rate of recovery from desensitization in unlabeled DRG neurons (24). These differences suggest that ASIC2 subunits play a lesser role in determining the properties of H+-gated currents in skeletal muscle afferents than in other sensory neurons. Together, our data suggest that the composition of ASICs in skeletal muscle afferents is unique compared with that of other populations of neurons, and we speculate that their properties are tuned to sense the particular interstitial changes that occur in skeletal muscle during exercise and disease states.
What is the composition of ASICs in muscle afferents? Our data demonstrate that ASIC1a, ASIC2, and ASIC3 are constituents of most channels. In fact, the properties of wild-type muscle afferents match well the properties of heteromeric channels formed by coexpression of ASIC1a, ASIC2a, and ASIC3 (Table 1). It is also possible that native ASICs are a mix of heteromeric channels formed by two subunits. For example, ASICs in wild-type muscle afferents might be a combination of ASIC1a/3 and ASIC2a/3 channels. However, if this were the case, we would have expected that the properties of acid-evoked currents from each of the single ASIC−/− mice to at least in part be consistent with homomers. However, we found no evidence for homomeric channels. For example, the kinetics of desensitization of currents from ASIC1a−/− and ASIC2−/− muscle afferents were faster than those of any of the homomeric channels. In addition, the properties of acid-evoked currents in ASIC3−/− muscle afferents best match the properties of ASIC1a/2a heteromers; the channels recovered from desensitization ∼10-fold faster than ASIC1a homomers and were ∼20-fold more pH-sensitive than ASIC2a homomers. What about the ASIC1 splice variant ASIC1b? The ASIC1a-null targeting strategy deleted the ASIC1a-specific exon, and thus the ASIC1b variant should be intact (33). Still, we found no ASIC-like currents from ASIC1a/2/3 triple-null mice, and the properties from ASIC1a/3 double-null mice matched those of ASIC2a homomers rather then ASIC1b/2a heteromers (26), suggesting that ASIC1b is not a major constituent of native ASICs in muscle afferents. On the whole, our data suggest that ASIC1a/2a/3 heterotrimers are the principle channel in native murine muscle afferents.
Whereas our findings provide important insight into the composition of ASICs in muscle afferents, it should be recognized that this molecular makeup is most certainly dynamic. For example, inflammatory mediators including nerve growth factor, serotonin, interleukin-1, and bradykinin increase ASIC transcription in DRG neurons (41). Likewise, ASIC-like current density in sensory neurons is increased in models of hind paw inflammation (42), nerve injury (43), and stomach ulcers (44). Regarding skeletal muscle, ASIC3 protein expression in rat muscle afferents is increased after muscle ischemia, and this increase correlated with potentiated reflex blood pressure responses generated by muscle injection of lactate (45). Similarly, ASIC2a and ASIC3 but not ASIC1a mRNA increased 10-fold in DRGs in a mouse model of muscle inflammation (40). Using this same mouse model, we previously showed that this increase correlated with an increase in ASIC-like current amplitudes in labeled muscle afferents, as well as with a shift in the pH dose response and the rate of recovery from desensitization (34). Taken together, these data suggest that muscle inflammation and associated disease states can selectively up-regulate specific ASIC subunits. Such changes will not only increase current amplitude but also alter the subunit composition of ASICs, leading to changes in their biophysical properties, both of which could have profound effects on neuronal excitability.
Understanding the subunit composition of ASICs in skeletal muscle afferents has important implications. First, ASIC−/− mice have been important tools in deciphering the function of ASICs in various physiological and pathophysiological conditions. Realizing that ASICs in muscle afferents are heteromeric channels composed of multiple different subunits and that genetic deletion of any one subunit does not abolish ASICs but rather alters their biophysical properties is critically important for interpreting data from ASIC−/− mice. Moreover, these data provide a clear molecular target for rational genetic and pharmacological interventions in our future efforts to understand the role of ASICs in muscle afferents. Second, as important mediators of muscle pain and perhaps pathological autonomic regulation in muscle disease states, pharmacological inhibitors of ASICs are being considered as clinical therapies. However, many of the known ASIC inhibitors are specific to particular subunits. For example, psalmotoxin is a peptide extracted from the tarantula Psalmopoeus cambridgei that specifically blocks ASIC1a homomeric channels (46). Another peptide, isolated from the sea anemone Anthopleura elegantissima toxin, APETx2, is a potent and selective inhibitor of ASIC3-containing channels (47). Thus, if we are to develop drugs to target ASICs in muscle afferents, it is imperative we understand their molecular nature.
Acknowledgments
The authors thank Margaret P. Price and Michael J. Welsh (University of Iowa, Howard Hughes Medical Institute, Iowa City, IA, USA) for providing ASIC double- and triple-null mice and Anne Marie S. Harding for technical support.
This work was supported by the U.S. National Institutes of Health National Heart, Lung, and Blood Institute and the U.S. Department of Veterans Affairs.
Footnotes
- ASIC
- acid-sensing ion channel
- CNS
- central nervous system
- DiI
- 1,1-dioctadecyl-3,3,3,3 tetramethylindocarbocyanine perchlorate
- DRG
- dorsal root ganglion
- TKO
- triple knockout
REFERENCES
- 1. Bangsbo J., Johansen L., Graham T., Saltin B. (1993) Lactate and H+ effluxes from human skeletal muscles during intense, dynamic exercise. J. Physiol. 462, 115–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Street D., Bangsbo J., Juel C. (2001) Interstitial pH in human skeletal muscle during and after dynamic graded exercise. J. Physiol. 537, 993–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohen R., Woods H. (1983) Lactic acidosis revisited. Diabetes 32, 181–191 [DOI] [PubMed] [Google Scholar]
- 4. Benson C. J., Eckert S. P., McCleskey E. W. (1999) Acid-evoked currents in cardiac sensory neurons: a possible mediator of myocardial ischemic sensation. Circ. Res. 84, 921–928 [DOI] [PubMed] [Google Scholar]
- 5. Molliver D. C., Immke D. C., Fierro L., Pare M., Rice F. L., McCleskey E. W. (2005) ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol. Pain 1, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Price M. P., Lewin G. R., McIlwrath S. L., Cheng C., Xie J., Heppenstall P. A., Stucky C. L., Mannsfeldt A. G., Brennan T. J., Drummond H. A., Qiao J., Benson C. J., Tarr D. E., Hrstka R. F., Yang B., Williamson R. A., Welsh M. J. (2000) The mammalian sodium channel BNC1 is required for normal touch sensation. Nature 407, 1007–1011 [DOI] [PubMed] [Google Scholar]
- 7. Wemmie J. A., Askwith C. C., Lamani E., Cassell M. D., Freeman J. H., Jr., Welsh M. J. (2003) Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J. Neurosci. 23, 5496–5502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xing J., Sinoway L., Li J. (2008) Differential responses of sensory neurones innervating glycolytic and oxidative muscle to protons and capsaicin. J. Physiol. 586, 3245–3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Connor M., Naves L. A., McCleskey E. W. (2005) Contrasting phenotypes of putative proprioceptive and nociceptive trigeminal neurons innervating jaw muscle in rat. Mol. Pain 1, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Light A. R., Hughen R. W., Zhang J., Rainier J., Liu Z., Lee J. (2008) Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J. Neurophysiol. 100, 1184–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Price M. P., McIlwrath S. L., Xie J., Cheng C., Qiao J., Tarr D. E., Sluka K. A., Brennan T. J., Lewin G. R., Welsh M. J. (2001) The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 32, 1071–1083 [DOI] [PubMed] [Google Scholar]
- 12. Sluka K. A., Price M. P., Breese N. M., Stucky C. L., Wemmie J. A., Welsh M. J. (2003) Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain 106, 229–239 [DOI] [PubMed] [Google Scholar]
- 13. Sluka K. A., Radhakrishnan R., Benson C. J., Eshcol J. O., Price M. P., Babinski K., Audette K. M., Yeomans D. C., Wilson S. P. (2007) ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain 129, 102–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walder R. Y., Gautam M., Wilson S. P., Benson C. J., Sluka K. A. (2011) Selective targeting of ASIC3 using artificial miRNAs inhibits primary and secondary hyperalgesia after muscle inflammation. Pain 152, 2348–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaufman M. P., Hayes S. G. (2002) The exercise pressor reflex. Clin. Auton. Res. 12, 429–439 [DOI] [PubMed] [Google Scholar]
- 16. McCloskey D. I., Mitchell J. H. (1972) Reflex cardiovascular and respiratory responses originating in exercising muscle. J. Physiol. 224, 173–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alam M., Smirk R. F. (1937) Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J. Physiol. 89, 372–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao Z., Henig O., Kehoe V., Sinoway L. I., Li J. (2006) Vanilloid type 1 receptor and the acid-sensing ion channel mediate acid phosphate activation of muscle afferent nerves in rats. J. Appl. Physiol. 100, 421–426 [DOI] [PubMed] [Google Scholar]
- 19. Hayes S. G., Kindig A. E., Kaufman M. P. (2007) Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. J. Physiol. 581, 1271–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li J., Maile M. D., Sinoway A. N., Sinoway L. I. (2004) Muscle pressor reflex: potential role of vanilloid type 1 receptor and acid-sensing ion channel. J. Appl. Physiol. 97, 1709–1714 [DOI] [PubMed] [Google Scholar]
- 21. Hayes S. G., McCord J. L., Rainier J., Liu Z., Kaufman M. P. (2008) Role played by acid-sensitive ion channels in evoking the exercise pressor reflex. Am. J. Physiol. Heart Circ. Physiol. 295, H1720–H1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Birdsong W. T., Fierro L., Williams F. G., Spelta V., Naves L. A., Knowles M., Marsh-Haffner J., Adelman J. P., Almers W., Elde R. P., McCleskey E. W. (2010) Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron 68, 739–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Immke D. C., McCleskey E. W. (2001) Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat. Neurosci. 4, 869–870 [DOI] [PubMed] [Google Scholar]
- 24. Benson C. J., Xie J., Wemmie J. A., Price M. P., Henss J. M., Welsh M. J., Snyder P. M. (2002) Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc. Natl. Acad. Sci. U. S. A. 99, 2338–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xie J., Price M. P., Berger A. L., Welsh M. J. (2002) DRASIC contributes to pH-gated currents in large dorsal root ganglion sensory neurons by forming heteromultimeric channels. J. Neurophysiol. 87, 2835–2843 [DOI] [PubMed] [Google Scholar]
- 26. Hesselager M., Timmermann D. B., Ahring P. K. (2004) pH Dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J. Biol. Chem. 279, 11006–11015 [DOI] [PubMed] [Google Scholar]
- 27. Lingueglia E., de Weille J. R., Bassilana F., Heurteaux C., Sakai H., Waldmann R., Lazdunski M. (1997) A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J. Biol. Chem. 272, 29778–29783 [DOI] [PubMed] [Google Scholar]
- 28. Donier E., Rugiero F., Jacob C., Wood J. N. (2008) Regulation of ASIC activity by ASIC4—new insights into ASIC channel function revealed by a yeast two-hybrid assay. Eur. J. Neurosci. 28, 74–86 [DOI] [PubMed] [Google Scholar]
- 29. Wu L. J., Duan B., Mei Y. D., Gao J., Chen J. G., Zhuo M., Xu L., Wu M., Xu T. L. (2004) Characterization of acid-sensing ion channels in dorsal horn neurons of rat spinal cord. J. Biol. Chem. 279, 43716–43724 [DOI] [PubMed] [Google Scholar]
- 30. Askwith C. C., Wemmie J. A., Price M. P., Rokhlina T., Welsh M. J. (2004) Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J. Biol. Chem. 279, 18296–18305 [DOI] [PubMed] [Google Scholar]
- 31. Baron A., Waldmann R., Lazdunski M. (2002) ASIC-like, proton-activated currents in rat hippocampal neurons. J. Physiol. 539, 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hattori T., Chen J., Harding A. M., Price M. P., Lu Y., Abboud F. M., Benson C. J. (2009) ASIC2a and ASIC3 heteromultimerize to form pH-sensitive channels in mouse cardiac dorsal root ganglia neurons. Circ. Res. 105, 279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wemmie J. A., Chen J., Askwith C. C., Hruska-Hageman A. M., Price M. P., Nolan B. C., Yoder P. G., Lamani E., Hoshi T., Freeman J. H., Jr., Welsh M. J. (2002) The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron 34, 463–477 [DOI] [PubMed] [Google Scholar]
- 34. Gautam M., Benson C. J., Sluka K. A. (2010) Increased response of muscle sensory neurons to decreases in pH after muscle inflammation. Neuroscience 170, 893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chu X. P., Wemmie J. A., Wang W. Z., Zhu X. M., Saugstad J. A., Price M. P., Simon R. P., Xiong Z. G. (2004) Subunit-dependent high-affinity zinc inhibition of acid-sensing ion channels. J. Neurosci. 24, 8678–8689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jiang Q., Inoue K., Wu X., Papasian C. J., Wang J. Q., Xiong Z. G., Chu X. P. (2011) Cysteine 149 in the extracellular finger domain of acid-sensing ion channel 1b subunit is critical for zinc-mediated inhibition. Neuroscience 193, 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang Q., Papasian C. J., Wang J. Q., Xiong Z. G., Chu X. P. (2010) Inhibitory regulation of acid-sensing ion channel 3 by zinc. Neuroscience 169, 574–583 [DOI] [PubMed] [Google Scholar]
- 38. Baron A., Schaefer L., Lingueglia E., Champigny G., Lazdunski M. (2001) Zn2+ and H+ are coactivators of acid-sensing ion channels. J. Biol. Chem. 276, 35361–35367 [DOI] [PubMed] [Google Scholar]
- 39. Lu Y., Ma X., Sabharwal R., Snitsarev V., Morgan D., Rahmouni K., Drummond H. A., Whiteis C. A., Costa V., Price M., Benson C., Welsh M. J., Chapleau M. W., Abboud F. M. (2009) The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron 64, 885–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walder R. Y., Rasmussen L. A., Rainier J. D., Light A. R., Wemmie J. A., Sluka K. A. (2010) ASIC1 and ASIC3 play different roles in the development of Hyperalgesia after inflammatory muscle injury. J. Pain 11, 210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mamet J., Baron A., Lazdunski M., Voilley N. (2002) Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J. Neurosci. 22, 10662–10670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Deval E., Noel J., Lay N., Alloui A., Diochot S., Friend V., Jodar M., Lazdunski M., Lingueglia E. (2008) ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 27, 3047–3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Poirot O., Berta T., Decosterd I., Kellenberger S. (2006) Distinct ASIC currents are expressed in rat putative nociceptors and are modulated by nerve injury. J. Physiol. 576, 215–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sugiura T., Dang K., Lamb K., Bielefeldt K., Gebhart G. F. (2005) Acid-sensing properties in rat gastric sensory neurons from normal and ulcerated stomach. J. Neurosci. 25, 2617–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu J., Gao Z., Li J. (2010) Femoral artery occlusion increases expression of ASIC3 in dorsal root ganglion neurons. Am. J. Physiol. Heart Circ. Physiol. 299, H1357–H1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mazzuca M., Heurteaux C., Alloui A., Diochot S., Baron A., Voilley N., Blondeau N., Escoubas P., Gelot A., Cupo A., Zimmer A., Zimmer A. M., Eschalier A., Lazdunski M. (2007) A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nat. Neurosci. 10, 943–945 [DOI] [PubMed] [Google Scholar]
- 47. Diochot S., Baron A., Rash L. D., Deval E., Escoubas P., Scarzello S., Salinas M., Lazdunski M. (2004) A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. 23, 1516–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]