Abstract
Epidemiological and preclinical studies indicate that polyphenol intake from moderate consumption of red wines may lower the relative risk for developing Alzheimer's disease (AD) dementia. There is limited information regarding the specific biological activities and cellular and molecular mechanisms by which wine polyphenolic components might modulate AD. We assessed accumulations of polyphenols in the rat brain following oral dosage with a Cabernet Sauvignon red wine and tested brain-targeted polyphenols for potential beneficial AD disease-modifying activities. We identified accumulations of select polyphenolic metabolites in the brain. We demonstrated that, in comparison to vehicle-control treatment, one of the brain-targeted polyphenol metabolites, quercetin-3-O-glucuronide, significantly reduced the generation of β-amyloid (Aβ) peptides by primary neuron cultures generated from the Tg2576 AD mouse model. Another brain-targeted metabolite, malvidin-3-O-glucoside, had no detectable effect on Aβ generation. Moreover, in an in vitro analysis using the photo-induced cross-linking of unmodified proteins (PICUP) technique, we found that quercetin-3-O-glucuronide is also capable of interfering with the initial protein-protein interaction of Aβ1–40 and Aβ1–42 that is necessary for the formation of neurotoxic oligomeric Aβ species. Lastly, we found that quercetin-3-O-glucuronide treatment, compared to vehicle-control treatment, significantly improved AD-type deficits in hippocampal formation basal synaptic transmission and long-term potentiation, possibly through mechanisms involving the activation of the c-Jun N-terminal kinases and the mitogen-activated protein kinase signaling pathways. Brain-targeted quercetin-3-O-glucuronide may simultaneously modulate multiple independent AD disease-modifying mechanisms and, as such, may contribute to the benefits of dietary supplementation with red wines as an effective intervention for AD.—Ho, L., Ferruzzi, M. G., Janle, E. M., Wang, J., Gong, B., Chen, T.-Y., Lobo, J., Cooper, B., Wu, Q. L., Talcott, S. T., Percival, S. S., Simon, J. E., Pasinetti, G. M. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer's disease.
Keywords: polyphenol bioavailability, cognitive deterioration, neuroplasticity
Alzheimer's disease (AD) is a major cause of functional disability in older adults, inflicting massive costs and suffering on individuals with the disease, their family members, caregivers, and on society as a whole (1). Approximately 5.4 million people in the United States currently have AD (1), and up to 16 million people in the United States are projected to be affected by AD by the middle of this century if effective therapies are not developed (2). In 2012 alone, the estimated direct health care cost associated with AD in the United States is $200 billion, with an additional $210 billion in unpaid costs to caregivers (1). The emotional consequences for caregivers are similarly high, with major effects on stress levels and quality of life (3–6). While genetic factors contribute to early-onset AD, they play less of a role in late-onset, sporadic AD, which is the most common form (7). There is currently no cure for AD. Delaying AD onset alone by just a few years would lead to significant reductions in disease prevalence, and, consequently, its burden on health care systems, and would mitigate the social cost to both AD patients themselves and their respective families. Given the increasing aged population and the effects of AD care on health care costs in an era of impending health care reform, it is important to find treatments that may help reduce the burden and cost of the disease.
Epidemiological studies indicate that moderate consumption of red wine may lower the relative risk for AD dementia (8–13). Consistent with this, we previously observed that long-term supplementation with a red Cabernet Sauvignon wine in an AD mouse model, using a dosage that is equivalent to moderate daily wine consumption in humans, is effective at attenuating the development of AD-type β-amyloid (Aβ) neuropathology and spatial memory decline (14). There is a general consensus that the health benefits of red wines are attributable, in part, to the high polyphenolic content of wines (15–17). Consistent with this, evidence from our Cabernet Sauvignon study suggests that polyphenol components in red wines may contribute to AD disease-modifying activity by reducing the generation of Aβ peptides that are critical for disease onset and progression (14).
A key issue in translating the observed benefits of red wine and other grape-derived dietary products in AD into clinical applications is the tremendous variability in the content and composition profile of polyphenols and, thereby, the potential bioactivity of polyphenol components among these products (18, 19). This stems from a combination of differences in grape varieties (20–22), degree of grape ripening (23), agroclimatic conditions in which the grapes were grown (20, 24–27), and variations in processing, storage, and utilization of the finished wine products (28–31). Being able to effectively incorporate individual grape-derived products into therapeutic applications for AD demands a better understanding of specific dietary polyphenolic components that are capable of exerting beneficial disease-modifying activities and their mechanisms of action.
Red wines, including the Cabernet Sauvignon wine that we described in our previous study (14), contain a wide variety of polyphenolic compounds, including phenolic acids, flavonols, proanthocyanidins, and anthocyanins. The majority of the flavonoids, including anthocyanins, are present in conjugated glycosidic forms (32, 33). While epidemiological evidence (8–13) and experimental evidence (14, 18) indicate that red wine consumption may beneficially modulate AD, only limited information currently exists regarding the identities of specific polyphenolic components in wine that are actually capable of penetrating the blood-brain barrier (BBB) to reach the target tissue of interest for AD. In addition, there is no information regarding specific biological activities and cellular/molecular mechanisms by which BBB-crossing polyphenolic components from red wine might beneficially modulate AD-type neuropathology and/or cognitive dysfunction. In this study, we used Sprague-Dawley rats as an experimental model for assessing bioavailability of dietary polyphenols, and we explored the pharmacokinetic behavior and brain accumulation of select polyphenol components from the red Cabernet Sauvignon wine. After identifying specific polyphenolic metabolites that accumulate in the brain, we studied these brain-targeted polyphenol components for disease-modifying activities that may contribute to the benefit of dietary supplementation with the Cabernet Sauvignon in terms of modulating AD phenotypes. There is a growing interest in the development of dietary polyphenolic compounds for preventing and/or treating AD, as well as other neurodegenerative conditions (34–40). Outcomes from our studies provide important information necessary for optimizing potential applications of dietary polyphenols to prevent and/or manage AD and other neurodegenerative disorders.
MATERIALS AND METHODS
Cabernet Sauvignon and polyphenolic extraction
Cabernet Sauvignon was generated from Vitis vinifera at the University of Florida as described previously (41). Cabernet Sauvignon grapes from Fresno, California, were shipped to Gainesville by air freight; grapes were crushed, destemmed, and allowed to ferment on the skins for 7 d at 13°C. The must was pressed and allowed to finish fermenting to dryness (<0.05% reducing sugar) at 13°C. Wines were then treated with 100 mg/L of potassium metabisulfite, cold-stabilized at 3°C for 2 mo, filtered, and stored at 13°C for ∼26 mo. The Cabernet Sauvignon wine contained ∼12% alcohol, as determined by ebulliometry (42) and had a titratable acidity (as tartaric acid) of 6 g/L and a pH of 3.6.
A Cabernet Sauvignon total polyphenol extract was then prepared using C18 solid-phase extraction cartridges (Waters Corp., Milford, MA, USA). Cabernet Sauvignon was diluted 1:4 in acidified water (water buffered to pH 2.4 with o-phosphoric acid) to optimize preservation of the polyphenol compounds in the wine. Diluted wine was loaded onto C18 cartridges, followed by washing the columns with 3 vol of acidified water. Bound organic compounds were eluted in 12% methanol diluted in acidified water. Polyphenol components recovered were concentrated by vacuum centrifugation, which also removed volatile organic components, including ethanol from the wine, as well as organic solvents used in the extraction.
Chemical analysis of Cabernet Sauvignon wine or extract
Major polyphenolic components in Cabernet Sauvignon wine and in the wine extract were characterized as described previously by Talcott and Lee (43). Polyphenols were separated by high-performance liquid chromatography (HPLC) using the Waters 2695 HPLC system with a Waters Spherisorb octadecyl silane column (4.6×250 mm) using a binary water/acidified methanol solvent gradient system. Compounds were detected using a Water 996 photodiode array detector, and polyphenols were identified by UV/visual spectral interpretation, retention time, and comparison to authentic standards, when available (Sigma Chemical, St. Louis, MO, USA).
Liquid chromatography–ultraviolet–mass spectrometry (LC-UV-MS) methodologies were used for more extensive chemical profiling and chemical structure analysis of polyphenols in Cabernet Sauvignon, as we have previously described (33). Briefly, polyphenols were separated on an Agilent 1100 LC-MS system (Agilent Technologies, Palo Alto, CA, USA) using a Varian C18 amide column (3 μm, 150×2.1 mm; Varian, Palo Alto, CA. USA) and a trifluoroacetic acid/acetonitrile or a formic acid/acetonitrile binary solvent gradient system. Following separation, the column effluents were monitored by electrospray-ionization mass spectrometry (ESI-MS) under positive ion mode for assessments of anthocyanins and flavonols. ESI capillary voltage was 3.5 kV, nebulizer gas pressure was set at 60 psig; gas temperature was 350°C, and drying gas flow rate was 12 L/min with helium as collision gas. UV detector was set at 254, 280, 370, and 520 nm, and the samples were scanned from m/z 100 to 1200 for MS detection. Software of HP ChemStation (Hewlett-Packard, Palo Alto, CA, USA), Bruker Daltonics 4.2 (Bruker Corp., Billerica, MA, USA), and Data Analysis 4.2 (SAS Institute, Cary, NC, USA)were used. Anthocyanins were quantified against authentic standards, when available. Flavonoids were quantified in quercetin equivalents. Peaks were identified on the basis of UV and MS data.
Bioavailability studies
Bioavailability of Cabernet Sauvignon polyphenolic constituents was assessed using male Sprague-Dawley rats weighing between 275 and 300 g that were obtained from Harlan Sprague Dawley (Indianapolis, IN, USA). All animal studies were conducted under guidance and with protocols reviewed and approved by the Purdue University Animal Care and Use Committee. On arrival, rats were placed on a polyphenol-free AIN-93M diet (Dyets, Bethlehem, PA, USA) and given deionized water ad libitum during a 3-d acclimation period. Following acclimation, anesthesia was induced with isoflurane (3–5%) in an anesthesia chamber and maintained with a mask (1.5–3% isoflurane). A polyethylene catheter was implanted into the jugular vein. Burenorphine (0.01–0.5 mg/kg)was administered prior to animals regaining consciousness to alleviate pain. Catheters were kept patent by flushing with heparinized saline containing 100 U heparin/ml every 12 h. Animals were allowed 24 h of recovery time postsurgery.
Prior to initiation of pharmacokinetic studies, food was removed for 7 h and was offered 2 h after administration of the Cabernet Sauvignon polyphenol extract. For single-dose acute pharmacokinetic studies, the Cabernet Sauvignon polyphenolic preparation was solubilized in 1.0 ml of distilled water to deliver 150 mg total polyphenolics/kg body weight (BW) and administered by intragastric gavage. For the repeated exposure study, rats were dosed by oral gavage for 10 d with the Cabernet Sauvignon polyphenolic preparation. On d 10, pharmacokinetics were assessed following administration of the last Cabernet Sauvignon polyphenolic preparation (150 mg/kg BW) by collecting ∼400 μl of blood at 0, 0.5, 1, 2, 4, 6, and 8 h postgavage from the jugular catheter into heparinized tubes and centrifuged at 4000 rpm for 10 min. Two hundred microliters of resulting plasma was collected and combined with 50 μl acidified saline (1% ascorbic acid w/w), purged with N2, and stored at −80°C until analysis. The day following the pharmacokinetic study, another dose (150 mg/kg BW) was administered, and rats were euthanized 1 h after dose. Rats were perfused with ice-cold saline to remove possible blood contamination; brain tissues were harvested, placed in 0.2% ascorbic acid in saline, and stored at −80°C until analysis.
Anthocyanins and flavonols were extracted from homogenized plasma and brain tissue samples by solid-phase extraction. Acidified plasma or crude methanolic brain extract wsd brought up to 0.5 ml with acidified saline (0.1% formic acid v/v) and loaded onto a preconditioned 1-cc Waters Oasis HLB SPE cartridge. Three milliliters of 2% aqueous formic acid (v/v) was used to wash the cartridges. Anthocyanins and flavonols were eluted with 2 ml of 2% formic acid in methanol (v/v) and dried under vacuum at 37°C. Dried extracts were resolubilized in 100 μl of 2% aqueous formic acid (v/v) for LC-MS/MS analysis.
Analysis of brain and plasma anthocyanin and quercetin metabolites was performed using both an Agilent HPLC MSD time-of-flight (TOF) mass spectrometer and a 6400 triple-quadrupole (QQQ) mass spectrometer under multiple reaction monitoring (MRM) mode. Both systems were equipped with an ESI source. A Waters XTerra RP-C18 column (2.1×100 mm, 3.5 μm particle size) was used for all analyses. A binary mobile-phase system consisting of mobile phase A, 0.1% aqueous formic acid (v/v), and mobile phase B, 0.1% formic acid in acetonitrile (v/v), was used for quercetin analysis. For anthocyanin analysis, mobile phase A was 2% aqueous formic acid (v/v), and mobile phase B was 0.1% formic acid in acetonitrile (v/v). Mass spectra were obtained under negative polarity scanning for quercetin analysis between 100 and 1000 m/z on MS-TOF. Mass spectra were obtained under positive polarity with QQQ. MRM mass transitions were 493 → 317 for methyl-quercetin-glucuronide, 479 → 303 for quercetin-glucuronide, and 301 → 153 for quercetin aglycone under positive polarity. Quantification of quercetin metabolites was accomplished by using a calibration curve constructed with quercetin-3-O-glucuronide standard (Carboxynth, Compton, UK). MRM transitions were 493 → 331 for malvidin-glucoside, 479 → 317 for petunidin-glucoside, 465 → 303 for delphinidin-glucoside, 463 → 301 for peonidin-glucoside, and 449 → 287 for cyanidin glucoside. Quantification of all anthocyanin glucosides was estimated by using calibration curves of malvidin-3-O-glucoside (Sigma, St. Louis, MO, USA).
Cell cultures and treatments with Cabernet Sauvignon polyphenolic extracts or brain-targeted polyphenolic components
Embryonic day 14–16 corticohippocampal neuronal cultures (Tg2576 neurons) were prepared from heterozygous Tg2576 transgenic AD mice (44). In brief, embryonic brain tissue was mechanically triturated and centrifuged. Neurons were seeded onto poly-d-lysine-coated 24-well plates at 5 × 105 cells/well and cultured in serum-free, chemically defined Neurobasal medium supplemented with 2% B27, 0.5 mM l-glutamine, and 1% penicillin-streptomycin (Gibco BRL, Carlsbad, CA, USA). The absence of astrocytes (<2%) was confirmed by the virtual absence of glial fibrillary acidic protein immunostaining (data not shown). Cells were treated with select polyphenolic extract or polyphenolic components, followed by analysis of conditioned medium for contents of Aβ peptides, or cell extracts for cellular modulators of the cAMP response element-binding (CREB) protein signaling pathway.
Photo-induced cross-linking of unmodified proteins (PICUP) assay
Freshly isolated low-molecular-weight Aβ1–42 (25 μM) or Aβ1–40 (25 μM) peptide (18 μl) was mixed with 1 μl of 1 mm tris-(2,2′-bipyridyl)dichlororuthenium(II) [Ru(Bpy)] and 1 μl of 20 mM ammonium persulfate in the presence or absence of 25 μM quercetin-3-O-glucuronide in 10 mM phosphate (pH 7.4). The mixture was irradiated for 1 s, quenched immediately with 10 μl of Tricine sample buffer (Invitrogen) containing 5% β-mercaptoethanol (45), and subjected to SDS-PAGE. Monomeric and multimeric Aβ peptide species were visualized by silver staining (SilverXpress; Invitrogen).
Measurement of Aβ peptides and CREB signaling pathway
Contents of Aβ1–40, 1–42 peptides in cultured medium were measured by ELISA, as we have previously reported (14). Luminex xMAP multiplex immunoassays (Millipore, Billerica, MA, USA) were used to evaluate the levels of activated, [Ser133]-phosphorylated CREB (P-CREB), as well as activated mediators of CREB signaling: [Ser473]-phosphorylated AKt (P-AKt), [Thr185/Tyr187]-phosphorylated Erk1/2 (P-Erk1/2) and [Thr183/Tyr185]-phosphorylated JNK (P-JNK). Cells were lysed with buffer provided by the manufacturer, and the supernatants (20 μg protein) were used for the assay according to the procedure provided by the manufacturer.
Assessments of basal synaptic transmission and long-term potentiation (LTP) in ex vivo hippocampal slice culture
Electrophysiological measurements from ex vivo hippocampal slice cultures were conducted as described in Gong et al. (46). In brief, mice were euthanized by decapitation. Hippocampal slices (350 μm) isolated from freshly isolated brain specimens were placed into oxygenated artificial cerebrospinal fluid (ACSF) at room temperature for a minimum of 2 h for acclimatization. Slices were then transferred to a recording chamber (Fine Science Tools, Foster City, CA, USA) and perfused continuously with oxygenated ACSF at 32°C. For extracellular recordings, Schaffer collaterals were stimulated with a monopolar stainless-steel electrode for activation of the CA1. A recording borosilicate glass electrode, filled with 3 M NaCl with 1–2 MΩ resistance, was placed in the CA1 region to record field excitatory postsynaptic potentials (fEPSPs). Constant current pulses (150 μs, 20–30 μA) were delivered using a stimulus isolation unit (A310 Accupulser; World Precision Instruments, Sarasota, FL, USA), and evoked fEPSPs were recorded with an amplifier and monitored on a digital oscilloscope. Responses were elicited every 1 min, digitized, stored, and analyzed using Axon pCLAMP 10 software (Axon Instruments, Foster City, CA, USA). Basal synaptic transmission was assessed by determining the input/output function of the fEPSP slope by increasing the stimulation intensity, between minimum and maximum responses. Baseline responses were then recorded for 10 min followed by high-frequency stimulation (HFS; 5 trains of 200 Hz, 100 ms duration, 10 s intertrain intervals) to induce LTP, and fEPSPs were monitored for 60 min to assess the magnitude of potentiation.
Statistical analysis
All values are expressed as means ± se. Differences between means were analyzed using either 2-way repeated-measures ANOVA or 2-tailed Student's t test. In all analyses, the null hypothesis was rejected at the 0.05 level. All statistical analyses were performed using the Prism Stat program (GraphPad Software, San Diego, CA, USA).
RESULTS
Select polyphenol components from Cabernet Sauvignon exert Aβ-lowering activity
We previously demonstrated that polyphenolic components from Cabernet Sauvignon wine may help modulate AD phenotypes by interfering with the generation of Aβ peptides (14). The red wine used in that study, as well as other commercially available red wines (18,33), contains a wide and complex variety of polyphenol compounds (19). We tested the possibility that select polyphenolic components in the wine may be responsible for Aβ-lowering activity. Using a solid-phase extraction procedure (Fig. 1A), we separated Cabernet Sauvignon polyphenol components into 3 subfractions, referred to as iolate I (polar tannins and low molecular weights phenolics), iolate II (condensed tannins, phenolic acids, and flavan-3-ols), and iolate III (anthocyanins). Chemical analysis confirmed that the wine polyphenol subfractions were characterized by distinct polyphenolic compositions (Fig. 1B). The Cabernet Sauvignon wine that we used in our studies contains low contents of resveratrol (14), and most of the resveratrol content in the wine was extracted into iolate II (Fig. 1B, middle panel); there was no detectable content of resveratrol in iolate III (Fig. 1B, bottom panel). Testing individual wine polyphenol subfractions for potential Aβ-lowering activities, we found that iolate III significantly reduced the generation of Aβ1–42 peptides in primary corticohippocampal neurons from Tg2576 mice (Fig. 1C). Moreover, almost all of the Aβ-lowering activity in the intact Cabernet Sauvignon was recovered in iolate III (Fig. 1C). No detectable Aβ-lowering activity was observed from iolate II (Fig. 1C).
Figure 1.
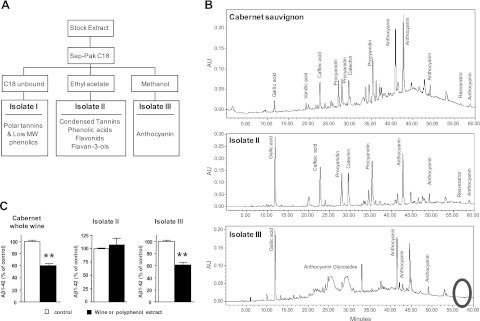
Selective polyphenolic preparation from Cabernet Sauvignon exerts Aβ-lowering activity in vitro in Tg2576 corticohippocampal primary neuron cultures. Polyphenolic contents in Cabernet Sauvignon were fractionated using a solid-phase extraction procedure and Aβ-lowering activity of recovered isolates were assessed in vitro, using primary corticohippocampal neuron cultures from Tg2576 AD mice. A) Schematics of the extraction procedure. Ethanol was removed from the red wine under reduced pressure at 35°C. Polyphenols from the dealcoholated stock extract was absorbed onto a C18 column. Unbound components were recovered as isolate I, which comprises polar tannins and low-molecular-weight phenolics. Thereafter, the column was eluted in series with ethyl acetate to recover the majority of nonanthocyanin polyphenolics (e.g., phenolic acids, flavonols, and flavanols; isolate II), followed by elution with methanol to recover anthocyanin and other polyphenolics on the column (isolate III). B) Polyphenol component analysis by HPLC using a binary water/acidified methanol solvent gradient system. Compounds were detected using a Waters 996 photodiode array detector. Top panel: Cabernet Sauvignon wine. Middle panel: isolate II. Bottom panel: isolate III. Circle indicates the position where resveratrol should be detected. C) Assessments of intact Cabernet Sauvignon, isolate II, and isolate III for Aβ-lowering activity in primary cortico-hippocampal neuron cultures. Bar graphs represents mean ± se; n = 3 cultures/treatment group. **P < 0.001.
Plasma and brain bioavailability of Cabernet Sauvignon polyphenolic components
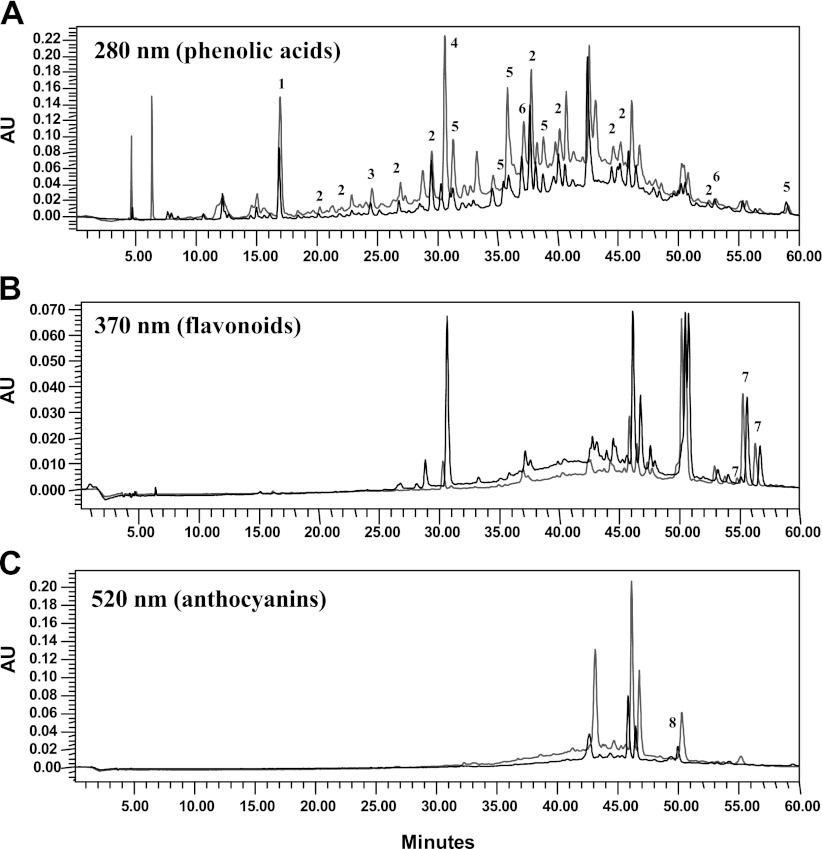
The potential benefits of dietary polyphenols depend on the delivery of biologically active polyphenol components to target tissues. Since the brain is the key target tissue for therapeutic interventions in AD, we next investigated the ability of dietary Cabernet Sauvignon polyphenolic components to accumulate in brain tissues, followed by an exploration of whether concentrations of polyphenolic components found accumulated in the brain may be sufficient for biological activities consistent with interventions in AD. We prepared a total polyphenolic extract from the wine, which we used for bioavailability studies. Chemical analysis confirmed that almost all of the polyphenol compounds in the red Cabernet Sauvignon wine were recovered in the total polyphenolic extract (Fig. 2). In Table 1, we list some of the most abundant anthocyanins, flavonols, proanthocyanidins, and phenolic acids and their respective concentrations in the red wine. We note that the most abundant anthocyanins in the wine are malvidin derivatives, including malvidin-glucoside, malvidin-glucoside-pyruvate, malvidin acetyl-glucoside-pyruvate, and malvidin-acetyl-glucoside. Figure 3 shows a representative chromatogram identifying the detection of these malvidin derivatives in the wine polyphenolic extract. The most abundant flavonols in the red wine were quercetin and syringetin-glucoside. Proanthocyanidins in the wine were primarily in the form of monomeric or dimeric catechin/epicatechins. Finally, multiple phenolic acids were also present in abundance in the red wine.
Figure 2.
Chemical analysis of Cabernet Sauvignon polyphenol extract. Polyphenolic component compositions of the Cabernet Sauvignon wine and the Cabernet Sauvignon total polyphenolic extract preparation were analyzed by HPLC using a binary water/acidified methanol solvent gradient system. Compounds were detected using a Waters 996 photodiode array detector. Spectrograms resulted from analysis of Cabernet Sauvignon (gray spectra) and the total polyphenol extract (black spectra). A) Detection of phenolic acid compounds at 280 nm. B) Detection of flavonoids at 370 nm. C) Detection of anthocyanins at 520 mm. Some of the peaks (indicated by numbers above selected peaks) in each of the three spectra were identified based on photodioarray spectroscopic interpretations from 200 to 600 nm; the names of the corresponding compounds and their glycosidic derivatives are listed below. Most of the flavonoids and anthocyanins are in a glycosidic form. We note that the maximal absorbance of resveratrol is 510 mm and is, therefore, detected in the 520 mm spectra (peak 8 in panel C), which, in general, is optimal for detection of anthocyanins. Peak ID: 1, gallic acid (280 nm); 2, procyanidin (280 nm); 3, protocatachuic acid (280 nm); 4, caffeic acid (280 nm); 5, p-coumaric acid (280 nm); 6, ferulic acid (280 nm); 7 flavonol (370 nm); 8, resveratrol (520 nm).
Table 1.
Highly abundant polyphenolic compounds in the red Cabernet Sauvignon wine and bioavailability of these compounds in blood
| Compound | Content in wine (μM) | Detection in plasma |
|---|---|---|
| Anthocyanins | ||
| Malvidin-glucoside | 585.09 | + |
| Malvidin-glucuronide | + | |
| Malvidin-glucoside-pyruvate | 334.86 | + |
| Malvidin-acetyl-glucoside-pyruvate | 139.5 | |
| Malvidin-acetyl-glucoside | 151.2 | |
| Total (μM) | 1210.65 | |
| Total (mg/L) | 640.11 | |
| Flavonoids | ||
| Syringetin-3-glucoside | 298.68 | |
| Quercetin | 593 | |
| Quercetin-3-glucuronide | + | |
| Methyl quercetin-glucuronide | + | |
| Total (μM) | 891.68 | |
| Total (mg/L) | 330.82 | |
| Proanthocyanidins | ||
| Catechin | 1351.09 | + |
| Epicatechin | 399.92 | + |
| Proanthocyanidin dimer | 4055.37 | |
| Total (μM) | 5806.37 | |
| Total (mg/L) | 2851.79 | |
| Phenolic acids | ||
| Gallic acid | 251 | + |
| Cafteric acid | 352.81 | |
| Vanillic acid | 729.95 | + |
| p-Coumaric acid | 1354.23 | + |
| 4-HBA | 471.92 | |
| Caffeic acid | 572.93 | + |
| Total (μM) | 3732.84 | |
| Total (mg/L) | 665.72 |
Polyphenolic components in the wine were analyzed by LC-UV-MS methodologies. Listed are some of the most abundant anthocyanins, flavonols, proanthocyanidins, and phenolic acid compounds and their concentrations in the red wine. + denotes detection in the plasma, as detected by LC-MS/MS following oral administration of the total wine polyphenolic extract.
Figure 3.
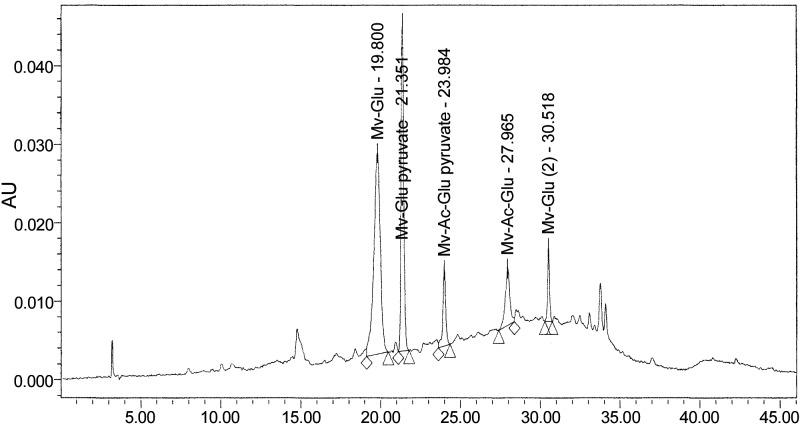
Analysis of malvidin derivatives in Cabernet Sauvignon. Polyphenolic component compositions of the Cabernet Sauvignon total polyphenolic extract preparation were analyzed by HPLC using a binary water/acidified methanol solvent gradient system. Malvidin derivatives were detected by UV (520 nm). Mv-glu, malvidin-glucoside; Mv-glu-pyruvate, malvidin-glucoside-pyruvate; Mv-Ac-glu-pyruvate; malvidin-acetyl-glucoside-pyruvate; Mv-Ac-glu; malvidin-acetyl-glucoside.
On the basis of in vitro evidence (Fig. 1C) that anthocyanin components in our red Cabernet Sauvignon wine are capable of inhibiting the generation of Aβ peptides, that malvidins are the most abundant anthocyanins in the wine, as well as recent in vitro evidence that malvidin and one of its derivatives, malvidin-3-O-glucuronide, may protect against Aβ-mediated toxicity (47), we continued to explore the bioavailability of Cabernet Sauvignon anthocyanins, particularly malvidins, in the blood and in the brain. For polyphenol bioavailability studies, animals were treated daily with an oral dose of 150 mg/kg BW of the total wine polyphenolic extract (administered by gavage) for 10 consecutive days to simulate chronic exposure. Following administration of the last dose of wine polyphenolic extract, blood pharmacokinetics and brain accumulations of individual polyphenolic compounds were examined. Among the four malvidin derivatives in the red wine, we found detectable accumulations of malvidin-glucoside and malvidin-glucoside-pyruvate in the plasma (Table 1; Fig. 4A); no detectable plasma accumulations of malvidin-acetyl-glucoside-pyruvate and malvidin-acetyl glucoside were observed (Table 1). Moreover, we detected plasma accumulations of a third malvidin derivative, malvidin-3-O-glucuronide, which is not originally found in the red wine but is formed as a result of phase II xenobiotic metabolism (Table 1 and Fig. 4A).
Figure 4.
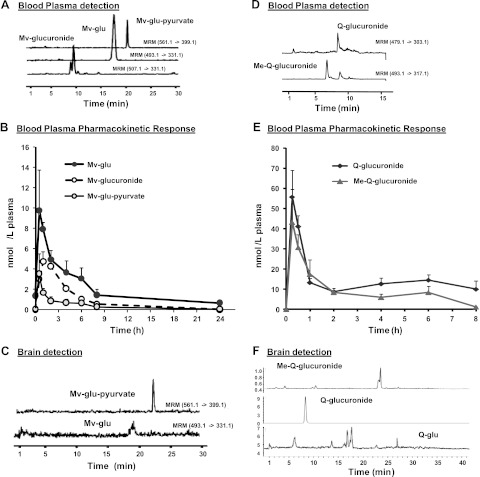
Plasma pharmacokinetics and brain levels of malvidin and quercetin derivatives. Contents of malvidin and quercetin derivatives in plasma and in the brain were assessed following repeated oral treatment of rats with the total polyphenolic extract prepared from the Cabernet Sauvignon wine. Tissue contents of anthocyanin and quercetin derivatives were monitored by LC-UV-MS methodologies. A, D) Detection of malvidin derivatives (A) and quercetin derivatives (D) in the plasma by LC-MS/MS. B, E) Plasma pharmacokinetic profile of major malvidin (B) and quercetin (F) components over time. C, F) Concentrations of malvidin (C) and quercetin (F) derivatives in brain tissue following 10 d of treatment. Mv-glu, malvidin-glucoside; Mv-glucuronide, malvidin-glucuronide; Mv-glu-pyruvate, malvidin-glucoside-pyruvate; Q-glu, quercetin-glucoside; Q-glucuronide, quercetin-glucuronide; Me-Q-glucuronide, methyl-quercetin-glucuronide.
Plasma pharmacokinetic responses of malvidin derivatives from acute on chronic dosing of the wine extract are shown in Fig. 4B. Absorption of the three malvidin derivatives was rapid, with an observed Tmax of ∼30 min, indicating rapid absorption from the gastrointestinal tract. Responses were greater for malvidin-glucoside compared to the malvidin-glucose-pyruvate derivative in plasma, reaching levels of ∼10 nM. Malvindin-3′-glucuronide was determined to be the primary plasma metabolite from red wine. Among the three malvidin derivatives that are bioavailable in the plasma, we detected accumulations of 12.3 ± 3.85 and 6.4 ± 5.2 pmol/g malvdin-3-glucoside and malvdin-3-glucopyruvate, respectively, in the perfused brain tissues (Fig. 4C); no detectable contents of malvidin-3-O-glucuronide were found in brain specimens (not shown).
While in vitro evidence from our group (Fig. 4C) and from Shih et al. (47) highlighted the potential role of anthocyanins in protecting against AD, recent in vitro evidence indicated that a select flavonoid, quercetin, may also protect against Aβ-mediated neurotoxic mechanisms by interfering with the assembly of Aβ peptides into neurotoxic oligomeric Aβ aggregates (80). On the basis of this and our LC-UV-MS profiling, indicating the presence of a significant quantity of quercetin in the Cabernet Sauvignon wine (Table 1), we extended our bioavailability studies and explored the plasma pharmacokinetic profile and brain accumulation of quercetin components. In the wine extract used in these studies, quercetin was found present primarily as quercetin aglycone (nonglycosylated quercetin; Table 1). In contrast, in rodent plasma, quercetin was detected primarily as quercetin-3-O-glucuronide and methyl-quercetin-3-O-glucuronide (Fig. 4D), common phase II conjugate metabolites derived from the native quercetin aglycone. These metabolites were tentatively identified by cochromatography with authentic standards (quercetin-3-O-glucuronide) and by MS-TOF spectra (data not shown). Appearance of quercetin-3-O-glucuronide and methyl-quercetin-3-O-glucuronide in plasma was rapid (Tmax∼15–30 min), with maximum plasma concentrations of 60.1 ± 34.7 and 44.2 ± 25.5 nM plasma, respectively (Fig. 4E). In the brain, we found accumulations primarily of quercetin-3-O-glucuronide (Fig. 4F). Methyl-quercetin-3-O-glucuronide was detected but not quantifiable in brain tissue extracts (Fig. 4F). The concentration of quercetin-3-O-glucuronide in brain tissues was found to be 0.91 ± 0.13, pmol/g brain tissue.
Exploring the potential role of brain-targeted quercetin and malvidin derivatives in modulating the generation of Aβ peptides
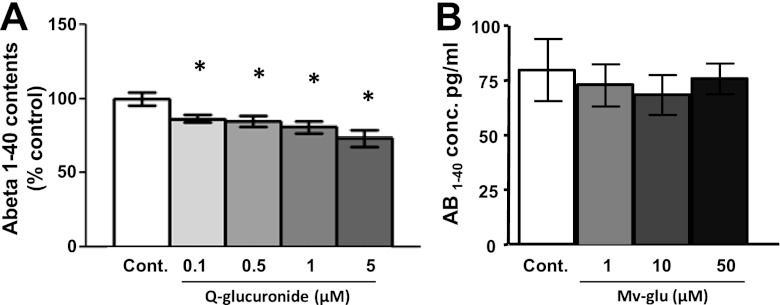
Among the multiple malvidin and quercetin derivatives that we detected in the brain, only malvidin-3-O-glucoside and quercetin-3-O-glucuronide are presently available (through commercial sources) for assessments of bioactivities relevant to AD interventions. Exploring the potential beneficial roles of malvidin-3-glucoside and quercetin-3-O-glucuronide in modulating Aβ neuropathological mechanisms, we found that treatments of primary Tg2576 corticohippocampal neurons with submicromolar concentrations of quercetin-3-O-glucuronide attenuated the generation of Aβ peptides (Fig. 5A); treatments with higher contents of quercetin-3-O-glucuronide lead to increasing inhibition of Aβ generation in a dose-responsive manner (Fig. 5A). In contrast, malvidin-3-glucoside had no detectable effects on Aβ generation by Tg2576 primary neuron cultures (Fig. 5B).
Figure 5.
Brain-targeted quercetin-3-O-glucuronide significantly inhibits generation of Aβ1–40 peptides from Tg2576 primary neuron cultures. The potential efficacy of brain-targeted quercetin-3-O-glucuronide (A) or malvidin-3-O-glucoside (B) in modulating the generation of Aβ1–40 peptides were assessed using primary Tg2576 neuron cultures. Cells were treated with varying doses of the polyphenols as indicated; control cells were treated with the vehicle. Aβ (A) and Aβ1–42 (B) contents in the conditioned medium. Q-glucuronide, quercetin-glucuronide; Mv-glu, malvidin-glucoside. Bar graphs represent mean ± se; n = 3 cultures/treatment group; values are expressed as percentage of control. *P < 0.05.
Quercetin-3-O-glucuronide interferes with Aβ aggregation
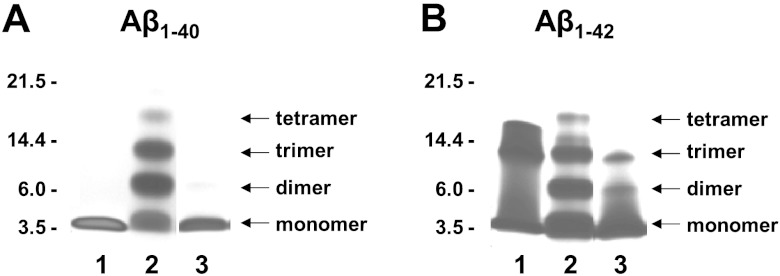
Similar to the nonglycosylated form of quercetin (quercetin aglycone; ref. 80), we found that the glycosylated brain-targeting quercetin metabolite, quercetin-3-O-glucuronide, is capable of interfering with the assembly of Aβ peptides into neurotoxic oligomeric Aβ aggregates (Fig. 6). We used an in vitro PICUP assay to explore the initial protein-protein interaction that is necessary for the assembly of monomeric Aβ peptides into oligomeric Aβ species. In the absence of cross-linking, only Aβ1–40 monomers (Fig. 6A, lane 1) and Aβ1–42 monomers, trimers and tetramers (Fig. 6B, lane 1) were observed. The Aβ1–42 trimers and tetramer bands under noncrosslinking condition (Fig. 6B, lane 1) are known SDS-PAGE-induced artifacts (49, 50). As expected, a mixture of dimeric, trimeric, and tetrameric aggregates are observed for Aβ1–40 (Fig. 6A, lane 2) and Aβ1–42 (Fig. 6B, lane 2) following cross-linking. The presence of quercetin-3-O-glucuronide at an equal molar concentration with Aβ1–40 completely blocked the formation of dimeric, trimeric, and tetrameric Aβ1–40 species (Fig. 6A, lane 3). Quercetin-3-O-glucuronide at 1:1 molar ratio with Aβ1–42 also significantly reduced the formation of higher-order Aβ1–42 species (Fig. 6B, lane 3).
Figure 6.
Brain-targeting quercetin-3-O-glucuronide potently interferes with aggregations of Aβ peptides, in vitro. We used the PICUP assay to explore initial protein-protein interactions necessary for the assembly of monomeric Aβ peptides into neurotoxic oligomeric Aβ species. Aβ1–40 (25 μM; A) or Aβ1–42 (25 μM; B) was cross-linked in the presence or absence of an equal molar concentration (25 μM) of quercetin-3-O-glucuronide. PICUP products were resolved by SDS-PAGE and visualized using silver staining. Lane 1, control non-cross-linked Aβ peptides; lanes 2 and 3, cross-linked Aβ peptide in the absence (lane 2) or presence (lane 3) of quercetin-3-O-glucuronide. Aβ1–42 trimers and tetramer bands under non-cross-linking conditions (B, lane 1) are known SDS-PAGE-induced artifacts (49, 50).
Quercetin-3-O-glucuronide promotes neuroplasticity mechanisms in the brain
LTP is one of the major neuronal plasticity mechanisms that are crucial for learning and memory functions (51). Evidence suggests that cellular CREB signaling plays a central role in LTP and memory formation (52, 53). Certain polyphenolics might be capable of promoting neuronal plasticity mechanisms (48, 54). For example, recent in vitro evidence from neuroblastoma cells (55), primary neuron cultures (48, 79), and HEK293 kidney cells (56) suggests that quercetin aglycone may promote CREB signaling. Moreover, quercetin aglycone has also been shown to protect against ischemia-mediated or lead exposure-mediated inhibition of LTP in rat models (57, 58). However, there is no information regarding the potential role of quercetin metabolites present in the brain in the regulation of CREB signaling and/or LTP responses. On the basis of our evidence that quercetin-3-O-glucuronide penetrated the blood-brain barrier and was detected in perfused brain tissue, we continued to explore the potential impact of quercetin-3-O-glucuronide on the regulation of CREB signaling. We treated primary corticohippocampal neuron cultures with quercetin-3-O-glucuronide and quantified cellular contents of P-CREB as a direct reflection of CREB activation. We found that quercetin-3-O-glucuronide treatments significantly increased cellular levels of activated P-CREB protein by ∼2-fold, compared to vehicle-treated controls (Fig. 7A).
Figure 7.
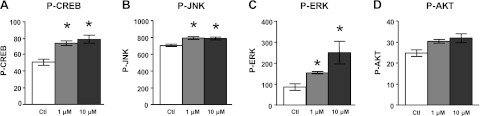
Brain-targeting quercetin-3-O-glucuronide promotes P-CREB in primary cortico-hippocampal neurons. Activated P-CREB (A), as well as P-JNK (B), P-Erk (C), and P-AKT (D), which are important for modulating P-CREB signaling, were assayed by a multiplex assay. Bar graphs represent means ± se; n = 3 cultures/treatment group; values are expressed as percentage of control. *P < 0.05.
The phosphorylation, and thereby the activation, of CREB is modulated by multiple cellular signaling pathways, including the JNK pathway in response to stress, the PI3K/AKT and the MEK/MAPK-Erk pathways in response to growth factors, and the protein kinase A (PKA) and the Ca2+/calmodulin-dependent protein kinases (CaMKs) pathways in response to neurotransmitters (59). Using a multiplex pathway signaling ELISA assay, we found that quercetin-3-O-glucuronide-mediated induction of cellular P-CREB was associated with significantly increased cellular contents of P-JNK (Fig. 7B) and P-Erk1/2 (Fig. 7C); no detectable change in cellular P-AKT was observed in primary neuron cultures in response to quercetin-3-O-glucuronide treatment (Fig. 7D).
On the basis of evidence that quercetin-3-O-glucuronide is effective in promoting P-CREB signaling, we continued to explore the potential role of quercetin-3-O-glucuronide in modulating LTP responses in ex vivo hippocampal slices from old (13–14 mo of age) Tg2576 mice, an age when this AD mouse model is typically characterized by severe cognitive impairment and impaired behavioral functions (60). Published evidence has revealed that, coinciding with cognitive impairments, old Tg2576 mice are characterized by deficits in hippocampal formation basal synaptic transmission and LTP responses (61). Consistent with this, we observed reduced basal neuronal transmission (Fig. 8A, B; 2-way ANOVA, P<0.01) and LTP (Fig. 8C, D; 2-way ANOVA, P<0.01) activities in ex vivo hippocampal slices from old Tg2576 mice. Consistent with our observed efficacy of quercetin-3-O-glucuronide in promoting CREB signaling in primary neuron cultures, we found that treatment of ex vivo hippocampal slices from old Tg2576 mice with submicromolar concentrations of quercetin-3-O-glucuronide significantly rescued deficits in basal neuronal transmission (Fig. 8A; 2-way ANOVA, P<0.01), as well as LTP (Fig. 8B;2-way ANOVA, P<0.01) in the CA1 region of hippocampal slices, compared to vehicle-treated hippocampal slices from old Tg2576 mice. In parallel control studies, we confirmed that treatment with comparable concentrations of another dietary polyphenol, epicatechin, did not lead to any detectable promotion of basal neuronal transmission or LTP in ex vivo hippocampal slices from old Tg2576 mice (data not shown).
Figure 8.
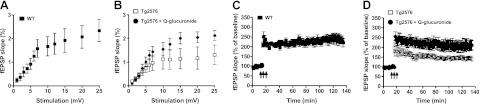
Brain-targeted quercetin-3-O-glucuronide promotes basal transmission and LTP. Assessment of basal synaptic transmission and LTP from ex vivo hippocampal slices. A, B) Treatment with quercetin-glucuronide-rescued basal synaptic transmission deficits in hippocampal slices from 12-mo-old Tg2576 AD mice. A) Basal synaptic transmission recordings from age-matched WT mice. B) Basal synaptic transmission recordings from Tg2576 mice in the absence or presence of treatment with 400 nM quercetin-3-O-glucuronide. Two-way ANOVA; P < 0.01 for WT vs. Tg2576 or Tg2576 vs. Tg2576+quercetin-3-O-glucuronide; no statistical difference observed for WT vs. Tg2576 + quercetin-3-O-glucuronide. C, D) Treatment with quercetin-3-O-glucuronide rescued LTP deficits in hippocampal slices from 12 mo-old Tg2576 AD mice. C) LTP recordings from age-matched WT mice. D) LTP recordings from Tg2576 mice in the absence or presence of treatment with 400 nM quercetin-3-O-glucuronide. Arrows indicate the beginning of tetanus used to induce LTP. Two-way ANOVA; P < 0.01 for WT vs. Tg2576 or Tg2576 vs. Tg2576+quercetin 3-glucuronide; no statistical difference was observed for WT vs. Tg2576+quercetin-3-O-glucuronide. Q-glucuronide, quercetin-3-O-glucuronide.
DISCUSSION
The potential health benefits of wine consumption have been, in general, ascribed to the polyphenol compounds in the wines (62, 63). Red wine and other grape-based products are known for their high concentrations of phytochemicals, including numerous and diverse polyphenols. The predominant source of antioxidant and bioactive polyphenols in these products is in the broad category of flavonoids, including anthocyanins (i.e., malvidin), flavonols (i.e., quercetin and kaempferol), and flavanols (i.e., catechin, epicatechin, and condensed tannins, which are biosynthetic polymers of either catechin or epicatechin; polymers easily reach in excess of 25 more units).
More recently, studies on the health benefits of red wine have been focused on resveratrol, a polyphenol component found in variable, but relatively low, concentrations in many commercial red wines. Evidence from our in vitro studies revealed that Aβ-lowering activity in the red Cabernet Sauvignon wine that we used in our study is mediated by a polyphenolic subfraction that contains no detectable contents of resveratrol. Our observation is consistent with accumulating evidence (35, 47, 48, 64–68) supporting the exploration of nonresveratrol polyphenol components in wine or in other dietary polyphenol sources for the prevention and/or treatment of AD.
Dietary polyphenols are primarily present in plant tissues and foods in glycosylated forms (32, 33). Moreover, following absorption, some polyphenols are rapidly metabolized to conjugated derivatives, including glucuronides and methylated metabolites (32). Therefore, a major consideration for the potential development of polyphenolics for treating neurodegenerative disorders is bioavailability and access of specific polyphenolic metabolites or naturally polyphenolic glycosylated derivatives to brain tissues. Thus, bioactivity studies exploring the physiological impacts of dietary polyphenols need to use the specific metabolite forms that have been identified in target tissues.
We previously showed that dietary supplementation with a Cabernet Sauvignon red wine interferes with the onset and progression of Aβ-mediated AD-type neuropathology and cognitive deterioration in a transgenic AD mouse model (14). However, there is no information regarding specific polyphenolic components that may contribute to the benefits the red wine in modulating AD phenotypes. We presently identified multiple malvidin and quercetin derivatives that are bioavailable in circulating plasma. More notably, we demonstrated that several malvidin and quercetin derivatives found in the plasma are also found accumulated in brain tissues, which is the key target tissue for AD interventions. Several studies have previously investigated the bioavailability of malvidin and quercetin in the brain, but questions remain on the capability of malvidin and quercetin to penetrate into the central nervous system. For example, Andres-Lacuev et al. (69) reported the detection of multiple malvidin derivative, including malvidin-3-glucoside, in brain specimens of rats following 10 wk of dietary supplementation with a blueberry extract. However, brain specimens were collected from nonperfused animals, and therefore, detections of malvidin derivatives from these brain specimens are confounded by potential blood contaminations. A similar issue arises regarding the penetration of quercetin from dietary sources into the central nervous system and the formulations of quercetin that are accumulated in the brain. For example, previous studies have reported detection of quercetin in rat (70, 71) or pig (71) brain specimens following dietary supplementation with quercetin or quercetin-rich preparations. However, there, studies were conducted using brain specimens collected from nonperfused animals, and therefore, it is uncertain whether detection of quercetin from these brain specimens might be derived from blood contaminations. Moreover, in these studies, polyphenol compounds in tissues were deconjugated prior to analysis, so these studies were not able to distinguish accumulation of individual quercetin derivatives, such as quercetin3-O-glucuronide, in the brain. In another related study on the accumulation of quercetin in the brain, Rivera et al. (72) delivered quercetin directly into the bloodstream of rats by intravenous injection and, thereafter, perfused the animals with saline and reported detection of micromolar concentrations of quercetin in brain specimens. However, this evidence does not necessarily provide guidance on the bioavailability of quercetins in the diet, since quercetin was administered intravenously, which bypassed gastrointestinal metabolism, that play major roles in the conversion of dietary polyphenols into polyphenol metabolites that are absorbed and utilize in vivo. Our present evidence from analysis of perfused rat brain specimens is the first conclusive demonstration that select malvidin and quercetin metabolites are capable of penetrating into the brain and provides critical information, implicating specific polyphenolic derivative that might contribute the beneficial AD disease-modifying activities.
A number of in vitro studies have implicated the potential health benefits of quercetin and malvidin. Notably, the majority of these studies was conducted using the aglycone form of quercetin or malvidin. However, biological activities of quercetin and malvidin metabolites are distinct from that of their corresponding quercetin and malvidin aglycone. For example, malvidin, but not malvidin-3-O-glucoside, is effective in inhibiting the growth of A431 human vulva carcinoma cells (73). As another example, both quercetin and quercetin3-O-glucuronide exhibit free radical scavenging activities (74). However, unlike quercetin aglycone, quercetin glucuronide is not able to inhibit xanthine oxidase or lipoxygenase in vitro (75). As an additional example, Spencer et al. (76) showed that micromolar concentrations of quercetin aglycone is neurotoxic for primary neurons. However, examining the quercetin metabolites that are found in vivo showed that methylated quercetin derivatives are much less neurotoxic compared to quercetin and a quercetin glucuronide metabolite is not toxic to primary neurons (76). Consistent with distinct toxicity neurotoxicity profiles among quercetin and quercetin metabolites, Vafeiadou et al. (77) also reported that the neurotoxicity of quercetin is reduced by conversion of quercetin into quercetin metabolites. Thus, these observations emphasize the importance of targeting specific quercetin and malvidin metabolites that are found in the brain for characterization of potential beneficial AD disease-modifying activities.
We explored two of the polyphenolic derivatives present in brain tissues, malvidin 3-glucoside and quercetin-3-O-glucuronide, for their potential impacts on AD. Our evidence suggests that quercetin-3-O-glucuronide derivatives found accumulated in the brain are capable of interfering with the generation of Aβ peptides. In comparison, malvidin-3-O-glucoside had no detectable impact on Aβ generation. Previous studies have reported the capability of quercetin aglycone in inhibiting the generation of Aβ peptides (78), interfering with Aβ aggregation (80), as well as promoting CREB signaling (55, 56, 79) and rescuing stress-induced impairments in LTP responses (57, 58). However, since the aglycone form of quercetin is not bioavailable in vivo, particularly in the brain, this information likely does not have direct physiological relevance to AD.
In addition to modulating Aβ generation, we found the brain-targeted quercetin-3-O-glucuronide is a potent inhibitor of Aβ aggregation, interfering with the initial protein-protein interaction that is necessary for the assembly of monomeric Aβ1–40 and Aβ1–42 peptides into neurotoxic oligomeric Aβ species. Lastly, our evidence using ex vivo hippocampal slices from old Tg2576 mice demonstrated the efficacy of quercetin-3-O-glucuronide in rescuing deficits in basal synaptic transmission and LTP, possibly through mechanisms involving activation of the JNK and the MAK-Erk signaling pathways, which are typically responsive to, respectively, stress and growth factors. Collectively, our evidence demonstrated that quercetin-3-O-glucuronide in the brain may simultaneously modulate multiple independent AD disease-modifying mechanisms and, as such, may contribute to the benefit of dietary supplementation with red wines as an effective intervention for AD. Outcomes from our studies clarify the processes by which dietary quercetins may beneficially modulate AD. Future studies with other brain-targeting polyphenols that we have identified may lead to the identification and characterization of additional dietary polyphenolic components that may exert beneficial disease-modifying activities.
There is a growing interest in the development of dietary polyphenolic compounds for preventing and/or treating AD, as well as other neurodegenerative conditions (34–40). Collectively, outcomes from our studies provide insights on specific dietary polyphenolic components that may benefit brain functions and clarify some of the underlying mechanisms involved. This important information provides the logical basis for developing novel therapeutic strategies incorporating dietary polyphenols, or novel pharmacological approaches using brain-targeted polyphenolic components, either individually or in combination, to modulate the onset and progress of AD, as well as other neurodegenerative disorders.
Acknowledgments
These studies were supported by the U.S. National Institutes of Health [grant PO1 AT004511; National Center for Complementary and Alternative Medicine (NCCAM) Project 1 to L.H. and Project 3 to G.M.P.].
The authors thank Dr. Kenjiro Ono (Kanazawa University Graduate School of Medical Science, Takara-Machi, Kanazawa, Japan), for valuable assistance and support with the PICUP analysis.
Footnotes
- Aβ
- β-amyloid
- ACSF
- artificial cerebrospinal fluid
- AD
- Alzheimer's disease
- BBB
- blood-brain barrier
- CREB
- cAMP response element binding
- ESI
- electrospray ionization
- fEPSP
- field excitatory postsynaptic potential
- HFS
- high-frequency stimulation
- HPLC
- high-performance liquid chromatography
- LC
- liquid chromatography
- LTP
- long-term potentiation
- MRM
- multiple reaction monitoring
- MS
- mass spectrometry
- P-AKt
- [Ser473]-phosphorylated AKt
- P-CREB
- [Ser133]-phosphorylated CREB
- P-Erk1/2
- [Thr185/Tyr-87]-phosphorylated Erk1/2
- PICUP
- photo-induced cross-linking of unmodified proteins
- P-JNK
- [Thr183/Tyr185]-phosphorylated JNK
- QQQ
- triple quadrupole
- TOF
- time of flight
REFERENCES
- 1. Alzheimer's Association (2012) 2012 Alzheimer's disease facts and figures. Alzheimers Dement. 8, 131–168 [DOI] [PubMed] [Google Scholar]
- 2. Thies W., Bleiler L. (2011) 2011 Alzheimer's disease facts and figures. Alzheimers Dement. 7, 208–244 [DOI] [PubMed] [Google Scholar]
- 3. Germain S., Adam S., Olivier C., Cash H., Ousset P. J., Andrieu S., Vellas B., Meulemans T., Reynish E., Salmon E. (2009) Does cognitive impairment influence burden in caregivers of patients with Alzheimer's disease? J. Alzheimers Dis. 17, 105–114 [DOI] [PubMed] [Google Scholar]
- 4. Gonzalez E. W., Polansky M., Lippa C. F., Walker D., Feng D. (2011) Family caregivers at risk: who are they? Issues Ment. Health Nurs. 32, 528–536 [DOI] [PubMed] [Google Scholar]
- 5. Mausbach B. T., Chattillion E. A., Roepke S. K., Patterson T., Grant I. (2012) A comparison of psychosocial outcomes in elderly Alzheimer caregivers and noncaregivers. [E-pub ahead of print] Am. J. Geriatr. Psychiatry doi: 10.1097/JGP.0b013e31824472d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mohamed S., Rosenheck R., Lyketsos C. G., Schneider L. S. (2010) Caregiver burden in Alzheimer disease: cross-sectional and longitudinal patient correlates. Am. J. Geriatr. Psychiatry 18, 917–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cummings J. L. (2004) Alzheimer's disease. N. Engl. J. Med. 351, 56–67 [DOI] [PubMed] [Google Scholar]
- 8. Dorozynski A. (1997) Wine may prevent dementia. BMJ 314, 997. [PMC free article] [PubMed] [Google Scholar]
- 9. Larrieu S., Letenneur L., Helmer C., Dartigues J. F., Barberger-Gateau P. (2004) Nutritional factors and risk of incident dementia in the PAQUID longitudinal cohort. J. Nutr. Health Aging 8, 150–154 [PubMed] [Google Scholar]
- 10. Lindsay J., Laurin D., Verreault R., Hebert R., Helliwell B., Hill G. B., McDowell I. (2002) Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 156, 445–453 [DOI] [PubMed] [Google Scholar]
- 11. Luchsinger J. A., Tang M. X., Siddiqui M., Shea S., Mayeux R. (2004) Alcohol intake and risk of dementia. J. Am. Geriatr. Soc. 52, 540–546 [DOI] [PubMed] [Google Scholar]
- 12. Orgogozo J. M., Dartigues J. F., Lafont S., Letenneur L., Commenges D., Salamon R., Renaud S., Breteler M. B. (1997) Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev. Neurol. (Paris) 153, 185–192 [PubMed] [Google Scholar]
- 13. Truelsen T., Thudium D., Gronbaek M. (2002) Amount and type of alcohol and risk of dementia: the Copenhagen City Heart Study. Neurology 59, 1313–1319 [DOI] [PubMed] [Google Scholar]
- 14. Wang J., Ho L., Zhao Z., Seror I., Humala N., Dickstein D. L., Thiyagarajan M., Percival S. S., Talcott S. T., Pasinetti G. M. (2006) Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer's disease. FASEB J. 20, 2313–2320 [DOI] [PubMed] [Google Scholar]
- 15. German J. B., Walzem R. L. (2000) The health benefits of wine. Annu. Rev. Nutr. 20, 561–593 [DOI] [PubMed] [Google Scholar]
- 16. Quideau S., Deffieux D., Douat-Casassus C., Pouysegu L. (2011) Plant polyphenols: chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. Engl. 50, 586–621 [DOI] [PubMed] [Google Scholar]
- 17. Zenebe W., Pechanova O. (2002) Effects of red wine polyphenolic compounds on the cardiovascular system. Bratisl. Lek. Listy. 103, 159–165 [PubMed] [Google Scholar]
- 18. Ho L., Chen L. H., Wang J., Zhao W., Talcott S. T., Ono K., Teplow D., Humala N., Cheng A., Percival S. S., Ferruzzi M., Janle E., Dickstein D. L., Pasinetti G. M. (2009) Heterogeneity in red wine polyphenolic contents differentially influences Alzheimer's disease-type neuropathology and cognitive deterioration. J. Alzheimers Dis. 16, 59–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thimothe J., Bonsi I. A., Padilla-Zakour O. I., Koo H. (2007) Chemical characterization of red wine grape (Vitis vinifera and Vitis interspecific hybrids) and pomace phenolic extracts and their biological activity against Streptococcus mutans. J. Agric. Food Chem. 55, 10200–10207 [DOI] [PubMed] [Google Scholar]
- 20. Cantos E., Espin J. C., Tomas-Barberan F. A. (2002) Varietal differences among the polyphenol profiles of seven table grape cultivars studied by LC-DAD-MS-MS. J. Agric. Food Chem. 50, 5691–5696 [DOI] [PubMed] [Google Scholar]
- 21. Masa A., Vilanova M., Pomar F. (2007) Varietal differences among the flavonoid profiles of white grape cultivars studied by high-performance liquid chromatography. J. Chromatogr. A 1164, 291–297 [DOI] [PubMed] [Google Scholar]
- 22. Onso Borbalan A. M., Zorro L., Guillen D. A., Barroso C. G. (2003) Study of the polyphenol content of red and white grape varieties by liquid chromatography-mass spectrometry and its relationship to antioxidant power. J. Chromatogr. A 1012, 31–38 [DOI] [PubMed] [Google Scholar]
- 23. Perez-Magarino S., Gonzalez-San Jose M. L. (2004) Evolution of flavanols, anthocyanins, and their derivatives during the aging of red wines elaborated from grapes harvested at different stages of ripening. J. Agric. Food Chem. 52, 1181–1189 [DOI] [PubMed] [Google Scholar]
- 24. De La Hera Orts M. L., Martinez-Cutillas A., Lopez Roca J. M., Perez-Prieto E., Gomez-Plaze E. (2005) Effect of deficit irrigation on anthocyanin content of Monastrell grapes and wines. J. Intl. Sci. Vigne Vin 39, 47–55 [Google Scholar]
- 25. Esteban M. A., Villanueva M. J., Lissarrague J. R. (2001) Effect of irrigation on changes in the anthocyanin composition of the skin of cv Tempranillo (Vitis vinifera L.) grape berries during ripening. J. Sci. Food Agric. 81, 409–420 [Google Scholar]
- 26. Radovanovic B. C., Radovanovic A. N., Souquet J. M. (2010) Phenolic profile and free radical-scavenging activity of Cabernet Sauvignon wines of different geographical origins from the Balkan region. J. Sci. Food Agric. 90, 2455–2461 [DOI] [PubMed] [Google Scholar]
- 27. Yokotsuka K., Nagao A., Nakazawa K., Sato M. (1999) Changes in anthocyanins in berry skins of Merlot and Cabernet Sauvignon grapes grown in two soils modified with limestone or oyster shell versus a native soil over two years. Am. J. Enol. Viticult. 50, 1–12 [Google Scholar]
- 28. Brouillard R., George F., Fougerousse A. (1997) Polyphenols produced during red wine ageing. Biofactors 6, 403–410 [DOI] [PubMed] [Google Scholar]
- 29. Ginjom I. R., D'Arcy B. R., Caffin N. A., Gidley M. J. (2010) Phenolic contents and antioxidant activities of major Australian red wines throughout the winemaking process. J. Agric. Food Chem. 58, 10133–10142 [DOI] [PubMed] [Google Scholar]
- 30. Lee J. E., Hwang G. S., Van Den B. F., Lee C. H., Hong Y. S. (2009) Evidence of vintage effects on grape wines using 1H NMR-based metabolomic study. Anal. Chim. Acta 648, 71–76 [DOI] [PubMed] [Google Scholar]
- 31. Manach C., Scalbert A., Morand C., Remesy C., Jimenez L. (2004) Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 79, 727–747 [DOI] [PubMed] [Google Scholar]
- 32. Han X., Shan T., Lou H. (2007) Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 8, 47–53 [Google Scholar]
- 33. Xu Y., Simon J. E., Welch C., Wightman J. D., Ferruzzi M. G., Ho L., Passinetti G. M., Wu Q. (2011) Survey of polyphenol constituents in grapes and grape-derived products. J. Agric. Food Chem. 59, 10586–10593 [DOI] [PubMed] [Google Scholar]
- 34. Craggs L., Kalaria R. N. (2011) Revisiting dietary antioxidants, neurodegeneration and dementia. Neuroreport 22, 1–3 [DOI] [PubMed] [Google Scholar]
- 35. Ho L., Pasinetti G. M. (2010) Polyphenolic compounds for treating neurodegenerative disorders involving protein misfolding. Expert Rev. Proteom. 7, 579–589 [DOI] [PubMed] [Google Scholar]
- 36. Joseph J., Cole G., Head E., Ingram D. (2009) Nutrition, brain aging, and neurodegeneration. J. Neurosci. 29, 12795–12801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Magrone T., Jirillo E. (2011) Potential application of dietary polyphenols from red wine to attaining healthy ageing. Curr. Top. Med. Chem. 11, 1780–1796 [DOI] [PubMed] [Google Scholar]
- 38. Mandel S. A., Weinreb O., Amit T., Youdim M. B. (2012) Molecular mechanisms of the neuroprotective/neurorescue action of multi-target green tea polyphenols. Front. Biosci. 4, 581–598 [DOI] [PubMed] [Google Scholar]
- 39. Ramesh B. N., Rao T. S., Prakasam A., Sambamurti K., Rao K. S. (2010) Neuronutrition and Alzheimer's disease. J. Alzheimers Dis. 19, 1123–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vafeiadou K., Vauzour D., Spencer J. P. (2007) Neuroinflammation and its modulation by flavonoids. Endocr. Metab. Immune. Disord. Drug Targets 7, 211–224 [DOI] [PubMed] [Google Scholar]
- 41. Percival S. S., Sims C. A. (2000) Wine modifies the effects of alcohol on immune cells of mice. J. Nutr. 130, 1091–1094 [DOI] [PubMed] [Google Scholar]
- 42. Zoecklein B. W., Fugelsang K. C., Gump B. H., Nury F. S., Abraham S. K. (1990) Production Wine Analysis, Van Nostrand Reinhold, New York [Google Scholar]
- 43. Talcott S. T., Lee J. H. (2002) Ellagic acid and flavonoid antioxidant content of muscadine wine and juice. J. Agric. Food Chem. 50, 3186–3192 [DOI] [PubMed] [Google Scholar]
- 44. Kelley K. A., Ho L., Winger D., Freire-Moar J., Borelli C. B., Aisen P. S., Pasinetti G. M. (1999) Potentiation of excitotoxicity in transgenic mice overexpressing neuronal cyclooxygenase-2. Am. J. Pathol. 155, 995–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bitan G., Lomakin A., Teplow D. B. (2001) Amyloid beta-protein oligomerization: prenucleation interactions revealed by photo-induced cross-linking of unmodified proteins. J. Biol. Chem. 276, 35176–35184 [DOI] [PubMed] [Google Scholar]
- 46. Gong B., Vitolo O. V., Trinchese F., Liu S., Shelanski M., Arancio O. (2004) Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J. Clin. Invest. 114, 1624–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shih P. H., Wu C. H., Yeh C. T., Yen G. C. (2011) Protective effects of anthocyanins against amyloid beta-peptide-induced damage in neuro-2A cells. J. Agric. Food Chem. 59, 1683–1689 [DOI] [PubMed] [Google Scholar]
- 48. Hou Y., Aboukhatwa M. A., Lei D. L., Manaye K., Khan I., Luo Y. (2010) Anti-depressant natural flavonols modulate BDNF and beta amyloid in neurons and hippocampus of double TgAD mice. Neuropharmacology 58, 911–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bitan G., Kirkitadze M. D., Lomakin A., Vollers S. S., Benedek G. B., Teplow D. B. (2003) Amyloid beta-protein (Abeta) assembly: Abeta 40 and Abeta 42 oligomerize through distinct pathways. Proc. Natl. Acad. Sci. U. S. A. 100, 330–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bitan G., Fradinger E. A., Spring S. M., Teplow D. B. (2005) Neurotoxic protein oligomers—what you see is not always what you get. Amyloid 12, 88–95 [DOI] [PubMed] [Google Scholar]
- 51. Bliss T. V., Collingridge G. L. (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 [DOI] [PubMed] [Google Scholar]
- 52. Bartsch D., Casadio A., Karl K. A., Serodio P., Kandel E. R. (1998) CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell 95, 211–223 [DOI] [PubMed] [Google Scholar]
- 53. Bito H., Takemoto-Kimura S. (2003) Ca2+/CREB/CBP-dependent gene regulation: a shared mechanism critical in long-term synaptic plasticity and neuronal survival. Cell Calcium 34, 425–430 [DOI] [PubMed] [Google Scholar]
- 54. Guedj F., Sebrie C., Rivals I., Ledru A., Paly E., Bizot J. C., Smith D., Rubin E., Gillet B., Arbones M., Delabar J. M. (2009) Green tea polyphenols rescue of brain defects induced by overexpression of DYRK1A. PLoS One 4, e4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xu Y., Cui C., Pang C., Christen Y., Luo Y. (2007) Restoration of impaired phosphorylation of cyclic AMP response element-binding protein (CREB) by EGb 761 and its constituents in Aβ-expressing neuroblastoma cells. Eur. J. Neurosci. 26, 2931–2939 [DOI] [PubMed] [Google Scholar]
- 56. Ohta K., Mizuno A., Li S., Itoh M., Ueda M., Ohta E., Hida Y., Wang M. X., Furoi M., Tsuzuki Y., Sobajima M., Bohmoto Y., Fukushima T., Kobori M., Inuzuka T., Nakagawa T. (2011) Endoplasmic reticulum stress enhances gamma-secretase activity. Biochem. Biophys. Res. Commun. 416, 362–366 [DOI] [PubMed] [Google Scholar]
- 57. Yao Y., Han D. D., Zhang T., Yang Z. (2010) Quercetin improves cognitive deficits in rats with chronic cerebral ischemia and inhibits voltage-dependent sodium channels in hippocampal CA1 pyramidal neurons. Phytother. Res. 24, 136–140 [DOI] [PubMed] [Google Scholar]
- 58. Hu P., Wang M., Chen W. H., Liu J., Chen L., Yin S. T., Yong W., Chen J. T., Wang H. L., Ruan D. Y. (2008) Quercetin relieves chronic lead exposure-induced impairment of synaptic plasticity in rat dentate gyrus in vivo. Naunyn Schmiedebergs Arch. Pharmacol. 378, 43–51 [DOI] [PubMed] [Google Scholar]
- 59. Alberini C. M. (2005) Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 28, 51–56 [DOI] [PubMed] [Google Scholar]
- 60. Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., Yang F., Cole G. (1996) Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274, 99–102 [DOI] [PubMed] [Google Scholar]
- 61. Fitzjohn S. M., Morton R. A., Kuenzi F., Rosahl T. W., Shearman M., Lewis H., Smith D., Reynolds D. S., Davies C. H., Collingridge G. L., Seabrook G. R. (2001) Age-related impairment of synaptic transmission but normal long-term potentiation in transgenic mice that overexpress the human APP695SWE mutant form of amyloid precursor protein. J. Neurosci. 21, 4691–4698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Scalbert A., Johnson I. T., Saltmarsh M. (2005) Polyphenols: antioxidants and beyond. Am. J. Clin. Nutr. 81, 215S–217S [DOI] [PubMed] [Google Scholar]
- 63. Urquiaga I., Leighton F. (2000) Plant polyphenol antioxidants and oxidative stress. Biol. Res. 33, 55–64 [DOI] [PubMed] [Google Scholar]
- 64. Bastianetto S., Krantic S., Chabot J. G., Quirion R. (2011) Possible involvement of programmed cell death pathways in the neuroprotective action of polyphenols. Curr. Alzheimer Res. 8, 445–451 [DOI] [PubMed] [Google Scholar]
- 65. Darvesh A. S., Carroll R. T., Bishayee A., Geldenhuys W. J., Van der Schyf C. J. (2010) Oxidative stress and Alzheimer's disease: dietary polyphenols as potential therapeutic agents. Expert Rev. Neurother. 10, 729–745 [DOI] [PubMed] [Google Scholar]
- 66. Singh M., Arseneault M., Sanderson T., Murthy V., Ramassamy C. (2008) Challenges for research on polyphenols from foods in Alzheimer's disease: bioavailability, metabolism, and cellular and molecular mechanisms. J. Agric. Food Chem. 56, 4855–4873 [DOI] [PubMed] [Google Scholar]
- 67. Wang J., Santa-Maria I., Ho L., Ksiezak-Reding H., Ono K., Teplow D. B., Pasinetti G. M. (2010) Grape derived polyphenols attenuate tau neuropathology in a mouse model of Alzheimer's disease. J. Alzheimers Dis. 22, 653–661 [DOI] [PubMed] [Google Scholar]
- 68. Wang J., Ferruzzi M. G., Ho L., Blount J., Janle E. M., Gong B., Pan Y., Gowda G. A., Raftery D., rrieta-Cruz I., Sharma V., Cooper B., Lobo J., Simon J. E., Zhang C., Cheng A., Qian X., Ono K., Teplow D. B., Pavlides C., Dixon R. A., Pasinetti G. M. (2012) Brain-targeted proanthocyanidin metabolites for Alzheimer's disease treatment. J. Neurosci. 32, 5144–5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Andres-Lacueva C., Shukitt-Hale B., Galli R. L., Jauregui O., Lamuela-Raventos R. M., Joseph J. A. (2005) Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr. Neurosci. 8, 111–120 [DOI] [PubMed] [Google Scholar]
- 70. Rangel-Ordonez L., Noldner M., Schubert-Zsilavecz M., Wurglics M. (2010) Plasma levels and distribution of flavonoids in rat brain after single and repeated doses of standardized Ginkgo biloba extract EGb 761(R). Planta Med. 76, 1683–1690 [DOI] [PubMed] [Google Scholar]
- 71. De Boer V. C., Dihal A. A., van der Woude H., Arts I. C., Wolffram S., Alink G. M., Rietjens I. M., Keijer J., Hollman P. C. (2005) Tissue distribution of quercetin in rats and pigs. J. Nutr. 135, 1718–1725 [DOI] [PubMed] [Google Scholar]
- 72. Rivera F., Urbanavicius J., Gervaz E., Morquio A., Dajas F. (2004) Some aspects of the in vivo neuroprotective capacity of flavonoids: bioavailability and structure-activity relationship. Neurotox. Res. 6, 543–553 [DOI] [PubMed] [Google Scholar]
- 73. Meiers S., Kemeny M., Weyand U., Gastpar R., von A. E., Marko D. (2001) The anthocyanidins cyanidin and delphinidin are potent inhibitors of the epidermal growth-factor receptor. J. Agric. Food Chem. 49, 958–962 [DOI] [PubMed] [Google Scholar]
- 74. Moon J. H., Tsushida T., Nakahara K., Terao J. (2001) Identification of quercetin 3-O-beta-d-glucuronide as an antioxidative metabolite in rat plasma after oral administration of quercetin. Free Radic. Biol. Med. 30, 1274–1285 [DOI] [PubMed] [Google Scholar]
- 75. Day A. J., Gee J. M., DuPont M. S., Johnson I. T., Williamson G. (2003) Absorption of quercetin-3-glucoside and quercetin-4′-glucoside in the rat small intestine: the role of lactase phlorizin hydrolase and the sodium-dependent glucose transporter. Biochem. Pharmacol. 65, 1199–1206 [DOI] [PubMed] [Google Scholar]
- 76. Spencer J. P., Rice-Evans C., Williams R. J. (2003) Modulation of pro-survival Akt/protein kinase B and ERK1/2 signaling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J. Biol. Chem. 278, 34783–34793 [DOI] [PubMed] [Google Scholar]
- 77. Vafeiadou K., Vauzour D., Rodriguez-Mateos A., Whiteman M., Williams R. J., Spencer J. P. (2008) Glial metabolism of quercetin reduces its neurotoxic potential. Arch. Biochem. Biophys. 478, 195–200 [DOI] [PubMed] [Google Scholar]
- 78. Shimmyo Y., Kihara T., Akaike A., Niidome T., Sugimoto H. (2008) Flavonols and flavones as BACE-1 inhibitors: structure-activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim. Biophys. Acta 1780, 819–825 [DOI] [PubMed] [Google Scholar]
- 79. Tchantchou F., Lacor P. N., Cao Z., Lao L., Hou Y., Cui C., Klein W. L., Luo Y. (2009) Stimulation of neurogenesis and synaptogenesis by bilobalide and quercetin via common final pathway in hippocampal neurons. J. Alzheimers Dis. 18, 787–798 [DOI] [PubMed] [Google Scholar]
- 80. Porat Y., Abramowitz A., Gazit E. (2006) Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanisms. Chem. Biol. Drug Des. 67, 27037. [DOI] [PubMed] [Google Scholar]