Abstract
Mounting evidence has established a role for chronic inflammation in the development of obesity-induced insulin resistance, as genetic ablation of pro-inflammatory cytokines and chemokines elevated in obesity improves insulin signaling in vitro and in vivo. Recent evidence further highlights interleukin (IL)-12 family cytokines as prospective inflammatory mediators linking obesity to insulin resistance. In this study, we present empirical evidence demonstrating that IL-12 family related genes are expressed and regulated in insulin-responsive tissues under conditions of obesity. First, we report that respective mRNAs for each of the known members of this cytokine family are expressed within detectable ranges in WAT, skeletal muscle, liver and heart. Second, we show that these cytokines and their cognate receptors are divergently regulated with genetic obesity in a tissue-specific manner. Third, we demonstrate that select IL-12 family cytokines are regulated in WAT in a manner that is dependent on the developmental stage of obesity as well as the inflammatory progression associated with obesity. Fourth, we report that respective mRNAs for IL-12 cytokines and receptors are also expressed and divergently regulated in cultured adipocytes under conditions of inflammatory stress. To our knowledge, this report is the first study to systemically evaluated mRNA expression of all IL-12 family cytokines and receptors in any tissue under conditions of obesity highlighting select family members as potential mediators linking excess nutrient intake to metabolic diseases such as insulin resistance, diabetes and heart disease.
Keywords: obesity, adipose tissue, adipocyte, inflammation, cytokines
1. INTRODUCTION
Obesity and diabetes mellitus are major public health concerns worldwide leading to neuropathies, cardiovascular disease, hypertension, and stroke [6,17]. Mounting evidence has established a role for chronic, low-grade inflammation in the development of obesity-induced insulin resistance [14,20,28]. Several investigations have shown that chemokine and cytokine levels are increased during obesity and that ablation of these pro-inflammatory molecules improves insulin signaling in insulin-responsive tissues [33,40]. While WAT is recognized as a key site of cytokine expression in obesity, other insulin-responsive tissues, such as skeletal muscle and liver also experience increased inflammation in the obese state [1,4,5,13,14,32]. Therefore, obesity induces an insulin-resistant state in these tissues, resulting in an imbalance of systemic glucose homeostasis that contributes to the pathological metabolic disorders associated with obesity. While it is now well recognized that an array of inflammatory cytokines and chemokines are increased in obese tissues, extensive studies are underway to identify inflammatory mediators that contribute to inflammation and ultimately chronic diseases such as type 2 diabetes.
Recent evidence highlights IL-12 family cytokines as prospective regulators linking obesity to insulin resistance. While plasma levels of select IL-12 family members are elevated with obesity, diabetes, and metabolic syndrome [37,41–43], their cellular origin has not been fully determined. IL-12 family cytokines are mostly expressed in antigen-presenting cells and play critical roles during inflammatory stress. The members of the IL-12 family are heterodimeric proteins consisting of an alpha chain and a beta chain (Fig. 1). The alpha chains consist of p19, p28, or p35 that share structural homology with IL-6, whereas the beta chains, p40 or Epstein-Barr virus-induced gene 3 (EBI3), shares homology with soluble cytokine receptor chains, such as IL-6Rα [12,29]. Dimerization of specific alpha chain and beta chain subunits form the four known family members including IL-12 (i.e., p35 and p40), IL-23 (i.e., p19 and p40), IL-27 (i.e., p28 and EBI3), and the newly identified IL-35 (i.e., p35 and EBI3). Each cytokine signals through unique heterodimeric receptors that are composed of combinations of IL-12Rβ1, IL-12Rβ2, IL-23R, gp130, and WSX-1 subunits [7]. Current evidence suggests that alpha and beta subunits dimerize within the cell to produce and secrete biologically active cytokines known to play roles in linking innate resistance and adaptive immunity [39]. Although the pairing between cytokine subunits and receptors is conserved, the origin, activity, and kinetic expression are cell-type and condition-specific.
Fig. 1. Schematic of IL-12 family cytokines and receptors.
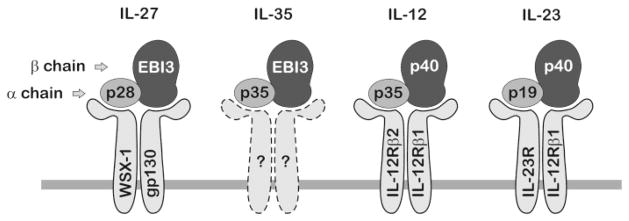
IL-12 family cytokines are heterodimers composed of shared alpha and beta chain subunits that dimerize to form IL-27, IL-35, IL-12, and IL-23. Each cytokine signals through unique heterodimeric cell surface receptors. Receptor composition for the newest member of the family, IL-35, has not been determined.
In this investigation, we examined the regulation of the IL-12 family cytokines and their cognate receptors in response to obesity in WAT, liver, skeletal muscle, and heart, as these insulin-responsive tissues centrally regulate glucose homeostasis and vascular function [13,19,24]. As we report that IL-12 family expression is most abundant in WAT, this report further investigated the developmental role of obesity on IL-12 family regulation in WAT as well as the regulation of these cytokines and receptors in cultured adipocytes. This report highlights select IL-12 family members as potential mediators linking excess nutrient intake to metabolic diseases such as insulin resistance, diabetes and heart disease.
2. MATERIALS AND METHODS
2.1. Materials
Dulbecco’s Modified Eagle’s Medium (DMEM), calf bovine serum (CS) and recombinant murine tumor necrosis factor alpha (TNFα) were purchased from Invitrogen. Fetal bovine serum (FBS) was obtained from HyClone.
2.2. Mice and experimental diets
Animals used for this study included genetically obese male B6.V-Lepob/J (B6-ob/ob) mice and their lean littermates as well as C57BL/6J mice rendered obese by dietary intervention and their lean controls. All mice were housed and treated by the supplier (Jackson Laboratories) until shipment 1 wk prior to tissue harvest. B6-ob/ob mice and lean littermates were purchased for experimentation at 6 wks and 10 wks of age and given free access to standard laboratory chow diet. C57BL/6J mice subjected to diet-induced obesity (DIO) were fed a high fat diet (HFD) consisting of 60% kcal from fat (Research Diets Inc., D12492) from 6 wks of age. Lean C57BL/6J control mice were fed a control diet consisting of 10% kcal from fat (Research Diet Inc., D12450B) from 6 wks of age. Both diets contained 10% kcal from protein with the balance in caloric intake provided by differences in carbohydrate content. Mice receiving both diets were given free access to food and shipped for experimentation at 18 wks and 24 wks of age. All animals were euthanized by CO2 gas asphyxiation and epididymal WAT, liver, calf skeletal muscle, and ventricular heart tissue collected and processed for total RNA isolation. Animal care and use was in compliance with the Institute of Laboratory Animal Research Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Use and Care Committee. All animal studies were conducted with 4–7 mice in each group.
2.3. Cell culture
The murine 3T3-L1 cell line was purchased from Howard Green, Harvard Medical School [10]. Cells were propagated in DMEM supplemented with 10% CS until reaching growth arrest as previously described [30]. At 2 days post-confluence, growth medium was replaced with DMEM supplemented with 10% FBS, 0.5 mM 1-methy-3-isobutylxanthane, 1 μM dexamethasone, and 1.7 μM insulin (MDI) for 2 days. Subsequently, cells were cultured in DMEM supplemented only with 10% FBS over the following six days as preadipocytes (PAs) differentiated into functionally and morphologically mature adipocytes (ADs). Adipocyte experiments described herein were conducted at the extremes of this process where undifferentiated PAs were compared to mature ADs. For reference, experiments were also conducted in confluent RAW 264.7 macrophage cells (gift from Mark Brown, Wake Forest University School of Medicine) under basal conditions with culture medium containing DMEM supplemented with 10% CS. All cell experiments in this study were performed in duplicate and repeated 2–3 times to validate results and ensure reliability.
2.4. Real-Time RT-PCR
For animal studies, total RNA was isolated from aforementioned insulin-responsive tissues utilizing Trizol reagent (Invitrogen) according to the manufacturer’s protocol and further processed as described by the Qiagen RNA clean-up protocol. For cell studies, total RNA was extracted and genomic DNA contamination was removed using the RNeasy Plus Mini Kit (Qiagen), according to the manufacturer’s protocol. RNA quality was assessed via integrity gels and quantified with a Nanodrop ND-1000 spectrophotometer. Total RNA was reverse-transcribed to cDNA in a 10 μl reaction volume using Applied Biosystems (ABI) High Capacity cDNA Reverse Transcription kit and the Gene Amp PCR System 9700 Thermal cycler following manufacturer’s protocol.
PCR amplification was run utilizing the ABI 7500 Fast System and the manufacturer’s Fast Protocol. All TaqMan primer probes used in this study were also purchased from ABI. Data were recorded and analyzed with ABI Sequence Detector Software and graphs visualized with SigmaPlot software. All data were presented as mean ± standard error of the mean (SEM) and representative of duplicate determinations. Data were normalized to 18S and presented as relative differences using the 2−Δ ΔCT method as previously described [11,26]. Statistical analyses were conducted using SPSS v18. Differences in gene expression between lean and obese animals as well as between PAs and ADs were determined via student’s t-test where a p-value of <0.05 was considered significant. Other data were analyzed using analysis of variance (ANOVA), with Tukey’s post-hoc analysis used when the p-value for the respective parameter was statistically significant (p < 0.05).
3. RESULTS
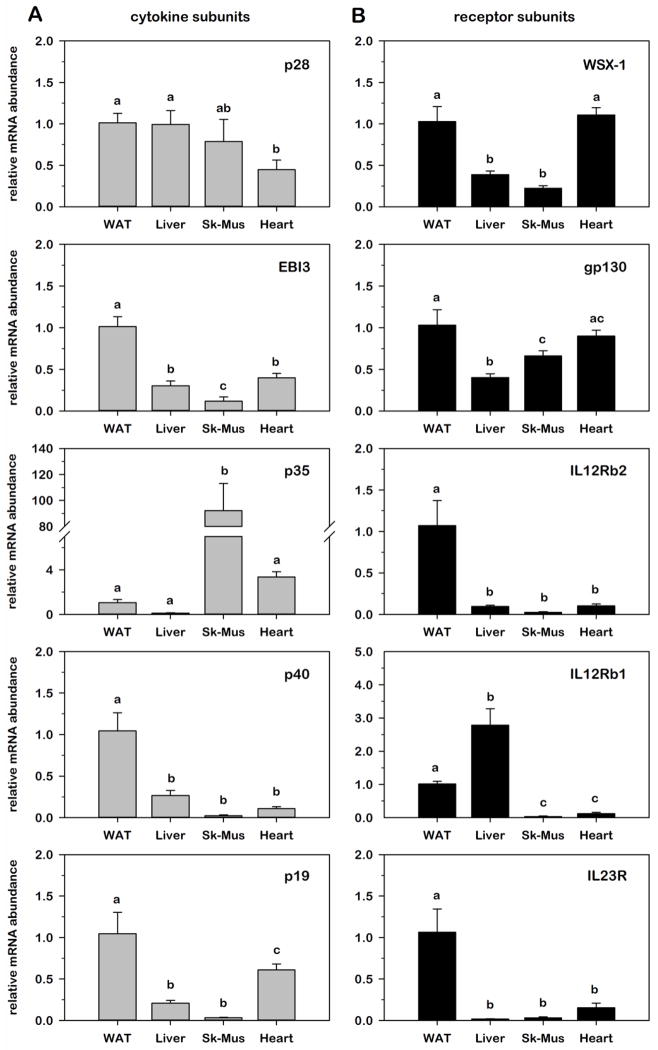
3.1. Relative IL-12 family cytokine and receptor gene expression across insulin-responsive tissues from wildtype C57BL/6J mice
As recent evidence has demonstrated a potential role for IL-12 family cytokines in obesity-induced IR [41,43], we began these studies by determining the gene expression profile of all IL-12 family genes across four major insulin-responsive tissues isolated from 10 wk old wildtype C57BL/6J mice given ad libitum access to standard chow. As illustrated in Table 1, all cytokine and receptor subunits were easily detectable in WAT with average Ct threshold values ranging from 21 to 32 cycles. As a point of reference, WAT Ct values varied by four cycles (i.e., 16-fold) or less from RAW 264.7 macrophage cells under basal conditions based on equal total RNA input of 100 ng/reaction. For comparison across tissues, relative mRNA abundance of each cytokine and receptor subunit in each tissue was normalized to 18S and expressed as fold-difference relative to WAT. As illustrated in Fig. 2, all 10 genes were detectable in all four tissues, but to varying degrees suggesting tissue-specific modes of regulation under normal lean conditions. Comparison between tissues also highlighted that 4 of 5 cytokine subunits and 4 of 5 receptor subunits were expressed in WAT at a level that was greater than or equal to other tissues with the exception of p35 that was ~90-fold more abundant in skeletal muscle and IL-12Rβ1 that was ~3-fold more abundant in liver. Comparable patterns of gene expression were also noted for EBI3, p40, p19, WSX-1 where relative mRNA was most abundant in WAT and least abundant in skeletal muscle as well as for IL-12Rβ2 and IL-23R that were markedly more abundant in WAT relative to all other tissues examined.
Table 1.
IL-12 family cytokine/receptor and inflammatory/adipocyte genes analyzed in this study.
| Name | Accession | ABI number | a WAT CT | b RAW CT | c PA CT | d AD CT | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| IL-12 cytokines | IL-27 | IL-35 | IL-12 | IL-23 | ||||||
| p28 | • | NM_145636 | Mm00461164_m1 | 32 | 28 | 34 | 28 | |||
| EBI3 | • | • | NM_015766 | Mm00469294_m1 | 28 | 24 | 32 | 34 | ||
| p35 | • | • | NM_008351 | Mm01208555_m1 | 32 | 35 | 36 | 35 | ||
| p40 | • | • | NM_008352 | Mm00434174_m1 | 30 | 33 | 36 | 36 | ||
| p19 | • | NM_031252 | Mm00518984_m1 | 27 | 25 | 30 | 31 | |||
| IL-12 receptors | ||||||||||
| WSX-1 | • | NM_016671 | Mm00497259_m1 | 28 | 34 | 33 | 36 | |||
| gp130 | • | NM_010560 | Mm00439665_m1 | 21 | 25 | 22 | 24 | |||
| IL-12Rβ2 | • | NM_008354 | Mm00434200_m1 | 29 | 33 | 35 | 36 | |||
| IL-12Rβ1 | • | • | NM_008353 | Mm00434189_m1 | 29 | 29 | 30 | 34 | ||
| IL-23R | • | NM_144548 | Mm00519943_m1 | 28 | 32 | 33 | 36 | |||
| adipocyte and inflammatory genes | ||||||||||
| Adipsin | NM_013456 | Mm00442664_m1 | 17 | ND | 35 | 14 | ||||
| MCP-1 | NM_011333 | Mm00441242_m1 | 27 | 17 | 23 | 27 | ||||
| IL-6 | NM_031168 | Mm99999064_m1 | 32 | 26 | 29 | 30 | ||||
| TNFα | NM_013693 | Mm99999068_m1 | 29 | 21 | 34 | 35 | ||||
| reference gene | ||||||||||
| 18S | X03205 | 4342930E | 9 | 7 | 7 | 8 | ||||
Average threshold cycle (CT) for white adipose tissue (WAT) from 10 wk old, lean C57BL/6J mice.
Average threshold cycle (CT) for confluent murine RAW 264.7 macrophage cells.
Average threshold cycle (CT) for confluent murine 3T3-L1 preadipocytes (PA).
Average threshold cycle (CT) for mature day 8 murine 3T3-L1 adipocytes (AD).
ND = non-detectable threshold cycle.
Fig. 2. Relative IL-12 family cytokine and receptor gene expression across insulin-responsive tissues from lean C57BL/6J mice.
Relative mRNA abundance of ligand (A) and receptor (B) subunits was determined by qRT-PCR from total RNA extracted from epididymal white adipose tissue (WAT), liver, calf skeletal muscle, and heart of 10 wk old lean C57BL/6J mice. Data were normalized to 18S and expressed as fold differences relative to WAT. Statistical differences were determined by ANOVA, with Tukey’s post-hoc analysis performed when the p-value for the respective parameter was statistically significant (p < 0.05).
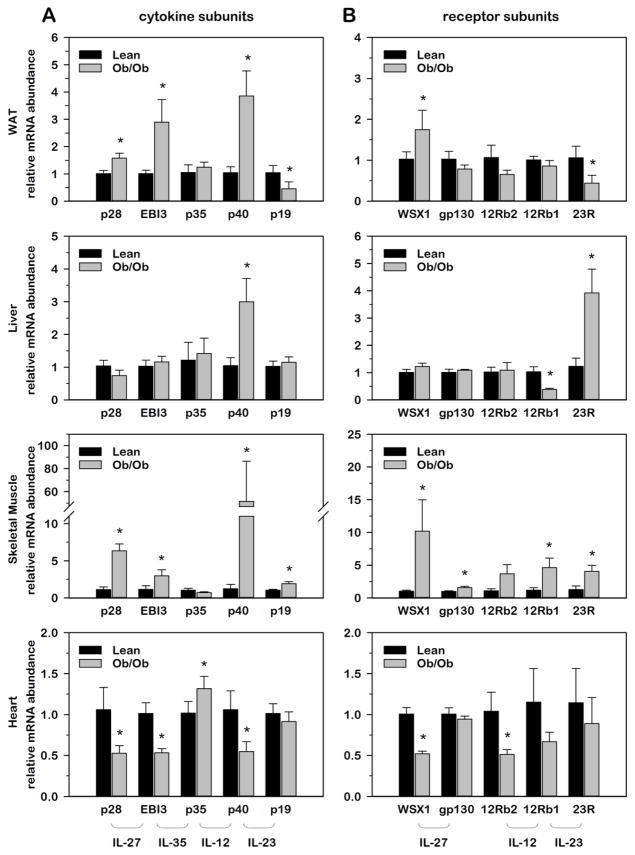
3.2. Impact of obesity on IL-12 family gene expression within insulin-responsive tissues comparing lean and genetically obese mice
While IL-12 family cytokines are well established in critical roles regarding inflammatory stress in antigen-presenting immune cells, the role of these cytokines regarding obesity-induced inflammation in metabolically active tissues remains poorly defined. As an initial step toward this goal, we examined the impact of genetic obesity on the expression of IL-12 family cytokines and receptors in each of the insulin-responsive tissues discussed above. While obesity is known to promote inflammation in WAT, skeletal muscle and liver, we purposely included heart tissue in these determinations as an insulin-responsive, metabolically active tissue that has no known role regarding the chronic inflammation that is commonly associated with excessive weight gain. For these determinations, we compared 10 wk old lean, wildtype mice to obese, leptin-deficient (ob/ob) mice given ad libitum access to standard chow. Using this genetic model of obesity, we determined relative mRNA abundance for each cytokine and receptor where obese values were expressed as fold-differences relative to lean values within each tissue. As shown in Fig. 3, WAT and skeletal muscle presented with similar profiles of gene expression where p28, EBI3 and p40 were significantly induced in obese relative to lean tissue. This is in striking contrast to heart tissue where the same three cytokine subunits and their cognate receptors were significantly suppressed with obesity. As an important target of inflammatory cytokine action regarding insulin-sensitivity, it was also interesting to note that skeletal muscle presented with the greatest obesity-induced increases in receptor subunit gene expression.
Fig. 3. Relative IL-12 family cytokine and receptor gene expression within insulin-responsive tissues comparing lean and obese Ob/Ob mice.
Relative mRNA abundance of ligand (A) and receptor (B) subunits was determined by qRT-PCR from total RNA extracted from white adipose tissue (WAT), liver, skeletal muscle and heart of 10 wk old lean and leptin-deficient (ob/ob) mice. Data were normalized to 18S and relative abundance determined for each cytokine and receptor where obese values were expressed as fold-differences relative to lean within each tissue. Differences in gene expression between lean and obese animals were determined via student’s t-test where a p-value of <0.05 was considered significant.
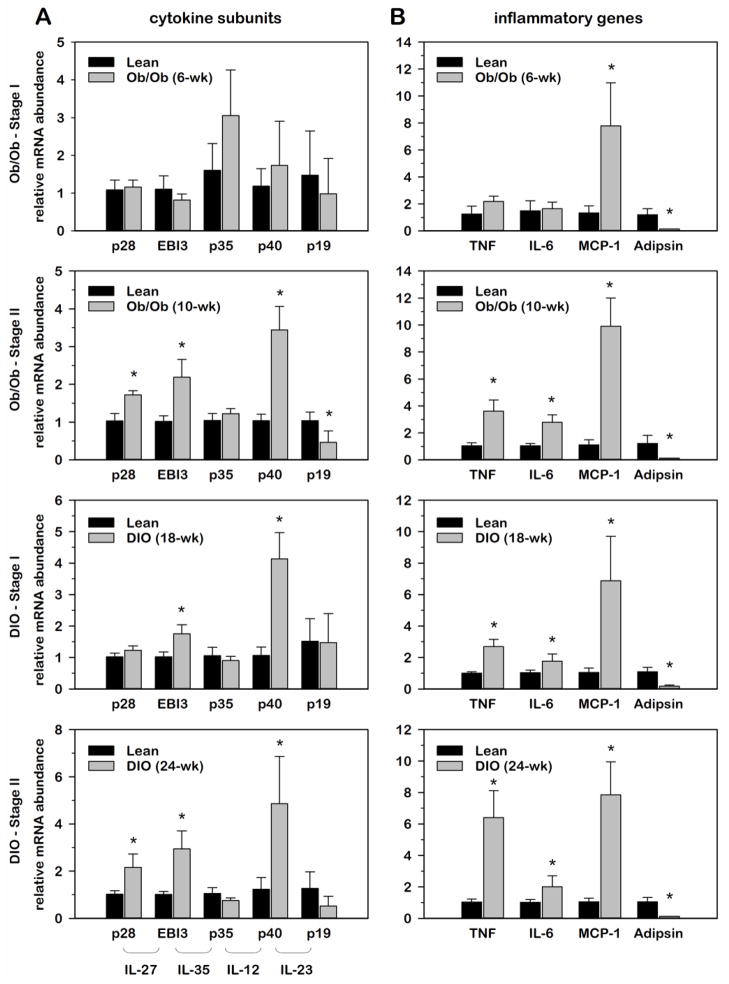
3.3. Relative IL-12 family cytokine gene expression in WAT during the progressive development of genetic and diet-induced obesity
It is well recognized that WAT is a key site regarding the origin of obesity-induced inflammation that leads to systemic IR [13,24]. As data presented above demonstrated that IL-12 family cytokines were abundantly expressed in WAT and induced with genetic obesity, we next examined the progressive development of obesity on IL-12 family cytokine expression in WAT using two distinct stages of development in two distinct models of obesity (genetic vs. diet-induced obesity). According to the supplier, B6-ob/ob mice exhibit adipocyte hyperplasia with ensuing obesity notable at 1 month of age and transient glucose intolerance that begins at ~6 wks of age and subsides after ~12 wks of age. Thus, experiments were conducted with 6 wk and 10 wk old B6-ob/ob mice and wildtype littermates, representing sequential, progressive stages of obesity with developing obesity-related metabolic disorders. For diet-induced obesity (DIO), C57BL/6J male mice were fed a high fat diet (HFD; 60% kcal from fat) by the supplier starting at 6 wks of age. Control littermates were fed a control diet containing 10% kcal from fat. Studies were conducted at 18 wks and 24 wks of age, representing 12 wks and 18 wks of dietary intervention, respectively. Progressive stages of obesity development in both models were arbitrarily designated as stage I and stage II. Using these distinct models of obesity, relative mRNA abundance for each IL-12 family cytokine in WAT was normalized to 18S and expressed as fold-differences relative to lean controls. To characterize the development of obesity-induced inflammation, we included the cytokines, TNFα and IL-6, as well as the chemokine, monocyte chemotactic protein-1 (MCP-1). Each of these inflammatory markers is known to play central roles in obesity-induced inflammation where WAT is a major site of expression. Adipsin was also included as a biochemical marker of obesity as others have documented a marked suppression of this gene in WAT of obese versus lean animals [8,22].
As illustrated in Fig. 4, mRNA expression of all IL-12 family cytokines showed no marked difference with stage I genetic (ob/ob) obesity where only MCP-1 mRNA was elevated. This was consistent with other inflammatory genes where TNFα and IL-6 were also not significantly different between lean and ob/ob mice at this stage of development. In contrast, relative mRNA abundance of p28, EBI3, and p40 was significantly induced in stage II ob/ob mice, paralleling the induction of TNFα and IL-6 mRNA expression. Although not apparent as illustrated, adipsin mRNA abundance progressively decreased as ob/ob mice transitioned from stage I (~40-fold reduction) to stage II (~80-fold reduction) obesity where the percent difference in body weight (data not shown) between lean and obese mice increased from 67% to 83%. Interestingly, the same three IL-12 cytokines were also elevated with DIO that correlated with the degree of inflammatory gene expression. While EBI3 and p40 were elevated in stage I DIO with increased TNFα expression, p28 was not increased until the mice reached stage II of development that presented with a greater increase in TNFα expression. This extended time of high fat feeding was also characterized by a progressive decrease in adipsin expression as well as an increase in percent difference in body weight from 13% to 26%. Collectively, these data demonstrated that p28, EBI-3 and p40 are induced at the level of gene expression in WAT concurrent with the progressive development of inflammation associated with sequential stages of obesity development.
Fig. 4. Relative IL-12 family cytokine gene expression in WAT during the progressive development of genetic and diet-induced obesity.
Relative mRNA abundance of IL-12 family cytokine (A) and inflammatory genes (B) was determined by qRT-PCR from total RNA extracted from white adipose tissue (WAT) at progressive stages of obesity from ob/ob mice as well as mice subject to diet-induced obesity (DIO). Data were normalized to 18S and relative abundance determined for each cytokine where obese values were expressed as fold-differences relative to lean within each group. Differences in gene expression between lean and obese animals were determined via student’s t-test where a p-value of <0.05 was considered significant.
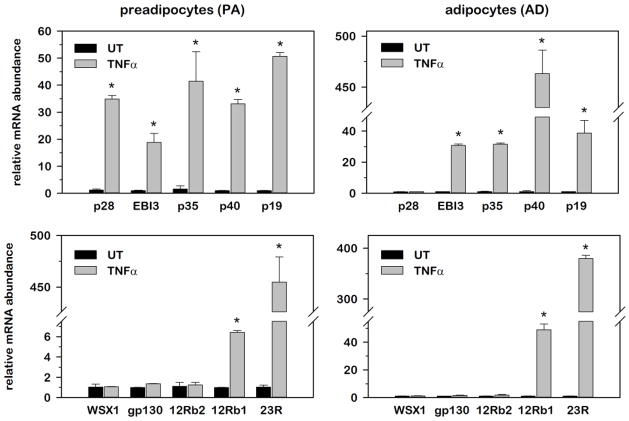
3.4. Relative IL-12 family cytokine and receptor gene expression in murine 3T3-L1 adipocytes under conditions of inflammatory stress
The progression of obesity involves enlargement of adipose tissue mass through an increase in size and number of adipocytes as well as recruitment of macrophages and other immune cells that contribute to systemic circulating inflammatory cytokines originating from adipose tissue. To investigate a potential role for adipocytes in IL-12 family gene expression as observed above for WAT, we next examined relative mRNA abundance for each cytokine and receptor subunit in 3T3-L1 murine undifferentiated preadipocytes (PA) and mature adipocytes (AD) overtime following stimulation with 100 pM TNFα. This concentration of TNFα was chosen as it approximated the ED50 for induction of MAPK and NF-kB signaling pathways that mediate inflammatory gene expression (data not shown). Both cell populations were rendered quiescent either by density arrest for PAs or by terminal growth arrest following 8 days of differentiation in ADs minimizing any influence of cell proliferation on gene expression. Data presented in Fig. 5 represent relative mRNA abundance at the time point of peak induction following TNFα stimulation for each gene, where mRNA was normalized to 18S and expressed as fold-differences relative to untreated (UT) controls. As illustrated in Fig. 5, TNFα stimulation significantly increased mRNA abundance for all five IL-12 cytokines from 20–50 fold in PAs, where peak induction was observed at 2hr for p19, 4hr for p28 and p40 and 12 hr for EBI-3 and p35. While similar kinetics of induction were observed in ADs, two notable differences were observed regarding the magnitude of induction. First, p40 was induced ~10-fold greater in ADs versus PAs and second, p28 was completely refractory to TNFα stimulation in ADs. In contrast to the inducible nature of IL-12 family cytokines, IL-12b1 and IL-23R were the only two receptors induced following TNFα stimulation where peak induction was observed at 6 hr post-stimulation. These data demonstrated 1) that all IL-12 cytokines and receptors were expressed in this adipocyte cell line that was devoid of immune cells such as monocytes and macrophages, 2) that IL-12 cytokines were collectively more inducible than receptors, and 3) that select cytokines (i.e., p28 and p40) presented with expression patterns that were dependent on the extent of adipocyte differentiation.
Fig. 5. Relative IL-12 family cytokine and receptor gene expression in murine 3T3-L1 adipocytes under conditions of inflammatory stress.
Relative mRNA abundance of cytokines and receptors was determined by qRT-PCR from total RNA extracted over time following 100 pM TNFα stimulation from undifferentiated preadipocytes (A) and mature adipocytes (B). Data were normalized to 18S and relative abundance determined for each phenotype where stimulated values were expressed as fold-differences relative to untreated (UT) controls. Differences in gene expression were determined via student’s t-test where a p-value of <0.05 was considered significant.
4. DISCUSSION
To our knowledge, this report is the first study to systemically evaluated mRNA expression of all IL-12 family cytokines and receptors in any tissue under conditions of obesity. Albeit limited to mRNA assessment, there is utility in observing the totality of all known members of this cytokine family in tissues and cell types that have not been reported previously. While much work has focused on activated inflammatory cells such as monocytes, macrophages, and dendritic cells [39], data presented here demonstrate that IL-12 family cytokines are also expressed and regulated at the mRNA level in insulin-responsive tissues with obesity-induced inflammation. While we did not examine activated immune cells, we do show that mRNA abundance within WAT from lean mice is comparable to that observed for quiescent macrophages. As most tissues are composed of multiple cell types that may include classic immune cells, it is plausible that observed changes in tissue gene expression presented in this report may reflect to some degree, changes in mRNA abundance of infiltrating macrophages. This could be particularly relevant for WAT as macrophage infiltration into this tissue during the progressive development of obesity has been widely observed [18,34,36,44]. On the other hand, we also present data demonstrating that genes encoding these cytokines and their receptors are expressed and regulated in an established murine adipocyte cell line that is devoid of macrophages or other classic inflammatory cells. As chronic inflammation is now considered an important element of the pathogenic mechanisms linking obesity and metabolic diseases, a potential role for IL-12 family cytokines seems plausible and should be addressed in future investigations.
It is generally well-established that production of biologically active IL-12 family cytokines depends on coordinate expression of genes encoding both heterodimeric partners in the same cell [39]. With this criterion in mind, our data highlight the expression of IL-27 as both p28 and EBI-3 genes were significantly elevated in WAT of genetic- and diet-induced obese mice, paralleling the onset of obesity-induced inflammation. We also demonstrate that both IL-27 genes were inducible in 3T3-L1 preadipocytes under conditions of inflammatory stress, but not in mature adipocytes as p28 becomes refractory to TNFα with differentiation. While the refractory nature of p28 that accompanies differentiation might suggests that IL-27 only plays a role in preadipocytes, we have also determined that constitutive, basal levels of p28 mRNA are elevated with differentiation and that IL-27 is secreted from both PAs as well as ADs under conditions of inflammatory stress (data not shown). While others have shown that p28 and EBI3 can be expressed independently in some cell types [27] and differentially regulated in response to various stimuli [35], the coordinate regulation of IL-27 heterodimeric gene expression and protein secretion highlight the possibility of a functional role for this cytokine in the sequelae of inflammatory mediators originating in WAT and linking obesity to metabolic diseases.
Historic literature identified IL-27 as a pro-inflammatory cytokine that is secreted mainly by macrophages and dendritic cells in response to microbial infection where it plays a role in autoimmune disease and host defense against infection. Dual functions for this cytokine have been reported more recently as IL-27 has also been shown to have anti-inflammatory properties in several murine disease models [21,23,48]. While underlying anti-inflammatory mechanisms are not yet clear, it has been observed that IL-27 can suppress inflammatory cytokines, such as TNFα, IL-6, and IL-17 [2,16,38,47]. Moreover, anti-inflammatory and anti-viral roles of IL-27 in cardiac muscle have been reported using mice with a cardiac-specific deletion of IL-27 receptor subunit gp130 [46]. We bring attention to this here as data presented in our study also demonstrate that both heterodimers for IL-27 are suppressed in cardiac tissue with obesity. Thus, it is also plausible that obesity-induced suppression of IL-27 in the heart may exacerbate the inflammatory response by inhibiting IL-27 suppression of TNFα and IL-6, two major players in vascular endothelial inflammation and atherosclerosis [15,25]. While this premise is presented hypothetically, it does support the notion that biologically relevant changes in IL-12 family gene expression could include those where genes are suppressed as well as those where genes are activated.
There is also evidence demonstrating that it is not inducibility in itself, but rather the magnitude of coordinated gene expression that dictates IL-12 family cytokine synthesis. Consistent with this premise, others have reported that alpha chains subunits (i.e., p35, p19, p28), are often constitutively expressed, whereas beta chain subunit (i.e., p40 and EBI-3) expression is highly regulated with external stimuli with synthesis of IL-12 family cytokines limited by the magnitude of constitutively expressed of alpha chain gene subunits [3,9,31,45]. In this regard, data presented in this report show that while p40 and EBI-3 were both induced with genetic obesity in skeletal muscle, p35 was not. However, we also demonstrate that constitutive expression of p35 was significantly elevated in skeletal muscle in lean mice relative to other tissues suggesting that the induction of p40 or EBI-3 may support the synthesis of IL-12 or IL-35, respectively.
In summary, this study provides the first comprehensive and systematic gene analysis of the IL-12 family cytokines under conditions of obesity in major insulin responsive tissues involved in metabolic homeostasis. While this study presents novel data regarding divergent regulation of gene expression in tissues and cell types not reported previously, the outcome should serve only as a platform for future investigations whereby cytokine secretion and functional activity can be evaluated for each individual IL-12 family member in a cell-, tissue-, and condition-specific manner.
Highlights.
IL-12 cytokines & receptors are regulated in insulin-responsive tissues by obesity.
IL-12 family genes divergently regulated with obesity in a tissue-specific manner.
IL-12 genes regulated in adipose tissue by developmental stage of obesity.
IL-12 genes regulated in adipose tissue by inflammatory stress of obesity.
IL-12 cytokines and receptors are regulated in adipocytes by inflammatory stress.
Acknowledgments
Funding: NIH R01-DK52968 (JMS), NIH R15-DK082799 (RFM)
The authors are grateful for superb technical assistance from Dr. Robin G. Hopkins (UNC Greensboro).
Footnotes
Disclosure: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51:212–223. doi: 10.1016/j.jhep.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker BJ, Park KW, Qin H, Ma X, Benveniste EN. IL-27 inhibits OSM-mediated TNF-alpha and iNOS gene expression in microglia. Glia. 2010;58:1082–1093. doi: 10.1002/glia.20989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beadling C, Slifka MK. Regulation of innate and adaptive immune responses by the related cytokines IL-12, IL-23, and IL-27. Arch Immunol Ther Exp (Warsz) 2006;54:15–24. doi: 10.1007/s00005-006-0002-6. [DOI] [PubMed] [Google Scholar]
- 4.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey AL, Bruce CR, Sacchetti M, Anderson MJ, Olsen DB, Saltin B, Hawley JA, Febbraio MA. Interleukin-6 and tumor necrosis factor-alpha are not increased in patients with Type 2 diabetes: evidence that plasma interleukin-6 is related to fat mass and not insulin responsiveness. Diabetologia. 2004;47:1029–1037. doi: 10.1007/s00125-004-1403-x. [DOI] [PubMed] [Google Scholar]
- 6.Caterson ID, Gill TP. Obesity: epidemiology and possible prevention. Best Pract Res Clin Endocrinol Metab. 2002;16:595–610. doi: 10.1053/beem.2002.0228. [DOI] [PubMed] [Google Scholar]
- 7.Collison LW, Vignali DA. Interleukin-35: odd one out or part of the family? Immunol Rev. 2008;226:248–262. doi: 10.1111/j.1600-065X.2008.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook KS, Min HY, Johnson D, Chaplinsky RJ, Flier JS, Hunt CR, Spiegelman BM. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 1987;237:402–405. doi: 10.1126/science.3299705. [DOI] [PubMed] [Google Scholar]
- 9.D’Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste M, Chan SH, Kobayashi M, Young D, Nickbarg E. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Djian P, Phillips M, Green H. The activation of specific gene transcription in the adipose conversion of 3T3 cells. J Cell Physiol. 1985;124:554–556. doi: 10.1002/jcp.1041240327. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson BS, Nam H, Hopkins RG, Morrison RF. Impact of reference gene selection for target gene normalization on experimental outcome using real-time qRT-PCR in adipocytes. PLoS One. 2010;5:e15208. doi: 10.1371/journal.pone.0015208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goriely S, Goldman M. Interleukin-12 family members and the balance between rejection and tolerance. Curr Opin Organ Transplant. 2008;13:4–9. doi: 10.1097/MOT.0b013e3282f406c4. [DOI] [PubMed] [Google Scholar]
- 13.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 14.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010;17:332–341. doi: 10.5551/jat.3939. [DOI] [PubMed] [Google Scholar]
- 16.Hamano S, Himeno K, Miyazaki Y, Ishii K, Yamanaka A, Takeda A, Zhang M, Hisaeda H, Mak TW, Yoshimura A, Yoshida H. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19:657–667. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- 17.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 18.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 19.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 21.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 22.Johnson PR, Spiegelman B, Rosen B, Turkenkopf I, Ree H, Greenwood MR. Reduced adipsin mRNA and circulating adipsin protein are modulated by adrenal steroids in obese Zucker rats. Am J Physiol. 1990;259:R184–R188. doi: 10.1152/ajpregu.1990.259.1.R184. [DOI] [PubMed] [Google Scholar]
- 23.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 24.Keller MP, Attie AD. Physiological insights gained from gene expression analysis in obesity and diabetes. Annu Rev Nutr. 2010;30:341–364. doi: 10.1146/annurev.nutr.012809.104747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res. 2008;79:360–376. doi: 10.1093/cvr/cvn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Maaser C, Egan LJ, Birkenbach MP, Eckmann L, Kagnoff MF. Expression of Epstein-Barr virus-induced gene 3 and other interleukin-12-related molecules by human intestinal epithelium. Immunology. 2004;112:437–445. doi: 10.1111/j.1365-2567.2004.01895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maury E, Noel L, Detry R, Brichard SM. In vitro hyperresponsiveness to tumor necrosis factor-alpha contributes to adipokine dysregulation in omental adipocytes of obese subjects. J Clin Endocrinol Metab. 2009;94:1393–1400. doi: 10.1210/jc.2008-2196. [DOI] [PubMed] [Google Scholar]
- 29.Merberg DM, Wolf SF, Clark SC. Sequence similarity between NKSF and the IL-6/G-CSF family. Immunol Today. 1992;13:77–78. doi: 10.1016/0167-5699(92)90140-3. [DOI] [PubMed] [Google Scholar]
- 30.Morrison RF, Farmer SR. Role of PPARgamma in regulating a cascade expression of cyclin-dependent kinase inhibitors, p18(INK4c) and p21(Waf1/Cip1), during adipogenesis. J Biol Chem. 1999;274:17088–17097. doi: 10.1074/jbc.274.24.17088. [DOI] [PubMed] [Google Scholar]
- 31.Murphy TL, Cleveland MG, Kulesza P, Magram J, Murphy KM. Regulation of interleukin 12 p40 expression through an NF-kappa B half-site. Mol Cell Biol. 1995;15:5258–5267. doi: 10.1128/mcb.15.10.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouwens DM, Sell H, Greulich S, Eckel J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J Cell Mol Med. 2010;14:2223–2234. doi: 10.1111/j.1582-4934.2010.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59:1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonobe Y, Yawata I, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A. Production of IL-27 and other IL-12 family cytokines by microglia and their subpopulations. Brain Res. 2005;1040:202–207. doi: 10.1016/j.brainres.2005.01.100. [DOI] [PubMed] [Google Scholar]
- 36.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 37.Surendar J, Mohan V, Rao MM, Babu S, Aravindhan V. Increased levels of both Th1 and Th2 cytokines in subjects with metabolic syndrome (CURES-103) Diabetes Technol Ther. 2011;13:477–482. doi: 10.1089/dia.2010.0178. [DOI] [PubMed] [Google Scholar]
- 38.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 39.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 40.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 41.Wegner M, Winiarska H, Bobkiewicz-Kozlowska T, Dworacka M. IL-12 serum levels in patients with type 2 diabetes treated with sulphonylureas. Cytokine. 2008;42:312–316. doi: 10.1016/j.cyto.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 42.Wen Y, Gu J, Li SL, Reddy MA, Natarajan R, Nadler JL. Elevated glucose and diabetes promote interleukin-12 cytokine gene expression in mouse macrophages. Endocrinology. 2006;147:2518–2525. doi: 10.1210/en.2005-0519. [DOI] [PubMed] [Google Scholar]
- 43.Winkler G, Dworak O, Salamon F, Salamon D, Speer G, Cseh K. Increased interleukin-12 plasma concentrations in both, insulin-dependent and non-insulin-dependent diabetes mellitus. Diabetologia. 1998;41:488. doi: 10.1007/s001250050935. [DOI] [PubMed] [Google Scholar]
- 44.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu M, Mizoguchi I, Morishima N, Chiba Y, Mizuguchi J, Yoshimoto T. Regulation of antitumor immune responses by the IL-12 family cytokines, IL-12, IL-23, and IL-27. Clin Dev Immunol. 2010 doi: 10.1155/2010/832454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yajima T, Yasukawa H, Jeon ES, Xiong D, Dorner A, Iwatate M, Nara M, Zhou H, Summers-Torres D, Hoshijima M, Chien KR, Yoshimura A, Knowlton KU. Innate defense mechanism against virus infection within the cardiac myocyte requiring gp130-STAT3 signaling. Circulation. 2006;114:2364–2373. doi: 10.1161/CIRCULATIONAHA.106.642454. [DOI] [PubMed] [Google Scholar]
- 47.Yamanaka A, Hamano S, Miyazaki Y, Ishii K, Takeda A, Mak TW, Himeno K, Yoshimura A, Yoshida H. Hyperproduction of proinflammatory cytokines by WSX-1-deficient NKT cells in concanavalin A-induced hepatitis. J Immunol. 2004;172:3590–3596. doi: 10.4049/jimmunol.172.6.3590. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida H, Miyazaki Y. Regulation of immune responses by interleukin-27. Immunol Rev. 2008;226:234–247. doi: 10.1111/j.1600-065X.2008.00710.x. [DOI] [PubMed] [Google Scholar]