Abstract
Balding causes widespread psychological distress but is poorly controlled. The commonest treatment, minoxidil, was originally an antihypertensive drug that promoted unwanted hair. We hypothesized that another serendipitous discovery, increased eyelash growth side-effects of prostamide F2α-related eyedrops for glaucoma, may be relevant for scalp alopecias. Eyelash hairs and follicles are highly specialized and remain unaffected by androgens that inhibit scalp follicles and stimulate many others. Therefore, we investigated whether non-eyelash follicles could respond to bimatoprost, a prostamide F2α analog recently licensed for eyelash hypotrichosis. Bimatoprost, at pharmacologically selective concentrations, increased hair synthesis in scalp follicle organ culture and advanced mouse pelage hair regrowth in vivo compared to vehicle alone. A prostamide receptor antagonist blocked isolated follicle growth, confirming a direct, receptor-mediated mechanism within follicles; RT-PCR analysis identified 3 relevant receptor genes in scalp follicles in vivo. Receptors were located in the key follicle regulator, the dermal papilla, by analyzing individual follicular structures and immunohistochemistry. Thus, bimatoprost stimulates human scalp follicles in culture and rodent pelage follicles in vivo, mirroring eyelash behavior, and scalp follicles contain bimatoprost-sensitive prostamide receptors in vivo. This highlights a new follicular signaling system and confirms that bimatoprost offers a novel, low-risk therapeutic approach for scalp alopecias.—Khidhir, K. G., Woodward, D. F., Farjo, N. P., Farjo, B. K., Tang, E. S., Wang, J. W., Picksley, S. M., and Randall, V. A. The prostamide-related glaucoma therapy, bimatoprost, offers a novel approach for treating scalp alopecias.
Keywords: hair follicle, hair growth, balding, prostaglandins, human organ culture
Hair loss disorders, such as androgen-dependent male pattern baldness (androgenetic alopecia; ref. 1) or alopecia areata, a putative autoimmune disease of both sexes (2), cause significant psychological distress (3, 4). Negative effects on the quality of life are reported even in men who have never sought treatment (3) and are particularly pronounced in women and adolescents (5). This is not surprising, given hair's importance in human social and sexual communication in all cultures (6, 7). Androgen-stimulated increases in hair size on the axilla and pubis signal adulthood in both sexes, while those on the face produce the beard, advertising the sexually mature man (6, 8, 9). This can occur because of the unique regenerative power of hair follicles. Each follicle is an individual miniature organ that goes through regular cycles of growth, regression, rest, and shedding (10, 11) where the existing hair is replaced with a new one. The new hair may be similar to its predecessor or differ in size and color. In androgenetic alopecia, androgens cause the gradual replacement of long terminal scalp hairs with shorter, finer ones until they become the virtually invisible, vellus hairs of bald skin (1, 7).
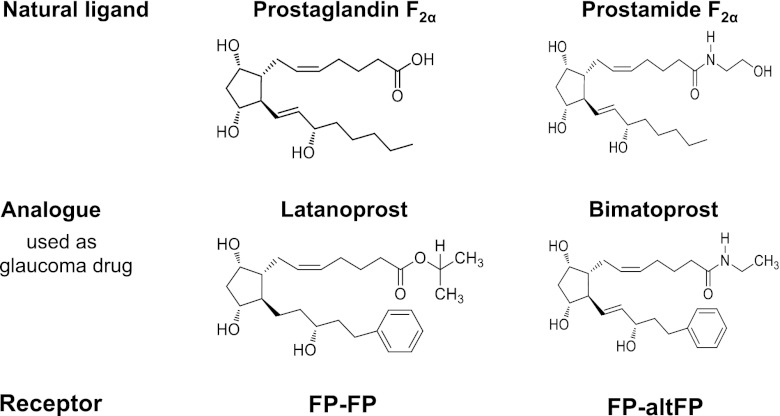
Unfortunately, hair loss disorders are currently poorly controlled; available therapies either have limited effectiveness or unwanted side effects (12, 13). Interestingly, the main therapies for androgenetic alopecia were developed for other conditions. Minoxidil (Rogaine), which opens ATP-sensitive potassium channels (14), is an example of a serendipitous drug; originally an oral antihypertensive, this application was discontinued because it stimulated unacceptable hair growth in many areas (15). Finasteride (Propecia), which reduces androgen effects by blocking testosterone metabolism to the more active 5α-dihydrotestosterone, was initially designed for prostate disorders (16, 17). More recently, increased eyelash growth has been reported as an unexpected side effect of certain eye drops used to reduce intraocular pressure in glaucoma (18). The drugs involved are some prostaglandin F2α (PGF2α)-related analogs, such as latanoprost, and the prostamide F2α-related analog, bimatoprost. The prostamides are a recently discovered group of biological lipids, which are related to prostaglandins, but which contain a terminal ethanolamide group and which target different receptors (refs. 19, 20; see Fig. 1). Whether they form a signaling system in the hair follicle is not known. Interestingly, not all prostaglandin F2α-related glaucoma drugs which successfully reduce intraocular pressure stimulate eyelash growth; some have mixed effects, even including some inhibition e.g., unoprostane isopropyl (Rescula; ref. 21), and others have no apparent effects e.g., PGF2α tromethanine salt (22). This variable response resembles the situation with antihypertensive potassium channel-opening drugs when taken orally; effects range from the major stimulation caused by minoxidil, through little effect in adults with diazoxide but increased growth in children, to no noticeable effect (reviewed in ref. 14). Bimatoprost (Latisse) was licensed for the treatment of hypotrichosis of the eyelashes by the FDA in December 2008 (NDA 022369). The ideal treatment for scalp alopecia is an externally applied (topical) substance, which would act locally to stimulate hair growth only in specific target areas with no side effects. If bimatoprost also stimulates scalp hair follicles, it could have great promise for use in scalp alopecias, as it causes marked stimulation of eyelash growth after local application; minoxidil's effect is much less topically than via the original oral ingestion (15).
Figure 1.
Comparison of PGF2α and prostamide F2α, their analogs, and receptors. The prostamides are electrochemically neutral biological lipids related to prostaglandins, but with a terminal ethanolamide group. PGF2α-related analogs, e.g., latanoprost, and the prostamide F2α-related analog, bimatoprost, are used for glaucoma. Alternative splicing variants of FP appear to heterodimerize with FP to create a bimatoprost-sensitive prostamide receptor (30).
Eyelashes are highly specialized, short hairs that have evolved to protect the eyes from foreign objects. They have marked differences to scalp hairs, and their follicles lack the normal arrector pili muscle (23). Nevertheless, eyelashes are produced by hair follicles and are replaced regularly via the hair follicle cycle (23) like all other hairs (10, 11). Human hair follicles also exhibit markedly different behaviors depending on their body site. For example, hair graying with age occurs first above the ears before gradually spreading over the scalp (24). There are also extreme differences in their hormonal response. Androgens stimulate hair growth in many areas, like the face or axilla, while inhibiting some scalp follicles, causing balding; they have no effect on eyelashes (7, 25). Therefore, it is not possible to extrapolate the effect of substances on eyelashes to scalp or other follicles. It is important to determine whether bimatoprost can stimulate the growth of other types of hair follicles.
Why eyelash stimulation occurs with this glaucoma therapy is unknown; possibilities include stimulating blood flow to the eyelashes, increasing the production of follicle stimulatory factors by other dermal components (26), or direct effects on the eyelash follicles themselves. Working on the hypothesis that bimatoprost will act directly on follicles, we initially investigated whether bimatoprost could stimulate growth in isolated scalp hair follicles in organ culture. Human scalp anagen hair follicles have an exciting and fascinating ability to grow in organ culture for several days, maintaining the epithelial-mesenchymal interactions (27, 28) and cell division necessary for the ordered synthesis of new pigmented hair seen in vivo (ref. 14; see Fig. 2); any growth stimulation under these conditions cannot be due to effects on the vasculature or other dermal components (26, 29). To determine whether non-eyelash follicles could also respond in vivo, bimatoprost effects were assessed in black mice. This approach takes advantage of the ability to see new black hair growth easily in the pink unpigmented skin, once the fully formed resting (telogen) hair is removed externally with clippers (26). Bimatoprost was applied topically, i.e., via external application to the skin, as this is the preferred approach for alopecia treatment. To clarify whether the bimatoprost effects were through specific prostamide receptors within the scalp follicles, the ability of the prostamide antagonist, AGN 211336 (19, 20) to block growth in isolated organ culture was also investigated.
Figure 2.
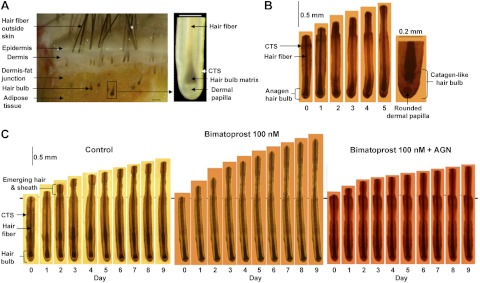
Sequential photomicrographs of human scalp hair follicles growing in organ culture. A) Isolation of hair follicles from scalp skin. Left panel: scalp skin, showing hair follicles and skin layers. Right panel: isolated hair follicle, demonstrating the hair fiber, hair matrix (pigmented in the upper hair bulb and unpigmented below), dermal papilla, and CTS. B, C) Sequential photomicrographs taken every 24 h for 9 d of individual scalp follicles in organ culture under various conditions showing growth of hair fiber, but not the CTS. B) Some follicles showed catagen-like changes in hair bulb morphology. By d 5, pigmentation had ceased, and the base of the hair fiber was retracting upward, leaving behind a ball of dermal papilla cells. C) Most follicles maintained bulb anagen morphology and increased in length over 9 d. Follicles were cultured in control medium (left), 100 nM bimatoprost (center), or 100 nM bimatoprost and AGN 211336 (AGN; 1 μM; right). Scale bars = 200 μm (A, B); 500 μm (C).
To relate these organ culture and living animal observations to human scalp in vivo, the expression of appropriate prostanoid receptor genes was examined in scalp hair follicles. These included the gene for the native PGF2α receptor, FP, and five alternative splicing variants of FP. One of these, FP splice variant 4 (altFP4), appears to heterodimerize with FP to create a bimatoprost-sensitive prostamide receptor (30). The glaucoma drugs darken eyelashes, as well as increase their length (18). This could be via separate actions on the keratinocytes, which make the hair, and the melanocytes that produce the color pigments; alternatively, it could occur via a single regulatory component that interprets the signals to other follicular cell types. The mesenchyme-derived dermal papilla, situated in the center of the hair bulb at the follicle base, regulates many aspects of follicular activity by producing paracrine signals to control other follicle cells (31, 32). Therefore, it may be the site of any coordinated response to a drug. To determine which mechanism is involved, the location of prostamide receptors in scalp hair follicles in vivo was investigated by immunohistochemistry on frozen skin sections and by reverse transcription–polymerase chain reaction (RT-PCR) using separately microdissected dermal papillae, hair bulb matrix (containing keratinocytes and melanocytes), the connective tissue sheath (CTS) surrounding the bulb, and other follicular components.
MATERIALS AND METHODS
Skin samples
Human scalp skin from nonbalding areas (occipital and parietal) was obtained from healthy individuals undergoing elective cosmetic surgery with written consent and approval by the University of Bradford Ethics Committee. For organ culture investigations, 1 woman and 14 men, aged 22 to 48, donated skin; samples were collected into sterile universal tubes (25 or 50 ml) containing basic culture medium: William's E medium supplemented with 10 μg/ml insulin, 10 ng/ml hydrocortisone, 2 mM l-glutamine (Life Technologies, Paisley, UK), and 10 U/ml penicillin. Unless specified, Sigma-Aldrich (Dorset, UK) supplied all materials. Supplies were transported on ice and stored at 4°C until hair follicles were isolated within 24 h of removal. For molecular biological investigations, small skin samples (∼1 cm3) from 7 men (aged 32–45) and a woman (aged 46) were placed into sterile universal tubes (10 ml) containing RNA stabilization solution, RNAlater, to inhibit RNases. They were transported on ice and kept at 4°C overnight to allow tissue penetration by RNAlater before storage at −20°C until analyzed. For immunohistochemical investigations, skin samples from 3 men (aged 36–42) and 2 women (aged 43 and 48) were collected as for organ culture, cut into small pieces, embedded in optimal cutting temperature (OCT) compound, and stored at −80°C.
Isolation of scalp hair follicles and individual follicular components
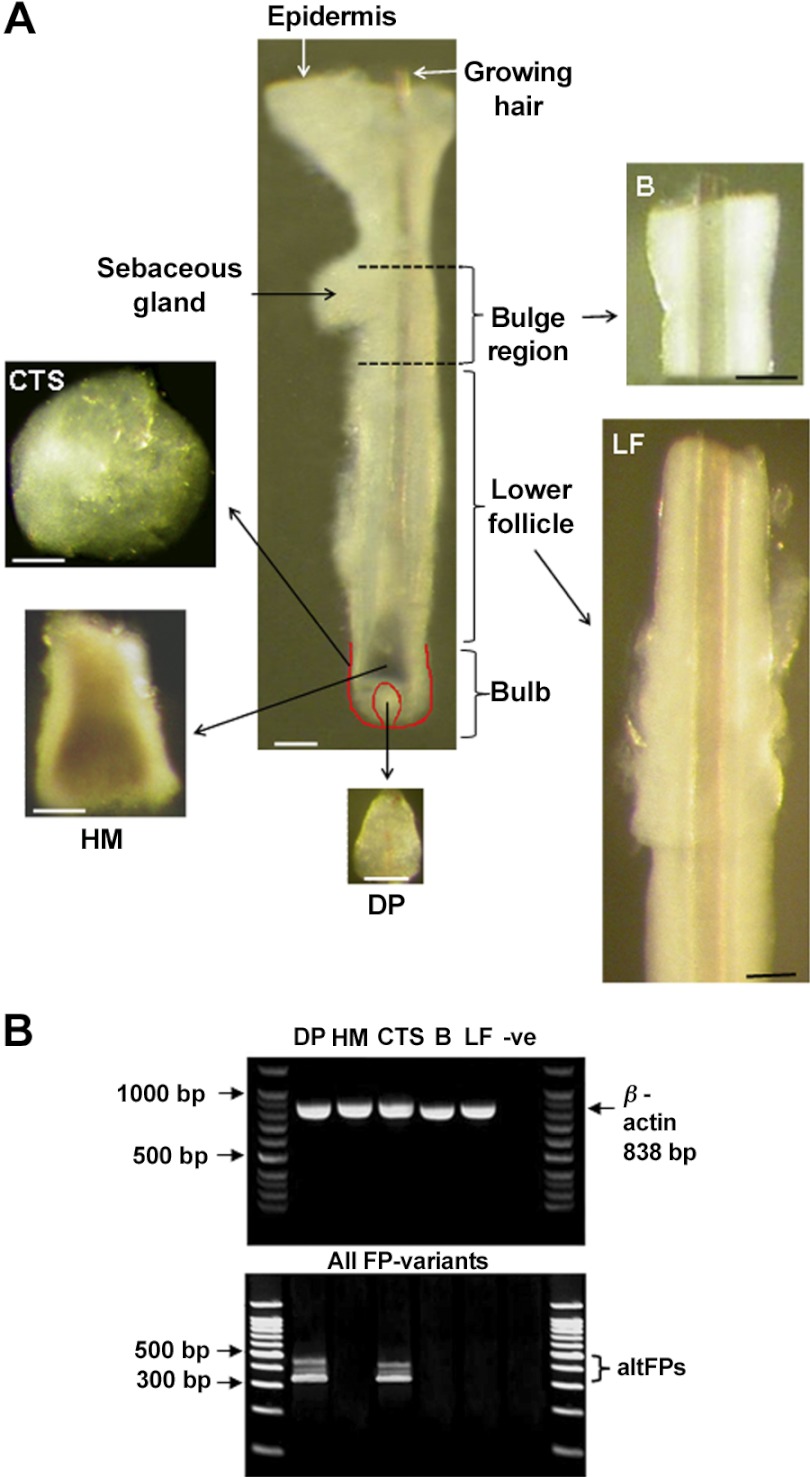
Anagen scalp hair follicles were microdissected individually from each sample under a Leica MZ8 dissecting microscope with fiberoptic cool illumination (Leica Microsystems, Wetzlar, Germany)using sterile equipment and plastic ware. Each sample was transferred to a petri dish containing sterile phosphate buffered saline (PBS; Oxoid, Basingstoke, UK) for organ culture or RNAlater at 4°C for molecular biological studies. Samples were cut at the level of the dermal-subcutaneous fat interface (see Fig. 2) using a scalpel blade, and intact lower follicles were then gently pulled from the subcutaneous fat using fine forceps. Isolated follicles were pooled into a fresh dish of cold PBS or RNAlater, and each follicle was gently cleaned of any attached dermis or subcutaneous fat using 27.5-gauge sterile syringe needles. Special care was taken to ensure that follicles were not damaged during isolation, as undamaged follicles are essential for successful culture (ref. 14; see Fig. 2). To investigate hair follicle gene expression, the lower follicles were microdissected from 60 follicles from each of 5 individual's samples; these were processed separately. To localize gene expression in specific follicle tissues, 125 follicles from each individual were further microdissected to obtain: the dermal papillae, the hair matrix, the CTS surrounding the hair bulb, and the follicle between the top of the bulb and the sebaceous gland attachment. This last part was then divided into the lower follicle above the bulb and the area containing the bulge, i.e., the epithelial and melanocyte stem cells (refs. 33, 34; see Fig. 6). Each component from each of 3 men was analyzed separately.
Figure 6.
Localization of three prostanoid receptor genes only in the scalp hair follicle dermal papilla and CTS. Amplified poly(A+)RNA from isolated scalp follicle components (A) from 3 individuals showed varying expression of prostanoid receptors despite equal expression of β-actin (B). With primers detecting native FP and all altFPs, three bands were seen only in isolated mesenchyme-derived tissues, the dermal papilla (DP), and the CTS surrounding the hair bulb (B). None were visible in the hair bulb matrix (M), the bulge (B), or the remaining lower follicle (LF). With specific primers for each gene, native FP, altFP1, and altFP4 were also only detected in the dermal papilla and CTS (see Table 1).
Hair follicle organ culture
Isolated follicles were carefully transferred to an individual well of a 24-well plate (Corning Glassworks, Corning, NY, USA) containing 1 ml of appropriate medium. Basic culture medium (see Skin Samples, above) was supplemented with either bimatoprost (10, 100 and 1000 nM) or prostamide antagonist AGN 211336 (1μM); both were dissolved in a stock solution of 0.001% dimethyl sulfoxide (DMSO) prepared using a sonicating water bath (Dawe Instruments, Middlesex, UK). Control medium was only supplemented with 0.001% DMSO. All media were sterile-filtered (0.2 μm; Sarstedt, Nümbrecht, Germany) before use. At least 6 hair follicles from each individual were cultured in each condition, maintained free-floating at 37°C in an atmosphere of 5% CO2 and 95% air in a humidified incubator. Medium was changed every 3 d, taking care not to damage the follicles.
Statistical assessment of hair follicle growth in culture
Hair follicles were assessed for follicle bulb morphology, photographed using a Nikon Coolpix 4500 digital camera (Nikon, Tokyo, Japan) and measured every 24 h for 9 d, using an inverted microscope (Leitz Labovert FS; Leica Microsystems). Follicles showing no growth after 3 d were classed “nonviable” and excluded. The mean value per person for each hair growth parameter under each treatment was determined before calculation of each sample mean. Data from each experimental group were analyzed for normal distribution using the Kolmogorov-Smirnof test. The effect of the different treatments with time on the daily rate of growth, percentage of follicles remaining in anagen, and total amount of hair follicle produced in culture was analyzed by 2-factor, within-subjects analysis of variance using the SPSS statistical analysis program (SPSS, Chicago, IL, USA). If the sample means of the different experimental groups differed significantly (P<0.05), they were compared using Student's paired t test with Sidak's correction for multiple comparisons.
Assessment of the effects of topical bimatoprost on mouse hair growth
Female C57BL/6J mice (cat. no. 000664; Jackson Laboratory, Bar Harbor, ME, USA), aged 7 wk, were randomly distributed into 4 groups to avoid any sibling bias and housed in groups of 5 with standard diet food pellets and water available ad libitum. Initially, dorsal hairs were removed externally by shaving (∼2×6 cm) using an electric trimmer (Wahl Stylique Designer/Liner pet trimmer; 919179; Petco, San Diego, CA, USA) revealing pink skin. From the next day, termed d 0, each mouse was treated topically with either the vehicle alone (ethanol:propylene glycol:water 3:5:2) or 0.03, 0.10, or 0.30% bimatoprost in the vehicle for 14 d; 10 mice were used for each condition. The appropriate solution (70 μl) was rubbed gently into the dorsal skin of each mouse daily; new gloves were worn for each treatment type. Hair growth was recorded daily for each animal for 42 d, and dorsal photographs were taken at d 0, 7, 14, 17, 21, 24, 28, 31, 35, and 42. The first day of anagen, defined as the first day when visible darkness could be seen that subsequently increased and progressed to visible black hair, was recorded for each animal. To calculate mean values, the first day of growth was designated as d 43 for any mouse showing no hair growth signs by the last day. The day when the shaved dorsal area was fully covered with new black hairs, i.e., there was no pink skin remaining and no visible difference in hair length to the adjacent unshaved areas, was also recorded.
Molecular biological investigations
Total RNA was isolated from scalp anagen follicles or isolated follicular components immediately after microdissection, as described previously (14) using a GenElute Mammalian Total RNA kit or RNeasy Mini Kit (Qiagen, Hilden, Germany), and quality was checked by agarose gel electrophoresis (1.5%) before further purification to isolate poly(A+)RNA using a GenElute mRNA Miniprep kit. Because of the limited amount of tissue components, component poly(A+)RNA was amplified before cDNA synthesis using the Smart mRNA amplification kit (Clontech Laboratories, Mountain View, CA, USA) following the manufacturer's instructions. Briefly, a modified oligo(dT) primer containing several additional nucleotides was used to initiate first-strand cDNA synthesis by Moloney murine leukemia virus (MMLV) reverse transcriptase; this sequence with additional sequences, primarily deoxycytidine, at its 5′ end, was used to generate double-stranded cDNA incorporating the additional sequence, containing a T7 RNA polymerase promoter. Finally, sense RNA was transcribed in vitro using T7 RNA polymerase; reinitiation and elongation continued in the presence of limiting amounts of ribonucleotides to produce linear amplification of mRNA.
RT-PCR was used to investigate the expression of mRNA for FP and splice variants of FP. To remove any contaminating genomic DNA, mRNA samples were treated with DNase I (Invitrogen, Paisley, UK), and cDNAs were synthesized immediately using the avian myeloblastosis virus (AMV) reverse-transcription system (Promega, Southampton, UK), as described previously (14); cDNA was portioned into 10 μl cDNA aliquots for storage at −20°C. PCR amplification was performed using 10 μl of cDNA in a 50 μl reaction volume containing 0.3 μM concentrations of forward and reverse primers (Sigma-Genosys Ltd., Pamisford, UK), 200 μM concentrations of each dNTP (Promega), 5 μl of 10× reaction buffer (200 mM Tris-HCl, pH 8.4, and 500 mM KCl; Invitrogen), 1.8 μM (FPs) or 2.5 mM (β-actin) MgCl2 (Invitrogen), and 2.5 U of recombinant TaqDNA polymerase (Invitrogen). Negative controls in which nuclease-free water replaced cDNA were run with each PCR reaction. Primers for β-actin were from Shorter et al. (14); those for all splice variants of FP and native FP (altFPs) were from Liang et al. (30); those for altFP2–5 were from U.S. patent 7320871 (SEQ ID no. 37 and 38); and those for FP and altFP1 were designed from the human FP gene (GenBank accession no. NC_000001.10) using the Ensembl Genome Browser (http://www.ensembl.org).
The primers were as follows: β-actin (GenBank NM_001101), forward 5′-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3′ and reverse 5′-CTCATACTCCTGCTTGCTGATCCACATCTGC-3′; altFPs, forward 5′-TGCAATGCAATCACAGGAAT-3′ and reverse 5′-CACTCCACAGCATTGACTGG-3′; altFP5, forward 5′-AGCTCCTGGCGATAATGTGT-3′ and reverse 5′-CCYYCHCAAYAHYCCYCCAA-3′; altFP4, forward 5′-AGCCCATTTCTGCGATAAGA-3′ and reverse 5′-GTTCTGGAGCCTCAGGTGTC-3′; altFP3 and altFP2, forward 5′-GAGCCCATTTCTGGGATACA-3′ and reverse 5′-AGTGCCTCTCTTCACCCTCA-3′; altFP1, forward 5′-GTGGTGTGTGCTTGTTTGCTG-3′ and reverse 5′-GCTAGGTGCTTGCTGATTTCTCTGC-3′; FP, forward 5′-CGATGCCATCATCACAGAAG-3′ and reverse 5′-CTGAGCAGCTTCTCTGGCTT-3′.
Initial denaturation of cDNA at 95°C for 5 min was followed by 35 cycles of PCR amplification. Cycling conditions for β-actin were 35 cycles of 95°C for 1 min, annealing at 55°C for 1 min, and 72°C for 1 min (14); cycling conditons for FP, altFPs, altFP4, and altFP1 were 95°C for 30 s, 53°C for 30 s, and 72°C for 30 s. A final extension period of 10 min at 72°C completed the thermocycling before cooling at 4°C (30). PCR products were analyzed by gel electrophoresis on a 1.5% Tris acetate-EDTA (TAE) agarose gel (Invitrogen), as described previously (14).
To confirm product identity, the PCR process was repeated with thin-walled PCR tubes (VWR International, Poole, UK) using the thermocycler with the hot lid. Products were separated on a low-melting-point 1.5% agarose gel, excised, and purified using the MinElute Gel Extraction kit (Qiagen, Crawley, UK), following the manufacturer's instructions, before sequencing by Geneblitz (Sunderland, UK). Results were compared with the known published sequences using the U.S. National Center for Biotechnology Information (NCBI) Basic Local Alignment Search Tool (BLAST) program (http://www.ncbi.nlm.nih.gov/blast/bl2seq/wblast2.cgi).
Immunohistochemistry
Immunohistochemistry was performed to confirm protein expression and localize FP in the hair follicle. Vertical frozen sections of human occipital scalp (5–7 μm thick) were cut using a Leica cryostat (CM 1800) and collected on poly-l-lysine-coated slides to increase adherence (14). Special care was needed during sectioning to orient the specimen to obtain longitudinal sections of follicles. Immunohistochemistry was performed as described previously (14), using 2 different polyclonal goat antibodies to FP, sc-5789 and sc-32461, and another for the nonrelevant potassium channel membrane protein, SUR2A (Santa Cruz Biotechnology, Santa Cruz, CA, USA; ref. 14), at 1:75 dilution at 4°C for 18 h in a moist chamber to avoid dehydration. Antibodies were diluted in 1.5% normal mouse serum in PBS. Sections were examined and photographed using a Nikon Eclipse 80i light microscope with a Nikon ACT-2U photographic system.
RESULTS
Bimatoprost-stimulated human scalp hair follicle growth in organ culture
To investigate whether bimatoprost could affect scalp hair follicle growth in the absence of any blood supply, isolated hair follicles (Fig. 2) were grown in organ culture for 9 d in serum-free medium with daily observation of their morphology, measurement, and photography. The number of follicles remaining in anagen and the increase in hair follicle length were recorded each day. The overall amount of hair actually produced by all the follicles was calculated using the final increase in length of each follicle on d 9, or on the last day the follicle maintained normal anagen morphology (Fig. 2B). Scalp follicles grew well in control medium, showing synthesis of new epithelial hair fiber and root sheath, but not the CTS (Fig. 2C). Most (∼70%) increased in length for 9 d (Figs. 2C and 3A), maintaining their anagen morphology (Figs. 2C and 3B). There was a gradual increase in the number showing catagen-like changes in the hair bulb with the hair fiber moving upward, losing contact with the regulatory mesenchyme-derived dermal papilla, which became rounded into a ball of cells (Fig. 2B).
Figure 3.
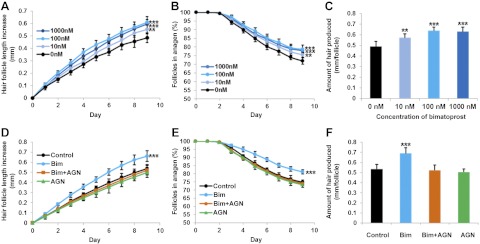
Bimatoprost stimulated scalp hair follicle growth in organ culture, and a prostamide F2α receptor antagonist blocked this stimulation. Anagen follicles were assessed daily for morphological changes and measured, while cultured with either vehicle alone (control), or bimatoprost (10, 100, and 1000 nM) with or without a specific antagonist. Values are means ± se of 5 individuals for each experiment; ≥6 follicles/person were examined for each condition. In control medium, hair follicles increased in length (A, D); the number of follicles remaining in anagen gradually declined (B, E). Bimatoprost increased scalp follicle growth rate at 10 nM (P<0.01) and 100 and 1000 nM (P<0.001) (A), number of anagen follicles at 10 nM (P<0.01) and 100 and 1000 nM (P<0.001) (B), and the total amount of hair produced at 10 nM (P<0.01) and 100 and 1000 nM (P<0.001) (C). The FP receptor antagonist AGN 211336 at 1 μM abolished the stimulatory effects of 100 nM bimatoprost on all parameters of scalp follicle growth in organ culture (P<0.01; D–F). *P < 0.05, **P < 0.01, ***P < 0.001 vs. control.
All three concentrations of bimatoprost stimulated human scalp hair follicles to grow faster in organ culture (Fig. 3A); 10 nM promoted growth significantly by ∼15% (P<0.01), while 100 and 1000 nM had a greater effect of ∼25% (P<0.001). They also prolonged anagen on d 9 by ∼7% with 10 nM bimatoprost (P<0.01) and ∼10% with 100 and 1000 nM (P<0.001) (Fig. 3B). Bimatoprost also stimulated the overall amount of hair synthesized in organ culture (Figs. 2C and 3C); this increased by ∼20% from 0.482 ± 0.021 mm/follicle (means±se) in control medium to 0.575 ± 0.023 mm/follicle with 10 nM (P<0.01), by ∼35% to 0.648 ± 0.024 mm/follicle with 100 nM (P<0.001) and by ∼33% to 0.639 ± 0.024 mm/follicle with 1000 nM (P<0.001).
Topical bimatoprost promoted new hair follicle growth in mice in vivo
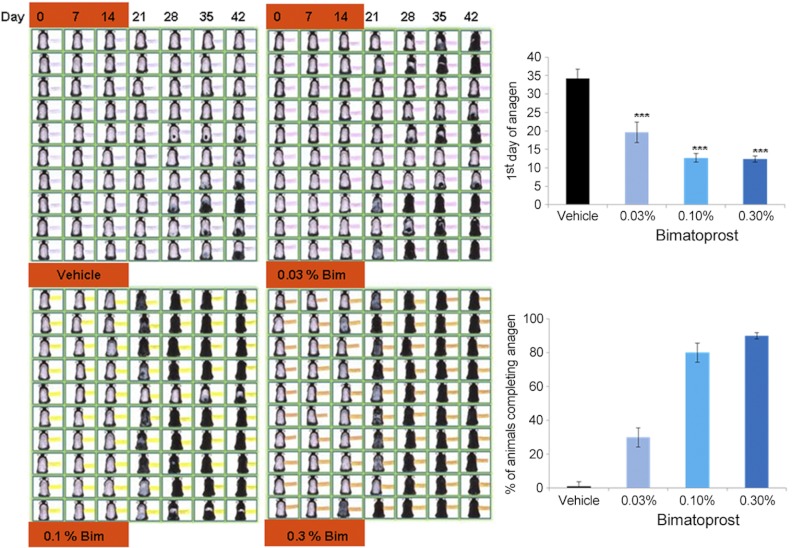
To establish whether bimatoprost could also stimulate non-eyelash follicles in vivo, particularly when supplied through the skin, the effects of topical bimatoprost on rodent pelage hair growth were assessed. After shaving, the dorsal skin of all mice remained pink, confirming that the hair follicles were in telogen (Fig. 4; d 0). Control animals receiving only the vehicle showed few skin changes until about d 28; 2 mice never showed any. In contrast, bimatoprost application for 14 d significantly (P<0.001) advanced the next hair cycle at all concentrations, with new hair growth clearly visible on d 14 in some mice given both higher amounts. The first sign of anagen occurred at 38.0 ± 4.23 d (mean±se) in control animals, while 0.03% bimatoprost brought this forward to 17.0 ± 0.85 d, and 0.10 and 0.3% even earlier, to 12.7 ± 1.16 and 12.4 ± 0.83 d, respectively (Fig. 4). This advancement of anagen extended across the dorsal area, despite treatment stopping after 14 d. Although no control mice had started anagen in all their dorsal follicles after 6 wk, 30% of the mice given the smallest amount of bimatoprost clearly exhibited anagen over the whole area, and this increased to 80 and 90% with the higher quantities. Overall, although the lowest dose of bimatoprost had a lesser effect, there was little difference between the two higher amounts.
Figure 4.
Topical application of bimatoprost promoted hair growth in mice in vivo. Bimatoprost applied topically to female mice for 2 wk stimulated resting (telogen) follicles to start to grow i.e., enter the next hair cycle (anagen). Pink skin visible in d 0 photographs confirms telogen status. Growing hairs are initially seen as darkening skin until black hair projects outside the skin (as seen in sequential photographs of individual animals). Bimatoprost (Bim) at 0.03, 0.10, and 0.30% significantly (P<0.001) advanced the first day at which anagen was visible, compared to the vehicle alone (top graph) and increased the percentage of animals in which all follicles on the back had grown at d 42 (bottom graph). n = 10/treatment. ***p < 0.001 vs. control.
Prostamide receptor antagonist, AGN 211336, blocked bimatoprost stimulation of scalp hair follicle growth
To determine whether the bimatoprost stimulation was a receptor-mediated response, the effect of the prostamide receptor antagonist, AGN 211336, on bimatoprost-stimulated hair follicle growth was investigated using follicles from 5 further people. The antagonist at 1 μM abolished the stimulatory effects of 100 nM bimatoprost on all parameters of scalp hair follicle growth in organ culture (P<0.01): the hair growth rate (Fig. 3D), the percentage of follicles in anagen (Fig. 3E), and the amount of hair synthesized (Figs. 2C and 3F). All remained at control levels.
Scalp hair follicles express the genes for prostanoid receptors in vivo
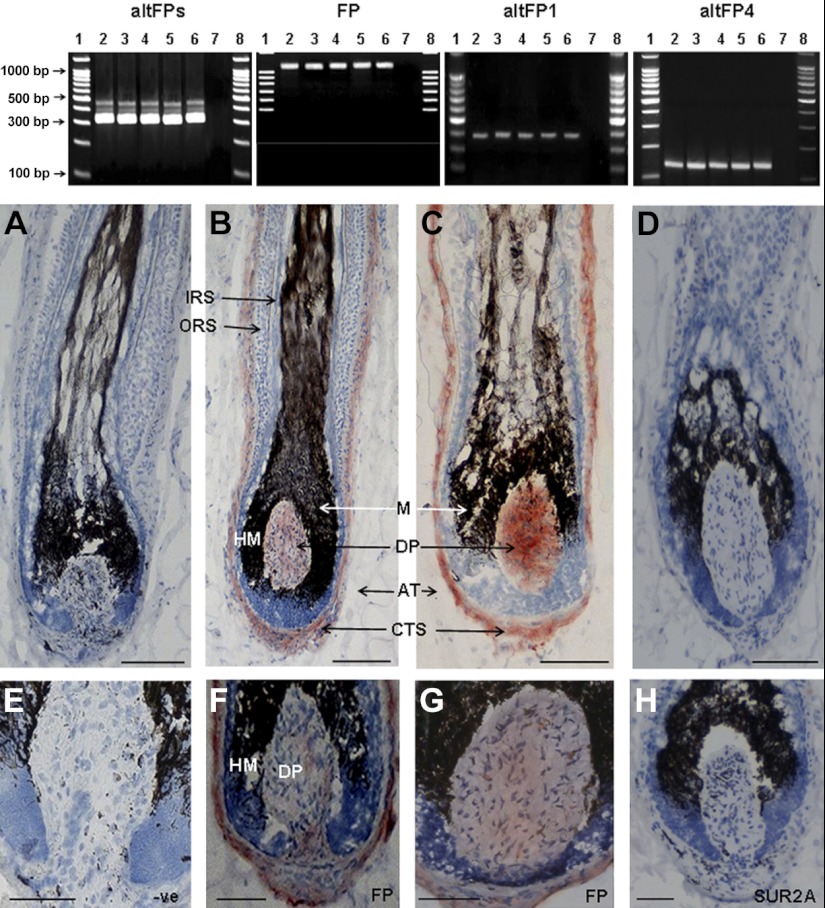
To determine whether human scalp hair follicles actually contain appropriate receptors in vivo to allow them to respond to bimatoprost, the expression of prostanoid receptor genes in scalp follicles was investigated by RT-PCR using skin samples collected and transported in a RNA stabilization reagent. Total and poly(A+)RNA were successfully extracted from the lower follicles of individually microdissected anagen scalp follicles from a further 5 people, and the quality of each individual's cDNA was confirmed by PCR using primers for β-actin, a highly expressed cytoskeletal protein (data not shown). When the expression of prostanoid receptor transcripts was examined using a primer pair which detected the PGF2α receptor, FP, and all the known prostamide receptor splice variants of FP (30), 3 bands were seen (Fig. 5). Their sizes suggested native FP, altFP4, and small amounts of another, possibly altFP1. Further RT-PCR reactions, using 6 sets of specific primers for FP and 5 splice variants, readily identified FP, altFP1, and altFP4 genes in scalp hair follicle cDNA from 5 individuals (Fig. 5); sequence analysis verified these genes against the relevant human sequence in GenBank. FP variants altFP2, altFP3, and altFP5 were not detected (data not shown).
Figure 5.
Human scalp anagen hair follicles expressed the genes and protein for three prostanoid receptors. Top panel: agarose gel electrophoresis of PCR products (30 μl) of hair follicle cDNAs from isolated scalp hair follicles from each of 5 individuals showed bands corresponding to 3 prostanoid receptors, with a primer pair for native FP and all known altFPs. These 3 bands correspond to the native FP receptor (∼321 bp) and 2 larger altFPs that include the same parts of the native FP sequence plus the relevant intron sequences. Specific primers for FP (1080 bp), altFP1 (392 bp), and altFP4 (141 bp) gave appropriately sized bands. AltFP2, altFP3, and altFP5 were not detected (data not shown). Lanes 1 and 8, DNA ladder (100-1500 bp); lanes 2–6, PCR products from 5 follicle cDNAs; lane 7, negative control without cDNA. Bottom panels: immunohistochemical analysis of scalp cryosections localized FP protein in the hair bulb in the dermal papilla and CTS with two primary antibodies for human FP (B, C, F, G). No staining occurred with omission of the primary antibody (A, E) nor with the nonrelevant antibody (D, H). Normal dark pigment (melanin) is visible in the hair bulb. DP, dermal papilla; CTS, connective tissue sheath; HM, hair matrix. Red, positive staining; blue, hematoxylin counterstain. Scale bars = 150 μm (A–D); 100 μm (E–H).
Location of prostanoid receptors in scalp hair follicles
Immunohistochemistry of frozen scalp skin using two different antibodies to FP showed strong staining in the dermal papillae and the CTS surrounding the hair bulb, but not in the epithelial keratinocytes or the melanocytes of the hair bulb (Fig. 5). The expression of relevant prostanoid receptor genes was also examined by RT-PCR in isolated components of 3 individuals' hair follicles (Fig. 6A). RT-PCR with β-actin primers confirmed that each component cDNA was of appropriate quality and quantity (Fig. 6B). When the primer pair for native FP and all splice variants of FP was used, only the dermal papillae and CTS samples exhibited any bands; each tissue produced 3 bands (Fig. 6B), resembling those seen in whole follicles (Fig. 5). Further analyses in each component from each individual using the specific primer sets also detected only FP and splice variants altFP1 and altFP4 in dermal papilla and CTS samples (verified by sequence analysis). No altFP2, altFP3, or altFP5 expression was detected in any sample (Table 1).
Table 1.
Expression of native FP and two splice variants was restricted to two components of scalp hair follicles
| Primers | Dermal papilla | Hair matrix | Bulb CTS | Lower follicle | Bulge region |
|---|---|---|---|---|---|
| All altFPs | ✓✓✓ | ××× | ✓✓✓ | ××× | ××× |
| AltFP1 | ✓✓✓ | ××× | ✓✓✓ | ××× | ××× |
| AltFP2 | ××× | ××× | ××× | ××× | ××× |
| AltFP3 | ××× | ××× | ××× | ××× | ××× |
| AltFP4 | ✓✓✓ | ××× | ✓✓✓ | ××× | ××× |
| AltFP5 | ××× | ××× | ××× | ××× | ××× |
| FP | ✓✓✓ | ××× | ✓✓✓ | ××× | ××× |
See Fig. 6.
DISCUSSION
The difference between producing a long or short human hair is primarily due to alterations in the length of anagen, the growing part of the hair growth cycle, rather than the rate of hair growth, which is relatively constant in vivo. Anagen varies from several years in large terminal scalp follicles forming long hairs, to only a few weeks for short finger hairs (35). During the androgenetic alopecia balding process, scalp follicle anagen is substantially reduced, and follicles spend much longer in the resting phase, telogen (36). Human hair follicle organ culture was used here to assess whether bimatoprost, a prostamide F2α analog, which stimulates eyelash growth as a side effect of glaucoma eyedrop treatment or when used as a topical treatment for eyelash hypotrichosis, can also act on scalp hair follicles. The method, based on Philpott et al. (37), was extended to include daily observation of follicle morphology and length measurement, modifications that reveal more detail. These allow assessment of any effects on anagen length and provide a more precise rate of growth, since the final hair length can be related to the number of days that the follicle was actually growing. They also enable a more accurate measurement of these increases, since false “growth” caused by upward movement of hairs when follicles enter the catagen-like stage is avoided. The overall alteration in hair length over the experimental period incorporates both the effects on anagen and growth rate.
Bimatoprost caused individual isolated scalp hair follicles from 10 different people to stay in anagen longer in organ culture, and about one-third more new hair was synthesized over 9 d with 100 and 1000 nM (Fig. 3). The effect was dose responsive, although raising the concentration to 1000 nM caused no further increases; this would concur with the saturation of a receptor-mediated effect, as reported in other tissues (19, 38, 39). Notably, these results demonstrate that bimatoprost can stimulate growth in human scalp hair follicles, as well as specialized eyelash follicles.
Bimatoprost eyedrops also stimulate mouse eyelash growth in vivo (40). Here, its promotion of early anagen in mouse dorsal skin follicles after topical application (Fig. 4) confirms that the bimatoprost response is also not restricted to the specialized eyelash follicles in vivo. This initiation of anagen is a significant aspect of the action from the clinical perspective because of the increased time androgenetic alopecia hair follicles spend in telogen (36) and the need to prompt early entry into anagen to reinitiate hair growth in patients after chemotherapy (41). Minoxidil is also reported to cause this effect (15). The ability to stimulate pelage hair growth by topical application in vivo is also an important asset. Although there are differences between human and mouse skin that prevent direct comparison, topical application is the most appropriate method for alopecia treatments, as the required areas can be targeted, reducing any potential side effects, such as increasing hair growth in inappropriate places, as occurred when minoxidil was initially given orally (15).
Because bimatoprost stimulated isolated scalp follicle growth in organ culture, where there is no possibility of involving alterations in blood supply, interactions with other skin tissues, or circulating cells, direct effects on follicles via specific receptors in hair follicle cells are the most likely method of action. The saturation of the effect at receptor-relevant concentrations (Fig. 3) and the ability of the potent prostamide antagonist, AGN 211336, with a pA2 of 7.6 against the prostamide receptor (20), to block the stimulatory effects of bimatoprost on both hair growth parameters in this dynamic bioassay (Fig. 3) also indicate a receptor-mediated response.
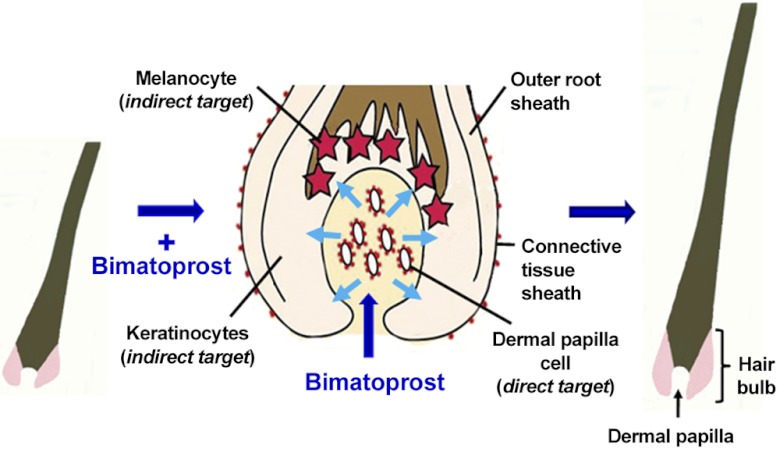
The presence of appropriate prostanoid receptors in human scalp hair follicles in vivo (Fig. 6 and Table 1), combined with the organ culture observations, strongly suggest that scalp follicles have the ability to respond with increased growth in vivo. The identification of genes for the known bimatoprost receptor, FP/altFP4 (30) in scalp hair follicles in vivo (Fig. 6 and Table 1), combined with the prostamide receptor antagonist's prevention of any effect (Fig. 3C), also indicate that bimatoprost acts directly on hair follicles via receptors within them. Studies in FP-deficient mice confirm FP gene expression is essential for bimatoprost effects on intraocular pressure in the eye (42). The location of FP protein (Fig. 5) and the gene expression of FP and splice variants altFP4 and altFP1 (Fig. 6 and Table 1) only in the mesenchyme-derived dermal papilla and CTS is particularly interesting. The dermal papilla determines the type of hair a follicle forms by producing paracrine signals to control other follicular functions (31, 32); the lower follicular CTS can replace this function (28). Reports of prostaglandin-metabolizing enzymes and FP in human scalp cultured dermal papilla cells (43, 44) support this localization in the dermal papilla. The absence of any relevant prostanoid receptors in the bulb keratinocytes, which form the hair or the melanocytes that produce the pigment and the bulge region, the site of epithelial (33) and melanocyte stem cells (34) strongly suggests that the dermal papilla is coordinating follicular responses of increased pigmentation and growth in anagen follicles. Both PGF2α and prostamide F2α bind to transmembrane Gαq protein-coupled receptors (Fig. 1), which trigger intracellular signaling, resulting in increased intracellular Ca2+ levels (30). Presumably, this will alter the gene expression of paracrine factors produced by the dermal papilla, which influence the activity of the bulb keratinocytes and melanocytes (see Fig. 7). This parallels the mechanism of androgen action in hair follicles (7, 25) and could include increasing production of stem cell factor (SCF), a pigment-stimulating factor reduced in androgenetic alopecia (45) and/or reducing that of TGF-β, an inhibitory factor associated with balding (46).
Figure 7.
Possible mechanism for the stimulation of hair growth by bimatoprost. Bimatoprost stimulates eyelash growth in vivo, human scalp hair growth in organ culture, and mouse pelage hair growth in vivo. In our hypothesis, these effects are due to bimatoprost binding to appropriate receptors on the plasma membrane of cells in the regulatory dermal papilla in the hair bulb (middle panel). This probably stimulates intracellular signaling pathways, which trigger alterations in the gene expression of paracrine signals and their extracellular release. Some of these factors would leave the dermal papilla, crossing the basement membrane, isolating it from the rest of the follicle, to stimulate the coordinated activity of the keratinocytes and melanocytes to produce increased hair growth and pigmentation. Red dots indicate FP and/or prostamide F2α receptors, blue arrows indicate direction of movement of paracrine factors.
Overall, these experiments demonstrate that the prostaglandin-related glaucoma drug bimatoprost can stimulate growth in non-eyelash hair follicles in mice in vivo and human scalp hair follicles in dynamic organ culture, i.e., mirroring the stimulation of eyelashes known to occur in vivo. These strong similarities in responses by eyelash and scalp follicles contrast with their differing physiology (23), biological responses to androgens (7), and aging pigmentary changes (23). The effects of bimatoprost and its antagonist in hair follicle organ culture suggest that the in vivo action is via direct effects on prostanoid receptors within the follicles themselves, rather than indirect effects involving the vasculature or other surrounding tissues. This is strongly supported by the gene expression of prostanoid receptors, FP, and its splicing variants, notably the altFP4 variant that forms a bimatoprost-sensitive activation site with FP (FP/altFP4; refs. 20, 30), in scalp hair follicles and isolated dermal papillae and CTS in vivo. These results indicate that prostamide receptors and prostamides may be part of a previously unrecognized signaling system within the follicle. Certainly, nonsteroidal anti-inflammatory drugs, such as ibuprofen, naproxen, and indomethacin, have been associated with hair loss for many years; these drugs inhibit the enzymes cyclooxygenase-1 and -2, which catalyze early steps in the synthesis of prostaglandins and prostamides (47, 48). Interestingly, the current main treatment for alopecia, minoxidil, increased prostaglandin synthesis in cultured dermal papilla cells (47). Since the development of new treatments for distressing hair growth disorders, such as alopecia and hirsutism, is hampered by our lack of understanding of hair follicle biology, the specific roles of prostaglandins and prostamides in hair follicles require further analysis. The hair follicle's regenerative capacity in adults, partially recapitulating embryonic development, with its unique ability to alter the next fully formed state in response to external signals (7) means this system may also have implications for developmental biology (49). Notably, these results demonstrate that bimatoprost offers a novel, and apparently low-risk, approach for treating scalp alopecias, which should reduce the significant problems associated with these distressing conditions.
Acknowledgments
The authors gratefully acknowledge a Research Scholarship from the Ministry of Higher Education and Scientific Research, Kurdistan, Iraq, to support K.G.K.
The authors also thank Allergan, Inc. (Irvine, CA, USA) for gifts of bimatoprost and AGN 211336. One of the authors of this article (V.A.R.) has informed The FASEB Journal that she has been asked to be an expert witness for Allergan and their coplaintiff Duke University in a legal matter. V.A.R. has acted as a consultant for Allergan, Inc., and has received unrestricted grants from Allergan, Inc., to support her research. E.S.T., J.W.W., and D.F.W. are Allergan employees.
Footnotes
- altFP
- prostaglandin F2α receptor splice variant
- CTS
- connective tissue sheath
- FP
- prostaglandin F2α receptor
- PBS
- phosphate buffered saline
- PGF2α
- prostaglandin F2α
- RT-PCR
- reverse transcription–polymerase chain reaction
REFERENCES
- 1. Hamilton J. B. (1951) Patterned loss of hair in man; types and incidence. Ann. N. Y. Acad. Sci. 53, 708–728 [DOI] [PubMed] [Google Scholar]
- 2. Randall V. A. (2001) Is alopecia areata an autoimmune disease? Lancet 358, 1922–1924 [DOI] [PubMed] [Google Scholar]
- 3. Girman C. J., Rhodes T., Lilly F. R., Guo S. S., Siervogel R. M., Patrick D. L., Chumlea W. C. (1998) Effects of self-perceived hair loss in a community sample of men. Dermatology 197, 223–229 [DOI] [PubMed] [Google Scholar]
- 4. Gulec A. T., Tanriverdi N., Duru C., Saray Y., Akcali C. (2004) The role of psychological factors in alopecia areata and the impact of the disease on the quality of life. Int. J. Dermatol. 43, 352–356 [DOI] [PubMed] [Google Scholar]
- 5. Cash T. F., Price V. H., Savin R. C. (1993) Psychological effects of androgenetic alopecia on women: comparisons with balding men and with female control subjects. J. Am. Acad. Dermatol. 29, 568–575 [DOI] [PubMed] [Google Scholar]
- 6. Jansen V. A., van Baalen M. (2006) Altruism through beard chromodynamics. Nature 440, 663–666 [DOI] [PubMed] [Google Scholar]
- 7. Randall V. A. (2007) Hormonal regulation of hair follicles exhibits a biological paradox. Semin. Cell Dev. Biol. 18, 274–285 [DOI] [PubMed] [Google Scholar]
- 8. Marshall W. A., Tanner J. M. (1969) Variations in pattern of pubertal change in girls. Arch. Dis. Child. 44, 291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marshall W. A., Tanner J. M. (1970) Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 45, 13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kligman A. G. (1959) The human hair cycle. J. Invest. Dermatol. 33, 307–316 [DOI] [PubMed] [Google Scholar]
- 11. Higgins C. A., Richardson G. D., Westgate G. E., Jahoda C. A. (2009) Exogen involves gradual release of the hair club fibre in the vibrissa follicle model. Exp. Dermatol. 18, 793–795 [DOI] [PubMed] [Google Scholar]
- 12. Garg S., Messenger A. G. (2009) Alopecia areata: evidence-based treatments. Semin. Cutan. Med. Surg. 28, 15–18 [DOI] [PubMed] [Google Scholar]
- 13. Rogers N. E., Avram M. R. (2008) Medical treatments for male and female pattern hair loss. J. Am. Acad. Dermatol. 59, 547–566; quiz 567–548 [DOI] [PubMed] [Google Scholar]
- 14. Shorter K., Farjo N. P., Picksley S. M., Randall V. A. (2008) Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil. FASEB J. 22, 1725–1736 [DOI] [PubMed] [Google Scholar]
- 15. Messenger A. G., Rundegren J. (2004) Minoxidil: mechanisms of action on hair growth. Br. J. Dermatol. 150, 186–194 [DOI] [PubMed] [Google Scholar]
- 16. Kaufman K. D., Olsen E. A., Whiting D., Savin R., DeVillez R., Bergfeld W., Price V. H., Van Neste D., Roberts J. L., Hordinsky M., Shapiro J., Binkowitz B., Gormley G. J. (1998) Finasteride in the treatment of men with androgenetic alopecia. Finasteride male pattern hair loss study group. J. Am. Acad. Dermatol. 39, 578–589 [DOI] [PubMed] [Google Scholar]
- 17. Whiting D. A., Olsen E. A., Savin R., Halper L., Rodgers A., Wang L., Hustad C., Palmisano J. (2003) Efficacy and tolerability of finasteride 1 mg in men aged 41 to 60 years with male pattern hair loss. Eur. J. Dermatol. 13, 150–160 [PubMed] [Google Scholar]
- 18. Curran M. P. (2009) Bimatoprost: a review of its use in open-angle glaucoma and ocular hypertension. Drugs Aging 26, 1049–1071 [DOI] [PubMed] [Google Scholar]
- 19. Woodward D. F., Carling R. W., Cornell C. L., Fliri H. G., Martos J. L., Pettit S. N., Liang Y., Wang J. W. (2008) The pharmacology and therapeutic relevance of endocannabinoid derived cyclo-oxygenase (COX)-2 products. Pharmacol. Ther. 120, 71–80 [DOI] [PubMed] [Google Scholar]
- 20. Woodward D. F., Liang Y., Wang J. W., Li C., Guzman V. M., Kharlamb A. B., Wheeler L. A., Garst M. E., Carling R. W., Cornell C. L., Fliri H. G., Martos J. L., Pettit S. N., Hopper D. L., DiMarzo V., Ortar G. (2011) Pharmacological differentiation of bimatoprost and latanoprost induced ocular hypotension by a second generation prostamide antagonist (AGN 211336). In Prostaglandins: Biochemistry, Functions, Types and Roles (Goodwin G. M., ed.) pp. 65–80, Nova Science Publishers, New York [Google Scholar]
- 21. McCarey B. E., Kapik B. M., Kane F. E. (2004) Low incidence of iris pigmentation and eyelash changes in 2 randomized clinical trials with unoprostone isopropyl 0.15%. Ophthalmology 111, 1480–1488 [DOI] [PubMed] [Google Scholar]
- 22. Lee P. Y., Shao H., Xu L. A., Qu C. K. (1988) The effect of prostaglandin F2 alpha on intraocular pressure in normotensive human subjects. Invest. Ophthalmol. Vis. Sci. 29, 1474–1477 [PubMed] [Google Scholar]
- 23. Thibaut S., De Becker E., Caisey L., Baras D., Karatas S., Jammayrac O., Pisella P. J., Bernard B. A. (2010) Human eyelash characterization. Br. J. Dermatol. 162, 304–310 [DOI] [PubMed] [Google Scholar]
- 24. Keogh E. V., Walsh R. J. (1965) Rate of greying of human hair. Nature 207, 877–878 [DOI] [PubMed] [Google Scholar]
- 25. Randall V. A. (2008) Androgens and hair growth. Dermatol. Ther. 21, 314–328 [DOI] [PubMed] [Google Scholar]
- 26. Plikus M. V., Chuong C. M. (2008) Complex hair cycle domain patterns and regenerative hair waves in living rodents. J. Invest. Dermatol. 128, 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rendl M., Lewis L., Fuchs E. (2005) Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 3, e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reynolds A. J., Lawrence C., Cserhalmi-Friedman P. B., Christiano A. M., Jahoda C. A. (1999) Trans-gender induction of hair follicles. Nature 402, 33–34 [DOI] [PubMed] [Google Scholar]
- 29. Plikus M. V., Baker R. E., Chen C. C., Fare C., de la Cruz D., Andl T., Maini P. K., Millar S. E., Widelitz R., Chuong C. M. (2011) Self-organizing and stochastic behaviors during the regeneration of hair stem cells. Science 332, 586–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liang Y., Woodward D. F., Guzman V. M., Li C., Scott D. F., Wang J. W., Wheeler L. A., Garst M. E., Landsverk K., Sachs G., Krauss A. H., Cornell C., Martos J., Pettit S., Fliri H. (2008) Identification and pharmacological characterization of the prostaglandin FP receptor and FP receptor variant complexes. Br. J. Pharmacol. 154, 1079–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jahoda C. A., Horne K. A., Oliver R. F. (1984) Induction of hair growth by implantation of cultured dermal papilla cells. Nature 311, 560–562 [DOI] [PubMed] [Google Scholar]
- 32. Jahoda C. A. (1992) Induction of follicle formation and hair growth by vibrissa dermal papillae implanted into rat ear wounds: vibrissa-type fibres are specified. Development 115, 1103–1109 [DOI] [PubMed] [Google Scholar]
- 33. Sotiropoulou P. A., Candi A., Mascre G., De Clercq S., Youssef K. K., Lapouge G., Dahl E., Semeraro C., Denecker G., Marine J. C., Blanpain C. (2010) Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat. Cell Biol. 12, 572–582 [DOI] [PubMed] [Google Scholar]
- 34. Nishimura E. K., Granter S. R., Fisher D. E. (2005) Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science 307, 720–724 [DOI] [PubMed] [Google Scholar]
- 35. Saitoh M., Uzuka M., Sakamoto M. (1970) Human hair cycle. J. Invest. Dermatol. 54, 65–81 [DOI] [PubMed] [Google Scholar]
- 36. Whiting D. A. (1993) Diagnostic and predictive value of horizontal sections of scalp biopsy specimens in male pattern androgenetic alopecia. J. Am. Acad. Dermatol. 28, 755–763 [DOI] [PubMed] [Google Scholar]
- 37. Philpott M. P., Green M. R., Kealey T. (1990) Human hair growth in vitro. J. Cell Sci. 97, 463–471 [DOI] [PubMed] [Google Scholar]
- 38. Liang Y., Li C., Guzman V. M., Evinger A. J., 3rd, Protzman C. E., Krauss A. H., Woodward D. F. (2003) Comparison of prostaglandin F2α, bimatoprost (prostamide), and butaprost (EP2 agonist) on Cyr61 and connective tissue growth factor gene expression. J. Biol. Chem. 278, 27267–27277 [DOI] [PubMed] [Google Scholar]
- 39. Woodward D. F., Krauss A. H., Chen J., Liang Y., Li C., Protzman C. E., Bogardus A., Chen R., Kedzie K. M., Krauss H. A., Gil D. W., Kharlamb A., Wheeler L. A., Babusis D., Welty D., Tang-Liu D. D., Cherukury M., Andrews S. W., Burk R. M., Garst M. E. (2003) Pharmacological characterization of a novel antiglaucoma agent, Bimatoprost (AGN 192024). J. Pharmacol. Exp. Ther. 305, 772–785 [DOI] [PubMed] [Google Scholar]
- 40. Tauchi M., Fuchs T. A., Kellenberger A. J., Woodward D. F., Paus R., Lutjen-Drecoll E. (2010) Characterization of an in vivo model for the study of eyelash biology and trichomegaly: mouse eyelash morphology, development, growth cycle, and anagen prolongation by bimatoprost. Br. J. Dermatol. 162, 1186–1197 [DOI] [PubMed] [Google Scholar]
- 41. Trüeb R. M. (2010) Chemotherapy-induced hair loss. Skin Ther. Lett. 15, 5–7 [PubMed] [Google Scholar]
- 42. Ota T., Aihara M., Narumiya S., Araie M. (2005) The effects of prostaglandin analogues on IOP in prostanoid FP-receptor-deficient mice. Invest. Ophthalmol. Vis. Sci. 46, 4159–4163 [DOI] [PubMed] [Google Scholar]
- 43. Colombe L., Michelet J. F., Bernard B. A. (2008) Prostanoid receptors in anagen human hair follicles. Exp. Dermatol. 17, 63–72 [DOI] [PubMed] [Google Scholar]
- 44. Colombe L., Vindrios A., Michelet J. F., Bernard B. A. (2007) Prostaglandin metabolism in human hair follicle. Exp. Dermatol. 16, 762–769 [DOI] [PubMed] [Google Scholar]
- 45. Randall V. A., Jenner T., Hibberts N., De Oliveira I., Vafaee T. (2008) Stem cell factor/c-kit signalling in normal and androgenetic alopecia hair follicles. J. Endocrinol. 197, 1–14 [DOI] [PubMed] [Google Scholar]
- 46. Inui S., Fukuzato Y., Nakajima T., Yoshikawa K., Itami S. (2003) Identification of androgen-inducible TGF-β1 derived from dermal papilla cells as a key mediator in androgenetic alopecia. J. Investig. Dermatol. Symp. Proc. 8, 69–71 [DOI] [PubMed] [Google Scholar]
- 47. Michelet J. F., Commo S., Billoni N., Mahe Y. F., Bernard B. A. (1997) Activation of cytoprotective prostaglandin synthase-1 by minoxidil as a possible explanation for its hair growth-stimulating effect. J. Invest. Dermatol. 108, 205–209 [DOI] [PubMed] [Google Scholar]
- 48. Piraccini B. M., Iorizzo M., Rech G., Tosti A. (2006) Drug-induced hair disorders. Curr. Drug Safety 1, 301–305 [DOI] [PubMed] [Google Scholar]
- 49. Chuong C. M., Randall V. A., Widelitz R. B., Wu P., Jiang T-X. (2012) Physiological regeneration of skin appendages and implication for regenerative medicine. Physiology 27, 61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]