Abstract
Development of spermatozoa in adult mammalian testis during spermatogenesis involves extensive cell migration and differentiation. Spermatogonia that reside at the basal compartment of the seminiferous epithelium differentiate into more advanced germ cell types that migrate toward the apical compartment until elongated spermatids are released into the tubule lumen during spermiation. Apical ectoplasmic specialization (ES; a testis-specific anchoring junction) is the only cell junction that anchors and maintains the polarity of elongating/elongated spermatids (step 8–19 spermatids) in the epithelium. Little is known regarding the signaling pathways that trigger the disassembly of the apical ES at spermiation. Here, we show that secreted Frizzled-related protein 1 (sFRP1), a putative tumor suppressor gene that is frequently down-regulated in multiple carcinomas, is a crucial regulatory protein for spermiation. The expression of sFRP1 is tightly regulated in adult rat testis to control spermatid adhesion and sperm release at spermiation. Down-regulation of sFRP1 during testicular development was found to coincide with the onset of the first wave of spermiation at approximately age 45 d postpartum, implying that sFRP1 might be correlated with elongated spermatid adhesion conferred by the apical ES before spermiation. Indeed, administration of sFRP1 recombinant protein to the testis in vivo delayed spermiation, which was accompanied by down-regulation of phosphorylated (p)-focal adhesion kinase (FAK)-Tyr397 and retention of nectin-3 adhesion protein at the apical ES. To further investigate the functional relationship between p-FAK-Tyr397 and localization of nectin-3, we overexpressed sFRP1 using lentiviral vectors in the Sertoli-germ cell coculture system. Consistent with the in vivo findings, overexpression of sFRP1 induced down-regulation of p-FAK-Tyr397, leading to a decline in phosphorylation of nectin-3. In summary, this report highlights the critical role of sFRP1 in regulating spermiation via its effects on the FAK signaling and retention of nectin-3 adhesion complex at the apical ES.—Wong, E. W. P., Lee, W. M., Cheng, C. Y. Secreted Frizzled-related protein 1 (sFRP1) regulates spermatid adhesion in the testis via dephosphorylation of focal adhesion kinase and the nectin-3 adhesion protein complex.
Keywords: spermatogenesis, seminiferous epithelial cycle, apical ectoplasmic specialization
Cell junction remodeling is fundamental to tissue homeostasis in multicellular organisms. Central to this process is the constant turnover of cell junction proteins at tight junctions (TJs), adherens junctions (AJs), and desmosomes (1–3). This phenomenon is manifested in the development of spermatozoa in the mammalian testis, which is accompanied with extensive junction restructuring. In rat testis, type B spermatogonia residing at the basal compartment of the seminiferous epithelium differentiate into preleptotene/leptotene spermatocytes, which in turn traverse the blood-testis barrier (BTB) at stage VIII of the seminiferous epithelial cycle to enter the adluminal compartment (4, 5). These germ cells further differentiate into pachytene spermatocytes and develop into step 1 round spermatids via meiosis I/II. Step 1 spermatids differentiate into step 19 spermatids (i.e., elongated spermatids) via spermiogenesis while migrating toward the lumen of the seminiferous epithelium. Between Sertoli cells and step 8–19 spermatids, a testis-specific AJ called apical ectoplasmic specialization (ES) is present to anchor and orientate spermatids onto the Sertoli cell epithelium (5, 6). Therefore, continuous remodeling of cell junctions between Sertoli cells and germ cells is needed to facilitate the migration of developing germ cells across the seminiferous epithelium. Apical ES is an atypical AJ and a hybrid cell junction because besides classic AJ proteins (e.g., cadherin and catenin), TJ [e.g., junctional adhesion molecule C (JAM-C)], and focal adhesion proteins (e.g., integrin and laminin) as well as their associated kinases [e.g., focal adhesion kinase (FAK)] are all present (7). Fully developed step 19 elongated spermatids are released into tubule lumen at late stage VIII via disassembly of the apical ES (i.e., spermiation; refs. 5, 6). Precise control of spermiation is required because premature release or retention of mature elongated spermatids can result in infertility. However, little is known about the signaling molecules that trigger the disassembly of apical ES at spermiation. Recent studies showed that protein kinases are involved in controlling spermiation. For instance, knockout mice of liver kinase B1 (LKB1), a serine/threonine protein kinase, displayed arrest in spermiation and consequently were fertile (8).
Secreted Frizzled-related proteins (sFRPs) are secreted glycoproteins that were initially identified due to sequence homology to the cysteine-rich domain (CRD) of the wingless integration (Wnt) receptors Frizzleds (9, 10). Subsequent studies identified additional members, and 5 members are now found in mammals, all of which contain a CRD (11). Phylogenetic and sequence analysis classified sFRP1, sFRP2, and sFRP5 into one subgroup, which is distinct from the one consisting of sFRP3 and sFRP4 (11, 12). In addition, functional redundancy can be observed in the sFRP1, sFRP2, and sFRP5 subgroup, where phenotypic changes were only observed after double or triple knockout of the genes (13, 14), which can partially be explained by sequence homology among the members. It has been shown that sFRPs are able to modulate Wnt signaling by interacting with both Wnts and Frizzleds, and their involvement in cellular functions independent of Wnt signaling is also emerging (11). These include apoptosis, osteoblastogenesis, and phosphate homeostasis (11, 15). Nevertheless, characterization of the functional roles of sFRPs is hindered by their complicated interactions with Wnts, Frizzleds, and/or other signaling molecules that are highly cell/tissue-context specific (16), including those found in the testis. In recent years, several lines of evidence have illustrated that sFRPs are tumor suppressor genes. Multiple studies have shown that the promoters of sFRPs are frequently hypermethylated in tumor cells from distinct histological origins (e.g., multiple myeloma and colorectal and breast cancers; refs. 17–19). In other studies, chromosome regions harboring sFRPs were often found deleted in cancer cells (20, 21). Although the functional significance of sFRPs as tumor suppressor genes is not well defined, there are studies implicating sFRPs in stabilizing cell junctions and decreasing the migratory ability of cancer cells (22–24).
In this study, the physiological roles of sFRP1 in spermatogenesis in normal rat testis are addressed. Previous studies have found that sFRP1 mRNA is present in both embryonic and adult testis (14, 25). sFRP1-knockout mice are normal and fertile (26) while double-homozygous male knockout mice of sFRP1 and sFRP2 have abnormal transabdominal descent of testes with fewer seminiferous cords (14). However, the expression profile of sFRP1 in developing testis and its involvement in spermatogenesis in adult testis are not known. We showed here that sFRP1 is important in regulating spermiation in adult rat testis via its effects on the phosphorylation of FAK and nectin-3 at the apical ES.
MATERIALS AND METHODS
In vivo administration of recombinant sFRP1 protein
Recombinant human sFRP1 protein [relative molecular mass (Mr) 37 kDa; R&D Systems, Minneapolis, MN, USA] was prepared in phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, and 1.47 mM KH2PO4, pH 7.4 at 22°C) at 250 μg/ml. The homology between the secreted region of human (Genbank accession no. NP_003003) and rat (Genbank XP_001072532.2) sFRP1 proteins is 97.5%. Adult Sprague-Dawley rats (90 d old) were injected intratesticularly with 2.5 μg/ml (∼67.6 nM) sFRP1. A dosage of 4 μg sFRP1 was used per testis, assuming a testicular volume of 1.6 ml. An equal amount of bovine serum albumin (BSA) was used as control. At specified time points, rats were euthanized by CO2 asphyxiation. Testes were either snap-frozen in liquid nitrogen for protein lysate preparation and to obtain frozen sections or fixed in Bouin's fixative and processed for paraffin section for histological analysis. Paraffin sections were deparaffinized in 3 changes of xylene for 5 min each, rehydrated in a graded series of ethanol, and washed once in distilled water. Nuclei were stained with Mayer's hematoxylin solution (Sigma, St. Louis, MO, USA) for 5 min. Sections were dehydrated before mounting with Poly-Mount (Polysciences, Warrington, PA, USA). Images were captured with a Nikon Eclipse 90i microscope with two built-in digital cameras (Nikon DS-Qi1Mc and DS-Fi1), and images were acquired using NIS Elements imaging software (Nikon, Tokyo, Japan). The use of animals was approved by the Rockefeller University Institutional Animal Care and Use Committee (protocol nos. 09016 and 12506).
Cloning of full-length sFRP1
Full-length sFRP1 coding sequence was amplified from 5-d-old rat testis cDNA with sense primer 5′-GGGAAGTTTGCAGCGGGA-3′ and antisense primer 5′-GCAACATGTGAACCCCAAGAGC-3′, using AccuPrime high fidelity Taq (Life Technologies, Carlsbad, CA, USA); 6% DMSO (v/v) was included in polymerase chain reaction (PCR) due to the high GC content at the 5′ end of sFRP1 coding sequence. Primers were designed according to the consensus sequences at the 5′ and 3′ noncoding regions from the predicted sequences of rat sFRP1 (GenBank XM_224987.5 and XM_001072532.2). Sequencing results from 3 testis samples showed that the full-length sequence of the sFRP1 cDNA matches the XM_001072532.2 record. The coding sequence of sFRP1 was cloned into pENTR/SD/D-TOPO (Gateway entry clone, Life Technologies) using sense primer 5′- CACCATGGGCGTCGGGCG-3′ and antisense primer 5′-CTTAAAAACAGACTGGAAGGTGGGACACTC-3′ with AccuPrime Pfx Taq (Life Technologies). pLenti7.3-sFRP1 was generated by standard Gateway LR recombination protocol from the entry clone and used to produce lentiviral vectors. Plasmids were prepared with Qiagen EndoFree Plasmid Maxi kit (Qiagen, Valencia, CA, USA). pLenti7.3-sFRP1 and pLenti7.3-β-galactosidase (LacZ; Life Technologies) were digested with Bst98I at 37°C for 2 h and analyzed by agarose gel electrophoresis to confirm there is no random recombination before use for lentiviral vector production.
Real-time quantitative PCR (qPCR) and semiquantitative PCR analysis
Total RNA from testes of different ages of rats (3–60 d old) was extracted by Trizol reagent (Life Technologies) with homogenization using Polytron (Kinematica, Luzern, Switzerland). Contaminated genomic DNA was eliminated by treatment with DNase I (Life Technologies). Reverse transcription was performed using M-MLV reverse transcriptase (Promega, Madison, WI, USA) with a mixture of random hexamers and oligo dT at a ratio of 4:1. PCR was performed using N′,N′-dimethyl-N-{4-[(E)-(3-methyl-1,3-benzothiazol-2-ylidene)methyl]-1-phenylquinolin-1-ium-2-yl}-N-propylpropane-1,3-diamine (SYBR green) real-time PCR master mix and ABI 7900HT real-time PCR machine (Life Technologies). The cycling parameters were 95°C for 10 min, 50 cycles of 95°C for 15 s, and 60°C for 1 min, followed by a slow increase to 95°C for dissociation curve analysis. A panel of housekeeping genes was tested [e.g., hypoxanthine-guanine phosphoribosyltransferase (Hprt), β-2-microglobulin (B2M), activating transcription factor 2 (ATF2), ribosomal protein S16 (S16), TATA box binding protein (TBP), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), peptidylproyl isomerase A (Ppia), and ribosomal protein L19 (Rpl19)], and GAPDH was chosen to normalize cDNA amount because its mRNA level remained most stable during the development of testis. Primers were designed by Primer Express 3.0 software (Life Technologies). The following primers were used: sFRP1, sense 5′-CGTCTGCATCGCCATGAC-3′ and antisense 5′-GATGGCCTCCGATTTCAACTC-3′; GAPDH (Genbank NM_017008.3), sense 5′-GCTGGTCATCAACGGGAAAC-3′ and antisense 5′-GGTGAAGACGCCAGTAGAC-3′. Amplification efficiencies of GAPDH and sFRP1 were determined to be approximately equal (∼100%) before calculation of fold change with comparative CT method. Semiquantitative PCR was performed using testis, Sertoli, and germ cell cDNA to determine the expression of sFRP1 mRNA. S16 (Genbank NM_001169146) was used as loading control and amplified by primers of sense 5′-TCCGCTGCAGTCCGTTCAAGTCTT-3′ and antisense 5′-GCCAAACTTCTTGGATTCGCAGCG-3′, using an ABI GeneAmp 2400 thermal cycler (Life Technologies). The identities of all PCR products were confirmed by nucleotide sequencing at Genewiz (South Plainfield, NJ, USA).
Production of lentiviral vectors
Lenti-X 293T cells (Clontech, Palo Alto, CA, USA) were maintained in high-glucose DMEM supplemented with 10% fetal bovine serum (FBS; Life Technologies), 3.7 mg/ml sodium bicarbonate, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM GlutaMAX, and 0.1 mM MEM nonessential amino acids (Life Technologies), pH 7.4 at 22°C. Cells were kept at 37°C in humidified atmosphere with 10% CO2:90% air (v/v) in CO2 incubators. Only cells of low passage number (<12) were used for high-titer lentiviral vector packaging. Several transfection reagents and methods [e.g., Fugene HD (Roche, Indianapolis, IN, USA), GeneJuice (EMD4Biosciences, Darmstadt, German), polyethylenimine (Polysciences), and calcium phosphate precipitation] were tested in pilot experiments for transfection efficiency and hence titer of lentiviral vectors. GeneJuice was found to give the highest titer, but calcium phosphate precipitation was selected because it was the most economic reagent and it produced satisfactory titer for transduction of high cell density primary Sertoli cell cultures. Briefly, three 150-mm plates of 95% confluence were split into twelve 0.001% poly-l-lysine/PBS (w/v) coated-150 mm plates in culture medium with 0.3% cholesterol (Life Technologies) 1 d before transfection. Before transfection, cells were replaced with fresh culture medium with 0.3% cholesterol and 25 μM chloroquine diphosphate. For twelve 150-mm plates, 270 μg pLenti7.3-lacZ or pLenti7.3-sFRP1, 180 μg gag-pol, and 90 μg pMD.G were used for transfection. Plasmids were combined with 1350 μl 2.5 M calcium chloride and made up to 13.5 ml in sterile Milli-Q water (Millipore, Bedford, MA, USA); 13.5 ml of 2× BBS {50 mM 2-[bis(2-hydroxyethyl)ammonio]ethanesulfonate (EMD4Biosciences), 280 mM NaCl, 1.5 mM Na2HPO4, pH 6.95 at 22°C} was added dropwise into the DNA mixture while vortexing and incubated at room temperature for 5 min. Calcium phosphate-precipitated DNA mixture (2.25 ml) was added to each plate. Cells were incubated at 37°C in humidified atmosphere with 3% CO2:97% air (v/v) for 16 h. Thereafter, the transfection reagent was replaced with serum-free UltraCulture medium (Lonza, Basel, Switzerland) with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM GlutaMAX, 0.1 mM MEM nonessential amino acids, 0.3% cholesterol, and 4 mM sodium butyrate and incubated for an additional 32 h in a humidified atmosphere at 10% CO2:90% air (v/v) before the first round of crude lentiviral vector was collected and stored at 4°C. The second round of crude lentiviral vectors was collected after an additional 24 h of incubation. The two collections were then combined, centrifuged at 2000 g for 10 min, and filtered through a 0.45-μm PVDF membrane filter (Millipore). To purify and concentrate lentiviral vector, 32 ml crude collection was first added to each ultracentrifuge tube and then underlayered by 3 ml 20% sucrose containing 1 mM EDTA in PBS and centrifuged at 70,000 g using a Beckman SW28 swing-bucket rotor (Beckman Coulter, Brea, CA, USA) for 2 h at 4°C. The supernatant was discarded, and the lentiviral vector pellets were resuspended gently in a total of 200 μl PBS containing 1 mM EDTA for 3 h at 4°C with occasional agitation. To measure the titer of lentiviral vectors, 0.5 × 105 Lenti-X 293T cells were seeded in each well of a 24-well plate. Cells were transduced with a 10-fold serial dilution of the concentrated lentiviral vectors 1 d later in the presence of 4 μg/ml polybrene (hexadimethrine bromide). At 2 d after transduction, cells were washed once with PBS, trypsinized at 37°C for 5 min, and centrifuged at 2000 g for 3 min. The cell pellet was resuspended in 1% BSA, 4 mM EDTA, 5 mM magnesium chloride, and 40 μg/ml DNaseI in PBS. Flow cytometry was performed by BD Calibur (BD Biosciences, San Jose, CA, USA), and the percentage of infected cells was analyzed by FlowJo 7.6.5 software (Tree Star, Ashland, OR, USA). An average titer of 1 × 109 transducing units/ml was routinely obtained.
Primary cell cultures and transduction by lentiviral vectors
Primary Sertoli cells were isolated from testes of 20-d-old rats and cultured as described earlier (27). Sertoli cells were seeded at a density of 0.5 × 106 cells/cm2 on Matrigel-coated culture dishes in F12/DMEM with supplements, as described previously (27). On d 4, when specialized junctions were formed (e.g., basal ES, TJ) that mimicked the Sertoli cell BTB in vivo, total germ cells [isolated as described previously (28) without the glasswool filtration step, so that the relative ratios of spermatogonia:spermatocytes:round spermatids:elongating/elongated spermatids in our germ cell preparation were similar to those in vivo (28, 29)] were added to the Sertoli cells in a ratio of Sertoli:germ cells at 1:5 to allow the assembly of specialized Sertoli-germ cell junctions, such as apical ES. To isolate primary total germ cells, a mechanical procedure without using enzymes, such as trypsin, was used (28). In brief, testes from 90-d-old rats were decapsulated, and seminiferous tubules were minced by sterile scalpels in a Petri dish with F12/DMEM for ∼15 min. Thereafter, total germ cells released into the supernatant were collected by centrifuge at 100 g for 2 min. Pelleted tubules (minced) were washed twice with fresh F12/DMEM to release remaining germ cells, and supernatants from these two washes (each centrifuged at 100 g for 2 min) containing total germ cells were collected and pooled. The supernatant was then filtered with Mira cloth (EMD Millipore) and a 100-μm cell strainer (BD Falcon; BD Biosciences). Total germ cells were pelleted at 500 g for 10 min, and the pelleted cells were washed 3 times with F12/DMEM. Pelleted cells were then reconstituted in F12/DMEM and filtered with a 40-μm cell strainer. Filtered cells were pelleted at 500 g for 10 min and resuspended in medium as for primary Sertoli cells (27) supplemented with 6 mM sodium lactate and 2 mM sodium pyruvate. At this time, recombinant sFRP1 was included in a concentration of 2.5 μg/ml. Sertoli-germ cell cocultures were terminated 2 d thereafter in immunoprecipitation lysis buffer (30). To overexpress sFRP1 in Sertoli-germ cell cocultures, Sertoli cells were seeded at a density of 0.3 × 106 cells/cm2. This cell density was selected as it gave the strongest overexpression, and TJ and basal ES were still maintained between Sertoli cells. Lentiviral vectors carrying sFRP1 or lacZ transgene were added at a multiplicity of infection of 2 in the presence of 4 μg/ml polybrene 2 d after Sertoli cell isolation. Fresh medium was replaced 3 h later. Sertoli cells were maintained for another 2 d before addition of total germ cells at a Sertoli:germ cell ratio of 1:5. Sertoli-germ cell cocultures were further incubated for 2 d before cell lysate and conditioned medium were harvested.
Immunoblotting and immunoprecipitation
Immunoblotting and immunoprecipitation were performed essentially the same as described previously (31). Protein lysate (25 μg) and Sertoli-germ cell coculture-conditioned medium (50 μl) were used for immunoblotting. For immunoprecipitation of nectin-3, 300 μg protein was used. All membranes were blocked in 5% nonfat dry milk (w/v) except for detection of p-FAK-Tyr397 and p-tyrosine (Tyr) where membranes were blocked in 5% BSA (w/v) and washed with TBS (15.2 mM Tris HCl, 4.6 mM Tris, and 150 mM NaCl, pH 7.6, at 22°C) containing 0.1% Tween 20. All the antibodies used in different experiments reported herein are listed in Table 1.
Table 1.
Antibodies used in this report
| Antibody | Vendor | Catalog no. | Application/working dilution |
|---|---|---|---|
| Rabbit anti-N-cadherin | Santa Cruz Biotechnology (Santa Cruz, CA, USA) | sc-7939 | IB (1:200) |
| Rabbit anti-α-catenin | Santa Cruz Biotechnology | sc-7894 | IB (1:200) |
| Rabbit anti-β-catenin | Santa Cruz Biotechnology | sc-7199 | IB (1:200) |
| Rabbit anti-nectin-3 | Santa Cruz Biotechnology | sc-28637 | IB (1:200) |
| IP (2 μg IgG/reaction) | |||
| Goat anti-nectin-3 | Santa Cruz Biotechnology | sc-14806 | IF (1:50) |
| Rabbit anti-afadin | Sigma (St. Louis, MO, USA) | A 0349 | IB (1:500) |
| Goat anti-JAM-C | R&D Systems (Minneapolis, MN, USA) | AF1213 | IB (1:500) |
| Rabbit anti- p-FAK-Tyr397 | Thermo Scientific (Waltham, MA, USA) | PA1-22001 | IB (1:1000) |
| IF (1:50) | |||
| Rabbit anti-FAK | Santa Cruz Biotechnology | sc-558 | IB (1:500) |
| Mouse anti-GAPDH | Abcam (Cambridge, MA, USA) | ab8245 | IB (1:1000) |
| Mouse anti-Arp3 | Sigma | A5979 | IB (1:3000) |
| IF (1:150) | |||
| Rabbit anti-p-Tyr | Life Technologies (Carlsbad, CA, USA) | 61-5800 | IB (1:500) |
| Rabbit anti-sFRP1a | Cell Signaling Technology (Danvers, MA, USA) | 4690 | IB (1:1000) |
| Rabbit anti-sFRP1 | Santa Cruz Biotechnology | sc-7425 | IB (1:200) |
| Rabbit anti-testin | Cheng et al. (73) | / | IB (1:1000) |
IB, immunoblotting; IP, immunoprecipitation; IF, immunofluorescence microscopy. aAnti-sFRP1 antibodies from Cell Signaling Technology and Santa Cruz Biotechnology detected overexpressed sFRP1 in both lysates and conditioned medium from Sertoli-germ cell cocultures; however, results shown in Fig. 6A were obtained using anti-sFRP1 antibody from Cell Signaling Technology.
Immunofluorescence microscopy
Frozen testis sections from sFRP1-treated or control (BSA-treated) rats were fixed and stained for the antibodies listed in Table 1. To colocalize nectin-3 with p-FAK-Tyr397, sections were fixed in methanol at −20°C for 10 min. For actin [phalloidin-fluorescein isothiocyanate (FITC), 1:100; Sigma] and Arp3 colocalization, sections were fixed in 4% paraformaldehyde/PBS (w/v) at room temperature for 10 min, followed by permeabilization with 0.2% Triton X-100 (v/v) for 5 min. Image acquisition and analysis were performed as described previously (30, 31).
Electron microscopy
The presence of functional junctions in the Sertoli-germ cell cocultures, in particular apical ES, was characterized by electron microscopy essentially as earlier described (29, 32). In brief, Sertoli cells cultured alone for 4 d at 0.5 × 106 cells/cm2 on Matrigel-coated 60-mm dishes (with a hypotonic treatment ∼36 h after isolation to lyse residual germ cells, as described in ref. 33) or cocultured with total germ cells using a Sertoli:germ cell ratio of 1:5 for an additional 2 d, were terminated by fixing in 2.5% (v/v) glutaraldehyde and 2.5% (w/v) paraformaldehyde in 0.1 M cacodylate buffer (pH 7.5 at 22°C) for 1 h. Cells were postfixed in OsO4 and stained with uranyl acetate. After dehydration in ascending graded ethanol, Sertoli cells or cocultured Sertoli and germ cells were detached from the 60-mm dishes by propylene oxide treatment and embedded in EPON blocks (Electron Microscopy Sciences, Fort Washington, PA, USA; ref. 34). Silver sections were obtained in a Reichert Ultracut E ultramicrotome (Reichert, Depew, NY, USA), and electron micrographs were obtained using a Jeol 100CXII electron microscope (Jeol USA, Peabody, MA, USA) at 80 kV. Electron microscopy was performed at the Rockefeller University Bio-Imaging Core Facility.
General methods
Protein estimation was determined by using DC protein assay kits (Bio-Rad, Hercules, CA, USA) and a Bio-Rad spectrophotometer (Model 680). The authenticity of cDNA clones and PCR products was confirmed by direct nucleotide sequencing at Genewiz.
Statistical analysis
All data presented here are results from ≥3 independent experiments using different animals and batches of testicular cells. Significance was determined by 1-way ANOVA followed by Dunnett's procedure using the GB-STAT 7.0 statistical analysis software package (Dynamic Microsystems, Silver Spring, MD, USA).
RESULTS
Expression of sFRP1 in the testis is developmentally regulated
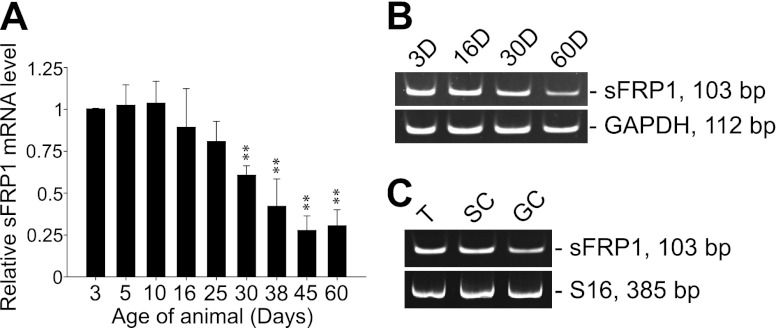
sFRP1 mRNA has been detected in both embryonic and adult testis (14, 25). However, it remains unknown whether there is change in the level of sFRP1 mRNA during postnatal development of the testis. Testes from 3- to 60-d-old rats were used for qPCR analysis, and a gradual decline in sFRP1 mRNA level was noted during development. Significant down-regulation of sFRP1 mRNA was detected beginning in 30-d-old testis when chromatin condensation was found in round spermatids while differentiating into elongating spermatids (ref. 35 and Fig. 1A). The lowest sFRP1 mRNA level in the testis was observed by 45 d of age, which persisted in 60-d-old testis (∼4-fold reduction in sFRP1 expression in 45- and 60-d-old vs. 3-d-old testes; Fig. 1A), coinciding with the first wave of spermiation that took place at 45 d of age (35), illustrating that sFRP1 down-regulation may be related to the onset of spermiation. Testicular sFRP1 mRNA level from selected ages of animals was also analyzed by PAGE following RT-PCR (Fig. 1B), consistent with findings shown in Fig. 1A. Next, we determined which cell types in the seminiferous epithelium expressed sFRP1 and we found that both Sertoli and germ cells expressed relatively similar steady-state levels of sFRP1 mRNA (Fig. 1C). However, by using two commercially available sFRP1 antibodies (see Table 1), we failed to detect sFRP1 protein in testis (including from 3-d-old rat), Sertoli and germ cells, and Sertoli cell- or germ cell-conditioned medium unless after sFRP1 overexpression in Sertoli-germ cell cocultures by lentiviral vectors (see below). We reasoned that the sFRP1 protein level under basal conditions in testicular cells was below the detection limits of these antibodies due to their low titers.
Figure 1.
Expression of sFRP1 in the testis is down-regulated during postnatal development, coinciding with the first wave of spermiation. A) RNA from 3- to 60-d-old rat testes was extracted, reverse transcribed, and analyzed for sFRP1 mRNA level by real-time qPCR. Steady-state level of sFRP1 mRNA was normalized to that of GAPDH. sFRP1 mRNA level from all ages of animals was compared with that from 3-d-old testis, which was arbitrarily set at 1. Significant decrease in sFRP1 mRNA was detected starting from 30-d-old testis, reached its lowest level and remained relatively unaltered in 45- and 60-d-old testis, coinciding with the first wave of spermiation that took place at 45 d of age. Three rats from each age were used to determine the amount of sFRP1 mRNA by qPCR with each sample analyzed in triplicates. Data points are means ± sd of n = 3 rats. **P < 0.01. B) RT-PCR products of sFRP1 and GAPDH from 4 representative time points were resolved by polyacrylamide gel. GAPDH served as a control. C) sFRP1 mRNA was detected in adult rat testes (T), Sertoli cells (SC; isolated from 20-d-old rat testes), and germ cells (GC; isolated from adult rat testes). S16 served as a loading control. The authenticity of the PCR products that confirmed the presence of sFRP1 in the testis and testicular cells was assessed by nucleotide sequencing at Genewiz.
sFRP1 delays spermiation in vivo
Since the findings shown in Fig. 1 implicate that down-regulation in sFRP1 might be involved in the control of spermiation, we therefore examined this role by administering recombinant sFRP1 protein intratesticularly in adult rats. A single dose of 4 μg sFRP1 recombinant protein was administered to each testis (2.5 μg/ml or 67.6 μM) in 90-d-old rats. Testes were obtained and processed for paraffin sections for morphological examinations at 6 h and 1, 2, and 4 d after treatment. The amount of sFRP1 used in our experiment is within the range to exert biological functions in the literature (36–38). Also, this concentration was selected based on initial in vitro pilot experiments using Sertoli-germ cell cocultures using different concentrations of recombinant sFRP1 to determine changes in the phosphorylation status of pFAK (see below). Thereafter, a concentration of 2.5 μg/ml (i.e., 67.6 mM) was selected for all in vivo experiments, which was found to induce morphological changes and was therefore used for subsequent in vivo experiments. About 800-900 tubules were randomly scored in cross-sections of testis per rat per time point (n=3 rats for each of the 5 time points) and the number of stage VIII, late VIII, and IX tubules was tabulated (Fig. 2). Tubules that had ≤50% elongated spermatids remaining in the seminiferous epithelium after spermiation were classified as late stage VIII tubule (Fig. 2A, B). After treatment of sFRP1 for 1 d, there was a significant increase in the number of tubules at stage VIII, without any sign of spermiation (5.4% in control vs. 7.8% in 1 d treatment group), and this pattern was similar to rat testes by 2 d after treatment (5.4% in control vs. 8.2% in 2 d treatment group; Fig. 2). As a result, the number of late stage VIII tubules in 1 d sFRP1-treated testis was lower (3.2% in control vs. 1.5% in 1 d treatment group; Fig. 2C). The phenotype of sFRP1 treatment group with spermiation arrest lasted until 2 d after treatment (stage VIII tubules: 5.4% in control vs. 8.2% in 2 d and late stage VIII tubules: 3.2% in control vs. 1.1% in 2 d; Fig. 2C). Eventually, spermiation took place by 4 d post-treatment, and the number of late stage VIII tubules returned to normal (3.2% in control vs. 2.4% in 4 d treatment group; Fig. 2C). Nonetheless, the number of stage IX tubules, in which spermiation had occurred and elongated spermatids was no longer found in the epithelium, was significantly lower 4 d after sFRP1 treatment (3.7% in control vs. 1.4% in 4 d treatment group; Fig. 2C), due to the delay in spermiation. It is noted that other than a delay in spermiation, the testes at 6 h and 1–4 d from rats after sFRP1 treatment displayed normal morphology, and spermatids at all other stages were not affected.
Figure 2.
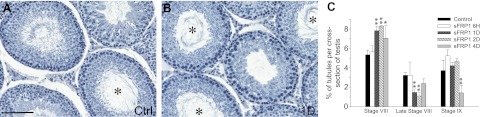
sFRP1 induces delay in spermiation. Ninety-day-old rats received 4 μg sFRP1 per testis (2.5 μg/ml, 67.6 μM; assuming a testicular volume of 1.6 ml and sFRP1 Mr at 37 kDa) in a volume of ∼200 μl at time 0 via intratesticular injection with a 28-gauge needle. Rats were terminated after 6 h and 1, 2, and 4 d with n = ∼3–5 rats/time point, including controls. Equal amount of BSA was administered to control rats. Testes were fixed in Bouin's fixative, embedded in paraffin, sectioned to 5 μm thickness using a microtome, and stained for hematoxylin for histological analysis. A, B) Testis sections from control (Ctrl; A) and 1 d after sFRP1 treatment (B). Asterisks indicate stage VIII tubules with their numbers significantly increased in testes treated with sFRP1 for 1 d. Scale bar = 60 μm. C) Numbers of stage VIII, late stage VIII, and stage IX tubules in each testis cross-section from control vs. sFRP1-treated rats were scored and represented as percentage of these staged tubules against total number of tubules in each cross-section. Late stage VIII was defined as tubules with ≥50% elongated spermatids left the tubules. Statistics were performed by comparing percentage of tubules from each stage in cross-sections of testes in rats from different treatment groups vs. rats in the control group. Results are data from n = 3 rats, with ∼800-900 tubules/testis/animal. At 1 and 2 d after sFRP1 injection, significantly more tubules were seen at stage VIII that failed to undergo spermiation. At 4 d after treatment, spermiation began to resume after sFRP1 was metabolically cleared, but the number of stage IX tubules remained significantly lower vs. control due to delay in spermiation. Bars represent means ± sd. *P < 0.05, **P < 0.01.
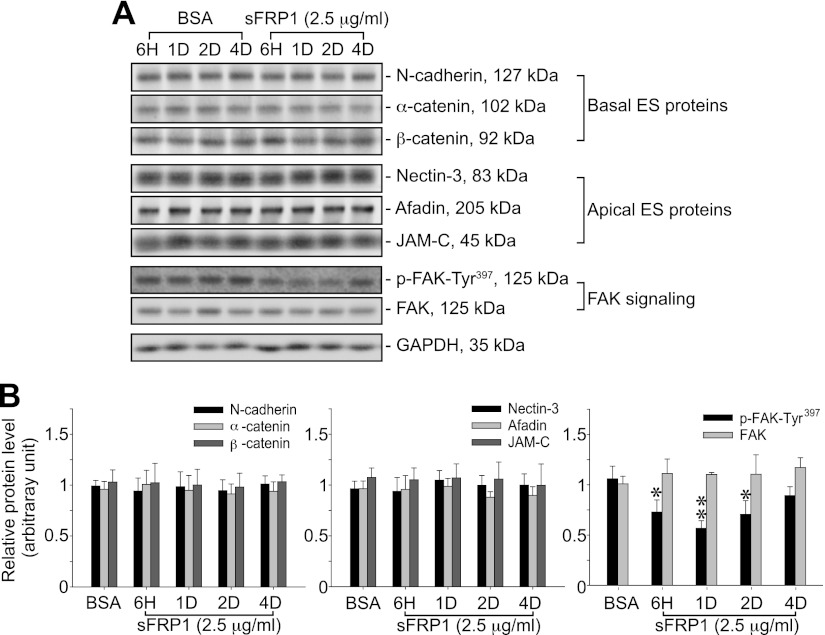
Down-regulation of p-FAK-Tyr397 during sFRP1-induced delay in spermiation
Since sFRP1 delayed spermiation, we next investigated whether there were any changes in the levels of apical ES proteins. Steady-state levels of several apical ES proteins (e.g., nectin-3, afadin, and JAM-C) did not change significantly after intratesticular injection of sFRP1 (Fig. 3). The levels of basal ES (e.g., N-cadherin, α-catenin, and β-catenin; Fig. 3) and TJ (e.g., occludin, CAR, JAM-A, and ZO-1; data not shown) proteins at the BTB also remain unchanged. Phosphorylation form of several protein kinases expressed at the apical ES [e.g., p-PKB-Ser473 (29), p-FAK-Tyr397, and p-ERK (39)] were also examined, and only p-FAK-Tyr397 was found to be significantly down-regulated as soon as 6 h after sFRP1 injection and persisted until 2 d (Fig. 3 and data not shown). Since the localization of p-FAK-Tyr397 in the seminiferous epithelium was limited to apical ES, displaying highly restricted spatiotemporal expression at the apical ES with its expression being highest at stage VII–VIII (ref. 40; see below), its down-regulation by sFRP1 implied that it may be the downstream signaling molecule of the sFRP1-induced defects in spermiation.
Figure 3.
sFRP1 inhibits phosphorylation of FAK-Tyr397. A) Testis lysates from BSA- or sFRP1-treated rats were analyzed by immunoblotting and probed for basal ES and apical ES proteins, and regulating proteins (e.g., p-FAK-Tyr397) known to affect spermiation. Steady-state levels of basal ES (N-cadherin, α-catenin, and β-catenin) and apical ES (nectin-3, afadin, and JAM-C) proteins remained unchanged. Phosphorylation of FAK-Tyr397 was inhibited by treatment of sFRP1 after 6 h and 1 and 2 d. GAPDH was used to ensure equal protein loading. B) Summarized composite results from ≥3 independent experiments. Protein levels of lysates from BSA control rat testis were averaged and set at 1, against which testis lysates from sFRP1-treated rats at the specified time points were compared. Bars represent means ± sd. *P < 0.05, **P < 0.01.
sFRP1 causes retention of adhesion protein complex at the apical ES
The delay in spermiation caused by sFRP1 following its administration in vivo suggested that the structures of the apical ES might be altered even though there were no changes in the steady-state levels of several apical ES proteins (Fig. 3). Immunofluorescence microscopy analysis revealed a significant change in the expression and localization of nectin-3, an apical ES protein, at the apical ES in stage VIII tubules after sFRP1 treatment (Fig. 4A). In control testis, nectin-3 was found to be strongest at stage VII at the apical ES (Fig. 4Aa–d), being used to confer cell adhesion, and its level considerably decreased to an almost undetectable level in stage VIII tubules (Fig. 4Ae–h), consistent with an earlier report (41). In contrast, nectin-3 remained at the apical ES in stage VIII tubules 1 d after sFRP1 administration (Fig. 4Am–p), wherein in stage VII tubules, the staining and localization of nectin-3 in the seminiferous epithelium were virtually indistinguishable between the control and treatment groups (Fig. 4Aa–d vs. i–l). On the other hand, laminin-γ3 chain, another cell adhesion protein at the apical ES, remained expressed on elongated spermatids even after they were depleting from the seminiferous epithelium (data not shown). To further investigate whether there were any changes in the adhesion complex at the apical ES, we performed dual-labeled immunofluorescence analysis for Arp3 and actin. Similar to our previous data (42), Arp3 was restricted to the concave side of the heads of elongating spermatids at stage VII, colocalizing with actin at the apical ES (Fig. 4Ba–c). As the elongated spermatids lined up at the adluminal edge of the tubule lumen at stage VIII before spermiation, staining of both Arp3 and actin diminished considerably to an almost undetectable level (Fig. 4Bd–f). Besides nectin-3, Arp3 and actin were also retained at the apical ES in stage VIII tubules 1 d after sFRP1 treatment, indicating retention of the apical adhesion complex (Fig. 4Bg–i). Previous studies have shown that p-FAK-Tyr397 expression was stage specific and localized to the apical ES at the dorsal (convex) side of the heads of elongating/elongated spermatids at stage VII–VIII (40, 43). Consistent with the previous reports, p-FAK-Tyr397 expression was up-regulated at stages VII–VIII at the apical ES in control testis (Fig. 4Bj, m). It was observed that nectin-3 colocalized with p-FAK-Tyr397 at stage VII (Fig. 4Bj–l), and most of the nectin-3 protein was absent from the apical ES site at stage VIII before spermiation (Fig. 4B, m-o). In line with the immunoblotting data, p-FAK-Tyr397 was found to be significantly weakened at the apical ES at stage VIII after sFRP1 intratesticular injection (Fig. 4Bp vs. j, m). Interestingly, weakened p-FAK-Tyr397 staining occurred concomitantly with abnormally strong nectin-3 staining in stage VIII tubules in sFRP1-treated testis (Fig. 4Bp–r).
Figure 4.
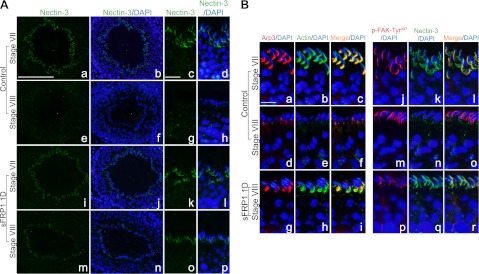
Delay in spermiation induced by sFRP1 is mediated by retention of apical ES complex. A) Frozen testis sections from BSA-treated control or sFRP1-treated rats for 1 d were fixed and stained for nectin-3 (green). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; blue). a–d) Nectin-3 was expressed at the apical ES site in stage VII tubules in control testis section. e–h) Just before spermiation in stage VIII, nectin-3 staining at the apical ES diminished considerably to an almost undetectable level. i–l) Level of nectin-3 did not change significantly in stage VII tubules after treatment of sFRP1 for 1 d. m–p) However, nectin-3 staining remained clearly visible at the apical ES in stage VIII tubules in sFRP1-treated testis. B) Dual-labeled immunofluorescence analysis was performed with Arp3 (red) and F-actin (green) (left panel) or with p-FAK-Tyr397 (red) and nectin-3 (green) (right panel). Nuclei were stained with DAPI (blue). a–c) Arp3 (red) colocalized with F-actin (green) at the ventral (concave) side of elongating spermatids in stage VII control tubule. b–f) Shortly before spermiation, Arp3 staining was considerably weakened with a concomitant disappearance of F-actin in stage VIII tubules, illustrating the disassembly of apical ES complex before spermiation. g–i) Arp3 and F-actin, however, remained at the concave side of elongated spermatids during sFRP1-induced delay in spermiation. j–l) p-FAK-Tyr397 (red) was found to express almost exclusively at the apical ES, colocalizing with nectin-3 (green) at the dorsal (convex) side of elongated spermatids. m–o) In control stage VIII tubule, p-FAK-Tyr397 was still detectable at the apical ES while nectin-3 staining was virtually lost. p–r) In contrast, p-FAK-Tyr397 at apical ES was considerably weaker at stage VIII in sFRP1-treated testis when compared with control, but nectin-3 staining was considerably stronger than the corresponding control. Scale bars = 60 μm (Aa, b, e, f, i, j, m, n); 20 μm (Ac, d, g, h, k, l, o, p); 12 μm (B).
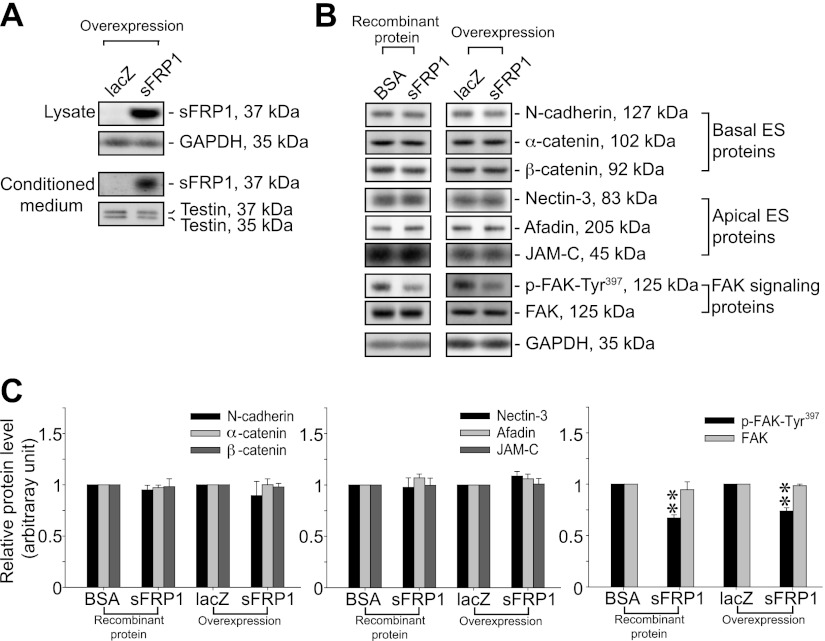
Overexpression of sFRP1 in Sertoli-germ cell cocultures with established apical ES leads to down-regulation of p-FAK-Tyr397
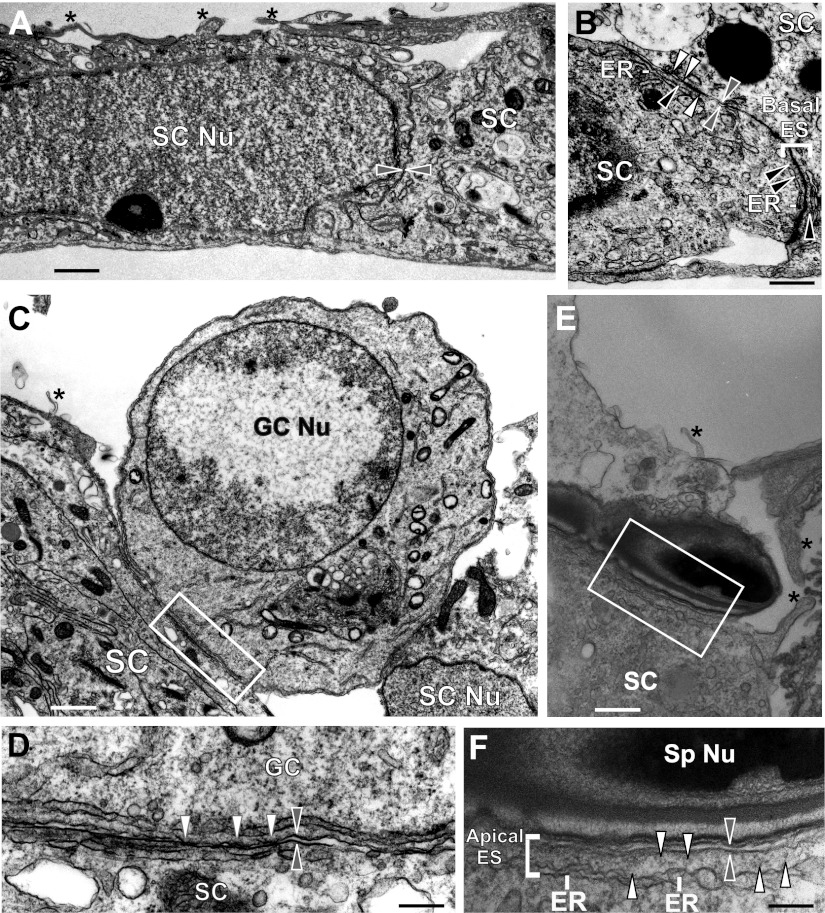
Based on findings reported in Figs. 2–4, it appeared that the persistent expression and localization of nectin-3 at the apical ES in stage VIII tubules following sFRP1 treatment were related to the down-regulation of p-FAK-Tyr397. We immunoprecipitated total nectin-3 using sFRP1-treated testis lysates and probed for p-Tyr to assess whether there were any changes in phosphorylated nectin-3 levels. However, this experiment did not yield consistent findings, since sFRP1-induced defects on spermiation were restricted to stage VIII tubules, which accounted for <10% of total tubules of the adult rat testis. Thus, changes in phosphorylation of nectin-3 at stage VIII could possibly be masked by nectin-3 in other stages. To overcome this challenge, we investigated the effects of sFRP1 in the Sertoli-germ cell coculture system in which sFRP1 protein was readily available to affect apical ES via the use of recombinant sFRP1 protein or its overexpression using lentiviral vectors. In addition, this coculture system that was characterized by electron microscopy shown in Fig. 5 eliminated the stage-specificity issue found in vivo. In the in vitro coculture system, primary Sertoli cells were cultured alone for 4 d in which TJ and basal ES were detected by electron microscopy that mimicked the Sertoli cell BTB in vivo (Fig. 5A, B) before the addition of adult total germ cells to allow desmosome assembly between Sertoli cells and spermatids (prestep 8), spermatocytes, and spermatogonia (Fig. 5C, D), as well as apical ES assembly between Sertoli cells and spermatids (step 8–19 spermatids) as shown in Fig. 5E, F. Recombinant sFRP1 protein (2.5 μg/ml) was added to the cocultures for 2 d to study its effect on the phosphorylation of FAK and nectin-3. Alternatively, sFRP1 was overexpressed by lentiviral vectors that had been shown to effectively produce high levels of recombinant proteins in Sertoli cells in vivo and in vitro (44, 45). As a secreted glycoprotein, sFRP1 was found in both Sertoli-germ cell coculture lysate and conditioned medium at high levels (Fig. 6A). It is noted that the endogenous sFRP1 protein was under the detection limit in control cells where lacZ transgene was overexpressed (Fig. 6A), perhaps due to the antibody titer. Similar to findings in vivo (Fig. 3), sFRP1 did not alter the steady-state levels of basal and apical ES proteins but significantly down-regulated p-FAK-Tyr397, but not total FAK, when recombinant sFRP1 protein was used or following its overexpression in Sertoli-germ cell cocultures (Fig. 6B, C). We did not perform electron microscopy to examine apical ES at the Sertoli-spermatid interface after addition of recombinant sFRP1 protein to the coculture or following lentiviral transfection since these treatments were noted to promote spermatid adhesion. Thus, this study was limited to biochemical analysis as shown in Fig. 6 and Fig. 7.
Figure 5.
Characterization of apical ES in Sertoli-germ cell cocultures. Sertoli cells were cultured at 0.5 × 106 cells/cm2 on Matrigel-coated 60-mm dishes in F12/DMEM with supplements at 35°C as described in Materials and Methods. About 36 h thereafter, cells were hypotonically treated to lyse residual germ cells as described previously (33). A) These highly purified Sertoli cells were cultured alone for 4 d, forming an intact epithelium (SC Nu, Sertoli cell nucleus). Two adjacent Sertoli cells (SC) with apposing Sertoli cell plasma membranes are annotated by apposing gray arrowheads. Cytoplasmic processes typical of Sertoli cell cultured in vitro were also seen (asterisks). B) Ultrastructures of tight junctions (white arrowheads) coexisted with basal ES [typified by the presence of actin filament bundles (black arrowheads) sandwiched between cisternae of endoplasmic reticulum (ER) of the apposing Sertoli cell plasma membranes (apposing gray arrowheads)], which mimicked the Sertoli cell BTB in vivo. C–F) At 4 d after Sertoli cells were cultured alone, total germ cells isolated from adult rat testis without the glass wool filtration steps to include elongating/elongated spermatids were added onto the Sertoli cell epithelium and cultured at a Sertoli:germ cell ratio of 1:5 for ∼2 d before termination for electron microscopy. C) Germ cell (see prominent germ cell nucleus, GC Nu) was attached to 2 Sertoli cells. Sertoli cell cytoplasmic process was seen (asterisk). D) Magnified view of boxed area in C. Desmosomes (white arrowheads) were typified by the presence of electron-dense substances at the Sertoli-germ cell interface (gray arrowheads indicate apposing Sertoli-germ cell plasma membranes). E) Functional apical ES was found between an elongating spermatid and a Sertoli cell (boxed area); asterisks indicate the typical cytoplasmic processes of Sertoli cells cultured in vitro. F) Magnified view of boxed area in E. Apical ES is typified by the presence of actin filament bundles (which laid perpendicular to the Sertoli cell plasma membrane, white arrowheads) sandwiched between cisternae of ER and the apposing plasma membranes of Sertoli cell and spermatid (apposing gray arrowheads). Sp Nu, spermatid nucleus. Scale bars = 5 μm (A); 1 μm (B); 3 μm (C); 0.5 μm (D); 2 μm (E); 0.5 μm (F).
Figure 6.
Inactivation of FAK by sFRP1 mediated via down-regulation of p-FAK-Tyr397 expression in Sertoli-germ cell cocultures. A) Sertoli cells (0.3×106 cells/cm2) were cultured for 2 d and transduced by lentiviral vectors (multiplicity of infection=2) to overexpress lacZ or sFRP1. Cells were incubated for 2 d after transduction before the addition of total germ cells (Sertoli:germ cell=1:5) including elongating/elongated spermatids and Sertoli cells to initiate apical ES assembly as described in Materials and Methods (see also Fig. 5). Sertoli-germ cocultures were harvested 2 d thereafter to obtain cell lysates and conditioned medium. High level of sFRP1 protein was found in both cell lysates and conditioned medium of Sertoli-germ cell cocultures, using GAPDH and testin as protein loading control, respectively. B) Left panel: Sertoli cells (0.5×106 cells/cm2) were cultured for 4 d before the addition of total germ cells (Sertoli:germ cell=1:5) and inclusion of recombinant sFRP1 (2.5 μg/ml). Protein lysates were harvested 2 d later in immunoprecipitation lysis buffer and probed for basal ES (e.g., N-cadherin, α-catenin and β-catenin) and apical ES (e.g., nectin-3, afadin and JAM-C) proteins, which were found to remain unchanged. Right panel: similarly, these protein levels did not change significantly in protein lysates from Sertoli-germ cell cocultures overexpressed with sFRP1 vs. lacZ. In contrast, phosphorylation of FAK-Tyr397 was significantly down-regulated in the presence of either recombinant or overexpressed sFRP1 protein. C) Bar graphs summarize results from 3 independent experiments in which each target protein was normalized against GAPDH and compared with the relative protein level in BSA or lacZ control, which was arbitrarily set at 1. Bars represent means ± sd. **P < 0.01.
Figure 7.
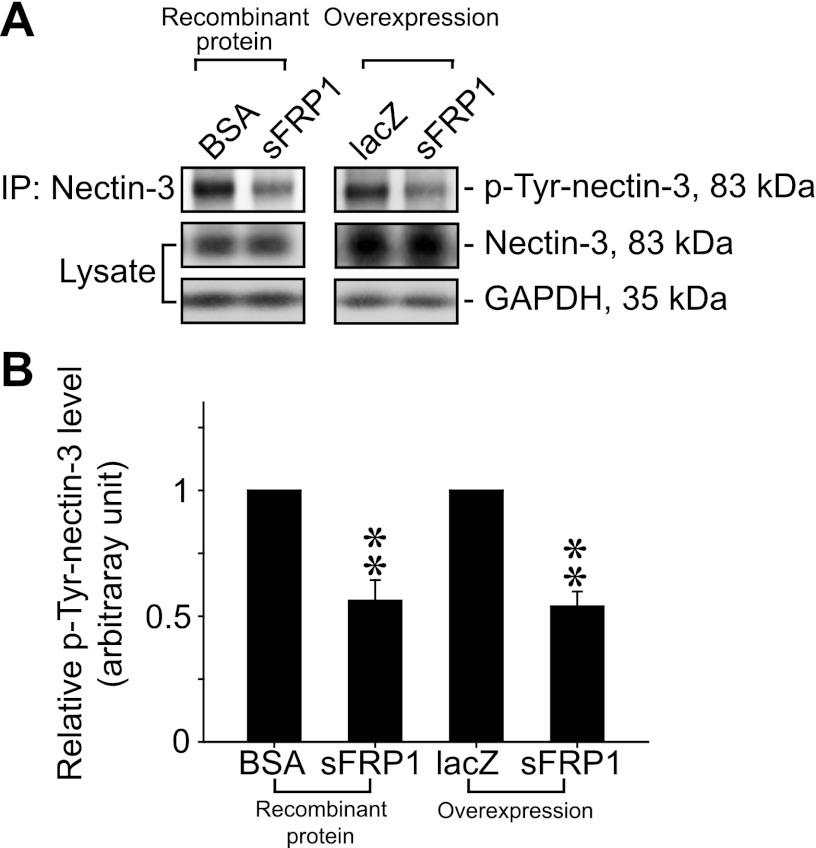
Decrease in phosphorylation of nectin-3 by sFRP1 in Sertoli-germ cell cocultures. A) Protein lysates from Sertoli-germ cell cocultures treated with recombinant sFRP1 (2.5 μg/ml; left panel) or following overexpression of sFRP1 by lentiviral transduction (right panel) were used for immunoprecipitation with nectin-3 antibody to obtain total nectin-3. Thereafter, the relative p-Tyr content of the immunoprecipitated nectin-3 was assessed by immunoblotting using an anti-phospho-Tyr antibody (p-Tyr-nectin-3). Total nectin-3 level and GAPDH did not change significantly in either treatment group vs. their corresponding controls. B) Histogram summarizes results from 3 independent experiments, illustrating that the presence of sFRP1 protein reduced tyrosine phosphorylation of nectin-3. Relative protein level of p-Tyr-nectin-3 in BSA or lacZ control was arbitrarily set at 1, against which statistical comparison was performed. Bars represent means ± sd. **P < 0.01.
sFRP1 induces a loss of Tyr phosphorylation in nectin-3
After treatment of Sertoli-germ cells with recombinant sFRP1 protein or transduction of cocultures with lentiviral vectors for sFRP1 overexpression, the phosphorylation state of nectin-3 at the apical ES was investigated. Anti-nectin-3 antibody was used to precipitate total nectin-3 protein and immunoblotted for p-Tyr protein. In line with our hypothesis, the level of p-Tyr-nectin-3 was significantly reduced by as much as 50% when recombinant sFRP1 was added to the Sertoli-germ cell cocultures or via its overexpression by lentiviral transduction (Fig. 7). Similar to findings in vivo (Fig. 3) and in vitro (Fig. 6), a change in p-Tyr-nectin-3 level shown in Fig. 7A was not a result of total nectin-3 level alteration.
DISCUSSION
Ultrastructures of apical ES were first discovered in the 1960s, and detailed morphological analysis of apical ES was completed in the 1970s in rodents (46–48). Under electron microscopy, apical ES is typified by the presence of hexagonally arranged actin filament bundles that lie perpendicular to the plasma membrane and sandwiched between the Sertoli cell plasma membrane and cisternae of endoplasmic reticulum, with no specific ultrastructure seen on the elongating/elongated spermatid (step 8–19) side (46–48). Since then, the apical ES has evolved from a sole adhesion structure to one that is also crucial for the orientation of elongating/elongated spermatids conferring spermatid polarity during spermiogenesis in the seminiferous epithelium (31). Several integral membrane adhesion complexes are found at the apical ES, which include N-cadherin-N-cadherin, β1-integrin-laminin-α3β3γ3, and nectin-2-nectin-3 (7). Information on the regulation and/or internalization of these integral membrane proteins mediating the disassembly of apical ES, in particularly at stage VIII at spermiation, is limited. Previous studies demonstrated that cytokines (e.g., tumor necrosis factor-α and transforming growth factor-β3) disrupt the adhesion between Sertoli cells and spermatids in vivo (49, 50). Similarly, suppression of testosterone in the testis causes detachment of spermatids from the seminiferous epithelium (51). Administration of a small molecule adjudin orally also preferentially disrupts the apical ES between elongating/elongated spermatids and Sertoli cells (7). Despite the fact that these studies commonly induce the loss of round spermatids besides elongating/elongated spermatids (7, 49–51), which is not observed during normal spermiation, they provide valuable information on the possible mechanisms that might initiate spermiation. In addition, recent studies also demonstrated the possible involvement of protein kinases at the apical ES in regulating spermiation. In LKB1-knockout mice, mature spermatozoa were retained in the seminiferous epithelium, where the apical ES failed to undergo disassembly and internalization (8). It is of interest to investigate whether the defects in spermiation are due to the effects of LKB1 on Wnt signaling (52, 53), which is also known to regulate cell junction restructuring (54). While there is no direct evidence that LKB1 regulates sFRPs or vice versa, sFRPs could possibly play a role in the regulation by LKB1 via modulating Wnts. Herein, we provided evidence that an elevated level of sFRP1 interferes with spermiation. Notably, no observable change was detected in tubules other than stage VIII when spermiation takes place in vivo. Expression of sFRP1 was found to be at its lowest after the onset of spermiation at 45 d postpartum in rats, and this diminished level of sFRP1 is likely required for the effective disassembly of adhesion complex at apical ES to facilitate spermiation. This also explains that sFRP1-knockout mice remain normal and fertile (26), because an inherent repression of sFRP1 is needed for normal spermatogenesis.
In this context, it is of interest to note that we did not include data that illustrate the relative protein levels of sFRP1 in Sertoli and germ cells, as well as its stage-specific expression and/or cellular localization in the seminiferous epithelium during the epithelial cycle. We attempted to quantify its levels in the testis and testicular cells by immunoblotting and its cellular distribution and stage-specific expression in the seminiferous epithelium by immunohistochemistry and immunofluorescence microscopy using different staining fixatives and approaches, including both paraffin sections plus different antigen retrieval methods (e.g., sodium citrate and EDTA buffer) and frozen sections with several commercially available antibodies (e.g., from Santa Cruz Biotechnology and Cell Signaling) without success. Yet, these antibodies were capable of recognizing recombinant sFRP1 protein (obtained from R&D Systems) and also sFRP1 overexpressed in Sertoli cells using a sFRP1 cDNA construct, as reported herein. Collectively, these experiments illustrate that the titers of these antibodies were low, and the endogenous protein levels of sFRP1 in the testis vs. Sertoli and germ cells were also low. However, it must be noted that the presence of sFRP1 in the testis and testicular cells and its relative expression in the testis during postnatal development were confirmed by both RT-PCR and/or qPCR, and its identity was validated by direct nucleotide sequencing.
It is noted that functional apical ES was detected in the Sertoli-germ cell cocultures used in this report based on analysis by electron microscopy as shown herein. However, we did not perform electron microscopy to assess whether “tighter” apical ES was formed, such as the presence of more actin filament bundles at the site, after addition or overexpression of sFRP1 in Sertoli-germ cell cocultures. Our biochemical data showed that an increase in actin filament bundles at the apical ES is probably not the only cause of the retention of elongating/elongated spermatids by sFRP1, since sFRP1 was found to down-regulate the expression of p-FAK-Tyr397 in the Sertoli-germ cell cocultures with functional apical ES, and p-FAK-Tyr397 was previously shown to display highly restrictive spatiotemporal expression at the apical ES at stage VII and VIII between step 18 and 19 elongated spermatids and Sertoli cells (40, 55). Instead, our findings suggest that sFRP1 possibly affects spermiation by delaying endocytosis of nectin-3 (via phosphorylation of FAK, such as mediated by p-FAK-Tyr397) at the apical ES based on our biochemical analysis. This postulate is also supported by other findings since extensive endocytic vesicle-mediated protein trafficking events are known to take place at the apical ES in stage VII-VIII tubules (56–58) that eventually leads to spermiation (59). Thus if sFRP1 only affects apical ES structurally and not stage specifically, one would expect to observe morphological changes in other stages of the epithelial cycle and spermatids (e.g., step 8–17 spermatids) where apical ES was also found, which we did not detect in our experiments.
As mentioned, previous models that were used to study apical ES dynamics provided useful information in identifying signaling molecules that are crucial to regulate spermiation. For instance, treatment of rats with adjudin (40) and suppression of testosterone in rats (60) were used as models to study apical ES dynamics (61–63), in which a transient increase in p-FAK-Tyr397 was detected in the testis when elongating/elongated spermatids were induced to deplete from the seminiferous epithelium. p-FAK-Tyr397 was found to be expressed exclusively at the apical ES, displaying highly restrictive spatiotemporal expression that was up-regulated at the apical ES in stage VII–VIII tubules (40, 43). Consistent with these earlier observations, we showed here that p-FAK-Tyr397 was highly expressed at stage VII and VIII of the epithelial cycle at apical ES in elongating/elongated spermatids, suggesting its possible role to phosphorylate adhesion proteins at the apical ES to elicit their internalization and hence to facilitate spermiation. Post-translational modification by phosphorylation is a common mechanism to regulate cellular localization of cell junction proteins, such as claudins and connexins (64, 65). On the other hand, knockout of endocytic recycling regulator Eps15 homology domain-containing protein 1 (EHD1) was shown to cause infertility in male offspring, with no mature spermatozoa in the epididymis (66). This further supports the importance of the endocytic pathway in the release of spermatids at spermiation (66). Indeed, our findings support this model in which sFRP1-induced down-regulation in p-FAK-Tyr397 would lead to a significant decline in Tyr-phosphorylation of nectin-3 and hence its internalization, leading to a delay in spermiation. p-FAK-Tyr397 was down-regulated following administration of sFRP1 recombinant protein in vivo or its overexpression in vitro. However, a reduced Tyr-phosphorylation of nectin-3 was only consistently detected in the in vitro Sertoli-germ cell coculture system. One possible explanation is that sFRP1 predominantly affects nectin-3 in stage VIII, which only accounts for ∼10% of tubules in any cross-sections of testis that were examined. Thus, changes in phosphorylation of nectin-3 would not readily be detected in the in vivo system unless stage VIII tubules were isolated for analysis. On the other hand, in vitro Sertoli-germ cell coculture has circumvented the stage-specific effect of sFRP1 on p-FAK-Tyr397 and nectin-3, in which significant decrease in Tyr-phosphorylation of nectin-3 was detected. In line with our observations, phosphorylation of FAK has been shown to be necessary for the entry of herpes simplex virus in mammalian epithelia cells via endocytosis of nectin-1 (67–69). During initial viral-cell contact, glycoprotein D of HSV first binds to nectin-1, which is one of the major receptors of HSV on mammalian cell surface, which subsequently triggers phosphorylation of FAK (68). The activation of FAK is essential for viral entry because overexpression of phosphorylation-defective mutant FAK (FAK Y397F) severely impeded viral infection (67). This is followed by rapid endocytosis of nectin-1 and virion to transport viral capsids to the nuclear pore to complete infection (69). This thus illustrates that internalization of nectin is mediated by phosphorylation of FAK.
Herein, we identified FAK as a downstream target for sFRP1-induced stabilization of cell junctions. Indeed, several studies have found that restoration of sFRPs in carcinomas inhibits their migratory ability (22, 24). For instance, expression of sFRPs in prostate and cervical cancer cells reverses their migratory mesenchymal phenotype to stationary epithelial phenotype (mesenchymal-epithelial transition) via increase in cell-cell contact and expression of E-cadherin (23, 24). Furthermore, epigenetic silencing of sFRPs often correlates with an increase in invasiveness and progression of tumor cells (23, 70). Conversely, Wnt has been shown to be activated in different carcinomas, inducing their migration (22, 71), which can be mediated by FAK (72). Collectively, findings from these other studies and the data reported here support the notion that sFRP1 is involved in spermatid adhesion and spermiation. In this context, it is noted that the low titer of the anti-sFRP1antibodies obtained from different vendors prohibited us from detecting the localization of sFRP1 in the seminiferous epithelium of rat testis during the epithelial cycle, as well as its expression by specific testicular cell types using immunoblotting, where we detected the expression of sFRP1 by using more sensitive techniques, such as RT-PCR and/or qPCR. Nonetheless, we have demonstrated unequivocally that overexpression or the use of exogenous sFRP1 promote spermatid adhesion in the epithelium and delay spermiation, via its effects on the expression and localization of nectin-3, in particular p-nectin-3, and by down-regulating p-FAK-Tyr397 at the apical ES. Thus, while these experiments do not establish a role for physiological levels of sFRP1 in regulating spermatid adhesion, this report supports the notion that sFRP1 is involved in spermatid adhesion, perhaps playing a crucial role in spermiation.
Acknowledgments
The authors thank Dr. Naama Kanarek for helpful discussion on the production of lentiviral vectors. The authors also appreciate the generous gifts of gag-pol and pMD.G plasmids from Prof. Yinon Ben-Neriah and Dr. Naama Kanarek (Hebrew University of Jerusalem, Jerusalem, Israel).
This work was supported by grants from the U.S. National Institutes of Health (National Institute of Child Health and Human Development, R01-HD-056034 to C.Y.C; U54-HD-029990 Project 5 to C.Y.C.).
Footnotes
- AJ
- adherens junction
- ATF2
- activating transcription factor 2
- B2M
- β-2-microglobulin
- BSA
- bovine serum albumin
- BTB
- blood-testis barrier
- CRD
- cysteine-rich domain
- ES
- ectoplasmic specialization
- FAK
- focal adhesion kinase
- p-FAK
- phosphorylated focal adhesion kinase
- FBS
- fetal bovine serum
- FITC
- fluorescein isothiocyanate
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- Hprt
- hypoxanthine-guanine phosphoribosyltransferase
- JAM-C
- juntional adhesion molecule C
- LacZ
- β-galactosidase
- LKB1
- liver kinase B1
- Mr
- relative molecular mass
- PBS
- phosphate-buffered saline
- PCR
- polymerase chain reaction
- Ppia
- peptidylproyl isomerase A
- qPCR
- quantitative polymerase chain reaction
- Rpl19
- ribosomal protein L19
- S16
- ribosomal protein S16
- sFRP
- secreted Frizzled-related protein
- SYBR green
- N′,N′-dimethyl-N-{4-[(E)-(3-methyl-1,3-benzothiazol-2-ylidene)methyl]-1-phenylquinolin-1-ium-2-yl}-N-propylpropane-1,3-diamine
- TJ
- tight junction
- TBP
- TATA box binding protein
- Tyr
- tyrosine
- Wnt
- wingless integration
REFERENCES
- 1. Baum B., Georgiou M. (2011) Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J. Cell Biol. 192, 907–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steed E., Balda M. S., Matter K. (2010) Dynamics and functions of tight junctions. Trends Cell Biol. 20, 142–149 [DOI] [PubMed] [Google Scholar]
- 3. Green K. J., Getsios S., Troyanovsky S., Godsel L. M. (2010) Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb. Perspect. Biol. 2, a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng C. Y., Mruk D. D. (2012) The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. 64, 16–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong E. W., Cheng C. Y. (2011) Impacts of environmental toxicants on male reproductive dysfunction. Trends Pharmacol. Sci. 32, 290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng C. Y., Mruk D. D. (2010) A local autocrine axis in the testes that regulates spermatogenesis. Nat. Rev. Endocrinol. 6, 380–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mruk D. D., Silvestrini B., Cheng C. Y. (2008) Anchoring junctions as drug targets: role in contraceptive development. Pharmacol. Rev. 60, 146–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Denison F. C., Smith L. B., Muckett P. J., O'Hara L., Carling D., Woods A. (2011) LKB1 is an essential regulator of spermatozoa release during spermiation in the mammalian testis. PLoS One 6, e28306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leyns L., Bouwmeester T., Kim S. H., Piccolo S., DeRobertis E. M. (1997) Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell 88, 747–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoang B., Moos M., Jr., Vukicevic S., Luyten F. P. (1996) Primary structure and tissue distribution of FRZB, a novel protein related to Drosophila frizzled, suggest a role in skeletal morphogenesis. J. Biol. Chem. 271, 26131–26137 [DOI] [PubMed] [Google Scholar]
- 11. Bovolenta P., Esteve P., Ruiz J. M., Cisneros E., Lopez-Rios J. (2008) Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J. Cell Sci. 121, 737–746 [DOI] [PubMed] [Google Scholar]
- 12. Kawano Y., Kypta R. (2003) Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 116, 2627–2634 [DOI] [PubMed] [Google Scholar]
- 13. Satoh W., Matsuyama M., Takemura H., Aizawa S., Shimono A. (2008) Sfrp1, Sfrp2, and Sfrp5 regulate the Wnt/beta-catenin and the planar cell polarity pathways during early trunk formation in mouse. Genesis 46, 92–103 [DOI] [PubMed] [Google Scholar]
- 14. Warr N., Siggers P., Bogani D., Brixey R., Pastorelli L., Yates L., Dean C. H., Wells S., Satoh W., Shimono A., Greenfield A. (2009) Sfrp1 and Sfrp2 are required for normal male sexual development in mice. Dev. Biol. 326, 273–284 [DOI] [PubMed] [Google Scholar]
- 15. Berndt T., Kumar R. (2007) Phosphatonins and the regulation of phosphate homeostasis. Annu. Rev. Physiol. 69, 341–359 [DOI] [PubMed] [Google Scholar]
- 16. Van Amerongen R., Nusse R. (2009) Towards an integrated view of Wnt signaling in development. Development 136, 3205–3214 [DOI] [PubMed] [Google Scholar]
- 17. Suzuki H., Gabrielson E., Chen W., Anbazhagan R., van Engeland M., Weijenberg M. P., Herman J. G., Baylin S. B. (2002) A genomic screen for genes upregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nat. Genet. 31, 141–149 [DOI] [PubMed] [Google Scholar]
- 18. Chim C. S., Pang R., Fung T. K., Choi C. L., Liang R. (2007) Epigenetic dysregulation of Wnt signaling pathway in multiple myeloma. Leukemia 21, 2527–2536 [DOI] [PubMed] [Google Scholar]
- 19. Veeck J., Niederacher D., An H., Klopocki E., Wiesmann F., Betz B., Galm O., Camara O., Durst M., Kristiansen G., Huszka C., Knuchel R., Dahl E. (2006) Aberrant methylation of the Wnt antagonist SFRP1 in breast cancer is associated with unfavourable prognosis. Oncogene 25, 3479–3488 [DOI] [PubMed] [Google Scholar]
- 20. Pole J. C., Courtay-Cahen C., Garcia M. J., Blood K. A., Cooke S. L., Alsop A. E., Tse D. M., Caldas C., Edwards P. A. (2006) High-resolution analysis of chromosome rearrangements on 8p in breast, colon and pancreatic cancer reveals a complex pattern of loss, gain and translocation. Oncogene 25, 5693–5706 [DOI] [PubMed] [Google Scholar]
- 21. Stoehr R., Wissmann C., Suzuki H., Knuechel R., Krieg R. C., Klopocki E., Dahl E., Wild P., Blaszyk H., Sauter G., Simon R., Schmitt R., Zaak D., Hofstaedter F., Rosenthal A., Baylin S. B., Pilarsky C., Hartmann A. (2004) Deletions of chromosome 8p and loss of sFRP1 expression are progression markers of papillary bladder cancer. Lab. Invest. 84, 465–478 [DOI] [PubMed] [Google Scholar]
- 22. Matsuda Y., Schlange T., Oakeley E. J., Boulay A., Hynes N. E. (2009) WNT signaling enhances breast cancer cell motility and blockade of the WNT pathway by sFRP1 suppresses MDA-MB-231 xenograft growth. Breast Cancer Res. 11, R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chung M. T., Lai H. C., Sytwu H. K., Yan M. D., Shih Y. L., Chang C. C., Yu M. H., Liu H. S., Chu D. W., Lin Y. W. (2009) SFRP1 and SFRP2 suppress the transformation and invasion abilities of cervical cancer cells through Wnt signal pathway. Gynecol. Oncol. 112, 646–653 [DOI] [PubMed] [Google Scholar]
- 24. Zi X., Guo Y., Simoneau A. R., Hope C., Xie J., Holcombe R. F., Hoang B. H. (2005) Expression of Frzb/secreted Frizzled-related protein 3, a secreted Wnt antagonist, in human androgen-independent prostate cancer PC-3 cells suppresses tumor growth and cellular invasiveness. Cancer Res. 65, 9762–9770 [DOI] [PubMed] [Google Scholar]
- 25. Finch P. W., He X., Kelley M. J., Uren A., Schaudies R. P., Popescu N. C., Rudikoff S., Aaronson S. A., Varmus H. E., Rubin J. S. (1997) Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action. Proc. Natl. Acad. Sci. U. S. A. 94, 6770–6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bodine P. V., Zhao W., Kharode Y. P., Bex F. J., Lambert A. J., Goad M. B., Gaur T., Stein G. S., Lian J. B., Komm B. S. (2004) The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol. Endocrinol. 18, 1222–1237 [DOI] [PubMed] [Google Scholar]
- 27. Mruk D. D., Cheng C. Y. (2011) An in vitro system to study Sertoli cell blood-testis barrier dynamics. Methods Mol. Biol. 763, 237–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aravindan G. R., Pineau C. P., Bardin C. W., Cheng C. Y. (1996) Ability of trypsin in mimicking germ cell factors that affect Sertoli cell secretory function. J. Cell. Physiol. 168, 123–133 [DOI] [PubMed] [Google Scholar]
- 29. Siu M. K., Wong C. H., Lee W. M., Cheng C. Y. (2005) Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J. Biol. Chem. 280, 25029–25047 [DOI] [PubMed] [Google Scholar]
- 30. Wong E. W., Mruk D. D., Lee W. M., Cheng C. Y. (2010) Regulation of blood-testis barrier dynamics by TGF-beta3 is a Cdc42-dependent protein trafficking event. Proc. Natl. Acad. Sci. U. S. A. 107, 11399–11404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wong E. W., Mruk D. D., Lee W. M., Cheng C. Y. (2008) Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc. Natl. Acad. Sci. U. S. A. 105, 9657–9662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li M. W. M., Mruk D. D., Lee W. M., Cheng C. Y. (2009) Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int. J. Biochem. Cell Biol. 41, 2302–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Galdieri M., Ziparo E., Palombi F., Russo M. A., Stefanini M. (1981) Pure Sertoli cell cultures: a new model for the study of somatic-germ cell interactions. J. Androl. 2, 249–254 [Google Scholar]
- 34. Brown W. J., Farquhar M. G. (1989) Immunoperoxidase methods for the localization of antigens in cultured cells and tissue sections by electron microscopy. Methods Cell Biol. 31, 553–569 [DOI] [PubMed] [Google Scholar]
- 35. Clermont Y., Perry B. (1957) Quantitative study of the cell population of the seminiferous tubules in immature rats. J. Anat. 100, 241–267 [DOI] [PubMed] [Google Scholar]
- 36. Kele J., Andersson E. R., Villaescusa J. C., Cajanek L., Parish C. L., Bonilla S., Toledo E. M., Bryja V., Rubin J. S., Shimono A., Arenas E. (2012) SFRP1 and SFRP2 dose-dependently regulate midbrain dopamine neuron development in vivo and in embryonic stem cells. Stem Cells 30, 865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Plisov S. Y., Yoshino K., Dove L. F., Higinbotham K. G., Rubin J. S., Perantoni A. O. (2001) TGF beta 2, LIF and FGF2 cooperate to induce nephrogenesis. Development 128, 1045–1057 [DOI] [PubMed] [Google Scholar]
- 38. Han X., Amar S. (2004) Secreted frizzled-related protein 1 (SFRP1) protects fibroblasts from ceramide-induced apoptosis. J. Biol. Chem. 279, 2832–2840 [DOI] [PubMed] [Google Scholar]
- 39. Xia W., Cheng C. Y. (2005) TGF-beta3 regulates anchoring junction dynamics in the seminiferous epithelium of the rat testis via the Ras/ERK signaling pathway: an in vivo study. Dev. Biol. 280, 321–343 [DOI] [PubMed] [Google Scholar]
- 40. Siu M. K., Mruk D. D., Lee W. M., Cheng C. Y. (2003) Adhering junction dynamics in the testis are regulated by an interplay of beta 1-integrin and focal adhesion complex-associated proteins. Endocrinology 144, 2141–2163 [DOI] [PubMed] [Google Scholar]
- 41. Ozaki-Kuroda K., Nakanishi H., Ohta H., Tanaka H., Kurihara H., Mueller S., Irie K., Ikeda W., Sakai T., Wimmer E., Nishimune Y., Takai Y. (2002) Nectin couples cell-cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr. Biol. 12, 1145–1150 [DOI] [PubMed] [Google Scholar]
- 42. Lie P. P., Chan A. Y., Mruk D. D., Lee W. M., Cheng C. Y. (2010) Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc. Natl. Acad. Sci. U. S. A. 107, 11411–11416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beardsley A., Robertson D. M., O'Donnell L. (2006) A complex containing alpha6beta1-integrin and phosphorylated focal adhesion kinase between Sertoli cells and elongated spermatids during spermatid release from the seminiferous epithelium. J. Endocrinol. 190, 759–770 [DOI] [PubMed] [Google Scholar]
- 44. Ikawa M., Tergaonkar V., Ogura A., Ogonuki N., Inoue K., Verma I. M. (2002) Restoration of spermatogenesis by lentiviral gene transfer: offspring from infertile mice. Proc. Natl. Acad. Sci. U. S. A. 99, 7524–7529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Froment P., Vigier M., Negre D., Fontaine I., Beghelli J., Cosset F. L., Holzenberger M., Durand P. (2007) Inactivation of the IGF-I receptor gene in primary Sertoli cells highlights the autocrine effects of IGF-I. J. Endocrinol. 194, 557–568 [DOI] [PubMed] [Google Scholar]
- 46. Brokelmann J. (1963) Fine structure of germ cells and Sertoli cells during the cycle of the seminiferous epithelium in the rat. Z. Zellforsch. Mikrosk. Anat. 59, 820–850 [PubMed] [Google Scholar]
- 47. Russell L. (1977) Observations on rat Sertoli ectoplasmic (“junctional”) specializations in their association with germ cells of the rat testis. Tissue Cell 9, 475–498 [DOI] [PubMed] [Google Scholar]
- 48. Russell L., Clermont Y. (1976) Anchoring device between Sertoli cells and late spermatids in rat seminiferous tubules. Anat. Rec. 185, 259–278 [DOI] [PubMed] [Google Scholar]
- 49. Xia W., Mruk D. D., Lee W. M., Cheng C. Y. (2006) Differential interactions between transforming growth factor-beta3/TbetaR1, TAB1, and CD2AP disrupt blood-testis barrier and Sertoli-germ cell adhesion. J. Biol. Chem. 281, 16799–16813 [DOI] [PubMed] [Google Scholar]
- 50. Li M. W., Xia W., Mruk D. D., Wang C. Q., Yan H. H., Siu M. K., Lui W. Y., Lee W. M., Cheng C. Y. (2006) Tumor necrosis factor α reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J. Endocrinol. 190, 313–329 [DOI] [PubMed] [Google Scholar]
- 51. O'Donnell L., McLachlan R. I., Wreford N. G., de Kretser D. M., Robertson D. M. (1996) Testosterone withdrawal promotes stage-specific detachment of round spermatids from the rat seminiferous epithelium. Biol. Reprod. 55, 895–901 [DOI] [PubMed] [Google Scholar]
- 52. Ossipova O., Bardeesy N., DePinho R. A., Green J. B. (2003) LKB1 (XEEK1) regulates Wnt signalling in vertebrate development. Nat. Cell Biol. 5, 889–894 [DOI] [PubMed] [Google Scholar]
- 53. Jacob L. S., Wu X., Dodge M. E., Fan C. W., Kulak O., Chen B., Tang W., Wang B., Amatruda J. F., Lum L. (2011) Genome-wide RNAi screen reveals disease-associated genes that are common to Hedgehog and Wnt signaling. Sci Signal 4, ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Amin N., Vincan E. (2012) The Wnt signaling pathways and cell adhesion. Front. Biosci. 17, 784–804 [DOI] [PubMed] [Google Scholar]
- 55. Lie P. P. Y., Mruk D. D., Mok K. W., Su L., Lee W. M., Cheng C. Y. (2012) Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc. Natl. Acad. Sci. U. S. A. 109, 12562–12567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Young J. S., Guttman J. A., Vaid K. S., Vogl A. W. (2009) Tubulobulbar complexes are intercellular podosome-like structures that internalize intact intercellular junctions during epithelial remodeling events in the rat testis. Biol. Reprod. 80, 162–174 [DOI] [PubMed] [Google Scholar]
- 57. Young J. S., Guttman J. A., Vaid K. S., Vogl A. W. (2009) Cortactin (CTTN), N-WASP (WASL), and clathrin (CLTC) are present at podosome-like tubulobulbar complexes in the rat testis. Biol. Reprod. 80, 153–161 [DOI] [PubMed] [Google Scholar]
- 58. Young J. S., Vogl A. W. (2012) Focal adhesion proteins zyxin and vinculin are co-distributed at tubulobulbar complexes. Spermatogenesis 2, 63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vaid K. S., Guttman J. A., Babyak N., Deng W., McNiven M. A., Mochizuki N., Finlay B. B., Vogl A. W. (2007) The role of dynamin 3 in the testis. J. Cell. Physiol. 210, 644–654 [DOI] [PubMed] [Google Scholar]
- 60. Wong C. H., Xia W., Lee N. P., Mruk D. D., Lee W. M., Cheng C. Y. (2005) Regulation of ectoplasmic specialization dynamics in the seminiferous epithelium by focal adhesion-associated proteins in testosterone-suppressed rat testes. Endocrinology 146, 1192–1204 [DOI] [PubMed] [Google Scholar]
- 61. Cheng C. Y., Lie P. P. Y., Wong E. W. P., Mruk D. D., Silvestrini B. (2011) Adjudin disrupts spermatogenesis via the action of some unlikely partners: Eps8, Arp2/3 complex, drebrin E, PAR6 and 14-3-3. Spermatogenesis 1, 291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cheng C. Y., Mruk D. D., Silvestrini B., Bonanomi M., Wong C. H., Siu M. K. Y., Lee N. P. Y., Mo M. Y. (2005) AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: A review of recent data. Contraception 72, 251–261 [DOI] [PubMed] [Google Scholar]
- 63. O'Donnell L., Nicholls P. K., O'Bryan M. K., McLachlan R. I., Stanton P. G. (2011) Spermiation: the process of sperm release. Spermatogenesis 1, 14–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Findley M. K., Koval M. (2009) Regulation and roles for claudin-family tight junction proteins. IUBMB Life 61, 431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Leithe E., Sirnes S., Fykerud T., Kjenseth A., Rivedal E. (2012) Endocytosis and post-endocytic sorting of connexins. Biochim. Biophys. Acta 1818, 1870–1879 [DOI] [PubMed] [Google Scholar]
- 66. Rainey M. A., George M., Ying G., Akakura R., Burgess D. J., Siefker E., Bargar T., Doglio L., Crawford S. E., Todd G. L., Govindarajan V., Hess R. A., Band V., Naramura M., Band H. (2010) The endocytic recycling regulator EHD1 is essential for spermatogenesis and male fertility in mice. BMC Dev. Biol. 10, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cheshenko N., Liu W., Satlin L. M., Herold B. C. (2005) Focal adhesion kinase plays a pivotal role in herpes simplex virus entry. J. Biol. Chem. 280, 31116–31125 [DOI] [PubMed] [Google Scholar]
- 68. Galen B., Cheshenko N., Tuyama A., Ramratnam B., Herold B. C. (2006) Access to nectin favors herpes simplex virus infection at the apical surface of polarized human epithelial cells. J. Virol. 80, 12209–12218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stiles K. M., Milne R. S., Cohen G. H., Eisenberg R. J., Krummenacher C. (2008) The herpes simplex virus receptor nectin-1 is down-regulated after trans-interaction with glycoprotein D. Virology 373, 98–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wong S. C., Lo S. F., Lee K. C., Yam J. W., Chan J. K., Hsiao W. L. (2002) Expression of frizzled-related protein and Wnt-signalling molecules in invasive human breast tumours. J. Pathol. 196, 145–153 [DOI] [PubMed] [Google Scholar]
- 71. Polakis P. (2012) Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol. 4, pii: a008052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ashton G. H., Morton J. P., Myant K., Phesse T. J., Ridgway R. A., Marsh V., Wilkins J. A., Athineos D., Muncan V., Kemp R., Neufeld K., Clevers H., Brunton V., Winton D. J., Wang X., Sears R. C., Clarke A. R., Frame M. C., Sansom O. J. (2010) Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev. Cell 19, 259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cheng C. Y., Grima J., Stahler M. S., Lockshin R. A., Bardin C. W. (1989) Testins are two structurally related Sertoli cell proteins whose secretion is tightly coupled to the presence of germ cells. J. Biol. Chem. 264, 21386–21393 [PubMed] [Google Scholar]