Abstract
Antithyroid medications are the preferred therapy for the treatment of Graves' disease during pregnancy. Propylthiouracil (PTU) is favored over methimazole (MMI) due to potential teratogenic concerns with MMI. This study was to determine the teratogenic potential of MMI and PTU using a validated Xenopus tropicalis embryo model. Embryos were exposed to 1 mM PTU (EC50=0.88 mM), 1 mM MMI, or vehicle control (water) from stages 2 to 45. Treated embryos were examined for gross morphological defects, ciliary function, and gene expression by in situ hybridization. Exposure to PTU, but not MMI, led to cardiac and gut looping defects and shortening along the anterior-posterior axis. PTU exposure during gastrulation (stage 8–12.5) was identified as the critical period of exposure leading to left-right (LR) patterning defects. Abnormal cilia polarization, abnormal cilia-driven leftward flow at the gastrocoel roof plate (GRP), and aberrant expression of both Coco and Pitx2c were associated with abnormal LR symmetry observed following PTU exposure. PTU is teratogenic during late blastula, gastrulation, and neurulation; whereas MMI is not. PTU alters ciliary-driven flow and disrupts the normal genetic program involved in LR axis determination. These studies have important implications for women taking PTU during early pregnancy.—Van Veenendaal, N.R., Ulmer, B., Boskovski, M.T., Fang, X., Khokha, M.K., Wendler, C.C., Blum, M., Rivkees, S.A. Embryonic exposure to propylthiouracil disrupts left-right patterning in Xenopus embryos.
Keywords: thyroid, Graves' disease, methimazole, situs, teratogenicity
Graves' disease (gd) is the most common cause of hyperthyroidism during pregnancy (1–3). Because of potential risks of radioactive iodine or surgery to the fetus, the use of antithyroid drugs (ATDs) is the recommended therapy during gestation (1, 2, 4–10). Of the two antithyroid drugs, 6-propyl-2-thiouracil [propylthiouracil (PTU)] or methimazole (MMI), PTU has long been preferred during pregnancy, a notion affirmed in 2007 by a consensus conference (11). This recommendation is based on a handful of reports of birth defects associated with MMI use and relatively scant reports of teratogenic effects of PTU (11); however, it is not clear whether the birth defects associated with MMI are due to the medication or the hyperthyroid state (11).
Despite widespread ATD use in pregnancy, formal studies of ATD teratogenic effects have not been performed (12). Some investigation of ATDs has been conducted in animal models on embryos, including studies of E9.5 to E11.5 rat embryos cultured with MMI, which revealed abnormal head morphology and defects in neural tube closure in some specimens (13). Treatment of rabbit and guinea pig fetuses with PTU led to thyroid enlargement but no congenital anomalies (14). Treatment of rats, mice, and rabbits after embryogenesis with ATDs did not cause congenital anomalies (15–17). In the most formal and comprehensive studies to date, PTU was recently found to be associated with delayed neural tube closure and cardiac problems, whereas no teratogenic effects of MMI were seen (18).
Supporting the notion that ATDs may alter normal left-right (LR) axis development, an association between PTU use during pregnancy and outflow tract defects, situs inversus, and dextrocardia in the offspring was observed (19). In contrast, MMI was found to be associated with choanal atresia and omphalocele (19). To further examine the teratogenic potential of PTU and MMI, we investigated potential teratogenicity during embryogenesis of the frog Xenopus tropicalis. The frog embryo is an excellent model for teratogenic studies, since embryos can be treated, independent of maternal effects, and studies can be performed at very early stages of development that are difficult to examine in mammals (20). As a tetrapod, much of the basic biology that establishes the body axis and organ primordia are conserved among mammals and frogs, as well (21).
In Xenopus, organ situs is established by cilia-driven leftward flow of extracellular fluid at the gastrocoel roof plate (GRP; ref. 21). The GRP is homologous to the posterior notochord/node region in mammals (22). Cilia-driven leftward flow induces asymmetric expression of genes across the LR axis. In the developing vertebrate embryo, the earliest known asymmetrically expressed gene, coco (dand5) is an extracellular nodal inhibitor that is an immediate molecular readout of cilia-driven leftward flow at the GRP (23). Subsequently Pitx2, the homeobox transcription factor, is expressed asymmetrically in the lateral plate mesoderm (LPM) and is required for normal situs development in fish, amphibians, and mammals. Using Xenopus embryos, we now report that PTU treatment affects embryogenesis, by altering LR axis development. We also show that cilia-driven leftward flow is diminished, and there is in aberrant expression of Coco and Pitx2c.
MATERIALS AND METHODS
Animals
Studies with Xenopus tropicalis were approved by the Yale University Animal Use and Care Committee. Mature West African clawed frog (X. tropicalis, inbred F12 generation) pairs were obtained from the animal research facility at Yale University and maintained in a recirculating system at 25 ± 1°C with 12-h light-dark cycle. Natural fertilization was induced as described previously (24). X. laevis work was also performed according to Sive et al. (25) and to the regulatory standards for experimental animal work as determined by the legal authorities in Germany.
Embryo treatments
Fertilized oocytes were washed in 3% cysteine (pH 7.9) for 2 min to partially remove the jelly coat. Embryos were collected and staged according to Nieuwkoop and Faber (26).
Experiments were conducted in 1/9× modified Ringers (MR) solution (20 mM NaCl, 0.4 mM KCl, 0.2 mM MgCl2, 0.4 mM CaCl2, and 1 mM HEPES, pH 7.2) supplemented with 10 mg/ml of gentamicin. Working solutions of PTU (Sigma-Aldrich, St. Louis, MO, USA) and MMI (Sigma-Aldrich) were prepared in 1/9× MR solution.
Embryos were cultured in 100-mm Petri dishes at 28°C, and the media and drugs were changed every 24 h. Each treatment group was tested 3 separate times using ≥30 randomly selected embryos per well. Embryos were treated with different compounds, different concentrations, and for different time periods, as detailed below. Embryos were evaluated at Nieuwkoop and Faber stage (st.) 45 for organ situs and length.
Embryos were paralyzed with benzocaine to facilitate scoring. Malformations were scored using standard criteria as described previously (20). Heart orientation was scored as right (D-looped), left (L-looped), or anterior (A-looped) (27). Head-to-tail length was determined using Image J (28) software to analyze photographs obtained using an Olympus C-5060 camera (Olympus, Tokyo, Japan) attached to a a Zeiss Stemi:2000C (Carl Zeiss, Oberkochen, Germany) dissecting microscope. An investigator who was unaware of treatment conditions performed all scoring.
Immunocytochemistry and in situ hybridization
Dorsal explants of embryos were prepared for studies of the GRP as reported previously (29). Immunohistochemistry and in situ hybridization (ISH) were performed as described previously (30, 31) with mouse anti-acetylated α-tubulin (Sigma-Aldrich) 1:250 and Cy3 anti-mouse IgG F(ab′)2 fragment (Sigma-Aldrich) 1:100. Actin cytoskeleton was visualized with Alexa Fluor 488 phalloidin (Life Technologies Corp., Carlsbad, CA, USA) according to the manufacturer's instructions. Pitx2c (32), Brachyury (33), Goosecoid (Gsc; ref. 34) Xnr3 (35), Foxj1 (36), Tektin2 (36), and Coco ISH, as well as Coco morpholino (23), were used as described by earlier research.
Flow analysis
Cilia-driven leftward flow was analyzed in dorsal explants by adding fluorescent beads, as described previously (29, 37). Time-lapse movies were processed to eliminate brownian movement of beads. Bead tracks were transformed into gradient time trails (GTTs) representing trajectories of 25 s each, which were color-coded to reveal directionality and velocity. The qualitative parameter ρ, which represents directionality (with a value of 1 in cases when all beads move in the same direction, and 0 when dislocation is random), was calculated as described previously (37).
Statistical analysis
Prism v.5.0 (GraphPad, La Jolla, CA, USA) was used for statistical analysis. Comparisons among groups were made by 2-way ANOVA. Comparisons among the different treatment conditions were made by the Bonferroni posttest analysis. One-way ANOVA was used to compare mean percentages of different treatment groups.
Concentration-response studies were carried out in triplicate. The EC50 of a specific defect was calculated using the background level of the defect in controls (%) as bottom/minimum exposure level. The maximum response was defined as the highest percentage defects of 1 mM PTU-treated embryos.
RESULTS
PTU induces heart and gut defects and impairs anterior-posterior development (tail elongation)
Concentration-response studies were performed using drugs from 1 nM to 10 mM. The concentration that killed 50% of embryos (EmLC50) for MMI was 8.70 ± 1.494 mM (n=463) and was 1.66 ± 2.025 mM for PTU (n=1269). PTU or MMI concentrations ≤ 1 mM did not adversely affect embryo viability (vehicle 98% viable, n=218; 1 mM MMI 97% viable, n=205; PTU 96% viable, n=164).
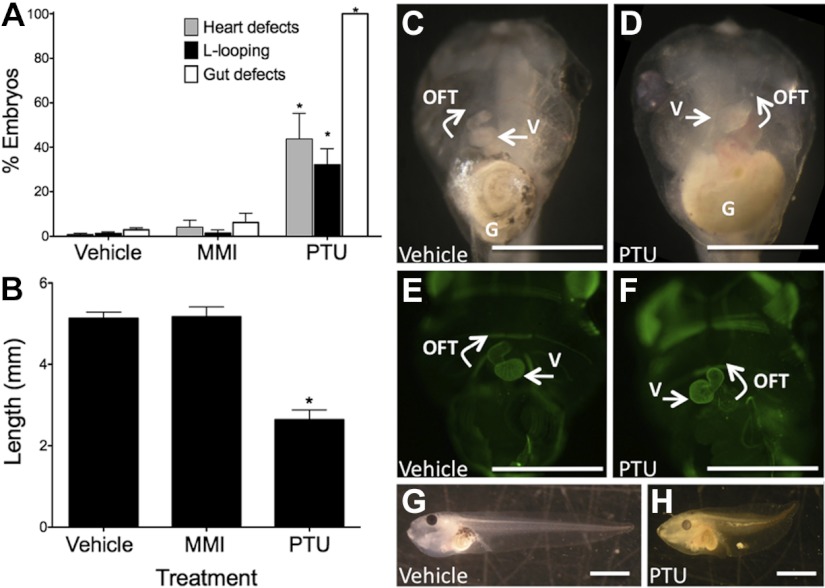
Based on dose-response studies (Supplemental Fig. S1) we treated embryos with 1 mM PTU, 1 mM MMI, or vehicle over st. 8–45 to assess the teratogenic potential of the drugs. MMI treatment resulted in the same malformation rate as vehicle. However, PTU exposure caused heart defects, gut defects, shortened body length, and an increase in the number of embryos that were stunted at st. 45. Stunted larvae did not develop far enough to score for cardiac-, gut-, and length defects; no beating heart could be visualized. PTU induced an array of cardiac looping defects. Only 56.3% of specimens exhibited normal D-looping, 24% were L-looped, and 19.7% of the hearts could not be classified as either L- or D-looped (undefined; Table 1 and Fig. 1). Gut defects were seen in the PTU-treated embryos, but not in the MMI- or vehicle-treated embryos. Miscoiled gut loops were the primary defects seen and were observed in 88.9% of PTU-treated embryos. 11.1% of PTU-treated embryos had more severe malformations of the endoderm, with an uncoiled, linear gut (Table 1). In addition, PTU treatment resulted in edema formation in >20% of embryos (Table 1).
Table 1.
Characteristics of PTU- and MMI-treated embryos
| Characteristic | Vehicle | MMI (1 mM) | PTU (1 mM) |
|---|---|---|---|
| Mortality (%) | 2.2 | 3.1 | 3.8 |
| Stunted larvae (%) | 0.3 | 1.8 | 24.2* |
| Heart defects (%) | 0.7 | 3.9 | 43.7* |
| l-loop | 0.0 | 1.4 | 24.0* |
| Undefined | 0.7 | 2.5 | 19.7* |
| Gut defects (%) | 2.8 | 6.2 | 100* |
| Miscoiled | 2.8 | 4.8 | 88.9* |
| Uncoiled linear | 0.0 | 0.7 | 11.1* |
| Situs inversus | 0.0 | 0.7 | 0 |
| Pigmentation | Normal | Normal | Decreased |
| Edema (%) | |||
| Cardiac | 0.7 | 3.7 | 23.2 |
| Abdominal | 0.7 | 2.3 | 22.7 |
| Facial | 0.7 | 5.1 | 5.4 |
| Optic | 0.7 | 3.4 | 6.5 |
| Length (mm) | 5.28 ± 0.22 | 5.17 ± 0.52 | 2.64 ± 0.38* |
| Embryos scored (n) | 212 | 196 | 122 |
| Total embryos (n) | 218 | 205 | 164 |
| Experiments (n) | 3 | 3 | 3 |
Note that one embryo can have different malformations. All malformations were scored and counted individually. Length values are means ± sd.
P < 0.001; 1-way ANOVA.
Figure 1.
PTU induces embryonic malformations. Embryos were treated from st.8 to 45 with vehicle (n=212), 1 mM PTU (n=122), or 1 mM MMI (n=196). Each experiment was carried out in triplicate, and embryos were examined at st.45. A) PTU treatment led to several developmental defects in the embryo, including heart defects, L-looped hearts, and gut defects, whereas vehicle and MMI treatment had no adverse effects. B) PTU also inhibited tail elongation. C, E, G) Vehicle-treated embryos displayed normal heart looping to the right, counterclockwise gut coiling, and proper tail elongation. D, F, H) Treatment with PTU caused specific malformations, including L-looping of the heart, gut defect (miscoiling), and inhibition of tail elongation. E, F) PTU-treated embryos were immunostained with antibodies to troponin-I, a heart muscle marker, to visualize heart looping. G, gut; n, number of embryos scored; OFT, outflow tract; V, ventricle. *P < 0.001.
PTU impaired tail elongation, whereas MMI- or vehicle-treated embryos were normal (Table 1 and Fig. 1B). Embryos treated with PTU had abnormally formed tails that were wavy, short, and/or kinked compared to vehicle (Fig. 1G, H). Primarily due to the malformed tails, the lengths of PTU-treated embryos were ∼50% less than vehicle-treated embryos. However, we observed, in concordance with earlier research (38), that higher doses of MMI (10 mM) caused a wavy or crimped notochord and shortened axis. But, no laterality defects were found in this group of embryos.
St. 8-12.5 is the critical period for PTU-induced heart and gut defects
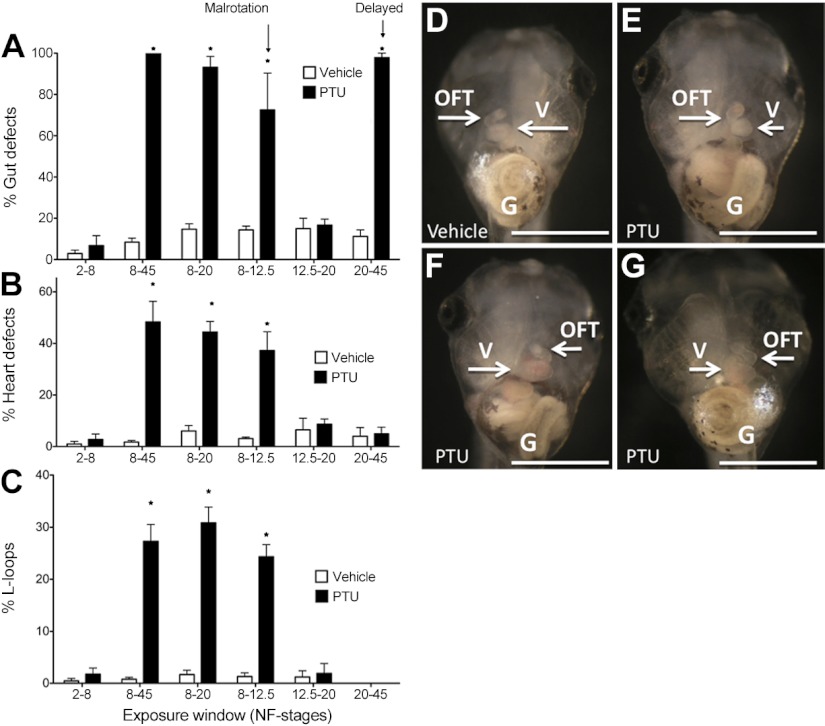
After discovering that PTU treatment causes morphological defects following exposure from st. 8-45, embryos were treated with PTU for shorter periods of time (st. 2-8, 8-12.5, 8-20, 20-45, or 8-45) and scored at st. 45. PTU (1 mM) induced equivalent amounts of heart defects in embryos treated from st. 8 to 45, 8 to 20, and 8 to 12.5, indicating that exposure to PTU between st. 8 and 12.5 was the critical window for inducing cardiac defects (Tables 2 and 3 and Fig. 2). When the gut was examined, we observed that PTU caused defects following exposure from st. 8 to 45, 8 to 20, 8 to 12.5, and 20 to 45, but not during st. 12.5 to 20 (Table 2 and Fig. 2). The most common gut defect was miscoiling (Table 2 and Fig. 2).
Table 2.
Embryo defects as related to stage of treatment
| Defect and treatment | Nieuwkoop-Faber embryonic stage |
||||
|---|---|---|---|---|---|
| 2–8 | 8–45 | 8–20 | 8–12.5 | 12.5–20 | |
| Heart (%) | |||||
| Control | 1.0 | 1.8 | 6.0 | 3.3 | 6.5 |
| PTU | 2.8 | 48.3 ** | 44.3 ** | 32.4 ** | 8.7 |
| L-looping (%) | |||||
| Control | 0.5 | 0.8 | 1.7 | 1.3 | 1.2 |
| PTU | 1.8 | 27.3** | 30.9** | 21.9** | 1.9 |
| Gut (%) | |||||
| Control | 2.9 | 7.1 | 17.4 | 14.3 | 15.0 |
| PTU | 6.8 | 100.0** | 92.0** | 72.5** | 16.7 |
| Miscoiling (%) | |||||
| Control | 2.5 | 6.4 | 15.6 | 14.3 | 10.7 |
| PTU | 3.8 | 96.3** | 89.8 ** | 65.1** | 16.0 |
| Length (mm) | |||||
| Control | 5.45 ± 0.13 | 5.40 ± 0.42 | 5.25 ± 0.04 | 5.20 ± 0.20 | 5.38 ± 0.14 |
| PTU | 5.44 ± 0.22 | 3.16 ± 0.22*** | 5.29 ± 0.29 | 5.20 ± 0.20 | 5.37 ± 0.12 |
| Embryos (n) | |||||
| Control | 170 | 385 | 151 | 228 | 98 |
| PTU | 164 | 268 | 141 | 239 | 139 |
| Experiments (n) | 4 | 7 | 4 | 3 | 3 |
PTU, 1 mM. Length values are means ± sd.
P < 0.0001, 2-way ANOVA;
P < 0.0001, paired t test.
Table 3.
Embryos exposed to treatment at different stages of development in Fig. 2 experiments
| Treatment | Exposure window (Nieuwkoop-Faber embryonic stage) |
|||||
|---|---|---|---|---|---|---|
| 2–8 | 8–45 | 8–20 | 8–12.5 | 12.5–20 | 20–45 | |
| Control (n) | 170 | 385 | 151 | 228 | 98 | 229 |
| PTU (n) | 164 | 268 | 141 | 239 | 139 | 162 |
| Experiments (n) | 4 | 7 | 4 | 3 | 3 | 5 |
Data apply to Fig. 2A–C.
Figure 2.
The critical window of vulnerability to PTU is from st. 8 to 12.5. Embryos were exposed to PTU (1 mM) or vehicle over different stages of development and scored at st. 45 for gut defects (A), heart defects (B), and L-looping (C); see Table 3. Tadpoles (st. 45) treated with PTU (1 mM) during st. 8–12.5 showed 3 types of abnormal phenotypes: gut miscoiling (E), L-looped heart and gut miscoiling (F), and complete situs inversus (G). Note that the vehicle picture was taken from a different angle to visualize the OFT and note that one embryo can have different malformations. All malformations were scored and counted individually. G, gut; OFT, outflow tract; V, ventricle. *P < 0.0001; 2-way ANOVA with Bonferroni posttest.
We also examined tadpole length. Length abnormalities were not seen when PTU (1 mM) was applied over st. 2-8, 8-12.5, 8-20, or 12.5-20. Rather, shortened length was identified in embryos exposed to PTU later in development over st. 8-45 or 20-45, with embryo lengths reduced by 50 or 42%, respectively, over these stages. These findings indicate that the sensitive period for PTU-induced length abnormalities was st. 20-45 (Table 2 and Supplemental Fig. S2).
Thyroid hormone fails to rescue PTU-induced LR defects
The Xenopus thyroid gland is present after st. 20 (38). To determine whether PTU-induced defects were potentially related to impaired thyroid hormone synthesis, different concentrations of triiodo-l-thyronine (T3; Sigma-Aldrich) were tested (1 nM to 10 mM) in the presence of PTU (1 mM). We observed that heart and gut defects were not prevented by the addition of T3 to the medium over st. 8–20 (Supplemental Fig. S3A–C). Tadpole length, however, seemed to be rescued on addition of T3 to the culture medium during st. 20–45, but this trend was not statistically significant (Supplemental Fig. S3D). Treatment with T3 alone did not result in defects over a broad range of concentrations (data not shown).
PTU alters asymmetric gene expression
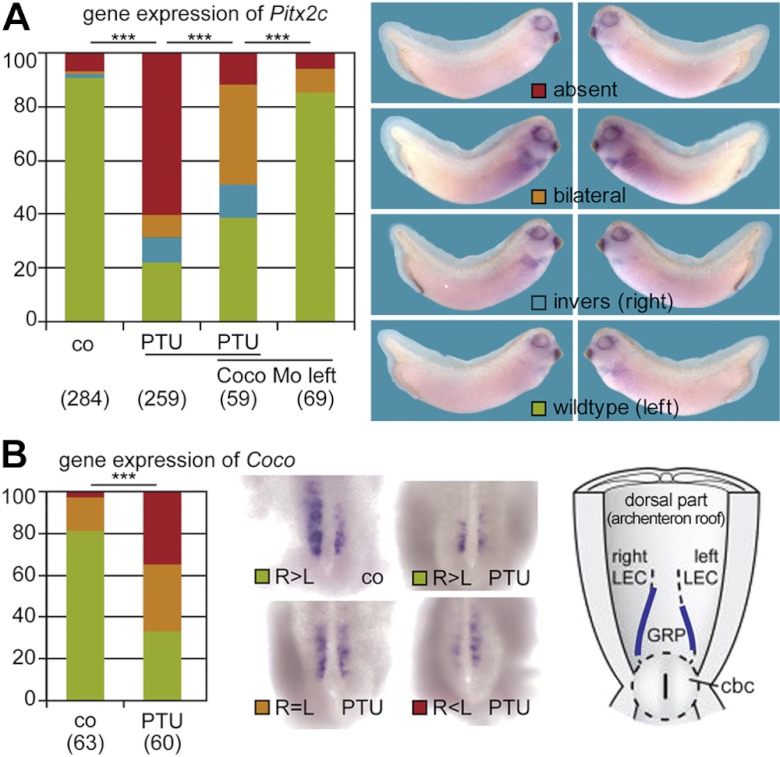
Because we observed defects in LR asymmetry with PTU, we assessed where in the LR asymmetry pathway PTU has its effects. To assess whether PTU affects Pitx2c asymmetry, embryos were exposed to PTU, and st. 29–31, tadpoles were fixed and processed for ISH. Figure 3A shows that Pitx2c expression was disturbed, with ∼70% of the specimens lacking left-asymmetric mRNA localization in the LPM.
Figure 3.
PTU impairs normal Pitx2c and Coco expression. A) PTU resulted in predominately absent Pitx2c expression in the left LPM. Note that parallel left-sided knockdown of Coco rescued left LPM expression of Pitx2c. PTU prevented left-sided down-regulation of Coco in postflow neurula stage embryos. Numbers in parentheses represent number of analyzed specimens, derived in each case from ≥3 independent experiments. B) ISH (st. 19) of dorsal explants for the early LR marker Coco. Embryos were treated with vehicle or PTU from st.8 to 19. Vehicle treatment resulted in normal expression of Coco (R>L). PTU treatment caused different expression of Coco. R > L, normal Coco expression; R = L, equal Coco expression; R < L, reversed Coco expression. ***P < 0.001; 1-way ANOVA.
Patterns of Coco expression in GRP dorsal explants were examined in embryos treated with PTU over st.8–20. Embryos exposed to PTU (1 mM) over st. 8–20 had significantly different patterns of Coco gene expression at st. 20 compared to vehicle-exposed embryos (Fig. 3B). R > L Coco expression was found in 81% of vehicle-treated embryos vs. 33% of PTU-treated embryos. Similar right and left (R=L) Coco expression was seen in 16% of vehicle-treated embryos vs. 32% of PTU-treated embryos, and L > R Coco expression was found in 3% of vehicle-treated embryos vs. 35% of PTU-treated embryos.
Rescue of localization defects
It has been shown that left-asymmetric LPM expression of Pitx2c can be restored even in the absence of flow by left-specific down-regulation of Coco via an antisense morpholino oligonucleotide (MO) (23). When PTU-treated embryos were injected with coco-MO, Pitx2c expression on the left side was rescued in ∼80% of cases (Fig. 3A). Bilateral Pitx2c expression was also observed in some cases. These observations suggest that flow is impaired by PTU (39).
Aberrant leftward flow in PTU-treated embryos
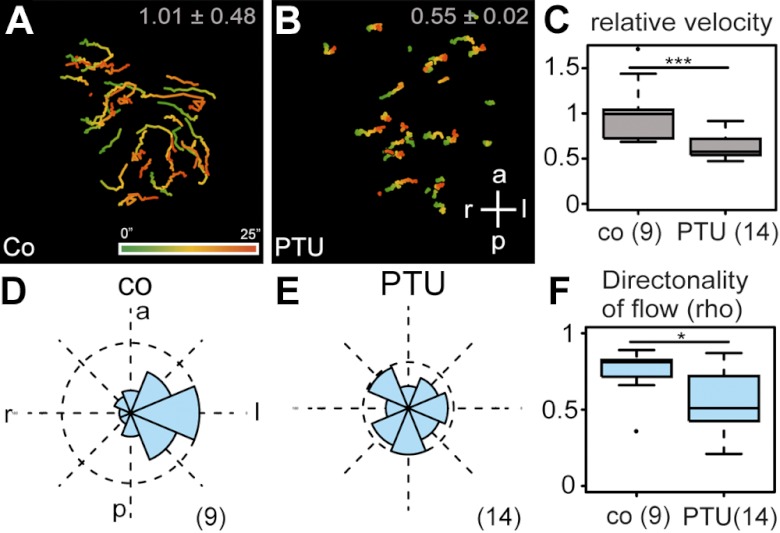
The altered expression patterns of genes involved in LR axis determination suggested that cilia-driven flow itself was impaired by PTU. Flow was analyzed in dorsal explants by adding fluorescent beads, as described previously (29, 37). Movies show representative wild-type and PTU-treated dorsal explants. Qualitative and quantitative analyses are depicted in Fig. 4. Beads in PTU-treated specimens moved at about half of the velocity of controls (Fig. 4A–C) and occurred in a random and undirected fashion (Fig. 4D, E). The qualitative parameter ρ, which represents directionality (with a value of 1 in cases when all beads move in the same direction, and 0 when bead movement is random), was 0.83 in vehicle-treated explants and decreased to 0.53 in PTU-treated explants (Fig. 4F).
Figure 4.
Aberrant flow in PTU-treated GRP explants. A, B) Flow as displayed by GTTs of 25 s length (cf. color bar; A) from representative control (A) and PTU-incubated (B) specimens. C) Bead velocity. Note that beads in PTU samples moved only at 60% of the velocity of control specimens, which were set to 1. D, E) Frequency distribution of trajectory angles (in 8 segments of 45° each), calculated from 9 control and 14 PTU-incubated time-lapse movies. F) Box plot of directionality, as indicated by the dimensionless number ρ. A value of 1 represents a case in which all beads move within the same 45° sector, while ρ = 0 indicates net random displacements of beads. a, anterior; co, control; l, left; p, posterior; r, right. Numbers in parentheses represent number of analyzed movies. *P < 0.05; ***P < 0.001.
It has been shown that aberrant flow can result from altered GRP morphology or an even earlier defect in the specification of the so-called superficial mesoderm (SM) during gastrulation (37, 40, 41). The SM involutes during gastrulation to form the GRP at early neurula stages (42). SM markers Foxj1 and Xnr3, as well as Gsc and Brachyury, which mark the organizer and the mesoderm, respectively, were unaltered on PTU treatment (Supplemental Fig. S4), demonstrating that PTU affects LR development after specification of the SM.
We therefore analyzed GRP morphology by immunohistochemistry of GRP explants. Cilia polarization to the posterior pole of cells, which is a prerequisite of directed flow, was seen in >85% of wild-type cells but only in ∼65% of PTU-treated specimens (Fig. 5A–C). Interestingly, biciliated cells were detected in 5.2% of PTU-exposed cells, a feature never encountered in control specimens (Fig. 5A, B). Cilia length (Fig. 5D) and ciliation rate (not shown) were not affected by PTU, and the overall morphology of the GRP appeared unchanged, with equal medial surface area of cells (Fig. 5E). The GRP marker gene Tekt2 (36), which localizes to ciliary and flagellar microtubules (43), was markedly down-regulated on PTU incubation (Fig. 5F, G), in line with altered GRP functionality. In summary, the analysis of PTU-induced LR-axis defects in Xenopus embryos demonstrate that altered flow resulted in absent Pitx2c expression in the left LPM and, consequently, altered organ situs in tadpoles.
Figure 5.
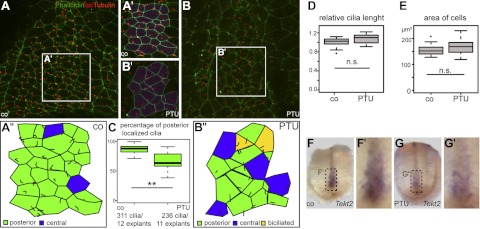
Ciliation defects in the GRP of PTU-treated embryos. A–B″) Cilia and cell boundaries were visualized in dorsal explants from control (A–A″) and PTU-incubated specimens (B–B″) by immunohistochemistry (acetylated α-tubulin, red) and phalloidin staining (actin, green), respectively. C–E) Note that posterior polarization of cilia was significantly reduced (cf. quantification in C), while cilia length (D) and cell surface area (E) were unaltered. F–G′) GRP marker gene Tekt2 was markedly reduced in PTU-treated (G, G′) vs. control (F, F′) explants.
DISCUSSION
Based on limited data, it has been long assumed that the antithyroid drug MMI is teratogenic, whereas PTU is not (11). In Xenopus embryos, a validated model for teratology studies (20), we observe that treatment with PTU during embryogenesis induces structural defects, whereas MMI does not. Defects induced by PTU included abnormal LR asymmetry establishment, edema formation, and a reduced length of the embryo.
Indicating that the observed effects were PTU-specific and a direct effect of PTU, the antithyroid drug MMI, which is 20 times more potent than PTU (44, 45), did not induce defects in the embryos. Thyroid hormone synthesis also begins well after the period of PTU vulnerability (38), supporting the notion that inhibition of thyroid hormone production is not the cause of the defects. Furthermore, the effects of PTU were not rescued by exogenous thyroid hormone in our experiments.
An important finding of our studies was that PTU resulted in abnormal establishment of LR orientation of the heart and abnormal coiling of the gut. PTU prevented the left-sided expression of Pitx2c in the LPM, and inhibited the down-regulation of Coco on the left side of the GRP. Remarkably, cilia-driven leftward flow, which is the symmetry-breaking event in Xenopus, was disrupted by PTU treatment. We cannot exclude that PTU has additional effects on ciliary beat frequency, integrity of the midline, or sensing of the flow, which we were not able to assess.
The morphology of the GRP was not grossly altered, as the specification of the SM, ciliation rate, and cell size appeared normal. A portion of the cells, though, lacked posterior localized monocilia, which play a role in leftward flow. In other studies, a decrease in the percentage of posterior localized cilia can disrupt flow and LR development (41, 46–48).
Time course studies narrowed the window of vulnerability to st. 8–12.5, which corresponds with the period of late blastula and gastrulation. The observation that PTU treatment beginning at st. 8 disrupts normal lateralization is consistent with the observation that the superficial mesoderm, from which the ciliated GRP is derived, gets specified during gastrulation, i.e., from st. 10 onward, and is in temporal proximity to onset of treatment at st. 8. In rodents, studies have been generally performed at developmental stages later than those employed in our current experiments. Our studies of mice treated with PTU over E7–E9 in early embryogenesis revealed the induction of neural tube and cardiac defects (18). However, treatment of pregnant mice over earlier periods of development, comparable to those of the Xenopus studies, did not result in situs defects. Fertilized murine embryos at such stages, though, have yet to implant (20, 49) and may not be exposed to drug.
Studies of ATD teratogenicity in humans are limited by the lack of information related to the timing of ATD therapy relative to pregnancy onset. In human studies, it is also difficult to directly attribute birth defects seen in GD to the antithyroid medication vs. the hyperthyroid state itself. Recently, Clementi et al. (19) performed a case-controlled study of the teratogenic defects of PTU vs. MMI. Interestingly, PTU, but not MMI, was found to be associated with an increased risk of situs inversus, an important observation that is consistent with our data.
We recognize that although studies of Xenopus embryos provide important insights into drug teratogenic potential, Xenopus embryos may not reflect teratogenic events that occur in mammals. However, the Xenopus model, as applied in this report, has been shown to provide important insights into mammalian and human biology (20, 27, 50–53). The concentrations of drugs that induce the defects in the frog embryo are also greater than human dosages, and the accessibility of the drug might be different.
Overall, we show that PTU is teratogenic during late blastula, gastrulation, and neurulation, whereas MMI is not. We find that the ability of PTU to induce laterality defects is limited to treatment during a specific window of early embryogenesis. We show that PTU may potentially induce birth defects by altering ciliary driven flow and disruption of the normal genetic program involved in LR axis establishment. Further studies are needed to evaluate the risk of PTU during pregnancy, especially for women taking PTU during very early pregnancy.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health grants 1R01DE018825 (to M.K.K.) and 1R01HD065200 (to S.A.R), by grant BL-285/10-1 from the Deutsche Forschungsgemeinschaft (to M.B.), and by The Nuffic Dutch Huygens Talent Scholarship (to N.R.V.V.).
The authors thank Michael Slocum for Xenopus husbandry, Sarah Kirschner for setting up Xenopus matings used in this study, and Isabelle Schneider, Thomas Thumberger, and Peter Walentek for help with GRP and flow analysis.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- ATD
- antithyroid drug
- GD
- Graves' disease
- GRP
- gastrocoel roof plate
- Gsc
- Goosecoid
- GTT
- gradient time trail
- ISH
- in situ hybridization
- LPM
- lateral plate mesoderm
- LR
- left-right
- MMI
- methimazole
- PTU
- 6-propyl-2-thiouracil (propylthiouracil)
- SM
- superficial mesoderm
- st.
- Nieuwkoop and Faber stage
- T3
- triiodo-l-thyronine
REFERENCES
- 1. Mestman J. H. (1998) Hyperthyroidism in pregnancy. Endocrinol. Metab. Clin. North Am. 27, 127–149 [DOI] [PubMed] [Google Scholar]
- 2. Glinoer D. (1998) Thyroid hyperfunction during pregnancy. Thyroid 8, 859–864 [DOI] [PubMed] [Google Scholar]
- 3. Fantz C. R., Dagogo-Jack S., Ladenson J. H., Gronowski A. M. (1999) Thyroid function during pregnancy. Clin. Chem. 45, 2250–2258 [PubMed] [Google Scholar]
- 4. Abalovich M., Amino N., Barbour L. A., Cobin R. H., De Groot L. J., Glinoer D., Mandel S. J., Stagnaro-Green A. (2007) Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 92, S1–S47 [DOI] [PubMed] [Google Scholar]
- 5. Okosieme O. E., Marx H., Lazarus J. H. (2008) Medical management of thyroid dysfunction in pregnancy and the postpartum. Expert Opin. Pharmacother. 9, 2281–2293 [DOI] [PubMed] [Google Scholar]
- 6. Marx H., Amin P., Lazarus J. H. (2008) Hyperthyroidism and pregnancy. BMJ 336, 663–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chattaway J. M., Klepser T. B. (2007) Propylthiouracil versus methimazole in treatment of Graves' disease during pregnancy. Ann. Pharmacother. 41, 1018–1022 [DOI] [PubMed] [Google Scholar]
- 8. Chan G. W., Mandel S. J. (2007) Therapy Insight: management of Graves' disease during pregnancy. Nat. Clin. Pract. Endocrinol. Metab. 3, 470–478 [DOI] [PubMed] [Google Scholar]
- 9. Burrow G. N. (1985) The management of thyrotoxicosis in pregnancy. N. Engl. J. Med. 313, 562–565 [DOI] [PubMed] [Google Scholar]
- 10. Roti E. (1996) Clinical review 80: management of hyperthyroidism and hypothyroidism in the pregnant woman. J. Clin. Endocrinol. Metabol. 81, 1679–1682 [DOI] [PubMed] [Google Scholar]
- 11. Bahn R. S., Burch H. S., Cooper D. S., Garber J. R., Greenlee C. M., Klein I. L., Laurberg P., McDougall I. R., Rivkees S. A., Ross D., Sosa J. A., Stan M. N. (2009) The role of propylthiouracil in the management of Graves' disease in adults: report of a meeting jointly sponsored by the American Thyroid Association and the Food and Drug Administration. Thyroid 19, 673–674 [DOI] [PubMed] [Google Scholar]
- 12. Koren G., Soldin O. (2006) Therapeutic drug monitoring of antithyroid drugs in pregnancy: the knowledge gaps. Ther. Drug Monit. 28, 12–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stanisstreet M., Herbert L. C., Pharoah P. O. (1990) Effects of thyroid antagonists on rat embryos cultured in vitro. Teratology 41, 721–729 [DOI] [PubMed] [Google Scholar]
- 14. Krementz E. T., Hooper R. G., Kempson R. L. (1957) The effect on the rabbit fetus of the maternal administration of propylthiouracil. Surgery 41, 619–631 [PubMed] [Google Scholar]
- 15. Goldey E. S., Kehn L. S., Rehnberg G. L., Crofton K. M. (1995) Effects of developmental hypothyroidism on auditory and motor function in the rat. Toxicol. Appl. Pharmacol. 135, 67–76 [DOI] [PubMed] [Google Scholar]
- 16. Calikoglu A. S., Gutierrez-Ospina G., D'Ercole A. J. (1996) Congenital hypothyroidism delays the formation and retards the growth of the mouse primary somatic sensory cortex (S1). Neurosci. Lett. 213, 132–136 [DOI] [PubMed] [Google Scholar]
- 17. Zolcinski. A., Heinrath T., Rzucidlo Z. (1964) Effect of methimazole on the development of rabbit fetuses. Ginekol. Pol. 35, 593–596 [PubMed] [Google Scholar]
- 18. Benavides V. C., Mallela M. K., Booth C. J., Wendler C. C., Rivkees S. A. (2012) Propylthiouracil is teratogenic in murine embryos. PLoS ONE 7, e35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clementi M., Di Gianantonio E., Cassina M., Leoncini E., Botto L. D., Mastroiacovo P., Castilla E. E., Bakker M K., Bianca S., Cocchi G., de Vigan C., Merlob P., Pierini A., Scarano G., Sipek A., Yamanaka M. (2010) Treatment of hyperthyroidism in pregnancy and birth defects. J. Clinl. Endocrinol. Metabol. 95, E337–E341 [DOI] [PubMed] [Google Scholar]
- 20. Mouche I., Malesic L., Gillardeaux O. (2011) FETAX assay for evaluation of developmental toxicity. Methods Mol. Biol. 691, 257–269 [DOI] [PubMed] [Google Scholar]
- 21. Schweickert A., Weber T., Beyer T., Vick P., Bogusch S., Feistel K., Blum M. (2007) Cilia-driven leftward flow determines laterality in Xenopus. Curr. Biol. 17, 60–66 [DOI] [PubMed] [Google Scholar]
- 22. Blum M., Andre P., Muders K., Schweickert A., Fischer A., Bitzer E., Bogusch S., Beyer T., van Straaten H. W., Viebahn C. (2007) Ciliation and gene expression distinguish between node and posterior notochord in the mammalian embryo. Differentiation 75, 133–146 [DOI] [PubMed] [Google Scholar]
- 23. Schweickert A., Vick P., Getwan M., Weber T., Schneider I., Eberhardt M., Beyer T., Pachur A., Blum M. (2010) The nodal inhibitor coco is a critical target of leftward flow in Xenopus. Curr. Biol. 20, 738–743 [DOI] [PubMed] [Google Scholar]
- 24. Sive H. L., Grainger R., Harland R. (2000) Early Development of Xenopus laevis: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA [Google Scholar]
- 25. Sive H. L., Grainger R. M., Harland R. M. (2010) Early Development of Xenopus laevis: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA [Google Scholar]
- 26. Nieuwkoop P. (1994) Normal Table of Xenopus laevis (Daudin), Garland Publishing, New York [Google Scholar]
- 27. Fakhro K. A., Choi M., Ware S. M., Belmont J. W., Towbin J. A., Lifton R. P., Khokha M. K., Brueckner M. (2011) Rare copy number variations in congenital heart disease patients identify unique genes in left-right patterning. Proc. Natl. Acad. Sci. U. S. A. 108, 2915–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abràmoff M., Magalhães P., Ram S. J. (2004) Image processing with ImageJ. Biophotonics Int. 11, 36–42 [Google Scholar]
- 29. Blum M., Beyer T., Weber T., Vick P., Andre P., Bitzer E., Schweickert A. (2009) Xenopus, an ideal model system to study vertebrate left-right asymmetry. Dev. Dyn. 238, 1215–1225 [DOI] [PubMed] [Google Scholar]
- 30. Khokha M. K., Chung C., Bustamante E. L., Gaw L. W., Trott K. A., Yeh J., Lim N., Lin J. C., Taverner N., Amaya E., Papalopulu N., Smith J. C., Zorn A. M., Harland R. M., Grammer T. C. (2002) Techniques and probes for the study of Xenopus tropicalis development. Dev. Dyn. 225, 499–510 [DOI] [PubMed] [Google Scholar]
- 31. Hemmati-Brivanlou A., Frank D., Bolce M. E., Brown B. D., Sive H. L., Harland R. M. (1990) Localization of specific mRNAs in Xenopus embryos by whole-mount in situ hybridization. Development 110, 325–330 [DOI] [PubMed] [Google Scholar]
- 32. Schweickert A., Campione M., Steinbeisser H., Blum M. (2000) Pitx2 isoforms: involvement of Pitx2c but not Pitx2a or Pitx2b in vertebrate left–right asymmetry. Mech. Dev. 90, 41–51 [DOI] [PubMed] [Google Scholar]
- 33. Smith J. C., Price B. M., Green J. B., Weigel D., Herrmann B. G. (1991) Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell 67, 79–87 [DOI] [PubMed] [Google Scholar]
- 34. Cho K. W., Blumberg B., Steinbeisser H., De Robertis E. M. (1991) Molecular nature of Spemann's organizer: the role of the Xenopus homeobox gene goosecoid. Cell 67, 1111–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Glinka A., Delius H., Blumenstock C., Niehrs C. (1996) Combinatorial signalling by Xwnt-11 and Xnr3 in the organizer ephithelium. Mech. Dev. 60, 221–231 [DOI] [PubMed] [Google Scholar]
- 36. Stubbs J. L., Oishi I., Izpisúa Belmonte J. C., Kintner C. (2008) The forkhead protein Foxj1 specifies node-like cilia in Xenopus and zebrafish embryos. Nat. Genet. 40, 1454–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vick P., Schweickert A., Weber T., Eberhardt M., Mencl S., Shcherbakov D., Beyer T., Blum M. (2009) Flow on the right side of the gastrocoel roof plate is dispensable for symmetry breakage in the frog Xenopus laevis. Dev. Biol. 331, 281–291 [DOI] [PubMed] [Google Scholar]
- 38. Tindall A. J., Morris I. D., Pownall M. E., Isaacs H. V. (2007) Expression of enzymes involved in thyroid hormone metabolism during the early development of Xenopus tropicalis. Biol. Cell 99, 151. [DOI] [PubMed] [Google Scholar]
- 39. Lenhart K. F., Lin S-Y, Titus T. A., Postlethwait J. H., Burdine R. D. (2011) Two additional midline barriers function with midline lefty1 expression to maintain asymmetric Nodal signaling during left-right axis specification in zebrafish. Development 138, 4405–4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Beyer T., Danilchik M., Thumberger T., Vick P., Tisler M., Schneider I., Bogusch S., Andre P., Ulmer B., Walentek P., Niesler B., Blum M., Schweickert A. (2012) Serotonin signaling is required for Wnt-dependent GRP specification and leftward flow in Xenopus. Curr. Biol. 22, 33–39 [DOI] [PubMed] [Google Scholar]
- 41. Maisonneuve C., Guilleret I., Vick P., Weber T., Andre P., Beyer T., Blum M., Constam D. B. (2009) Bicaudal C, a novel regulator of Dvl signaling abutting RNA-processing bodies, controls cilia orientation and leftward flow. Development 136, 3019–3030 [DOI] [PubMed] [Google Scholar]
- 42. Shook D. R., Majer C., Keller R. (2004) Pattern and morphogenesis of presumptive superficial mesoderm in two closely related species, Xenopus laevis and Xenopus tropicalis. Dev. Biol. 270, 163–185 [DOI] [PubMed] [Google Scholar]
- 43. Setter P. W., Malvey-Dorn E., Steffen W., Stephens R. E., Linck R. W. (2006) Tektin interactions and a model for molecular functions. Exp. Cell Res. 312, 2880–2896 [DOI] [PubMed] [Google Scholar]
- 44. Diav-Citrin O., Ornoy A. (2002) Teratogen update: antithyroid drugs-methimazole, carbimazole, and propylthiouracil. Teratology 65, 38–44 [DOI] [PubMed] [Google Scholar]
- 45. Cooper D. (2005) Antithyroid drugs. N. Engl. J. Med. 352, 905–917 [DOI] [PubMed] [Google Scholar]
- 46. Alten L., Schuster-Gossler K., Beckers A., Groos S., Ulmer B., Hegermann J., Ochs M., Gossler A. (2012) Differential regulation of node formation, nodal ciliogenesis and cilia positioning by Noto and Foxj1. Development 139, 1276–1284 [DOI] [PubMed] [Google Scholar]
- 47. Antic D., Stubbs J. L., Suyama K., Kintner C., Scott M. P., Axelrod J. D. (2010) Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis. PLoS ONE 5, e8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Song H., Hu J., Chen W., Elliott G., Andre P., Gao B., Yang Y. (2010) Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature 466, 378–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Braverman L. E., ed. (2003) Diseases of the Thyroid, Humana Press, New York Totowa, NJ, USA [Google Scholar]
- 50. Wang H., Dey S. K. (2006) Roadmap to embryo implantation: clues from mouse models. Nat. Rev. Genet. 7, 185–199 [DOI] [PubMed] [Google Scholar]
- 51. Kashiwagi K., Kashiwagi A., Kurabayashi A., Hanada H., Nakajima K., Okada M., Takase M., Yaoita Y. (2010) Xenopus tropicalis: an ideal experimental animal in amphibia. Exp. Anim. 59, 395–405 [DOI] [PubMed] [Google Scholar]
- 52. Shi H., Qian L., Guo S., Zhang X., Liu J., Cao Q. (2010) Teratogenic effects of tetrabromobisphenol A on Xenopus tropicalis embryos. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 152, 62–68 [DOI] [PubMed] [Google Scholar]
- 53. Wheeler G. N., Brändli A. W. (2009) Simple vertebrate models for chemical genetics and drug discovery screens: lessons from zebrafish and Xenopus. Dev. Dyn. 238, 1287–1308 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.