Abstract
Maintenance of female reproductive competence depends on the actions of several hormones and signaling factors. Recent reports suggest roles for bone morphogenetic proteins (BMPs) in early stages of folliculogenesis. A role for the type I BMP receptor BmprIB as a regulator of ovulation rates in sheep has been described recently, but little is known about the roles of BMP signaling pathways in other aspects of reproductive function. We report here that BMPRIB is essential for multiple aspects of female fertility. Mice deficient in BmprIB exhibit irregular estrous cycles and an impaired pseudopregnancy response. BmprIB mutants produce oocytes that can be fertilized in vitro, but defects in cumulus expansion prevent fertilization in vivo. This defect is associated with decreased levels of aromatase production in granulosa cells. Unexpectedly, levels of mRNA for cyclooxygenase 2, an enzyme required for cumulus expansion, are increased. BmprIB mutants also exhibit a failure in endometrial gland formation. The expression of BmprIB in uterine linings suggests that these defects are a direct consequence of loss of BMP signaling in this tissue. In summary, these studies demonstrate the importance of BMP signaling pathways for estrus cyclicity, estradiol biosynthesis, and cumulus cell expansion in vivo and reveal sites of action for BMP signaling pathways in reproductive tissues.
Members of the transforming growth factor β (TGF-β) superfamily transduce their signals via one of two distinct pathways: the activin/TGF-β, or the bone morphogenetic protein (BMP) pathway. TGF-βs and BMPs have diverse effects because the TGF-β/activin and BMP pathways activate different subsets of genes (reviewed in ref. 1). The pathway activated by individual ligands is based on affinities for specific type I receptors. For example, activins and TGF-βs recognize type I activin and TGF-β receptors, respectively, and activate the intracellular mediators SMADs 2 and 3, whereas BMPs recognize one of three type I BMP receptors (BMPRIA/ALK3, BMPRIB/ALK6, and ActRI/ALK2) and activate SMADs 1 and 5 (1, 2).
Several members of the TGF-β superfamily play essential roles in folliculogenesis in vivo. For example, BMP15/GDF9b and growth differentiation factor 9 (GDF9) are expressed in oocytes, and loss of either of these genes leads to defective preantral follicle development (3, 4). It is not known whether BMP15 and GDF9 use the activin/TGF-β, BMP, or an as-yet-unknown pathway. Therefore, although the fundamental role of the activin pathway has been clearly established in vivo (5, 6), much less is known about the potential functions of the BMP signaling pathway in female reproduction.
A recent in vitro study has demonstrated the existence of a functional BMP system in the ovary, showing that BMP4 and BMP7 can have positive and negative effects on follicle-stimulating hormone (FSH)-induced steroidogenesis in granulosa cells (7). Although these studies strongly support a function for the BMP pathway in responsiveness of granulosa cells to FSH, they do not address other potential roles for this pathway in female reproduction. It has been shown recently that the FecB allele, which increases ovulation rate and litter size in sheep, carries a point mutation in BmprIB (8, 9). Whether the FecB allele encodes a receptor with increased or decreased signaling activity or altered specificity is unknown.
To investigate the role(s) of BMP signaling pathways in female fertility, we have examined the reproductive phenotype of mice lacking BmprIB. Unlike mice lacking either of the other two type I BMP receptors (10–12), BmprIB−/− mice are viable. We show that BmprIB−/− females are infertile due to a constellation of defects, including irregular estrus cyclicity, impaired pseudopregnancy responses, severe defects in cumulus cell expansion, and insufficient uterine endometrial gland development. Thus, our results provide in vivo evidence for functions for BMP pathways in ovarian and uterine physiology.
Materials and Methods
Mating, Superovulation, and in Vitro Fertilization.
Generation of BmprIB-deficient mice was as described (13). BmprIB−/− or wild-type (WT) littermate virgin 5- to 6-week-old mice were used in natural matings. Immature (22–23 days old) mice were superovulated with pregnant mare serum gonadotropin (5 units, Sigma) and with human chorionic gonadotropin (5 units, Sigma) 48 h later (14). Oocytes and embryos were recovered from the uterus or oviduct and classified as described (14). In vitro fertilization of eggs obtained from superovulated females was performed on oocytes without surrounding cumulus cells as described (14) by using sperm from CD-1 males.
Histology, Electron Microscopy, and Hoechst Staining.
Paraffin sections (7 μm) of BmprIB−/− and WT tissues were stained with hematoxylin and eosin. Oocytes were staged according to the system of Pedersen and Peters (15). Ultrastructural analysis was performed on ovaries from 4-week-old mice by the University of California, Los Angeles, Electron Microscopy Core Facility. Chromatin patterns in oocytes were evaluated by staining with Hoechst 33258 and classified as described (16).
Vaginal Smears.
Estrous cycles were monitored by daily vaginal smears over a period of 2 months. A small drop of water was flushed into the vagina and then dried on a slide. Cells were fixed in methanol for 10 s, stained with 1% methylene blue for 2 min, and rinsed in tap water to remove excess stain. Smears were taken daily between 2 and 4 p.m. Smears were evaluated by an experienced observer blinded to the genotype of the mice.
In Situ Hybridization, Semiquantitative Reverse Transcription–PCR, and Slot-Blot Analysis.
In situ hybridization was performed by using a previously described BmprIB antisense RNA probe (13) and protocol (13) except that [α-33P]UTP was used and exposure was for 5 days. Nonradioactive in situ hybridization was performed as described (13). Total RNA from ovaries of immature (P22–23) mice 48 h after FSH treatment was prepared by using TRIzol (GIBCO/BRL). Slot blot analysis was performed as described by using probes for Cyp 19, Fshr, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (17–19). BmprIB, Cox-2, and EP2 levels were examined by semiquantitative reverse transcription–PCR on oligo(dT)-primed cDNA (Superscript, GIBCO/BRL) from ovarian total RNA using previously described primers for BmprIB (13), Cox-2 and EP2 (20), and GAPDH (21). Reactions were performed as described (13, 20, 21) for 18, 20, and 25 cycles. Quantitation of expression relative to GAPDH was performed by using imagequant software. Mean values and standard errors were calculated by using Microsoft EXCEL 98.
Results
BmrpIB−/− Females Are Infertile.
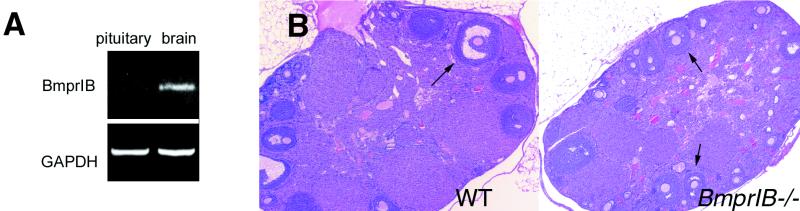
In over 30 matings of BmprIB−/− females over a period of 60 weeks, only two litters of one pup and five pups were generated from two different mothers. No differences were observed in the onset of mating as assessed by the time of appearance of the first copulatory plug (age at first plug: WT, 33.5 ± 3.6 days, n = 8; BmprIB mutants, 36.1 ± 4.3 days, n = 6). We next examined BmprIB expression in reproductive tissues of WT mice (Fig. 1). In ovaries, BmprIB is expressed in oocytes of maturing (type 6) follicles (Fig. 1 A and C), and in oocytes and granulosa cells of antral follicles (Fig. 1 B and C). No BmprIB transcripts are detected in granulosa cells of resting, primordial, developing (types 1–5b), or atretic follicles, corporal lutea, or thecal cells (Fig. 1 A–C). Our results are thus in agreement with studies of BmprIB expression in the rat (1). BmprIB also is expressed in uterine endometrium (Fig. 1D). Therefore, BmprIB is expressed in a pattern consistent with roles in folliculogenesis, fertilization, and/or implantation. No BmprIB transcripts are detected in the pituitary (Fig. 2A), suggesting that BmprIB does not play a direct role in this tissue in the regulation of FSH release. Histological analysis (Fig. 2B) and transmission electron microscopy (Fig. 3A) of ovaries were performed to examine whether mutants exhibit impaired follicle development. No obvious abnormalities or differences in the numbers of developing follicles or corpora lutea were observed in mutant vs. WT adult mice (Fig. 2B).
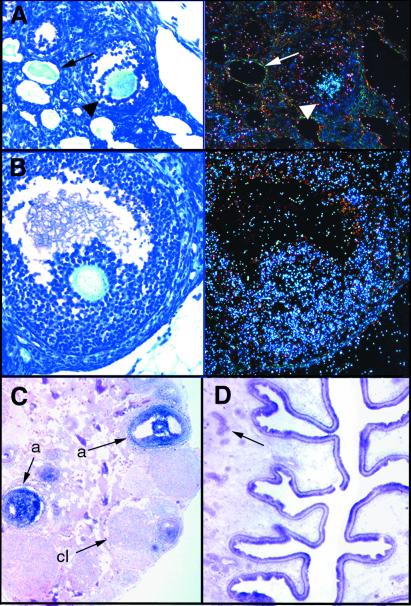
Figure 1.
Expression of BmprIB in adult ovary and uterus. (A and B) Bright-field (Left) and corresponding dark-field (Right) images of expression of BmprIB in (A) oocytes of maturing follicles (arrowhead) but not atretic follicles (arrow) and (B) antral follicles. (C and D) Nonradioactive in situ hybridization showing BmprIB expression in (C) antral follicles, but not in corpora lutea, and (D), in epithelium of the uterine endometrium and endometrial glands (arrow). a, antral follicle; cl, corpus luteum.
Figure 2.
Expression of BmprIB in pituitary and normal ovarian histology in BmprIB mutants. (A) Reverse transcription–PCR analysis of BmprIB expression in pituitary and brain of adult WT mice. (B) Sections through WT (Left) and mutant (Right) ovaries from 5-wk-old females. Ovaries from mutants and WT controls contain similar numbers of follicles at all stages of development, as well as corpora lutea, suggesting that ovulation takes place. Arrows point to antral follicles.
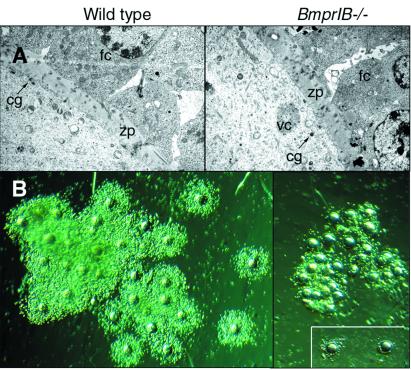
Figure 3.
Defective cumulus cell expansion in BmprIB mutants. (A) Transmission electron microscopy at the interface of the oocyte surface and granulosa cells in WT (Left) and BmprIB mutant (Right) mice. Cortical granules, a marker for oocyte maturation, are present in oocytes from mutants, and no differences were detected in the zona pellucida. (B) Cumulus cell-oocyte complexes from WT (Left) and mutant (Right) mice, photographed at the same magnification. (Inset) Two cumulus cell-oocyte complexes isolated from the cumulus mass. cg, cortical granules; g, granulosa cells; zp, zona pellucida.
Oocytes from BmprIB−/− Mice Can Be Fertilized in Vitro but Not in Vivo.
Because BmprIB is expressed in oocytes, we examined whether these were defective in mutants. Hoechst staining revealed normal chromatin configurations, germinal vesicle breakdown, and polar body formation in ovulated oocytes from mutants (data not shown). Ultrastructural analyses of oocytes within antral follicles revealed normal zona pellucidae and the presence of cortical granules (Fig. 3A). Therefore the infertility of BmprIB−/− females is unlikely to be attributable to a block in early stages of oocyte maturation. The recovery of similar numbers of oocytes from WT and mutant mice mated with fertile males at 0.5 days postcoitum (dpc) (Table 1), demonstrates that ovulation occurs in mutants. Moreover, mutants and WT mice display similar ovulatory responses to exogenous gonadotropins (Table 1). Therefore, events subsequent to ovulation were examined. At 1.5 dpc and 3.0 dpc, two-cell embryos and blastocysts, respectively, were recovered from reproductive tracts of WT females. However, only unfertilized oocytes were recovered from mutants (data not shown). Similar results were obtained at 0.5 dpc (Table 2). Therefore oocytes from BmprIB mutants fail to be fertilized in vivo, despite apparently normal responsiveness to gonadotropins.
Table 1.
Ovulation in WT and BmprIB−/− mice
| Ovulation | WT | BmprIB−/− | P value |
|---|---|---|---|
| Spontaneous | |||
| Mice ovulating | 3/3 (100%) | 4/4 (100%) | NS |
| Total eggs | 19 | 25 | |
| Eggs per mouse | 6.3 ± 3.2 | 6.3 ± 4.0 | NS |
| Hormonally induced | |||
| Mice ovulating | 12/12 (100%) | 12/14 (86%) | NS |
| Total eggs | 535 | 509 | |
| Eggs per mouse | 44.6 ± 19 | 36.4 ± 29 | NS |
Oocytes were recovered from spontaneously ovulating or hormonally induced females 0.5 days after mating to fertile males. Student's t test was used to assess statistical significance. NS, not significant.
Table 2.
Fertilization in WT and BmprIB−/− mice
| Fertilization | WT | BmprIB−/− | P value |
|---|---|---|---|
| In vivo | |||
| Eggs fertilized | 11/25 (44%) | 0/38 (0%) | <0.001 |
| (spontaneous ovulation) | (n = 3) | (n = 3) | |
| Eggs fertilized | 73/144 (51%) | 0/167 (0%) | <0.001 |
| (hormonally induced, 0.5 dpc) | (n = 4) | (n = 4) | |
| In vitro | |||
| Eggs fertilized | 84/274 (30.6%) | 35/173 (20.2%) | NS |
| (n = 12) | (n = 14) |
Oocytes or one-cell embryos were recovered from spontaneously ovulating or hormonally induced females 0.5 days after mating to fertile males. For in vitro fertilization, oocytes were collected from the oviducts of hormonally induced animals 19 hr after human chorionic gonadotrophin treatment. Cumulus cells were removed by treatment with hyaluronidase. In all experiments, oocytes had undergone germinal vesicle breakdown and extrusion of the first polar body. One-cell embryos (0.5 dpc) were classified as having two polar bodies and/or two pronuclei. Student's t test was used to assess statistical significance. NS, not significant.
We then performed in vitro fertilization experiments to determine whether oocytes from mutants are defective despite their apparently normal morphology and ultrastructure. Mutant oocytes can be fertilized in vitro, but not in vivo (Table 2). Eggs from BmprIB mutants fertilized in vitro progressed to the two-cell stage as efficiently as eggs from WT mice, providing further indication that oocyte quality is normal in mutants (data not shown). We therefore examined whether the viability or passage of sperm to the oviduct is defective in mutants. Examination of 0.5 dpc cumulus masses confirmed that normal numbers of motile sperm reach the isthmus of the oviduct in mutants (data not shown). Therefore, the absence of fertilization in mutants does not appear to be a result of an intrinsic defect in oocyte development or of a physical barrier preventing the union of eggs and sperm.
Defective Cumulus Expansion in BmprIB−/− Mice.
BmprIB is expressed in cumulus cells (Fig. 1B). Cumulus expansion promotes oocyte maturation and is essential for sperm penetration, and hence for fertilization (22). Therefore, we examined the morphology of cumulus-oocyte complexes recovered from the oviducts of WT and BmprIB mutant females after superovulation (Fig. 3B). Complexes from WT mice were expanded, and abundant cumulus cells were arranged radially around each oocyte. In contrast, complexes from BmprIB mutants were aggregated and retained fewer cumulus cells, indicating that loss of BmprIB prevents cumulus cell expansion in vivo. These data suggest that a primary cause of infertility in BmprIB mutants is a failure in cumulus cell expansion. This is consistent with the ability of oocytes from BmprIB mutants to be fertilized in vitro but not in vivo; cumulus cells improve fertilization rates but are not essential in vitro (23), and cumulus cells were removed in our experiments.
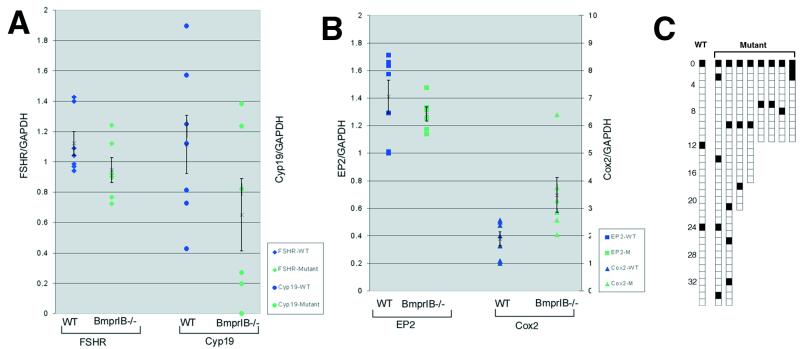
Cumulus cell expansion is thought to require the combined activity of FSH and a soluble factor(s) produced by the oocyte (24). We therefore examined levels of Fshr mRNA in ovaries from WT and mutant hormone-primed immature mice (before the onset of estrous cycles). Mean Fshr mRNA levels did not differ significantly between WT and mutant littermates (Fig. 4A), suggesting that defective cumulus expansion in mutants is not primarily due to decreased levels of Fshr expression. Several studies have indicated an essential role for prostaglandin E2 (PGE2) in cumulus expansion (20, 25), and this activity is mediated through the EP2 prostaglandin receptor (Ptgerep2) (23). Therefore, we examined the levels of expression of Ptgerep2 and cyclooxygenase 2 (Cox2;Ptgs2), the rate-limiting enzyme in the synthesis of prostaglandins. No significant differences in EP2 levels were observed, but levels of Cox2 mRNA were significantly increased in BmprIB mutants (Fig. 4B). This increase was unexpected given that prostanoids, the derivatives of COX-2 activity, are required for cumulus expansion in vivo (23). This result may reflect compensatory up-regulation of prostanoid signaling pathways due to the impairment in cumulus expansion and suggests that increased signaling through this pathway cannot compensate for loss of signaling through BMPRIB.
Figure 4.
Expression of Fshr, Cyp 19 (aromatase), prostaglandin receptor EP2, and Cox2, and pseudopregnancy responses. (A) Slot blot analysis of Fshr, and Cyp 19 (aromatase) expression normalized to GAPDH in ovaries from immature noncycling (P22–23) WT and BmprIB mutant mice 48 h after treatment with FSH. (B) Reverse transcription–PCR analysis of Ptgs2 (Cox2) and EP2 expression normalized to GAPDH in ovaries from immature noncycling (P22–23) WT and mutant mice 48 h after treatment with FSH. Mean values and standard errors are represented by an X and bars, respectively. Each square or triangle represents a measurement from an individual mouse. (C) Impaired pseudopregnancy responses in BmprIB−/− mice. Mating frequency in BmprIB−/− mice as assessed by the appearance of copulatory plugs. BmprIB−/− (mutant) and WT females housed individually with vasectomized males were checked daily for the presence of a vaginal plug, indicated by a filled box. Data for a single WT female are shown.
Impaired Estrus Cyclicity and Pseudopregnancy Responses in BmprIB−/− Mutants.
After mating with vasectomized males, females normally experience a pseudopregnancy response in reaction to stimulation of the cervix (26). During the subsequent 11–12 days, the females will not accept males for mating. Unexpectedly, BmprIB mutant females frequently mated over consecutive days (Fig. 4C), suggesting an impaired pseudopregnancy response. Moreover, monitoring of estrous cycles by vaginal smears revealed that 33% (n = 2/6) of BmprIB mutants exhibited an extended period of leukocytic smears, indicative of a prolonged di-estrous phase (data not shown).
The observation that mutant females display abnormal pseudopregnancy responses and ovulatory cycles characteristic of a prolonged di-estrous phase suggests delay or impairment in endogenous estradiol (E2) production. BmprIB and the gene product of the Cyp 19 gene, P450 aromatase, which converts androgens into E, are coexpressed in granulosa cells, raising the possibility that E2 production is impaired in mutants. Direct comparisons of E2 levels in WT and mutant mice cannot be made because mutants exhibit irregular cycles. Therefore, Cyp 19 expression was monitored in ovaries of immature mice in response to exogenous gonadotropin stimulation. Although mean Cyp 19 levels were not statistically different owing to variation among individuals of each genotype, mutants exhibited a clear trend toward decreased expression relative to WT littermates (Fig. 4A). This result suggests that BmprIB is required in granulosa cells for maximal induction of aromatase, and hence, for normal levels of E2 biosynthesis.
BmprIB Is Required for Endometrial Gland Formation.
During each estrous cycle, endometrial cells proliferate in response to increasing E2 levels (e.g., ref. 27). If E2 remains low, the endometrial lining fails to thicken, uterine glands are underdeveloped, and implantation cannot take place. When uterine horns from WT and mutant females were compared 0.5 days after mating, striking differences were observed (Fig. 5). The uterine linings were thin, and endometrial glands were absent or underdeveloped in 89% (8/9) of the mutants (Fig. 5). Therefore, BmprIB is required for endometrial gland formation. There is wide variation in the extent of endometrial development among mutants, ranging from a failure in gland formation (Fig. 5B) to inability to develop beyond a simple epithelium (Fig. 5C). The basis for the variable phenotype is unknown, but may reflect in part the effects of modifier genes segregating in the hybrid 129Sv × C57BL/6 background. BmprIB may have both direct and indirect actions on endometrial development. A direct action for BMPRIB signaling is supported by the observation that BmprIB, and its potential ligands are expressed in the endometrium and endometrial glands (Fig. 1D, refs. 28 and 29). An indirect action due to diminished E2 levels resulting from decreased aromatase expression is also possible; however, no morphological defects were seen in other E2-sensitive tissues, such as the oviducts (Fig. 1A, and data not shown).
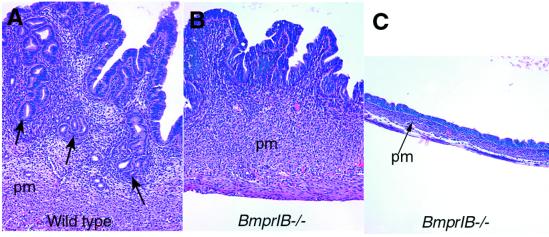
Figure 5.
Defective endometrial development in BmprIB mutants. Uteri from (A) WT and (B and C) mutant adult mice 0.5 days after mating. Numerous glands can be seen in the endometrium of the WT uterus in A (arrows). (B) Uterus from a mutant female, exhibiting some thickening of the endometrial stroma but no gland formation. The muscular layer and perimetrium appear normal. (C) A severely underdeveloped uterus from a mutant female. The epithelial endometrium is present, but has a flattened, cuboidal appearance. The stromal perimetrial and muscular layers are thinner than in WT mice. pm, perimetrium.
Discussion
The BMPs comprise the largest subgroup of the TGF-β superfamily, yet little is known about the potential roles of BMPs in reproductive function. Studies by Shimisaki et al. (7) demonstrate that granulosa cells respond to exogenous BMPs in vitro, raising the possibility that BMPs play an essential role in ovarian function. Our studies confirm that BMP signaling pathways are required for normal ovarian function in vivo. The prolonged di-estrus phase and decreased Cyp 19 expression observed in mutants suggests that circulating E2 levels may be insufficient for normal reproductive function. These results are consistent with those of Shimasaki et al. (7), showing that BMPs stimulate E2 production in granulosa cells in vitro, and indicate that BMPRIB is an essential receptor for the regulation of E2 production by BMPs in vivo. Our results contrast with those reported for sheep carrying the FecB allele of BmprIB. FecB acts in a dose-dependent manner to increase ovulation rate (8, 9), whereas the prolonged ovulatory cycles seen in BmprIB−/− mice imply a decreased ovulation rate. Moreover, FecB increases litter size (8, 9), whereas the two litters we have obtained from BmprIB females consisted of one pup and five pups, respectively (average litter size for WT littermates is ≈8–10). One interpretation for the different phenotypes is that the FecB allele represents an activating mutation in BmprIB, leading to increased signaling capacity. Consistent with this hypothesis, granulosa cells from FecB sheep exhibit increased Cyp 19 (aromatase) activity at a smaller follicular diameter than in WT sheep (30), whereas we observed decreased levels of Cyp 19 mRNA in BmprIB−/− mice. It will be interesting in future studies to examine whether the onset of Cyp 19 expression is delayed in follicles from BmprIB−/− mice.
Our results show that loss of BmprIB is associated with defective cumulus cell expansion, decreased levels of Cyp 19 (aromatase), and increased Ptgs2 (Cox2) expression. The expression of BmprIB in ovaries, but not in the pituitary, suggests that these defects result from insufficient BMP signaling in ovarian tissues. The expression of BmprIB in granulosa cells is consistent with a direct role for BMP signaling pathways in cumulus cell expansion. However, we cannot exclude the possibility that BmprIB acts in the oocyte, because BmprIB is expressed in oocytes, and factors secreted by oocytes are required for cumulus cell expansion (31). The molecular basis for the defective cumulus expansion in BmrpIB mutants is currently unknown. Cumulus expansion requires the activity of FSH, prostaglandins, and soluble oocyte factor(s); TGF-β can substitute for the oocyte-derived factor(s) (23, 24). BmprIB mutants do not exhibit decreased Fshr mRNA expression, suggesting that decreased activation of FSH receptor does not contribute to defective expansion. COX-2 is the rate-limiting enzyme responsible for the production of prostanoids, and mutations in the prostanoid receptor EP2 cause defective cumulus expansion (23). Although levels of EP2 receptor mRNA are unaffected, Cox-2 levels are significantly increased in BmprIB mutants. Therefore, defective cumulus expansion in BmprIB mutants is not a result of decreased expression of prostanoids or their receptors. BMPRIB may act in a parallel signaling pathway, and/or downstream of prostanoid action.
The ligands that activate BMPRIB in the ovary are currently unknown. To date, reproductive phenotypes have been associated with mutations in the genes encoding two BMP family members, Gdf9, and Bmp15 (3, 4). The receptors for these ligands have not been identified. GDF9 is capable of stimulating cumulus expansion in vitro (32), raising the possibility that this oocyte-specific ligand binds to BMPRIB in vivo. However, GDF9 is structurally divergent from ligands known to bind to BMPRIB, and folliculogenesis is blocked at an early stage in Gdf9−/− mice (3, 33, 34), but not in BmprIB mutants. Moreover, GDF9 increases Cox2 mRNA levels in vitro, whereas we observe increased levels of Cox2 in the absence of signaling through BMPRIB. In sheep, BMP15 affects follicle development in a dose-dependent manner, but does not elicit cumulus expansion in vitro (7, 32). Therefore, although BMPRIB may act as a receptor for GDF9 or BMP15 in vivo, these ligands are unlikely to mediate the effects of BMPRIB on cumulus expansion and Cox2 expression. It is possible that BMPRIB regulates levels of expression of these ligands and/or their receptor(s) in follicle cells. Other potential ligands for BMPRIB include BMPs 2, 4, and 7, which are expressed in thecal cells and are known to signal through BMPRIB in vitro (2, 7, 34, 35). GDF5 is the most potent stimulator of signal transduction through BMPRIB known to date (34). Although reproductive functions have not been described for GDF5, the related family members GDF6 and GDF7 may play roles in female fertility.
Recently, it has been shown that BMPRIB can act in vitro as a receptor for Müllerian inhibiting substance (MIS) (36). MIS is the TGF-β-related molecule responsible for the regression of the Müllerian duct (which develops into the uterus in females) in males. Although BmprIB males exhibit reduced fertility due to defective growth of seminal vesicles (unpublished data), we observe no remnants of the Müllerian duct in BmprIB mutant males (37). Our in vitro and in situ hybridization studies suggest that ALK2 rather than BMPRIB is a receptor for MIS in vivo (37). Although we find no evidence for a role for BMPRIB in regression of the Müllerian duct in males, our results do indicate that BMPRIB is required for normal uterine function in females. Although it is not possible at present to determine whether the uterine defects in BmprIB mutants are secondary to ovarian insufficiency, expression of BmprIB and BMP ligands in uterine epithelium (28, 29) argues for a primary role for BMPRIB in the preparation of the uterus for successful fertilization and implantation. Future studies examining cycle-specific expression of BmprIB in the uterus should help to clarify the role of BMP signaling in endometrial gland formation. It would also be of interest to examine endometrial gland formation in FecB sheep.
The targets of BMP signaling pathways in follicular and endometrial cells are unknown. The similar defects in cumulus expansion and endometrial development in mice lacking BmprIB, Ptgs2 (Cox-2) (38), or the prostanoid receptor EP2 (39, 40) suggest a potential link between BMP and prostanoid signaling pathways. However, despite increased Cox-2 expression, BmprIB mutants exhibit impairments in cumulus expansion and endometrial development. This finding suggests that BMPRIB may act in a parallel pathway in conjunction with prostaglandin pathways and/or downstream of prostanoid action in reproductive tissues. The infertility of BmprIB-deficient mice is reminiscent of female reproductive senescence. Estrous cycle length increases toward the end of reproductive life as a result of diminishing E2 production (41–43). Vaginal smear data indicated a prolonged estrous phase in a fraction of BmprIB mutants. Therefore, examination of BmprIB levels in granulosa cells from aging females will be warranted in future studies.
Acknowledgments
We thank K. Williams for assistance with hormone-priming and J. Lengyel and L. Birnbaumer for comments on the manuscript. This work was supported by National Institutes of Health Grant AR44528 to K.M.L.
Abbreviations
- BMP
bone morphogenetic protein
- BmprIB
BMP receptor type IB
- E2
estradiol
- TGF-β
transforming growth factor β
- GDF9
growth differentiation factor 9
- FSH
follicle-stimulating hormone
- WT
wild type
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- dpc
days postcoitum
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Massagué J. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 2.ten Dijke P, Yamashita H, Sampath T K, Reddi A H, Estevez M, Riddle D L, Ichijo H, Heldin C-H, Miyazono K. J Biol Chem. 1994;269:16985–16988. [PubMed] [Google Scholar]
- 3.Dong J, Albertini D F, Nishimori K, Kumar T R, Lu N, Matzuk M M. Nature (London) 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 4.Galloway S M, McNatty K P, Cambridge L M, Laitinen M P E, Juengel J L, Jokiranta T S, McLaren R J, Luiro K, Dodds K G, Montogmery G W, et al. Nat Genet. 2000;25:279–283. doi: 10.1038/77033. [DOI] [PubMed] [Google Scholar]
- 5.Matzuk M M, Kumar T R, Bradley A. Nature (London) 1995;374:356–360. doi: 10.1038/374356a0. [DOI] [PubMed] [Google Scholar]
- 6.Knight P G. Front Neuroendocrinol. 1996;17:476–509. doi: 10.1006/frne.1996.0013. [DOI] [PubMed] [Google Scholar]
- 7.Shimasaki S, Zachow R J, Li D, Kim H, Iemura S, Ueno N, Sampath K, Chang R J, Erickson G F. Proc Natl Acad Sci USA. 1999;96:7282–7287. doi: 10.1073/pnas.96.13.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson T, Wu X-Y, Juengel J L, Ross I K, Lumsden J M, Lord E A, Dodds K G, Walling G A, McEwan J C, O'Connell A R, et al. Biol Reprod. 2001;64:1225–1235. doi: 10.1095/biolreprod64.4.1225. [DOI] [PubMed] [Google Scholar]
- 9.Mulsant P, Lecerf F, Fabre S, Schibler L, Monget P, Lanneluc I, Pisselet C, Riquet J, Monniaux D, Callebaut I, et al. Proc Natl Acad Sci USA. 2001;98:5104–5109. doi: 10.1073/pnas.091577598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishina Y, Suzuki A, Ueno N, Behringer R R. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- 11.Mishina Y, Crmbie R, Bradley A, Behringer R R. Dev Biol. 1999;15:314–326. doi: 10.1006/dbio.1999.9378. [DOI] [PubMed] [Google Scholar]
- 12.Gu Z, Reynolds E M, Song J, Lei H, Feigen A, Yu L, He W, MacLaughlin D T, van den Eijnden-van Raaij J, Donahoe P K, et al. Development (Cambridge, UK) 1999;126:2551–2561. doi: 10.1242/dev.126.11.2551. [DOI] [PubMed] [Google Scholar]
- 13.Yi S E, Daluiski A, Pederson R, Rosen V, Lyons K M. Development (Cambridge, UK) 2000;127:621–630. doi: 10.1242/dev.127.3.621. [DOI] [PubMed] [Google Scholar]
- 14.Hogan B L M, Beddington R, Constatini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. [Google Scholar]
- 15.Pedersen T, Peters H. J Reprod Fertil. 1968;17:555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- 16.Mattson B A, Albertini D F. Mol Reprod Dev. 1990;25:374–383. doi: 10.1002/mrd.1080250411. [DOI] [PubMed] [Google Scholar]
- 17.Lyons K M, Graycar J L, Lee A, Hashmi S, Lindquist P B, Chen E Y, Hogan B L M, Derynck R. Proc Natl Acad Sci USA. 1989;86:4554–4558. doi: 10.1073/pnas.86.12.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hickey G J, Krasnow J S, Beattie W G, Richards J S. Mol Endocrinol. 1990;4:3–12. doi: 10.1210/mend-4-1-3. [DOI] [PubMed] [Google Scholar]
- 19.LaPolt P S, Tilly J S, Aihara T, Nishimori K, Hsueh A J W. Endocrinology. 1992;130:1289–1295. doi: 10.1210/endo.130.3.1537292. [DOI] [PubMed] [Google Scholar]
- 20.Elvin J A, Yan C, Matzuk M M. Proc Natl Acad Sci USA. 2000;97:10288–10293. doi: 10.1073/pnas.180295197. . (First Published August 15, 2000, 10.1073/pnas.180295197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bostrom K, Tintut Y, Kao S C, Stanford W P, Demer L L. J Cell Biochem. 2000;78:210–221. doi: 10.1002/(sici)1097-4644(20000801)78:2<210::aid-jcb4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 22.Eppig J. BioEssays. 1991;13:569–574. doi: 10.1002/bies.950131105. [DOI] [PubMed] [Google Scholar]
- 23.Hizaki H, Segi E, Sugimoto Y, Hirose M, Saji T, Ushikubi F, Matsuoka T, Noda Y, Tanaka T, Yoshida N, et al. Proc Natl Acad Sci USA. 1999;96:10501–10506. doi: 10.1073/pnas.96.18.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tirone E, D'Alessandris C, Hascall V C, Siracussa G, Salustri A. J Biol Chem. 1997;272:4787–4794. doi: 10.1074/jbc.272.8.4787. [DOI] [PubMed] [Google Scholar]
- 25.Eppig J J. Biol Reprod. 1981;25:191–195. doi: 10.1095/biolreprod25.1.191. [DOI] [PubMed] [Google Scholar]
- 26.Wuttke W. Rev Physiol Biochem Pharmacol. 1976;76:59–101. doi: 10.1007/BFb0027687. [DOI] [PubMed] [Google Scholar]
- 27.Safranski T J, Lamberson W R, Keisler D H. Biol Reprod. 1993;48:669–673. doi: 10.1095/biolreprod48.3.669. [DOI] [PubMed] [Google Scholar]
- 28.Ozkaynak E, Jin D F, Jelic M, Vukicevic S, Oppermann H. Biochem Biophys Res Commun. 1997;234:242–246. doi: 10.1006/bbrc.1997.6624. [DOI] [PubMed] [Google Scholar]
- 29.Zhao R, Lawler A M, Lee S-J. Dev Biol. 1999;212:68–79. doi: 10.1006/dbio.1999.9326. [DOI] [PubMed] [Google Scholar]
- 30.Montgomery G W, McNatty K P, Davis G H. Endocr Rev. 1992;13:309–328. doi: 10.1210/edrv-13-2-309. [DOI] [PubMed] [Google Scholar]
- 31.Eppig J J, Chesnel F, Hirao Y, O'Brien M, Pendola F L, Watanabe S, Wigglesworth K. Hum Reprod. 1997;12:127–132. [PubMed] [Google Scholar]
- 32.Elvin J A, Clark A T, Wang P, Wolfman N M, Matzuk M M. Mol Endocrinol. 1999;13:1035–1048. doi: 10.1210/mend.13.6.0310. [DOI] [PubMed] [Google Scholar]
- 33.Elvin J A, Yan C, Wang P, Nishimori K, Matzuk M M. Mol Endocrinol. 1999;13:1018–1034. doi: 10.1210/mend.13.6.0309. [DOI] [PubMed] [Google Scholar]
- 34.Nishitoh H, Ichijo H, Kimura M, Matsumoto T, Makishima F, Yamaguchi A, Yamashita H, Enomoto S, Miyazono K. J Biol Chem. 1996;271:21345–21352. doi: 10.1074/jbc.271.35.21345. [DOI] [PubMed] [Google Scholar]
- 35.Liu F, Ventura F, Doody J, Massagué J. Mol Cell Biol. 1995;15:3479–3486. doi: 10.1128/mcb.15.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gouedard L, Chen Y G, Thevenet L, Racine C, Borie S, Lamarre I, Josso N, Massague J, di Clemente N. J Biol Chem. 2000;275:27973–27978. doi: 10.1074/jbc.M002704200. [DOI] [PubMed] [Google Scholar]
- 37.Clarke, T. R., Hoshiya, Y., Yi, S. E., Liu, X., Lyons, K. M. & Donahoe, P. K. (2001) Endocrinology, in press. [DOI] [PubMed]
- 38.Lim H, Paria B C, Das S K, Dinchuk J E, Langenbach R, Trzaskos J M, Dey S K. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 39.Tilley S L, Audoly L P, Hicks E H, Kim H S, Flannery P J, Coffman T M, Koller B H. J Clin Invest. 1999;103:1539–1545. doi: 10.1172/JCI6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy C R, Zhang Y, Brandon S, Guan Y, Coffee K, Funk C D, Magnuson M A, Oates J A, Breyer M D, Breyer R M. Nat Med. 1999;5:217–220. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- 41.Lu J K, Anzalone C R, LaPolt P S. Neurobiol Aging. 1994;15:541–544. doi: 10.1016/0197-4580(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 42.Thung P J, Boot L M, Muhlbock O. Acta Endocrinol. 1956;23:8–32. doi: 10.1530/acta.0.0230008. [DOI] [PubMed] [Google Scholar]
- 43.Nelson J F, Felicio L S, Randall P K, Sims C, Finch C E. Biol Reprod. 1982;27:327–339. doi: 10.1095/biolreprod27.2.327. [DOI] [PubMed] [Google Scholar]