Abstract
BACKGROUND:
Celiac disease (CD) is a common autoimmune disorder of the small intestine that occurs in genetically predisposed individuals. Animal studies have suggested that the hedgehog (Hh) signalling pathway is involved in gut inflammation, injury and repair.
OBJECTIVE:
To examine the expression of components of the Hh signalling pathway in CD.
METHODS:
Children undergoing gastroscopy investigation for CD at Monash University (Victoria, Australia), and other children undergoing gastroscopy in whom small bowel pathology was not expected (ie, controls), were included in the present study. One histopathologist, who was blinded to the biopsy data, analyzed the biopsies and a diagnosis of CD was made according to standard Marsh criteria. From these samples, RNA was extracted and complementary DNA was synthesized using reverse transcription polymerase chain reaction. The levels of Hh ligand Sonic hh, Indian hh, protein patched homologue 1 (PTCH 1) and bone morphogenetic protein 4 (BMP4) messenger RNA were quantified by real-time polymerase chain reaction. Relative expression quantification was performed using the ΔΔCt method.
RESULTS:
Duodenal biopsies were collected from 37 children. There were 20 CD specimens and 17 normal controls. The relative expression of Sonic hh from CD patients was 58% lower than that of the controls; similarly, Indian hh expression was decreased in children with CD by 44%. Compared with controls, the expression of Hh receptor PTCH 1 decreased by 71% and the expression of the Hh target gene BMP4 by 42%.
CONCLUSIONS:
The expression of the Hh signalling pathway genes was consistently downregulated in untreated CD children. These results suggest that the Hh signalling pathway plays a role in the mucosal lesions encountered in CD.
Keywords: BMP4, Celiac disease, Duodenum, Hedgehog signalling, PTCH1
Abstract
HISTORIQUE :
La maladie cœliaque (MC) est un trouble auto-immun courant de l’intestin grêle qui se déclare chez des personnes prédisposées sur le plan génétique. Les études chez des animaux laissent croire que la voie de signalisation Hedgehog (Hh) participe à l’inflammation, aux lésions et à la réparation de l’intestin.
OBJECTIF :
Examiner l’expression des éléments de la voie Hh en cas de MC.
MÉTHODOLOGIE :
Les chercheurs ont inclus dans la présente étude des enfants ayant subi une gastroscopie en raison d’une MC à l’université Monash de Victoria, en Australie, ainsi que d’autres enfants ayant subi une gastroscopie, mais chez qui on ne prévoyait pas la présence d’une pathologie du grêle (sujets témoins). Un histopathologiste, qui n’était pas informé des données de biopsie, a analysé les biopsies et posé un diagnostic de MC conformément aux critères de Marsh standards. À partir de ces échantillons, les chercheurs ont extrait l’ARN et synthétisé l’ADN complémentaire au moyen de la réaction en chaîne de la polymérase par transcription inverse. Les chercheurs ont quantifié le taux d’ARN messager dans les ligands Hhde la protéine Sonic hh, la protéine Indian hh, la protéine homologue patched 1 (PTCH 1) et la protéine morphogénétique osseuse 4 (BMP4) par réaction en chaîne de la polymérase en temps réel. Ils ont quantifié l’expression relative au moyen de la méthode ΔΔ Ct.
RÉSULTATS :
Les chercheurs ont prélevé des biopsies duodénales chez 37 enfants. Ils comptaient 20 échantillons de MC et de 17 sujets témoins. L’expression relative de la protéine Sonichhchez les patients ayant la MC était 58 % plus faible que celle des sujets témoins. De même, l’expression de la protéine Indian hh était 44 % plus faible chez les enfants ayant la MC. Par rapport aux sujets témoins, l’expression du récepteur PTCH 1 diminuait de 71 % et celle du gène cible BMP4 Hh, de 42 %.
CONCLUSIONS :
Chez les enfants ayant une MC non traitée, on observait une régulation négative constante de l’expression de la voie Hh. D’après ces résultats, la voie Hh participe aux lésions muqueuses observées en cas de MC.
Celiac disease (CD) is a common autoimmune disorder of the small intestine that affects genetically predisposed individuals of all ages. The prevalence is 1% in Western populations. This disease is caused by an immune-mediated response to dietary gluten. The response leads to an enteropathy characterized by villous atrophy, crypt hyperplasia and increased numbers of intra-epithelial lymphocytes. Treatment consists of lifelong dietary gluten exclusion with resolution of the lesion(s) the expectation.
The hedgehog (Hh) signalling pathway is known to be essential in gastrointestinal patterning and development. Hh signalling stimulates mesenchymal growth in the developing digestive tract (1). This signalling is vital to the patterning of the intestinal crypt-villus axis in mice (2). In mice with Hh signalling pathway gene mutations, gastrointestinal anomalies, including duodenal stenosis, aganglionic colon, imperforate anus and anorectal malformation, have been observed (3–5).
In the mature gut, Hh is localized to the region of the crypts where intestinal stem cells reside and it is suspected that Hh plays a role in stem cell regulation and, thus, it may also be involved in epithelial proliferation (6). Hh signalling is also involved in the injury-induced repair of the bladder that originates from the hindgut during embryogenesis (7). Indian Hh (Ihh) regulates intestinal stem cell self-renewal and differentiation through epithelial-mesenchymal interactions during development (8). Abnormal regulation of the Hh signalling pathway has been linked to many human cancers, including gastrointestinal cancers, highlighting its role in promoting proliferation (9,10). Hh signalling is linked to repair of tissues such as skeletal muscle (11), bones (12), myocardium (13) and skin (14). The aim of the present study was to assess the expression of Hh signalling molecules in the enteropathy associated with CD before starting a gluten-free diet when compared with controls.
METHODS
Subjects and histological diagnosis
The present study was approved by the institutional human research ethics committee. Children undergoing gastroduodenoscopy for investigation of possible CD, including children with both symptoms and/ or serology consistent with the diagnosis as well as those who had been screened because of a high risk for CD, such as children with type I diabetes and found to have positive serology, were recruited for the study. Included as controls were age-matched children in whom CD or other small bowel pathology was not expected to be diagnosed. Informed written consent or assent was obtained as appropriate. Biopsies were taken and analyzed blindly by a pathologist; a diagnosis of CD was made according to the standard Marsh criteria. Additional duodenal biopsies were also taken from these children and stored; transported in RNAlater (Ambion, USA) and maintained at −80°C for later use.
RNA isolation and quality controls
Total RNA was extracted from tissue using a commercially available kit (RNeasy Mini Kit, Qiagen, USA). Homogenization was performed using 1.4 mm ceramic beads (Mobio, USA) in lysis buffer. Samples were shaken using a homogenizer (Precellys 24 Lysis and Homogenization machine, Precellys, USA). Optional DNase digestion was also performed. The concentration and quality of the isolated RNA were measured spectrophotometrically (Nanodrop, Thermo Fisher Scientific Inc, USA). The total RNA (<3 μg) was primed with oligo(dT)20 (Invitrogen, USA) and reverse transcribed to complementary DNA using Moloney murine leukemia virus reverse transcriptase (Invitrogen) in a total volume of 20 μL as per manufacturer’s instructions. Control reactions were performed using control RNA (HeLa) provided by the manufacturer.
Real-time polymerase chain reaction
Real-time polymerase chain reaction (PCR) reactions were run in triplicate in 384-well plates using the Applied Biosystems 6900HT Fast RTPCR System (Applied Biosystems, USA). Blank controls were included in parallel for each primer SYBR Green master mix (Applied Biosystems, USA). Each reaction contained 5 μL SYBR Green PCR master mix, 200 mM forward and reverse primers, 1 μL complementary DNA and distilled water up to a total volume of 10 μL.
The cycling conditions were as follows: initial template denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s, annealing and elongation at 58°C for 1 min. These cycles were followed by melt-curve analysis: 95°C for 15 s, 58°C for 15 s followed by 95°C for 15 s. The relative expression quantification was determined by reference to beta-actin expression using the ΔΔCt method.
Statistical analysis
All data were expressed as mean ± SE. Statistical data were collected and calculations performed using a spreadsheet (Excel, Microsoft Corporation, USA). The Student’s t test was applied for data analysis using GraphPad Prism software (GraphPad Software Inc, USA); P<0.05 was considered to be statistically significant.
RESULTS
Small bowel biopsies were obtained from 37 children including 20 children (median age six years, range two to 13 years) diagnosed with CD by histology and 17 children (median age seven years, range three to 15 years) who had normal small bowel histopathology (controls). There was no statistically significant difference in age between the two groups. The results of the expression data are summarized in Table 1.
TABLE 1.
Expression levels of hedgehog signalling pathway genes in celiac disease (CD) and normal controls
| Shh | Ihh | PTCH1 | BMP4 | |
|---|---|---|---|---|
| CD (n=20) | 0.52±0.074* | 0.48±0.055** | 0.24±0.036** | 0.57±0.053** |
| Control (n=17) | 0.89±0.082 | 0.85±0.052 | 0.84±0.14 | 0.99±0.071 |
Data presented as mean ± SE.
P<0.005;
P<0.0001. BMP4 Bone morphogenetic protein 4; Ihh Indian hedgehog; Shh Sonic hedghog; PTCH1 Protein patched homologue 1
Of the three Hh ligands present in humans, Sonic Hh (Shh), Ihh and Desert Hh, only Shh and Ihh are expressed in the gut. The mean (± SE) expression level of Shh in the CD group was found to be 58% lower (0.52±0.074) than that of the control group (0.89±0.082) (P<0.005). Similarly, the Ihh expression showed a decrease of 56% compared with that of the control group (0.48±0.055 versus 0.85±0.052) (P<0.0001).
Protein patched homologue 1 (PTCH1) is not only the inhibitor membrane receptor but also one of the main target genes in the Hh pathway. PTCH1 expression was down-regulated by 71% in the CD group (0.24±0.036 versus 0.84±0.14) (P<0.0001). Bone morphogenetic protein 4 (BMP4) is one of the target genes in Hh signalling. BMP4 expression decreased by 42% (0.57±0.053) compared with that of the control group (0.99±0.071) (P<0.0001) (Figure 1).
Figure 1).
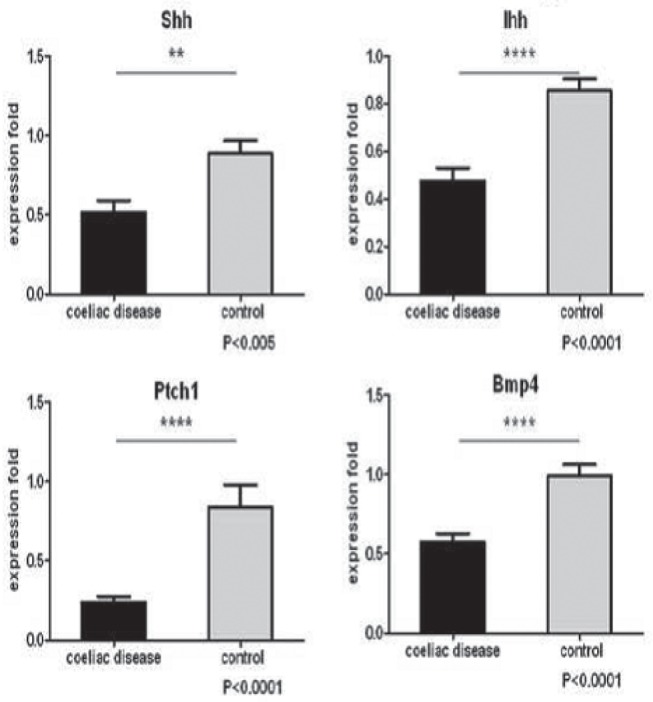
Expression levels of Sonic hedgehog (Shh), Indian hedgehog (Ihh), Protein patched homologue 1 (Ptch1) and bone morphogenetic protein 4 (Bmp4) in celiac disease and controls. **P<0.005; ****P<0.001
No relationship between the degree of villous atrophy and/or Marsh score as categorized by the histopathologist, and the expression level of any of the molecules assessed was observed in the present study.
DISCUSSION
The results of the present study demonstrated for the first time that Hh signalling pathway ligands and downstream transcriptional targets are significantly downregulated in the duodenal epithelia of children with untreated CD. This information will further expand the understanding of intestinal repair and injury in human subjects. While larger studies will be needed to confirm these findings, we believe that the magnitude of difference makes it likely to be reproducible. Furthermore, it is not clear at this stage whether expression would be downregulated in small bowel damage outside the setting of CD; we are planning future studies within our department to quantify Hh expression in other small bowel pathologies. This will help clarify whether the decreased expression of Ihh and Shh simply reflects a reduction in epithelial cells consequent to the villous atrophy. Our study remains, however, one of the first to investigate Hh signalling in small bowel pathology in human subjects.
Hh signalling has previously been linked to the repair of many tissues types, including the gut, and has been suggested to play a role in promoting proliferation in many human cancers. In a previous study by Nielsen et al (15), Hh signalling was shown to increase in response to intestinal injury. This finding is consistent with the results of a previous report (16), which showed that Hh signalling is involved in the repair of gastric ulcers. Our study suggests that in humans with CD, Hh signalling may be diminished in the setting of ongoing immune-mediated damage.
Recently, Zacharias et al (17) demonstrated that chronic suppression of gut Hh signalling in transgenic mice leads to villous atrophy and wasting. In addition, microarray data revealed upregulation of many genes that are usually involved in inflammation. Similarly, in another model using transgenic mice (18), prolonged inhibition of the Hh pathway led to leukocyte infiltration of the crypt area, blunting and loss of villi, and fibrosis (18). While the relationship between Hh signalling and the lesions of CD is not clear, our results appear to be consistent with these findings in animal models of small bowel damage.
It remains unclear, of course, as to what role Hh downregulation plays in the pathogenesis of CD and, equally, whether it would be present in other enteropathies. Nevertheless, downregulation of Hh is observed in many animal models of intestinal injury. Future studies investigating other small bowel damage would be extremely useful in this context. Ideally, we would have also liked to measure levels of Hh expression in children after commencement of a gluten-free diet to determine whether they returned to normal. However, as per European Society for Paediatric Gastroenterology, Heaptology and Nutrition (ESPGHAN) guidelines, it is no longer our practice to repeat biopsies where there has been a good clinical response with normalization of CD serology. Outside the setting of CD, our study does raise the question of whether local application of Hh analogues is feasible in modulating inflammation, in a similar way as local application of Shh reduced myocardial damage after infarction and accelerated wound healing in diabetic mice (13,14).
CONCLUSION
Our data showed that Hh signalling appears to be downregulated in children with CD when compared with controls. While larger studies are required to confirm this finding, Hh signalling may play a prominent role in the small intestinal lesion encountered in CD.
KEY MESSAGES.
The present study demonstrated, for the first time, that Hh signalling pathway ligands and downstream transcriptional targets are downregulated in the duodenal epithelia of children with untreated CD.
Information from the present study will further expand the understanding of intestinal repair and injury in human subjects.
Outside the setting of CD, it may be possible that local application of an Hh analogue could modulate gut inflammation similarly to how local application of Shh reduced myocardial damage after infarction and accelerated healing in mice.
Footnotes
DISCLOSURES: The authors have no financial disclosures or conflicts of interest to declare.
References
- 1.Mao J, Kim BM, Rajurkar M, et al. Hedgehog signaling controls mesenchymal growth in the developing mammalian digestive tract. Development. 2010;137:1721–9. doi: 10.1242/dev.044586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madison BB, Braunstein K, Kuizon E, et al. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132:279–89. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- 3.Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–72. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- 4.Sukegawa A, Narita T, Kameda T, et al. The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development. 2000;127:1971–80. doi: 10.1242/dev.127.9.1971. [DOI] [PubMed] [Google Scholar]
- 5.Ming JE, Roessler E, Muenke M. Human developmental disorders and the Sonic hedgehog pathway. Mol Med Today. 1998;4:343–9. doi: 10.1016/s1357-4310(98)01299-4. [DOI] [PubMed] [Google Scholar]
- 6.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 7.Shin K, Lee J, Guo N, et al. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472:110–4. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosinski C, Stange DE, Xu C, et al. Indian hedgehog regulates intestinal stem cell fate through epithelial-mesenchymal interactions during development. Gastroenterology. 2010;139:893–903. doi: 10.1053/j.gastro.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lees CW, Zacharias WJ, Tremelling M, et al. Analysis of germline GLI1 variation implicates hedgehog signalling in the regulation of intestinal inflammatory pathways. PLoS Med. 2008;5:e239. doi: 10.1371/journal.pmed.0050239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–31. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 11.Straface G, Aprahamian T, Flex A, et al. Sonic hedgehog regulates angiogenesis and myogenesis during post-natal skeletal muscle regeneration. J Cell Mol Med. 2009;13:2424–35. doi: 10.1111/j.1582-4934.2008.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyaji T, Nakase T, Iwasaki M, et al. Expression and distribution of transcripts for sonic hedgehog in the early phase of fracture repair. Histochem Cell Biol. 2003;119:233–7. doi: 10.1007/s00418-003-0501-z. [DOI] [PubMed] [Google Scholar]
- 13.Kusano KF, Pola R, Murayama T, et al. Sonic hedgehog myocardial gene therapy: Tissue repair through transient reconstitution of embryonic signaling. Nat Med. 2005;11:1197–204. doi: 10.1038/nm1313. [DOI] [PubMed] [Google Scholar]
- 14.Asai J, Takenaka H, Kusano KF, et al. Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell-mediated microvascular remodeling. Circulation. 2006;113:2413–24. doi: 10.1161/CIRCULATIONAHA.105.603167. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen CM, Williams J, van den Brink GR, et al. Hh pathway expression in human gut tissues and in inflammatory gut diseases. Lab Invest. 2004;84:1631–42. doi: 10.1038/labinvest.3700197. [DOI] [PubMed] [Google Scholar]
- 16.Kang DH, Han ME, Song MH, et al. The role of hedgehog signaling during gastric regeneration. J Gastroenterol. 2009;44:372–9. doi: 10.1007/s00535-009-0006-1. [DOI] [PubMed] [Google Scholar]
- 17.Zacharias WJ, Li X, Madison BB, et al. Hedgehog is an anti-inflammatory epithelial signal for the intestinal lamina propria. Gastroenterology. 2010;138:2368–77. doi: 10.1053/j.gastro.2010.02.057. 77 e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Dop WA, Heijmans J, Buller NV, et al. Loss of Indian hedgehog activates multiple aspects of a wound healing response in the mouse intestine. Gastroenterology. 2010;139:1665–76. doi: 10.1053/j.gastro.2010.07.045. 76;e1-10. [DOI] [PubMed] [Google Scholar]


