Abstract
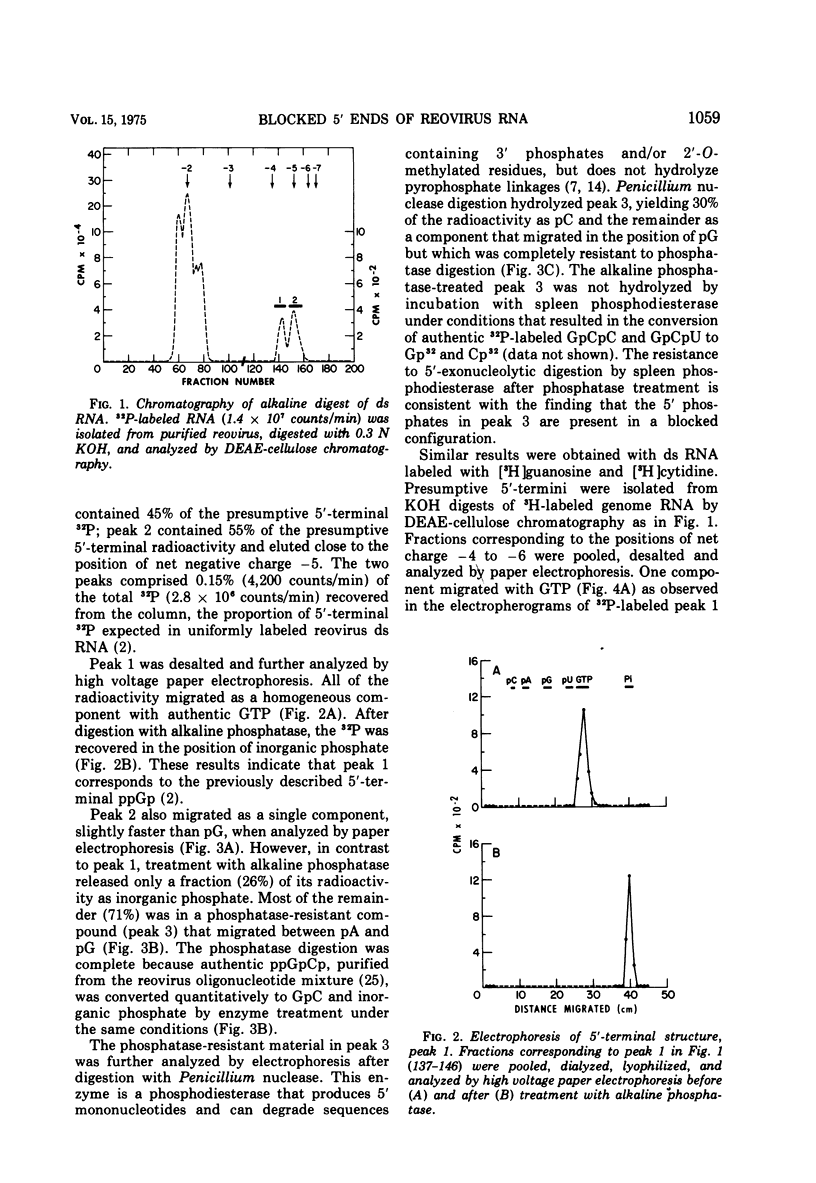
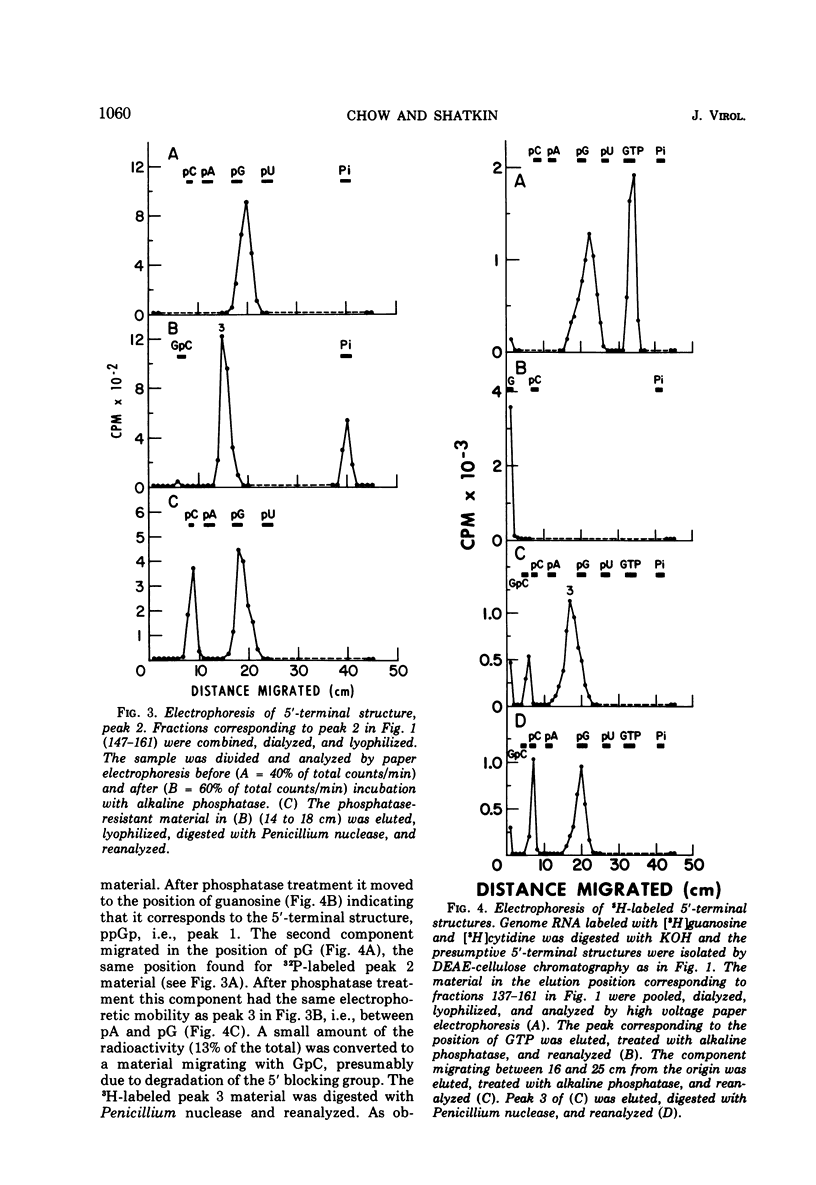
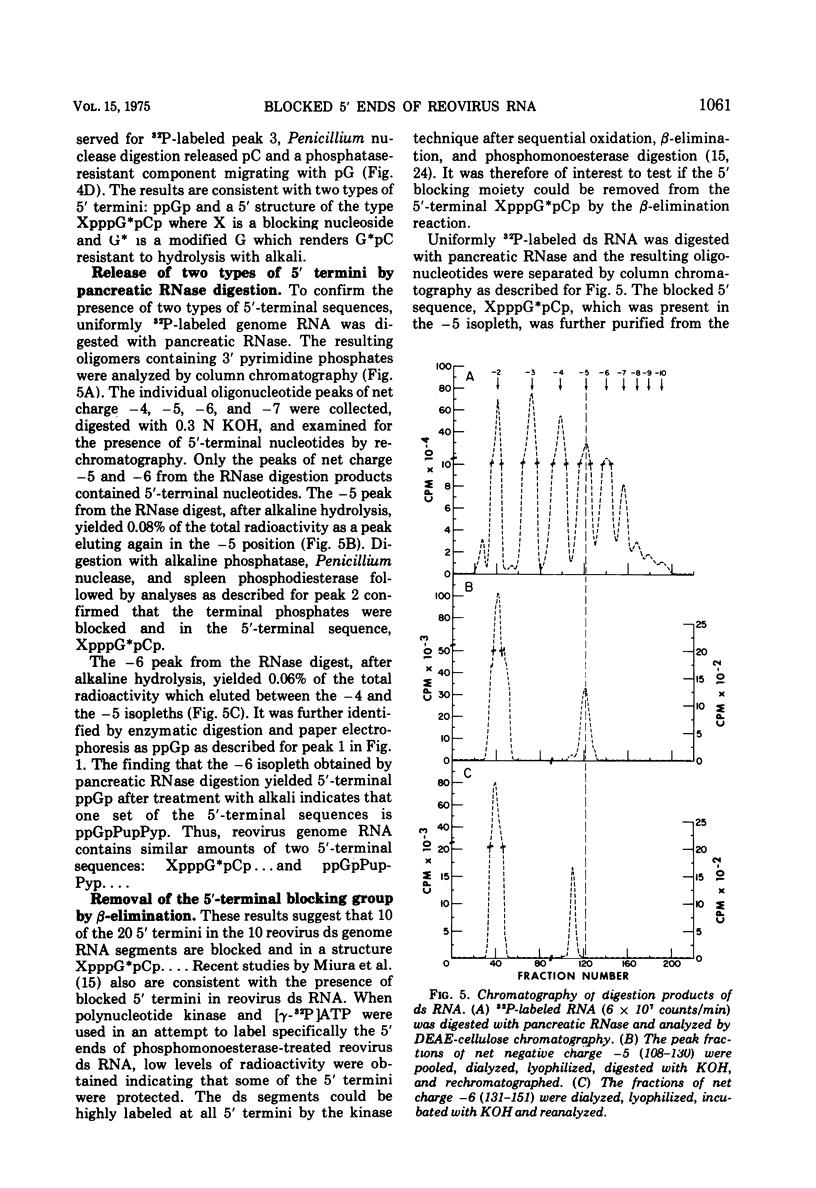
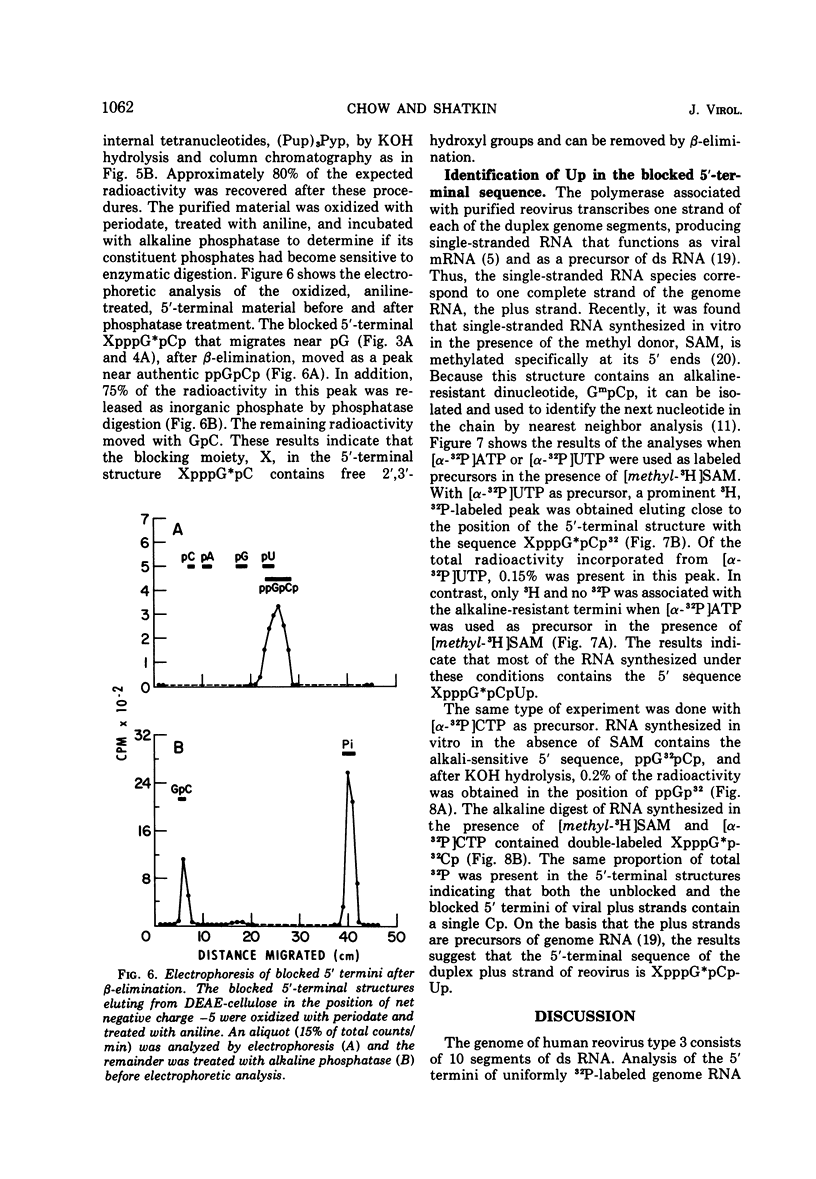
Uniformly 32P-labeled, double-stranded genome RNA isolated from purified reovirus contains two types of 5′-terminal sequences. One strand contains a phosphatase-resistant 5′-terminal structure, XpppG*pCpU, which is also present in the viral mRNA. The 5′ blocking group, X, is removed by β-elimination indicating that it is a nucleoside containing free 2′,3′-hydroxyls. G*pC is an alkaline-resistant, 2′-O-methylated sequence. The other strand contains a phosphatase-sensitive 5′ sequence, ppGpPupPyp. The results are discussed in relation to blocked 5′-terminal structures in other viral and cellular RNAs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K., Shatkin A. J. Guanosine-5'-diphosphate at the 5' termini of reovirus RNA: evidence for a segmented genome within the virion. J Mol Biol. 1971 Nov 14;61(3):643–653. doi: 10.1016/0022-2836(71)90069-6. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K., Shatkin A. J. Transcription in vitro by reovirus-associated ribonucleic acid-dependent polymerase. J Virol. 1970 Jul;6(1):1–11. doi: 10.1128/jvi.6.1.1-11.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. K., Ward R., Shatkin A. J. Initiation of reovirus mRNA synthesis in vitro. Nat New Biol. 1971 Apr 7;230(14):169–172. doi: 10.1038/newbio230169a0. [DOI] [PubMed] [Google Scholar]
- Borsa J., Graham A. F. Reovirus: RNA polymerase activity in purified virions. Biochem Biophys Res Commun. 1968 Dec 30;33(6):895–901. doi: 10.1016/0006-291x(68)90396-3. [DOI] [PubMed] [Google Scholar]
- Both G. W., Lavi S., Shatkin A. J. Synthesis of all the gene products of the reovirus genome in vivo and in vitro. Cell. 1975 Feb;4(2):173–180. doi: 10.1016/0092-8674(75)90124-5. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Miura K. A blocked structure at the 5' terminus of mRNA from cytoplasmic polyhedrosis virus. Nature. 1975 Jan 31;253(5490):374–375. doi: 10.1038/253374a0. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Muthukrishnan S., Shatkin A. J. Reovirus messenger RNA contains a methylated, blocked 5'-terminal structure: m-7G(5')ppp(5')G-MpCp-. Proc Natl Acad Sci U S A. 1975 Jan;72(1):362–366. doi: 10.1073/pnas.72.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOSSE J., KAISER A. D., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. VIII. Frequencies of nearest neighbor base sequences in deoxyribonucleic acid. J Biol Chem. 1961 Mar;236:864–875. [PubMed] [Google Scholar]
- Katz L., Penman S. The solvent denaturation of double-stranded RNA from poliovirus infected HeLa cells. Biochem Biophys Res Commun. 1966 May 25;23(4):557–560. doi: 10.1016/0006-291x(66)90765-0. [DOI] [PubMed] [Google Scholar]
- Levin D. H., Mendelsohn N., Schonberg M., Klett H., Silverstein S., Kapuler A. M., Acs G. Properties of RNA transcriptase in reovirus subviral particles. Proc Natl Acad Sci U S A. 1970 Jul;66(3):890–897. doi: 10.1073/pnas.66.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K., Watanabe K., Sugiura M. 5'-Terminal nucleotide sequences of the double-stranded RNA of silkworm cytoplasmic polyhedrosis virus. J Mol Biol. 1974 Jun 15;86(1):31–48. doi: 10.1016/s0022-2836(74)80005-7. [DOI] [PubMed] [Google Scholar]
- Miura K., Watanabe K., Sugiura M., Shatkin A. J. The 5'-terminal nucleotide sequences of the double-stranded RNA of human reovirus. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3979–3983. doi: 10.1073/pnas.71.10.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukrishnan S., Shatkin A. J. Reovirus genome RNA segments: resistance to S-1 nuclease. Virology. 1975 Mar;64(1):96–105. doi: 10.1016/0042-6822(75)90082-3. [DOI] [PubMed] [Google Scholar]
- Reddy R., Ro-Choi T. S., Henning D., Busch H. Primary sequence of U-1 nuclear ribonucleic acid of Novikoff hepatoma ascites cells. J Biol Chem. 1974 Oct 25;249(20):6486–6494. [PubMed] [Google Scholar]
- Rottman F., Shatkin A. J., Perry R. P. Sequences containing methylated nucleotides at the 5' termini of messenger RNAs: possible implications for processing. Cell. 1974 Nov;3(3):197–199. doi: 10.1016/0092-8674(74)90131-7. [DOI] [PubMed] [Google Scholar]
- Schonberg M., Silverstein S. C., Levin D. H., Acs G. Asynchronous synthesis of the complementary strands of the reovirus genome. Proc Natl Acad Sci U S A. 1971 Feb;68(2):505–508. doi: 10.1073/pnas.68.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J. Methylated messenger RNA synthesis in vitro by purified reovirus. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3204–3207. doi: 10.1073/pnas.71.8.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D., Loh P. Separation of ten reovirus genome segments by polyacrylamide gel electrophoresis. J Virol. 1968 Oct;2(10):986–991. doi: 10.1128/jvi.2.10.986-991.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D. RNA polymerase activity in purified reoviruses. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1462–1469. doi: 10.1073/pnas.61.4.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Joklik W. K. Studies on the in vitro transcription of reovirus RNA catalyzed by reovirus cores. Virology. 1969 Dec;39(4):822–831. doi: 10.1016/0042-6822(69)90019-1. [DOI] [PubMed] [Google Scholar]
- Steinschneider A., Fraenkel-Conrat H. Studies of nucleotide sequences in tobacco mosaic virus ribonucleic acid. IV. Use of aniline in stepwise degradation. Biochemistry. 1966 Aug;5(8):2735–2743. doi: 10.1021/bi00872a034. [DOI] [PubMed] [Google Scholar]
- Stoltzfus C. M., Banerjee A. K. Two oligonucleotide classes of single-stranded ribopolymers in reovirus A-rich RNA. Arch Biochem Biophys. 1972 Oct;152(2):733–743. doi: 10.1016/0003-9861(72)90269-x. [DOI] [PubMed] [Google Scholar]



